Abstract
Background
Preoperative radiation therapy with 5-fluorouracil chemotherapy is a standard of care for cT3-4 rectal cancer. Studies incorporating additional cytotoxic agents demonstrate increased morbidity with little benefit. We evaluate a template that: (1) includes the benefits of preoperative radiation therapy on local response/control; (2) provides preoperative multidrug chemotherapy; and (3) avoids the morbidity of concurrent radiation therapy and multidrug chemotherapy.
Methods and Materials
Patients with cT3-4, any N, any M rectal cancer were eligible. Patients were confirmed to be candidates for pelvic surgery, provided response was sufficient. Preoperative treatment was 5 fractions radiation therapy (25 Gy to involved mesorectum, 20 Gy to elective nodes), followed by 4 cycles of FOLFOX [5-fluorouracil, oxaliplatin, leucovorin]. Extirpative surgery was performed 4 to 9 weeks after preoperative chemotherapy. Postoperative chemotherapy was at the discretion of the medical oncologist. The principal objectives were to achieve T stage downstaging (ypT < cT) and preoperative grade 3+ gastrointestinal morbidity equal to or better than that of historical controls.
Results
76 evaluable cases included 7 cT4 and 69 cT3; 59 (78%) cN+, and 7 cM1. Grade 3 preoperative GI morbidity occurred in 7 cases (9%) (no grade 4 or 5). Sphincter-preserving surgery was performed on 57 (75%) patients. At surgery, 53 patients (70%) had ypT0-2 residual disease, including 21 (28%) ypT0 and 19 (25%) ypT0N0 (complete response); 24 (32%) were ypN+. At 30 months, local control for all evaluable cases and freedom from disease for M0 evaluable cases were, respectively, 95% (95% confidence interval [CI]: 89%–100%) and 87% (95% CI: 76%–98%). Cases were subanalyzed by whether disease met requirements for the recently activated PROSPECT trial for intermediate-risk rectal cancer. Thirty-eight patients met PROSPECT eligibility and achieved 16 ypT0 (42%), 15 ypT0N0 (39%), and 33 ypT0-2 (87%).
Conclusion
This regimen achieved response and morbidity rates that compare favorably with those of conventionally fractionated radiation therapy and concurrent chemotherapy.
Introduction
For clinical T3 or T4 rectal cancer, preoperative radiation therapy has been established as a standard of care. Randomized trials have established that preoperative radiation therapy provides significantly better locoregional control than surgery alone or surgery followed by postoperative radiation therapy (1–5).
In the late 1980s and early 1990s, it was also established that adjunctive chemotherapy significantly improved disease-free survival for rectal cancer. At that time, chemotherapy consisted of 5-fluorouracil (5FU) either alone or with a modifier. Radiation therapy has remained a component of treatment for patients with stage II or III rectal cancer, with phase 3 trials confirming that locoregional control is improved by adding radiation therapy to surgery and chemotherapy (6–9).
Cooperative group and single-institution studies have confirmed that the addition of concurrent 5FU (or a similar fluoropyrimidine) to conventionally fractionated preoperative radiation therapy significantly improves response rate (~10% to 15% by several different measures) and, to a lesser extent (~5%), locoregional control over preoperative radiation therapy alone (3, 4, 10, 11). The concurrent 5FU increased acute nonhematologic morbidity; however, it remained at an acceptable level of ~10% to 15%.
A frequently used sequence for clinical stage II or III rectal cancer is 45 to 50 Gy in 25 to 28 fractions (with concurrent 5FU) followed after ~4 to 8 weeks by extirpative surgery and then adjuvant chemotherapy. Postoperative chemotherapy is delayed by over 3 months in this sequence. With such an approach, locoregional control is over 90%, and freedom from disease relapse is ~60% to 70% (3–5, 10, 11), although subgroups with good and poor prognosis can be identified (10, 11).
Inasmuch as the greatest risk is the development of extrapelvic metastases, strategies to incorporate more effective systemic chemotherapy have been evaluated in several studies (12–14), adding either concurrent oxaliplatin (15, 16) or irinotecan (16–18) to preoperative 5FU/radiation therapy. However, the addition of these agents to standard 5FU/radiation therapy was associated with similar response and disease control rates and with a >30% higher incidence of grade 3 to 4 nonhematologic toxicities, twice the incidence for conventional 5FU/radiation therapy.
The potential downside to concurrent radiation therapy and multidrug chemotherapy is that the simultaneous chemotherapy may unduly enhance radiation injury to normal tissue. If toxicity is excessive, it might be better to give the modalities sequentially instead of concurrently.
With these concerns in mind, we initiated an institutional review board-approved, phase 2 study evaluating a preoperative regimen satisfying 3 constraints:
Radiation therapy delivered at doses and with sufficient time before surgery to elicit a tumor response.
Administer standard multidrug chemotherapy between the radiation therapy and surgery, but with sufficient time between modalities to avoid overlapping toxicities.
Preserve the interval between start of preoperative therapy and surgery at approximately 3 to 4 months.
The sequence of modalities in this trial was as follows:
Short-course pelvic radiation therapy, 5 fractions over 1 week.
Four cycles of every-other-week FOLFOX [5-fluorouracil, oxaliplatin, leucovorin] chemotherapy, beginning 2 weeks after completion of radiation therapy (details below).
Extirpative surgery 4 to 9 weeks after completion of chemotherapy.
Postoperative chemotherapy at the discretion of the treating medical oncologist. An additional 6 to 8 cycles of FOLFOX was recommended.
The primary objectives were as follows:
Demonstrate that this regimen will elicit a rate of T stage downstaging comparable to or better than seen with prolonged-course preoperative radiation therapy and chemotherapy. T stage downstaging is defined as clinical pretreatment American Joint Committee on Cancer (19) T stage (cT) being greater than pathologic T stage at surgery (ypT).
Demonstrate that this regimen will lead to preoperative gastrointestinal (GI) morbidity comparable to or better than seen with prolonged-course preoperative radiation therapy and chemotherapy.
Five-fraction radiation therapy has a long track record as a preoperative regimen, but usually with surgery immediately after radiation therapy (1, 2, 20–23). There have, however, been reports indicating that if surgery is delayed, tumors will respond about as much as they would to conventionally fractionated radiation therapy (24, 25). More recently, trials in the Netherlands (26) and Poland (27) have piloted regimens similar to ours.
Our selected interval between short-course pelvic radiation therapy and multidrug chemotherapy was informed by prior experience with a 5-fraction course of external beam radiation therapy followed 6–10 weeks later by endocavitary radiation therapy (28, 29). Typically, any acute proctitis would peak about 1 week after the external beam radiation therapy and resolve within a few days.
For these reasons, we decided that the planned regimen would be likely to achieve response rates comparable with those of conventionally fractionated radiation therapy with concurrent 5FU. The trial included mandatory interim evaluations to make certain that adequate response rates were being achieved. Response served not just as a short-term surrogate for local control (LC) (30) but also as an important endpoint in itself, because we wished to apply this regimen to all clinical T3 or T4 tumors, not just the readily resectable ones.
Methods and Materials
Eligibility and assessments
All protocol-mandated treatment was delivered at the Siteman Cancer Center of the Washington University School of Medicine. Because postoperative chemotherapy was neither mandated by protocol nor germane to the primary objectives, patients from outside facilities were allowed to receive postoperative chemotherapy with their outside medical oncologists, with suggestions from the Washington University oncologist. Eligible patients had biopsy-proven cT3 or cT4 rectal adenocarcinoma, were evaluated by a surgeon, and (assuming adequate response to preoperative treatment) were deemed candidates for extirpative surgery and medically fit for chemotherapy. Children, patients with other malignancies, and patients unable to give consent or tolerate chemotherapy or surgery were excluded. Patients with distant metastatic disease were eligible, provided they had an estimated survival of at least a year and were pelvic surgery candidates. Clinical evidence of T stage could be based on physical examination (lesion tethered to palpation), transrectal ultrasound (TRUS), magnetic resonance imaging (MRI), or computerized tomography (CT). Although TRUS or MRI was strongly recommended, assessment of clinical T stage on the basis of physical findings was allowed to avoid selecting a cohort with less advanced disease; very advanced lesions often cannot be traversed by the rigid ultrasound probe, and T stage is often difficult to assess by MRI for distally located tumors.
Pretreatment staging procedures included physical examination, CT or MRI of the abdomen and pelvis, chest imaging (chest x-ray or CT or 18F-fluorodeoxyglucose positron emission tomography). Complete colonoscopy or barium enema was required when feasible. For patients with partially obstructing tumors, evaluation of the large bowel proximal to the tumor could be deferred until just before surgery, provided there was no suggestion of a second colonic neoplasm in the other imaging studies. After treatment, follow-up was with physical examination and laboratory studies every 3 months for the first year and every 6 months thereafter. CT scan of the trunk was performed at least annually.
Treatment
The radiation therapy regimen consisted of 5 fractions delivered over 5 to 7 days with intensity modulated radiation therapy using 6- to 18-MV photons. All treatments were guided by daily orthogonal kilovoltage images and cone beam CT scans. The planning target volume (PTV) margin was 0.5 cm. There were 2 nested target volumes receiving a total of 20 and 25 Gy in the same 5 fractions. The clinical target volume 20 Gy (CTV20Gy) was an elective nodal target volume defined by the Radiation Therapy Oncology Group (RTOG) guidelines (31). CTV25Gy included the rectum and the mesorectal compartment over the length of involved rectum with a 1-cm margin cephalad and caudad. If CTV25Gy extended into the distal rectum, its lateral borders included the full thickness of the sphincteric musculature. Any clinically positive nodal regions within the pelvis were also included within CTV25Gy, including the mesorectum with involved perirectal nodes. If involved perirectal nodes continued more than 1 cm cephalad or caudad to the primary tumor, then the mesorectal coverage was appropriately extended. The 2-tiered dose scheme was chosen in an effort to limit the risk of radiation injury, given that our patients would also be receiving aggressive preoperative chemotherapy. The elective nodal dose was based on prior reports of 20 Gy in 5 fractions as an immediate preoperative regimen for patients with readily resectable lesions (20, 28, 29). The majority of these patients had ypT3 or ypN+ disease and received no further radiation therapy, but still achieved excellent local and overall disease control. Because the present study included patients with a need for downstaging, we opted to treat involved mesorectum and nodes to the higher dose of 25 Gy, which has been used in Northern European trials (1, 2, 21–27).
It was required that at least 95% of the PTV be covered by 95% of the prescription dose. Maximum allowed dose was 115% of prescription. Examples of PTV20Gy and PTV25Gy are shown in Figure 1.
Fig. 1.
Planning target volume (PTV) 20 Gy (light blue) and PTV 25 Gy (dark blue) with isodose distributions for a typical case.
Normal tissue constraints were that small bowel be kept below an absolute maximum of 25 Gy and that volume at or above 20 Gy (V20Gy) be kept as small as possible for small bowel (preferably <50 cc), uninvolved large bowel (defined as large bowel lying outside CTV20Gy), and femoral heads. Bowel was contoured tightly, as described in the RTOG normal tissue guidelines (32).
Standard chemotherapy with FOLFOX was initiated 9 to 12 working days after the completion of radiation therapy. Chemotherapy doses were as follows: oxaliplatin 85 mg/m2 intravenous (IV) with leucovorin 400 mg/m2 IV over 2 hours, 5FU IV bolus 400 mg/m2 followed by 5FU continuous infusion 2400 mg/m2 over 46 hours. One cycle was every 2 weeks for a total of 4 cycles. Standard dose modifications for FOLFOX were used. To avoid unlimited delays, the protocol mandated that if toxicity-mediated delays exceeded 3 weeks, the surgeon, the medical oncologist, and the radiation oncologist were required to reappraise the case and decide whether it would be best to proceed to surgery without further delay or to complete the full course of preoperative chemotherapy.
Statistics
The overall accrual objective was set at 80 cases, provided that at most 10% of cases were inevaluable. Assuming a target T stage downstaging rate of 65%, 72 evaluable cases would allow a 90% power at a significance level of 0.1 using a binomial noninferiority test with a noninferiority margin of 15% (33). The protocol required interim analyses after 25 and 50 evaluable cases. Stopping criteria were: (1) if the observed rate of T stage downstaging was unlikely to be consistent with a true rate of ≥50%; or (2) if the rate of grade 3 or higher GI toxicity was incompatible with a true rate of ≤20%. For protocol purposes, episodes of stomatitis, gastritis, and hepatopathy, although tracked, did not contribute to the second stopping criterion because they could not be attributed to pelvic radiation therapy.
Results
Patient characteristics
Between November 2009 and April 2012, 181 patients underwent radiation therapy for rectal cancer. Eighty met protocol eligibility and consented to be enrolled. Of these, 1 patient was inevaluable for both primary objectives because the patient withdrew consent after completing radiation therapy, refused chemotherapy, and underwent R0 resection of a ypT3N0 lesion 7 weeks after radiation therapy with no acute morbidity. Three other patients were inevaluable for response at surgery but are included in the review of preoperative treatment delivery and morbidity: 1 experienced a new medical condition, which rendered him medically unfit for extirpative surgery; 1 patient with cM1 disease experienced progression of extrapelvic disease (with radiographs showing pelvic response) and proceeded to second-line chemotherapy; and 1 patient (also with pelvic response) experienced complications of sepsis after 4 cycles of FOLFOX and never underwent surgery.
The characteristics of the 76 fully evaluable patients are presented in Table 1; 69 (91%) of the patients had clinical T3 tumors, and the remaining 7 (9%) patients had cT4; 59 (78%) tumors were cN+ and 7 (9%) were cM1. TRUS was performed on 45 patients, pelvic MRI on 37 patients, and neither on 9 patients.
Table 1.
Characteristics of 76 evaluable patients
| Characteristics | Total = 76 cases |
|---|---|
| Age | 56.4 ± 1.3 years |
| Sex | |
| Male | 54 (71%) |
| Female | 22 (29%) |
| Clinical T stage | |
| cT3 | 69 (91%) |
| cT4 | 7 (9%) |
| Clinical N stage | |
| cN0 | 17 (22%) |
| cN+ | 59 (78%) |
| Clinical M stage | |
| cM0 | 69 (91%) |
| cM1 | 7 (9%) |
| Staging pelvic disease includes | |
| Transrectal ultrasound (TRUS) | 45 |
| Magnetic resonance imaging (MRI) | 37 |
| Both TRUS and MRI | 15 |
| Neither (CT and clinical findings only) | 9 |
| PROSPECT eligible?* | |
| Y | 38 (50%) |
| N | 38 (50%) |
| Clinical score† | |
| CS0 | 18 (24%) |
| CS1 | 48 (63%) |
| CS2 | 10 (13%) |
Abbreviations: CT = computed tomography; 5FU = 5-fluorouracil; RT = radiation therapy.
Clinical score (CS) based on 4 adverse pretreatment findings: site <5 cm from verge, tethered or fixed to palpation, circumferential, near obstructing. CS0: 0 adverse factors; CS1: 1or 2 adverse factors; CS2: 3 or 4 adverse factors.
PROSPECT eligible: disease extent eligible for recently activated (N1048) trial (comparing preoperative RT+5FU vs FOLFOX): location at ≥5 cm, no disease ≤3 mm from mesorectal fascia, and cT <cT4.
Table 1 also presents the distribution of cases by 2 potential prognostic groupings. Half (38 of 76) of evaluable patients had pelvic disease that would have been eligible for the recently activated PROSPECT trial (N1048): tumors at least 5 cm above the verge, cT Stage < cT4, nodal stage < cN2, and no disease within 3 mm of the mesorectal fascia. We also characterized local presentations by a clinical scoring system previously reported (11). The clinical score (CS) is a 3-level scheme (CS0–CS2) based on physical findings and summarized in Table 1. The distribution of clinical scores in the present study is slightly more advanced than historical controls of 57% CS1 and 13% CS2 (11). It should also be noted that the 9 patients who did not undergo TRUS or MRI had more advanced lesions than the overall cohort with 7 (78%) PROSPECT ineligible, 4 (44%) CS2 and no CS0.
Treatment delivery
All patients completed radiation therapy without interruption. All dose reductions or delays of preoperative chemotherapy were protocol mandated for morbidity and are reviewed in Table 2. All but 4 of the 76 evaluable patients received 4 cycles of chemotherapy preoperatively. One patient was still deemed unresectable after 4 cycles of FOLFOX; the treating physicians and the principal investigator opted for an additional 4 cycles of FOLFOX plus bevacizumab, after which the patient underwent a R1 resection of ypT4 ypN0 residual disease. Three patients (all downstaged) received 2 cycles of preoperative chemotherapy because of chemotherapy toxicity.
Table 2.
Preoperative chemotherapy delivery*
| Preoperative chemotherapy timing and delivery | Target interval (weeks) |
Achieved interval (weeks) average ± SD |
Number of cases, % |
|---|---|---|---|
| Onset of chemotherapy† | |||
| Interval from last radiation treatment to day 1, cycle 1 of chemotherapy | 1.6–2.6 | 2.3 ± 0.6 | 2 (2.5%)‡ |
| Number of cases delayed ≥2 weeks beyond target‡ | |||
| Delivery of chemotherapy† | |||
| Duration: Interval from day 1, cycle 1 to day 1, last cycle chemotherapy | 6 | 6.4 ± 1.2 | 9 (12%)§ |
| Number of cases delayed ≥2 weeks beyond target duration | |||
| Dose: Number of cases with chemotherapy dose reduction | 19 (24%) | ||
| Number of cases with dose reduction and/or any delay (1 week delay alone allowed for first instance of grade 3 neutropenia) | 35 (44%) | ||
| Timing of surgery‖ | |||
| Interval from day 1, last cycle chemotherapy to surgery | 4–9 | 7.7 ± 2.8 | 4 (5%)§ |
| Number of cases delayed ≥2 weeks beyond target from last cycle chemotherapy to surgery | |||
| Interval from day 1 radiation therapy to surgery | 13–18 | 17.3 ± 2.9 | 8 (11%)§ |
| Number of cases delayed ≥2 weeks beyond target from day 1 radiation therapy to surgery |
All dose reductions or delays were caused by morbidity and were required by protocol.
Number of evaluable patients for chemotherapy delivery = 79 (see text).
Neither instance of delayed chemotherapy was caused by radiation therapy morbidity. One case was delayed by port site infection. Another case was delayed for the patient to recover after coronary stent placement (asymptomatic cardiac disease, detected on stress test).
Includes 1 patient who completed 4 cycles of full-dose FOLFOX (5-fluorouracil, oxaliplatin, leucovorin) on schedule, was found to still have unresectable disease, and therefore received additional chemotherapy before undergoing surgery (see text).
Number of evaluable patients for surgery = 76 (see text).
The 76 evaluable patients underwent extirpative surgery. Sphincter-preserving surgery was performed on 57 (75%) patients (all but 1 located ≥5 cm from the verge), the remainder (all but 1 at < 5 cm) underwent abdominoperiteal resection. Four (5%) of the patients who underwent resection had microscopically positive or close (<1-mm) margins (R1). The frequency of R1 resections is comparable to our historical controls of 7% (11). Three of the R1 resection patients had CS2. All of the patients with R1 resections would have been PROSPECT ineligible. Although it was not mandatory, all but 2 of the evaluable patients received post-operative chemotherapy, and of these, all but 9 received additional FOLFOX.
Response
Table 3 gives the details of pathologic findings. There were a total of 21 (28%) ypT0 (including 19 [25%] ypT0N0—complete response), and overall, 53 (70%) of the patients were ypT0-2. Including 1 cT4 to ypT3 case, the overall T stage downstaging rate was 71%. Twenty-four (32%) of the patients had residual nodal involvement (ypN+) compared with 59 (78%) initially cN+. It should be noted that the 67 patients who underwent MRI and/or TRUS had a T stage response of 51 (76%) ypT0-2 and 20 (30%) ypT0. Table 3 also shows that lower clinical score and PROSPECT eligibility both correlated with response. The 38 PROSPECT eligible patients were particularly responsive, with 16 (42%) ypT0, 15 (39%) ypT0N0, and 33 (87%) ypT0-2 (vs PROSPECT ineligible: P = .009, .004, and .002, respectively).
Table 3.
Comparison of pretreatment clinical T stage (cT) with pathologic stage at extirpative surgery (ypT) for 76 evaluable cases.
| Group\Number | n | ypT0 | ypT0-2 |
|---|---|---|---|
| All | 76 | 21 (28%) | 53 (70%) |
| cT stage | |||
| cT3 | 69 | 20 (29%) | 49 (71%) |
| cT4 | 7 | 1 (14%) P=NS | 4 (57%) P=NS |
| Interval from day 1 radiation therapy to surgery | |||
| ≤17 weeks | 35 | 10 (29%) | 26 (74%) |
| >17 weeks | 41 | 11 (27%) P=NS | 27 (66%) P=NS |
| PROSPECT eligible?* | |||
| Y | 38 | 16 (42%) | 33 (87%) |
| N | 38 | 5 (13%) P=.009 | 20 (53%) P=.002 |
| Clinical score‡ | |||
| 0 | 18 | 7 (39%) | 16 (89%) |
| 1 | 48 | 13 (27%) | 35 (73%) |
| 2 | 10 | 1 (10%) P=NS | 2 (20%) P=.0008 CS2 vs CS0,1 |
Statistical significance estimated by Fisher exact test.
PROSPECT eligible: disease extent eligible for recently activated (N1048) trial (comparing preoperative RT+5FU vs FOLFOX): location at ≥5 cm, no disease ≤3 mm from mesorectal fascia, and cT <cT4.
Clinical score (CS) based on 4 adverse pretreatment findings: site <5 cm from verge, tethered or fixed to palpation, circumferential, near obstructing. CS0: 0 adverse factors; CS1: 1or 2 adverse factors; CS2: 3 or 4 adverse factors.
Outcome
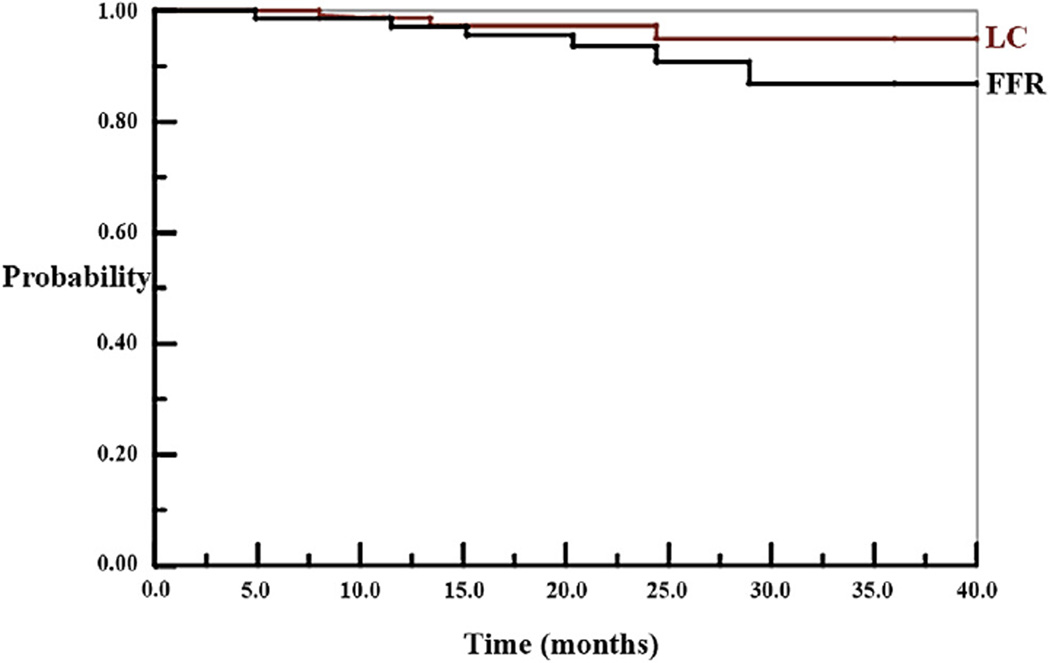
To date, the evaluable patients have been followed up for an average of 26 ± 8 months. There have been 3 deaths (2 resulting from disease and 1 from a late perforation), 3 local failures (2 occurring in cM1 patients with R1 resections), and (among M0 patients) 5 extrapelvic failures. Figure 2 shows Kaplan-Meier (19) projections of LC (for the 76 evaluable patients) and freedom from disease relapse (FFR) (for 69 cM0 evaluable patients). At last event, LC is 95% (95% confidence interval [CI]: 89%–100%) and FFR is 87% (95% CI: 76%–98%) (Systat version 8.0, SPSS Inc, Chicago).
Fig. 2.
Local control (LC) and freedom from disease relapse (FFR) by Kaplan-Meier method. LC is for the 76 evaluable cases; FFR for the 69 cM0 evaluable cases. Probabilities after last event are LC 95% (95% CI: 89%–100%) and FFR 87% (95% CI: 76%–98%).
Tolerance
The preoperative morbidity among the 79 patients who received preoperative treatment according to the protocol is summarized in Table 4. Overall, 16 (20%) experienced nonhematologic grade ≥3 preoperative morbidity (including 2 with grade 4). These included 7 (9%) with grade 3 GI morbidity with no higher grade. Preoperative grade ≥3 hematologic morbidity occurred in 21 (27%) patients, including 10 (13%) with grade 4. After the completion of all treatment (including postoperative chemotherapy) there have been 21 patients with late grade ≥3 morbidities, including 1 fatality: perforation with peritonitis 3 months after the completion of chemotherapy and ileostomy takedown. Eight of the remaining late events are unrelated to radiation therapy (peripheral neuropathy, renal failure, delayed anterior abdominal wound healing involving unirradiated tissue) and 13 are potentially related (small bowel obstruction managed conservatively, abscess, pain, urinary stricture, symptomatic bone injury).
Table 4.
Incidence of preoperative morbidities among 79 patients who received preoperative treatment per protocol (see text)*
| Grade 3+ preoperative morbidity/number (of 79 cases) |
Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|
| All nonhematologic (including GI) | 14 (18%)† | 2 (3%) | 0 |
| Gastrointestinal | 7 (9%)† | 0 | 0 |
| Hematologic | 11 (14%) | 10 (13%) | 0 |
Abbreviation: GI = gastrointestinal.
The number of cases (19) with chemotherapy dose reductions in Table 2 is less than the total incidence of grade 3 or 4 morbidity because the first instance of grade 3 neutropenia could be managed with a 1 week delay (per protocol) and because some instances developed after the last cycle of preoperative chemotherapy.
Includes 1 grade 3 GI (dehydration) after radiation therapy (chemotherapy not delayed) and 1 grade 3 radiation dermatitis (chemotherapy delayed 1 week).
Discussion
This trial sought to evaluate a template in which patients receive both multidrug chemotherapy and preoperative radiation therapy with sufficient time for tumor response/downstaging before surgery. The use of short-course radiation therapy before chemotherapy was intended to avoid delaying surgery substantially beyond when it occurs with preoperative long-course radiation therapy and concurrent single-drug chemotherapy.
The long-term hope is that, as Figure 2 suggests, this regimen will achieve excellent FFR (by initiating multidrug chemotherapy early in the course of treatment) while retaining the level of LC of long-course radiation therapy with 5FU. The immediate objectives were to demonstrate acceptable response rates and toxicity. In this regard, the overall response, by several measures, was good: 28% ypT0, 70% ypT0-2, 71% T stage downstaging, 25% ypT0N0. The response rate seen in our trial is comparable with that reported by Garcia-Aguilar et al (34) in the third arm of a prospective trial evaluating prolonged-course radiation therapy followed by 0, 2, and 4 cycles of FOLFOX. Both response and preoperative GI morbiditity (9% grade 3) compare well with historical controls (3–5, 10, 11, 15–19). GI morbidity was substantially better than when 2 cytotoxic drugs are attempted concurrently with radiation therapy (15–19). Groups in the Netherlands and Poland have independently developed similar regimens, with results comparable to ours (26, 27).
We note that patients who would have been eligible for the recently activated PROSPECT trial had an excellent response: 42% ypT0, 87% ypT0-2, 39% ypT0N0, 100% R0 resections. The PROSPECT trial (N1048) is being conducted in North America and compares preoperative FOLFOX with no radiation therapy (unless tumor progresses locally) versus conventionally fractionated preoperative radiation therapy with concurrent 5FU (with FOLFOX postoperatively). A potential future conundrum is what to do if the no radiation therapy arm of this trial shows superior extrapelvic control but inferior LC. If this is demonstrated and the response rate is less than that of the present study, then a regimen like ours would be a potential arm of a successor study.
For the more advanced PROSPECT-ineligible cases, there is another recently activated international trial. The RAPIDO (Rectal cancer and pre-operative Induction therapy followed by dedicated operation) trial is being conducted in Europe and compares the Dutch regimen—25 Gy in 5 fractions followed by capecitabine/oxaliplatin (26)—with conventionally fractionated radiation therapy with capecitabine. The RAPIDO trial’s eligibility complements the PROSPECT trial: patients must have cT4 disease or mesorectal fascia involvement or vascular involvement or N2 disease. There are subtle differences in radiation therapy target volume definitions from our study, including our use of a lower-dose elective nodal volume (PTV20Gy) and our routine coverage of the internal iliac region (to at least 20 Gy). These small differences may be worth revisiting, depending on morbidity and disease control in the RAPIDO trial.
Patients with CS2 lesions achieved significantly inferior T stage downstaging (Table 3) and had 3 of 4 R1 resections. They might be candidates for a clinical trial evaluating intensified preoperative treatment.
Summary.
A regimen of 5 fractions of pelvic radiation therapy followed by 4 cycles of FOLFOX [5-fluorouracil, oxaliplatin, leucovorin] was evaluated as a preoperative regimen for cT3-4 rectal cancer in a prospective phase 2 trial. Response rates, morbidity, and disease control compare favorably with those of historical controls.
Acknowledgments
Supported by the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Conflict of interest: none.
References
- 1.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 3.Gerard J, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 4.Bosset J, Collett L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, et al. Preoperative vs. adjuvant chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 7.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Wolmark N, Rockette H, et al. Postoperative radiation therapy for rectal cancer: Results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Crane CH, Phan T, Skibber J, et al. The addition of continuous infusion 5-FU to preoperative radiotherapy increases response, sphincter preservation, and overall survival in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2003;57:84–89. doi: 10.1016/s0360-3016(03)00532-7. [DOI] [PubMed] [Google Scholar]
- 11.Myerson RJ, Singh A, Birnbaum E, et al. Pretreatment clinical findings predict outcome for patients receiving preoperative radiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2001;50:665–674. doi: 10.1016/s0360-3016(01)01476-6. [DOI] [PubMed] [Google Scholar]
- 12.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Gerard JP, Chapet O, Nemoz C, et al. Advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: The Lyon R0–04 phase II trial. J Clin Oncol. 2003;21:1119–1124. doi: 10.1200/JCO.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Wong SJ, Winter K, Meropol NJ, et al. Radiation Therapy Oncology Group 0247: A randomized phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1367–1375. doi: 10.1016/j.ijrobp.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan BR, Thomas F, Myerson RJ, et al. Thymidylate Synthase Genotype-Directed Neoadjuvant Chemoradiation for Patients With Rectal Adenocarcinoma. J Clin Oncol. 2011;29:875–883. doi: 10.1200/JCO.2010.32.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta VK, Cho C, Ford JM, et al. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int J Radiat Oncol Biol Phys. 2003;55:132–137. doi: 10.1016/s0360-3016(02)03863-4. [DOI] [PubMed] [Google Scholar]
- 19.Cancer Staging Manual. 7th ed. New York: Springer; 2010. American Joint Committee on Cancer. [Google Scholar]
- 20.Myerson RJ, Genovesi D, Lockett M, et al. Five fractions of preoperative radiotherapy for selected cases of rectal carcinoma: Long-term tumor control and tolerance to treatment. Int J Radiat Oncol Biol Phys. 1999;43:537–543. doi: 10.1016/s0360-3016(98)00435-0. [DOI] [PubMed] [Google Scholar]
- 21.Pahlman L, Glimelius B. Pre or postoperative radiotherapy in recetal and rectosigmoid carcinoma: Report from a randomized multicenter trial. Ann Surg. 1990;211:187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bujko K, Nowacki MP, Nasierowska-Guttmejerb A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24. doi: 10.1016/j.radonc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 24.Radua C, Berglunda A, Pahlman L, et al. Short-course preoperative radiotherapy with delayed surgery in rectal cancer: A retrospective study. Radiother Oncol. 2008;87:343–349. doi: 10.1016/j.radonc.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Hatfield P, Hingorani M, Radhakrishna G, et al. Short-course radiotherapy, with elective delay before surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol. 2009;92:210–214. doi: 10.1016/j.radonc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.van Dijk TH, Tamas J, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24:1762–1769. doi: 10.1093/annonc/mdt124. [DOI] [PubMed] [Google Scholar]
- 27.Bujko K, Nasierowska-Guttmejer A, Wyrwicz L, et al. Neoadjuvant treatment for unresectable rectal cancer: An interim analysis of a multicentre randomized study. Radiother Oncol. 2013;107:171–177. doi: 10.1016/j.radonc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: Results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys. 2001;51:363–370. doi: 10.1016/s0360-3016(01)01677-7. [DOI] [PubMed] [Google Scholar]
- 29.Myerson RJ, Hunt SR. Conservative alternatives to extirpative surgery for rectal cancer. Clinical Oncology (R Coll Radiol) 2007;19:682–686. doi: 10.1016/j.clon.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 31.Myerson RJ, MC Garafalo MC, ElNaqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: A Radiation Therapy Oncology Group consensus panel contouring atlas. Int J Rad Onc Biol Phys. 2009;74:824–831. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gay HA, Barthold J, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson PK. Conditional power calculations as an aid in the decision whether to continue a clinical trial. Control Clin Trials. 1987;8:67–74. doi: 10.1016/0197-2456(87)90027-4. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Aguilar J, Marcet J, Coutsoftides T, et al. Impact of neo-adjuvant chemotherapy following chemoradiation on tumor response, adverse events, and surgical complications in patients with advanced rectal cancer treated with TME. Ann Surg. 2011;254:97–102. [Google Scholar]