Abstract
Background
Understanding the mechanisms underlying deep tissue pain in the postoperative period is critical to improve therapies. Using the in vitro plantar flexor digitorum brevis (FDB) muscle-nerve preparation and patch-clamp recordings from cultured dorsal root ganglia (DRG) neurons innervating incised and unincised muscle, we investigated responses to various pH changes.
Methods
Incision including the plantar FDB muscle or sham operation was made in the rat hindpaw. On postoperative day one, in vitro single fiber recording was undertaken. Based on previous studies, we recorded from at least 40 fibers per group. Also Di-I labeled DRG innervating muscle from rats undergoing incision and a sham operation were cultured and tested for acid responses using whole cell patch-clamp recordings.
Results
The prevalence of responsive group IV afferents to lactic acid pH 6.5 in the incision group (15 of 67, 22.3%) was greater than that in the control group (2 of 35, 5.7%, p=0.022). In DRG neurons innervating muscle, incision increased mean current amplitudes of acid-evoked currents; the acid-sensing ion channel blocker, amiloride 300 μM, inhibited more than 75% of the acid-evoked current, whereas the transient receptor vanilloid receptor 1 blocker (AMG9810 1 μM) did not cause significant inhibition.
Conclusion
Our experiments demonstrated that incision increases the responses of FDB muscle afferent fibers to weak acid solutions, and increased acid-evoked currents in DRG innervating muscle. Our data suggest that upregulation of acid-sensing ion channels might underlie this increased chemosensitivity caused by surgery.
Introduction
Pain mechanism associated with deep tissue injury is of increasing significance for evaluating clinical pathologic pain conditions and improving its treatment. Deep tissues, which include muscle, joint, bone, and their afferents have unique responses to various stimuli and distinctively respond to injury. However, the mechanism for deep tissue pain still remains incompletely understood despite its high clinical significance.
We previously generated an in vivo rat model of post-operative pain 1 and demonstrated that incision in skin plus fascia and muscle tissue caused much stronger guarding pain, and a greater prevalence and greater rate of spontaneous discharges from nociceptors and dorsal horn neurons, compared to skin incision alone 2-4. The mechanism for pain caused by muscle incision is important for advancing perioperative pain control.
Previously we examined the mechanisms underlying deep tissue pain by measuring tissue pH and lactate after incision. The maximal decrease in pH (pH ~ 6.8) 5, and the maximal increase in lactate concentration (6 mM) 6 correlated with a peak in pain behaviors at 1 to 2 days after incision. Additionally, we used an in vitro plantar flexor digitorum brevis (FDB) muscle-nerve preparation, and found that incision increased heat sensitivity, mechanosensitivity, and chemosensitivity (lactic acid, pH 6.0) of muscle afferent fibers7.
In the present study, we used an in vitro FDB muscle-nerve preparation to investigate the properties of mechanosensitive group III and group IV afferents of incised and unincised muscle, and explored response of the muscle to more modest decreases in pH (pH 6.5 and 7.0) and an increase in pH to 7.6. We emphasized responses to pH 6.5 and 7.0 because a small number of fibers were tested previously7. We also examined responses of dorsal root ganglia (DRG) neurons that innervated normal and incised muscle to various pH changes with patch clamp recordings. The effects of antagonists to acid sensing ion channels (ASIC) and transient receptor vanilloid receptor 1 (TRPV1) were tested against these acid responses in cultured DRG neurons innervating muscle.
Materials and Methods
General
All experiments were reviewed and approved by The University of Iowa Animal Care and Use Committee (Iowa City, Iowa). One-hundred and nine adult male Sprague-Dawley rats (250–300 g, Harlan, Indianapolis, IN) were used. Rats were housed under a 12-h light-dark schedule. Food and water were available ad libitum.
Plantar incision
Hindpaw plantar incision was performed as previously described1. Briefly, rats were anesthetized with 1.5-2% isoflurane delivered via a nose cone. The right hindpaw was prepared in a sterile manner. A 10 mm longitudinal incision was made through the skin, underlying fascia and the FDB muscle with a #11 surgical blade. Blunt curved forceps were then inserted through the incision into the FDB to divide and retract the muscle. The skin was closed with three subcutaneous mattress sutures with 6-0 nylon on a P-1 needle and covered with topical antibiotic ointment. The control sham operated rats underwent anesthesia and sterile preparation and no incision. Primary afferent fiber recordings were performed 1 day after incision or sham control incision.
Primary afferent fiber recordings
Preparation
The rat FDB in vitro muscle-nerve preparation has been described7. In brief, rats were anesthetized with isoflurane and the tibial nerve from the malleolus to the mid-thigh was dissected free. Then rats were euthanized with carbon dioxide and the FDB muscle was isolated from the skin and other connective tissue. It was carefully dissected at its tendons proximally at the heel and distally at the metatarsal phalangeal joints with the medial plantar nerve, a branch of the tibial nerve. The lengths from the edge of the proximal tendon to the distal three tendons of the muscle were measured before excision. Throughout the surgery, the tissue was saturated with a synthetic interstitial fluid (SIF; in mM: 107 NaCl, 26.2 NaHCO3, 9.64 sodium gluconate, 5.5 glucose, 7.6 sucrose, 3.48 KCl, 1.67 NaH2PO4, 1.53 CaCl2, 0.69 MgSO4), which had been oxygenated with a mixture of 95% O2 and 5% CO2. The dissection was usually accomplished in approximately 40 minutes.
The isolated preparation was immediately placed in an organ bath, which was continuously superfused with the oxygenated SIF solution (Fig. 1). The temperature of the bath solution was maintained at 36 ± 1.0 °C. The FDB muscle was positioned with its plantar side down and pinned at the proximal tendon and three distal tendons approximating its rest length. The tibial nerve was drawn through a small hole to the recording chamber, which was filled with paraffin oil. The nerve was placed on a mirror, desheathed, and filaments were repeatedly teased and placed on a platinum electrode until single-unit activity could be recorded. Action potentials were amplified (DAM50, Harvard Apparatus, Holliston, MA), filtered, and displayed using standard techniques. Amplified signals were led to an oscilloscope and an audio monitor, and recorded on a personal computer via a data acquisition system (spike2/CED1401 program; Cambridge Electronic Design, Cambridge, United Kingdom).
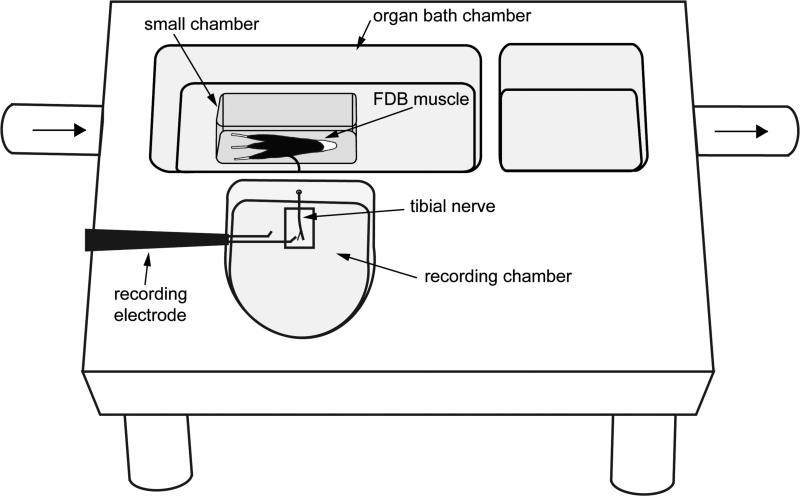
Figure 1.
Schematic of the flexor digitorum brevis (FDB) muscle-nerve preparation. The isolated FDB muscle and tibial nerve were placed in the organ bath chamber, which was continuously perfused with oxygenated synthetic interstitial fluid solution. The distal part of tibial nerve was drawn through a small hole to the recording chamber, which was filled with mineral oil. Following repeated dissection, single fiber activity was recorded. When afferent responses to chemical application were tested, a smaller chamber was used to isolate the muscle. The synthetic interstitial fluid (SIF) solution inside the small chamber was replaced with test solutions.
Recording protocol
The receptive fields of mechanosensitive afferents were investigated by probing the muscle with a fire-polished glass rod; thus, all were mechanosensitive fibers. The afferents that had receptive fields on tendons or were rapidly adapting or responded only to stretch of the distal tendons were excluded. Units were accepted for further study also had a clearly distinguished signal to noise ratio (greater than 2:1). We successfully collected complete recordings from 74 preparations; however, in 35 preparations complete recordings were not obtained from any afferents. No more than two afferents were recorded in the same preparation. Seventy-one units (4 group III and 67 group IV afferents) from 44 incised rats and 40 units (5 group III and 35 group IV afferents) from 30 control, sham-operated rats were tested.
Ongoing activity
Once the receptive field was identified, the muscle was isolated by placing a rectangular chamber (30 mm long, 15 mm wide) over the muscle. The attached tibial nerve was positioned on a notch in the base of a sidewall of the chamber. The small chamber made a seal at the bottom of the organ bath by its own weight and inert silicone grease added to its rim to prevent leakage from the bath. Next, baseline ongoing activity of the unit was recorded for 5 min. The activity during the period was averaged and analyzed. An afferent with a mean discharge rate of at least 0.1 imp/sec was categorized as spontaneously active.
Lactic acid application
After ongoing activity was evaluated, chemosensitivity was examined using lactic acid solutions with varying pH. Lactic acid solutions were made by replacing NaHCO3 in the SIF solution with 15 mM L-lactic acid (Sigma, St. Louis, MO). The pH was titrated to 6.5 or 7.0 with NaOH. These unoxygenated solutions were placed in a sealed vial at 36°C. The pH was stable throughout the day. Unoxygenated SIF pH 7.6 (no lactate) solution containing NaHCO3 was sealed in a vial at 36°C and the pH was stable. The oxygenated SIF solution inside the small chamber was removed with a syringe. Then 15 mM lactic acid (36 °C) pH 6.5 and 7.0, and pH 7.6 (no lactate), was sequentially applied to the muscle for 5 min to each unit and the responses were measured. Following each test solution application, the solution was removed the preparation was perfused with oxygenated SIF for a 2 min washout. We previously showed that repeated applications of acidic lactate solutions at the same pH did not induce either potentiation or tachyphylaxis of subsequent applications7.
An afferent was considered responsive to lactic acid when the mean discharge rate was greater than 0.1 impulse/s during the application if ongoing activity was absent. If background activity was present, the afferent was defined as acid-responsive if the average activity was increased at least two standard deviations greater than the average of baseline ongoing activity for 10 sec.
Mechanical stimulation
After chemical stimulation, mechanosensitivity was assessed using calibrated von Frey filaments with forces of 1.5mN, 58mN and 255mN. Following baseline recording for 2 min, each von Frey filament was sequentially applied to the most sensitive spot of the receptive field for approximately 3 s. The interval between each application was 1 min. Action potentials were counted during the first 1 second of approximately a 3 second application. The ongoing activity was included in the total discharge measurement. Complete mechanical data were obtained from 36 of 40 and 56 of 71 units from the control and the incision groups, respectively. In some fibers, mechanical stimulation was not possible because the filament could not be precisely positioned due to the uneven shape of the muscle. Units were classified as mechanosensitive nociceptors on the basis of their threshold (> 1mN), slowly adapting responses and increased response to greater forces into the noxious range. The area of the receptive field was estimated using the 255 mN filament. We previously studied computer controlled, constant force mechanical stimuli in more than 120 fibers. Because the protocol emphasized acid responses, semiquantitative monofilaments were used to demonstrate we were recording mechanonociceptors.
Conduction velocity
The conduction velocity was measured at the end of the experiments. Electrical stimulation (5 – 20 V, 0.1 – 2.0 ms duration, 0.2 – 1.0 Hz) was applied to the most mechanosensitive point in the receptive field through a monopolar or bipolar electrode to evoke action potentials in the afferent. The distance between receptive field and the recording electrode was measured and divided by the latency of the evoked action potential. A fiber was classified as either a group III or group IV afferent if the conduction velocity was between 2.5 and 30 m/sec, or slower than 2.5 m/sec, respectively. Rapidly adapting afferents and the fast conducting (greater than 30 m/s) were excluded.
Patch-clamp studies of labeled muscle afferents
Labeling of muscle sensory neurons
Sensory neurons innervating muscle were fluorescently labeled using the retrograde tracer DiI (1,1-dioctadecyl-3,3,3,3 tetramethylindocarbocyanine perchlorate; 50 mg/ml dissolved in DMSO)8. Animals were anesthetized with 2–5% isoflurane, a small incision was made in skin over the left gastrocnemius muscle, and 8ul (4ul in each head of gastrocnemius muscle) DiI solution was injected into the muscle as previously described9. After injection, two small drops of cyanoacrylate glue were placed on the puncture site to prevent leaking of DiI. Two to three minutes later, the skin was sutured and rats were allowed to recover for 2 weeks.
Culture of rat DRG neurons
Rat lumbar DRG neurons were cultured as previously described10. Briefly, one day after plantar incision or sham surgery, rats were euthanized and the DRG neurons (L4-5) were collected, dissociated with papain and collagenase/dispase, plated on poly-D-lysine/laminin-coated 35mm plastic dishes, and stored at 37°C in F12 medium supplemented with nerve growth factor. Muscle afferents were identified by fluorescence microscopy, and were studied 18–48 h after plating.
Electrophysiology
Whole-cell patch-clamp recordings (at -70 mV) of DiI-labeled muscle sensory neurons were performed at room temperature with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and were acquired and analyzed with Clampex 8.2 (Axon instruments, Union city, CA) and IGOR PRO 6.01 (WaveMetrics, Lake Oswego, OR) software. Currents were filtered at 1 kHz and sampled at 2 kHz. Micropipettes (3-5 MΩ) were filled with internal solution (mmol/L): 100 KCl, 10 EGTA, 40 HEPES, and 5 MgCl2, pH 7.4 with KOH. External solution contained (mmol/L): 120 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 MES; pH was adjusted with tetramethylammonium hydroxide, and osmolarity adjusted with tetramethylammonium chloride. Rapid extracellular solutions exchanges were made using a computer-driven BPS 8 (ALA scientific, Westbury NY) system. Kinetics of desensitization were fit to with single exponential equations and time constants (τ) reported. pH activation curves were fit using the Hill equation: Fraction of open channels = 1/(1 +(pH10/pH5010)n), where pH50 is the pH at which half of the channels are opened.
Statistical analysis
The Kolmogorov-Smirnov test was used for test of normality. χ2-test was performed to analyze prevalence of ongoing activity and lactic acid-responsive units between groups. Relationships among ongoing activity and acid responsiveness on muscle afferent responses were also examined with χ2-test. When multiple χ2-tests were performed, corrections were made using Bonferonni’s method. Student’s t test was used for comparing mean rate of ongoing activity. Analysis of the mechanical stimulus–response functions between the control and incision group was performed using two-way ANOVA with repeated measures. The magnitude of acid responses in muscle afferent fibers was analyzed by Friedman’s test followed by Dunn’s post hoc test. For patch-clamp studies, data are medians and interquartile range. Statistical significance was assessed using Friedman’s test for pH dependence then Mann-Whitney test for comparisons between incised and sham groups. Multiple measures, in both afferent fibers and DRG neurons, were considered independent from each other. Data on pharmacological inhibitors was assessed using paired Student’s t-test. These results are expressed as mean ± standard error of the mean (SEM). A P value less than 0.05 (two tailed) was considered statistically significant. All tests were performed with GraphPad Prism software (GraphPad, San Diego, CA).
Results
General properties of muscle afferents
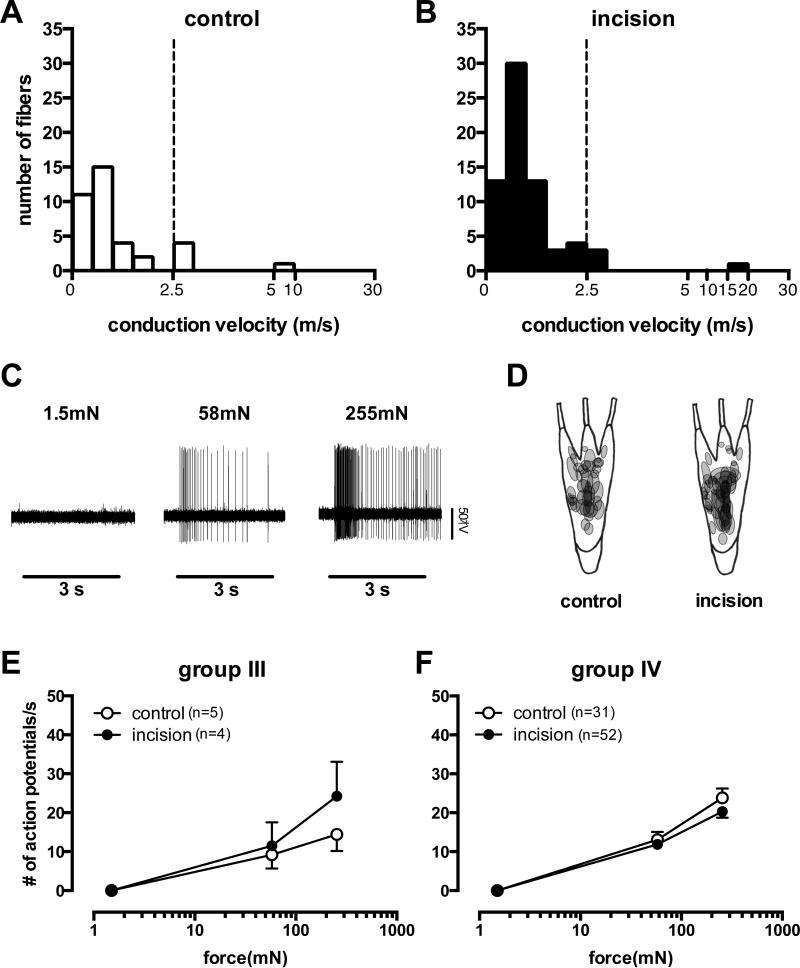
Of 111 mechanosensitive afferents recorded from 74 rats, 71 units (4 group III and 67 group IV) were studied from the incision group and 40 units (5 group III and 35 group IV) were studied from the control group. The average conduction velocity of group III afferents was 6.27 ± 7.17 m/s and 3.21 ± 1.44 m/s from the control and incision groups, respectively and the average of conduction velocity for group IV afferents was 0.82 ± 0.41 m/s vs. 0.81 ± 0.37 m/s in these two groups (Fig. 2A, B). There was no significant difference in the conduction velocity of afferents between the control and incision groups.
Figure 2.
The conduction velocity distribution histogram of muscle afferents in the control (A) and incision (B) groups. The conduction velocity of group III was greater than 2.5 m/s and equal or less than 30 m/s, as a group IV afferent if the conduction velocity was equal or less than 2.5 m/s. The dashed lines separate the two groups. An example recording showing the responses of a group IV afferent from an incised muscle to the indicated von Frey filaments (C). Distribution of the mechanical receptive fields of group III and IV afferents from the control and the incision groups (D). Mechanical stimulus – response function of group III afferents in control (n=5) vs. incision group (n=4) (E). Mechanical stimulus – response function of group IV afferents in control (n=31) and incision group(n=52) (F). m/s=meters per second, mN=millenewtons, s=second.
Response to mechanical stimulation
The response of a slowly adapting mechanoreceptive group IV muscle afferent that did not respond to a weak monofilament but was excited by stronger filaments is shown in Fig. 2C. Altogether, both group III and IV afferents increased responses to greater forces. In the group III afferents (n=9), the average number of action potentials during the first second of mechanical stimulation was 0±0, 10.2±9.3 and 18.8±13.7 imp/sec for 1.5, 58 and 255 mN filaments, respectively. There was a force-dependent increase in both the control (p= 0.0063) and incision (p = 0.0268) groups. For group IV afferents (n=83), the average number of action potentials was 0±0, 11.2±8.8 and 20.8±12.3 imp/sec for 1.5, 58 and 255 mN, respectively. As in group III fibers, increasing force produced greater activation in group IV fibers from both the control (p < 0.0001) and the incision groups (p < 0.0001) indicating these fibers were mechanonociceptors. Shapes of receptive fields were round or oval and the sizes varied (Fig. 2D). We estimated most of the receptive field areas were less than 4 mm2. The mechanoreceptive fields were commonly found in the middle of the FDB muscle or around the area where the medial plantar nerve enters the muscle. In the incised group, the mechanosensitive sites were often located close to the incision (Fig. 2D). There were no differences in responses to the various filaments between sham and incision groups in both group III (Fig. 2E, p = 0.462) and group IV afferents (Fig. 2F, p = 0.083). Mechanical responses could also be elicited from the tendons, the connective tissues surrounding the nerve, and the distal ends of transected nerves, however these afferents were not studied.
Ongoing activity
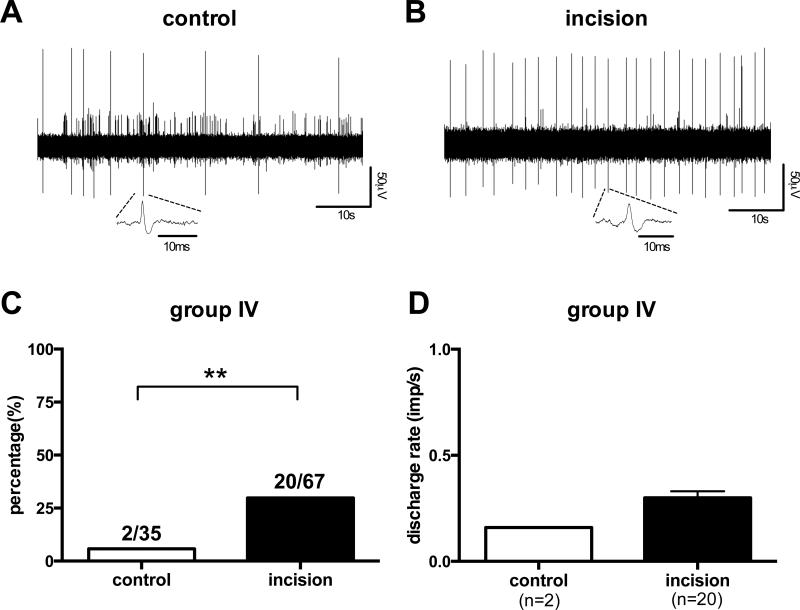
Of the 9 group III afferents, no ongoing activity was found in any of the fibers from the control (n=5) or incision (n=4) groups. In group IV afferents, the prevalence of fibers with ongoing activity (Fig.3A and B) was significantly greater in the incised group (20 of 67, 29.9%) compared to the control group (2 of 35, 5.7%) (Fig. 3C, p =0.0049). The mean rate of ongoing activity in group IV afferents was not different between the control (0.16 ± 0.04 imp/s) and incision (0.29 ± 0.03 imp/s) groups (Fig. 3D).
Figure 3.
Digitized oscilloscope trace of spontaneous action potentials of a group IV afferent from the control group (A) and the incision group (B). The percentage of group IV afferents(C) with ongoing activity (**p =0.0049 χ2-test) and mean rate of spontaneous activity (D) of group IV afferents in each group. s=seconds, ms=msec. imp=impulse, μV=microvolt.
Response to lactic acid
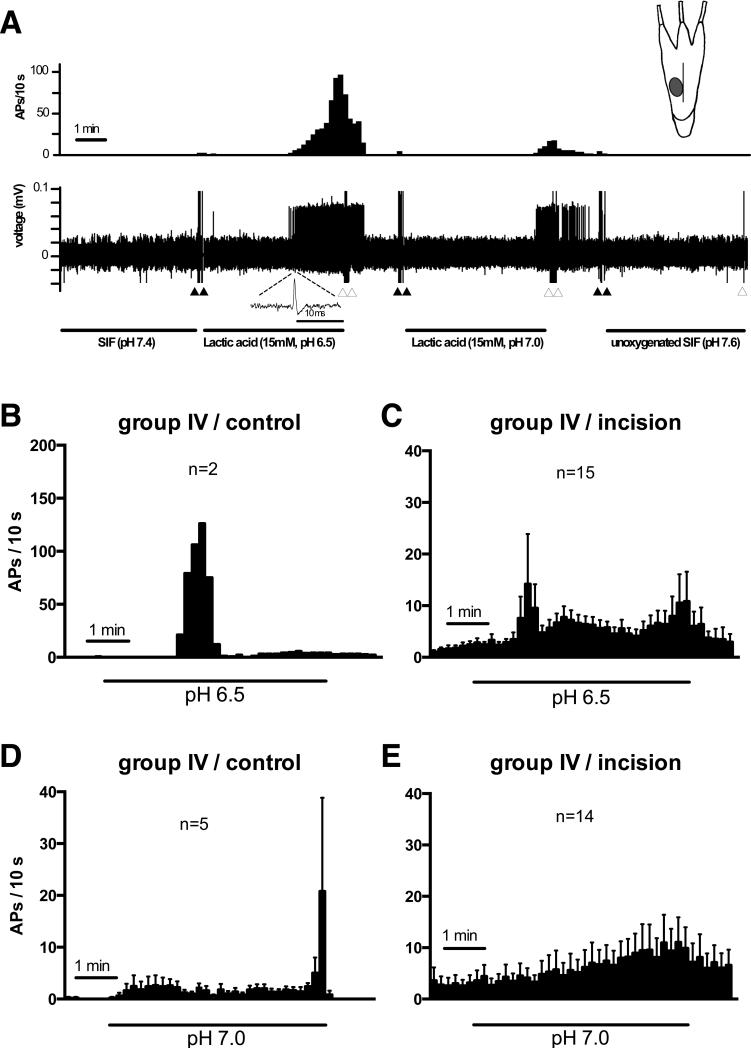
Figure 4A shows an example recording of an acid-responsive group IV afferent from the incision group and the location of the receptive field. The afferent began to fire action potentials 3 min after application of pH 6.5, 4 min after application of pH 7.0, and did not respond to pH 7.6. Mean spike density histograms during exposure to pH 6.5 and 7.0 lactic acid solutions on group IV acid-responsive afferents revealed distinct patterns (Fig. 4B-E). During pH 6.5 application, some fibers from both the control and incision groups had a marked increase in activity approximately 2 min after acid application (Fig. 4B and C). In response to pH 7.0 (Fig. 4D and E), activity did not abruptly increase upon acid application; a few of the control group fibers displaying a marked increase in activity toward the conclusion of the application. For group IV afferents in incised muscle, the increase in activity from pH 7.0 was gradual and throughout the application. Activity slowly returned to baseline after removal of acid and replacement with the neutral oxygenated SIF (not shown).
Figure 4.
Responses of a mechanosensitive muscle afferent fiber from an incised muscle to 15 mM lactic acid with varying pH (pH 6.5, 7.0) and unoxygenated synthetic interstitial fluid (SIF, pH 7.6). (A) An example recording of the response of a group IV afferent to lactic acid. The upper panel shows a spike density histogram (bin width = 10 s) and lower panel shows the digitized oscilloscope tracing. Filled arrowheads mark artifacts generated by the addition of the acid solution; the open arrowheads mark artifacts created during removal of the solution. The receptive field is depicted in the inset. The mean spike density histograms of group IV afferents from the control group (B, D) and incision group (C, E) during 5-min lactic acid exposure at pH 6.5 or 7.0. s=seconds. APs= action potentials. mV=millevolts, mM=millimolar, min=minute.
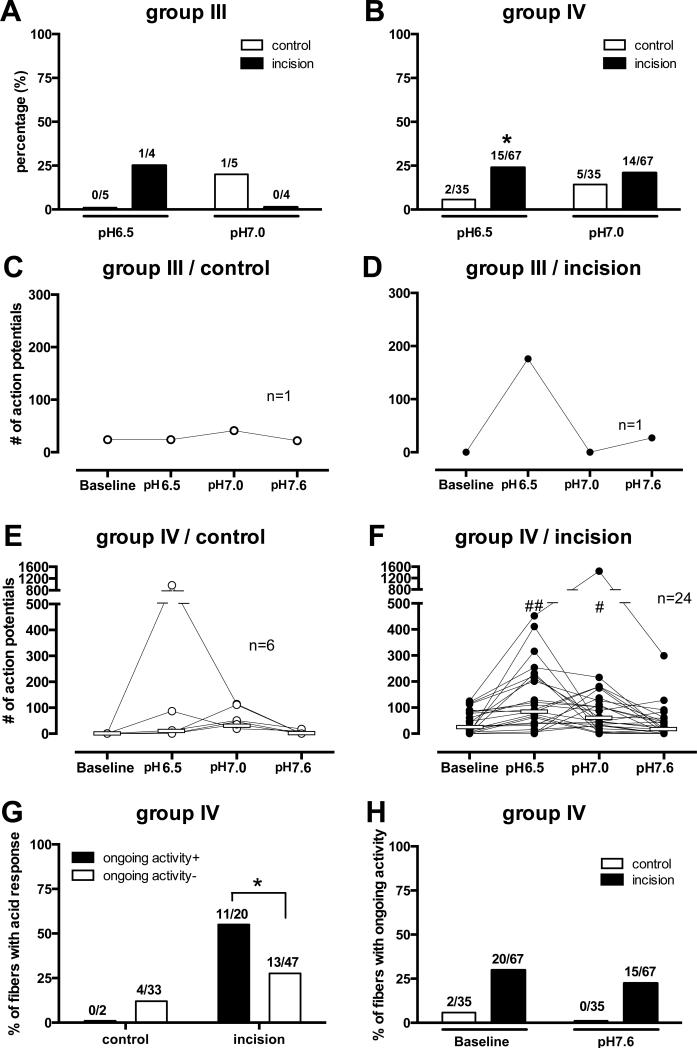
In the group III afferents, 1of 4 units from the incision group was activated by lactic acid pH 6.5 and 1 of 5 units from the control group was activated by pH 7.0 (Fig. 5A). In the group IV afferents, the prevalence of responsive afferents to lactic acid pH 6.5 in the incision group (15 of 67, 22.3%) was greater than that in the control group (Fig. 5B; 2 of 35, 5.7%, p=0.022); the proportion responding to pH 7.0 was not different (p = 0.416).
Figure 5.
Percentage of group III (A) and group IV (B) afferents activated by lactic acid at each pH level. More group IV afferents in the incision group responded to pH6.5 than the control group (* p=0.022 χ2-test). The total number of action potentials for each acid-responsive group III (C, control; D, incision) and group IV (E, control; F, incision) afferents. White horizontal lines in each graph indicate median values. There was a pH-dependent response (E, p = 0.013; F, p < 0.0001 Friedman’s test), and significant differences at pH 6.5 (## p < 0.001 vs. Baseline) and 7.0 (# p< 0.01 vs. Baseline, Dunn’s test) in the incision group (F). (G) The relation between acid responses and ongoing activity in group IV afferents from control and incision groups (* p=0.032 χ2-test). (H) Percentage of group IV afferents from control and incision groups with ongoing activity before and after pH 7.6 application. #=number
The total number of action potentials of each acid-responsive afferent at three different pH levels are shown in Fig. 5C-F. In the unincised group IV afferents (Fig. 5E), there was no difference between baseline activity and total activity during each pH application (n=6). The median number of action potentials during the 5 min application period was 0.5, 8.5, 48.5, and 0 for pH 7.4 (baseline), 6.5, 7.0 and 7.6, respectively.
In the incised group (Fig. 5F), the median number of action potential during the 5 min application period was 28.0, 89.0, 55.0, and 20.5, for baseline, pH 6.5 (p < 0.001 vs. baseline), 7.0 (p < 0.01 vs. baseline), and 7.6, respectively. Greater acidity did not necessarily produce greater responses in all fibers. In both the sham and the incision groups, many fibers responded to pH 6.5, but some only responded to pH 7.0, suggesting heterogeneity among afferent responses to acid.
For the group IV afferents from incised muscle, a greater percentage of units with ongoing activity were activated by lactic acid (11 of 20; 55%) compared to units without ongoing activity (13 of 47; 27.6%; p=0.032) (Fig. 5G). Application of pH 7.6 did not decrease the percentage of fibers with ongoing activity (21.6% before versus 14.7 % after pH 7.6; p = 0.203; Fig. 5H), nor did it reduce ongoing action potential frequency (data not shown).
Acid-evoked currents recorded from isolated DRG innervating muscle are potentiated by muscle incision
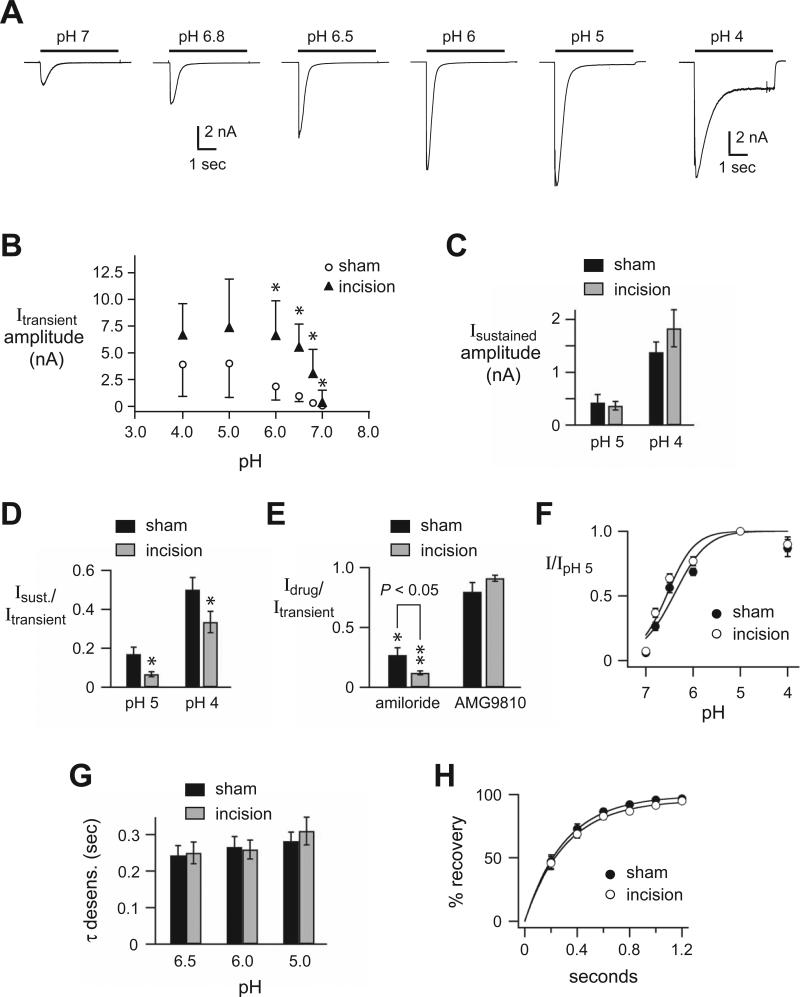
As a correlate of our data from the muscle-nerve preparation, we used whole-cell patch clamp to study the pH responses of isolated DRG neurons innervating muscle. These had been labeled by injection of a retrograde tracer dye (DiI) into the gastrocnemius muscle in rats that had undergone either sham operation or incision of gastrocnemius muscle. We used a larger muscle, the rat gastrocnemius muscle to study DRG innervating muscle because maintaining DiI injections within the flexor digitorum brevis muscle was not possible. Figure 6A shows typical acid currents evoked by the various indicated pH solutions from a DRG from a rat that underwent muscle incision. The currents typically displayed a rapid activation and then desensitized in the continued presence of acidic solution (transient current), followed by a variable persistent activation (sustained current) that is more prominent at more acidic solutions. Acid-evoked currents (> 60 pA) were recorded from a large percentage of DRG innervating muscle from sham rats (33 of 40; 84%; n =5 rats), and this was not different than data in DRG innervating muscle from incised rats (31 of 37; 84%; n =5 rats). However, the peak current amplitudes, typically the transient components, (Fig. 6B) were significantly greater in muscle DRG from incised rats (p<0.001, incision vs sham). It is generally understood that the transient components of the acid-evoked currents in mammalian sensory neurons are generated by ASICs, and that the sustained components, particularly those generated at lower acidic pH solutions can be attributed to TRPV1, ASICs, and perhaps other channels. The sustained current amplitudes were not different between DRG innervating sham versus incised muscle (Fig. 6C), and the ratios of the sustained to peak current amplitudes were significantly smaller in incision compared to sham DRG (Fig. 6D).
Figure 6. Acid-evoked currents recorded by whole-cell patch-clamp from muscle afferents are potentiated after muscle incision.
A, Representative currents evoked by indicated pH solutions from a control solution of pH 7.4 in labeled skeletal muscle afferents in primary dissociated culture of dorsal root ganglia (DRG) neurons collected two weeks after injection of 1,1-dioctadecyl-3,3,3,3 tetramethylindocarbocyanine perchlorate (DiI) into the rat gastrocnemius muscle. B, Median (interquartile range) peak amplitudes evoked by the indicated pH solutions in muscle afferents from control (sham) rats and rats having undergone muscle incision (n = 27 total cells from 5 sham rats, and n = 28 total cells from 5 incision rats). *P < 0.05 compared to sham data at the same pH. DRG of afferents from incised rats were different than sham for pH 6.0 (P= 0.021), pH 6.5 (P=0.039), pH 6.8 (P=0.028) and pH 7.0 (P=0.018). C, Mean amplitudes of the sustained current evoked by the indicated pH solutions (measured at the end of a 5 sec application) from sham and incision muscle afferents (n = 33). D, The mean ratios of the sustained to the peak current amplitudes evoked by the indicated pH solutions from sham and incision muscle afferents (n = 28). *P < 0.05 compared to sham data at the same pH. E, The mean ratios of pH 5-evoked current amplitudes in the presence of the acid sensing ion channels (ASIC) antagonist (amiloride 300 μM) or the transient receptor vanilloid receptor 1 (TRPV1) antagonist (AMG9810 1 μM) compared to the amplitude of the previous pH 5 application in the absence of drug from sham and incision muscle afferents (n = 12). *P < 0.05 and **P < 0.01 compared to the previous pH 5 application in the absence of drug. P < 0.05 comparing amiloride inhibition in sham vs. incision muscle afferents. F, pH dose-response data for pH-evoked currents in sham and incision muscle afferents. Data were normalized to the peak currents evoked by pH 5. Lines are fits of the Hill equation of the means (n ≥ 28). G, Mean time constants (τ) of desensitization as measured from single exponential fits to the falling phase of the transient currents evoked by the indicated pH solutions in sham and incision muscle afferents (n ≥ 28). H, Mean recovery from desensitization data for sham and incision muscle afferents (n ≥ 12). Current was desensitized with a 7-sec application of pH 6. Cells were then exposed to pH 7.4 solution for the indicated times before they were stimulated again with pH 6. Recovery is the percentage of current evoked by the second pH 6 application compared to the first. Lines are fits of single exponentials of the means (τ = 0.31 sec and 0.30 sec for sham and incision data, respectively). Sec=second. nA=nanoamp. I=current. Sust=sustained. Desens.=desensitization.
To further evaluate the molecular nature of the channels underlying the acid currents, we tested the effect of the ASIC blocker (amiloride 300 μM) or the TRPV1 blocker (AMG9810 1 μM) on pH 5-evoked currents (Fig. 6E). Amiloride blocked greater than 75% of the current on average, whereas AMG9810 did not cause significant inhibition of current. Interestingly, amiloride caused a larger inhibition of currents from DRG innervating incised muscle compared to that from sham DRG innervating control, sham-operated muscle. We then tested if other biophysical properties of the acid-evoked currents were altered after incision. The pH sensitivity of activation as measured by pH dose-response (Fig. 6F), the kinetics of desensitization as measure by fitting the falling phase of the transient currents to a single exponential (Fig. 6G), and the time it takes for the currents to recover from desensitization (Fig. 6H) were not different in DRG innervating sham and incised muscle.
Discussion
Deep tissue injury has a critical role in the generation of postoperative pain. In this study, using a rat FDB muscle-nerve preparation, we demonstrate that deep tissue incision causes a greater proportion of group IV mechanonociceptive afferents to respond to pH 6.5 lactic acid and have spontaneous activity in vitro compared to a sham-operated group. Moreover, these group IV afferents with spontaneous activity are more likely to respond to modest reductions in pH after incision. In addition, we found that acid-evoked currents recorded from labeled muscle afferents in DRG culture preparations were potentiated after muscle incision. These currents displayed properties consistent with ASICs. These results offer cellular and molecular insight into the mechanisms underlying nociception associated with deep tissue incision.
Ongoing activity in muscle afferents
In agreement with our previous study 7, deep tissue incision increased the ongoing activity of muscle afferents in vitro. In this study, the prevalence of ongoing activity of group III and group IV afferents in the control group was 5.0%, whereas in the incision group the prevalence of ongoing activity was 28.2%. In vivo, after deep muscle incision, both the prevalence of afferents with ongoing activity and unprovoked guarding pain in the deep muscle incision group were greater than after skin incision only3. Together these data support the importance of studying mechanisms for deep tissue afferent activation by incisions as a mechanism for pain at rest after surgery. Even though spontaneous activity predicts responses to acid, exposure to alkaline pH 7.6 did not decrease the percentage of afferents with spontaneous activity or magnitude of spontaneous activity.
Response of muscle afferents to lactic acid
Few studies have examined the response of primary afferent fibers to weak acid solutions. The present study demonstrates that application of pH 7.0 and 6.5 activated 14.3% and 5.7% of group IV afferents in the normal, non-incised condition. These results are similar to findings of Wenk and McCleskey who examined the acid sensitivity of afferents using a mouse muscle-nerve preparation. Mouse muscle afferents that were acid sensitive were activated when the pH reached approximately 6.711.
Importantly, deep tissue incision increased the prevalence of afferents responding to muscle acidification. Previous studies in incised rat flexor digitorum brevis muscle demonstrated a 6-fold increase in the percentage group IV afferents that responded to pH 6.5 compared to the unincised preparation, and this increased responsiveness did not occur if the incision was made in vitro immediately before testing acid 7. Similarly, in the present study we found a 4-fold increase in the percentage of group IV fibers responsive to pH 6.5 in incised muscle.
These results and the findings from our previous study suggest that sensory terminal-tissue interface in the incisional area is sensitized to lactic acid. We used lactic acid to produce tissue acidosis because we have previously demonstrated that one to four day after incision the muscle lactate concentration increases to approximately 6 mM when pH decreases to 6.8 5,6. In addition, 15 mM lactic acid potentiates responses of ASIC3 to mild acidosis 12,13. Future studies will determine the role of lactate in enhancing the response to acid in incised muscle afferents.
The responses to acidification vary among the group IV afferents. The pH responses to weak acid solutions in some cases showed a delayed onset, but then excitation was maintained throughout the remaining application period. For some fibers, pH 6.5 elicits the greatest response; in other afferents, pH 7.0 produces a greater response than pH 6.5. A similar seemingly paradoxical response was previously described in isolated rat sensory neurons, where application of mildly acidic solutions (pH 6.8) was found to cause firing of more action potentials than a more acidic (pH 6.5) solution14. This result might reflect inactivation of voltage-gated channels caused by greater depolarization at more acidic pH, but also these responses might reflect differences in activation patterns of pH sensitive ion channels at varying pH. For example, pH 7.0 causes a persistent sustained current through ASIC3 channels, whereas pH 6.5 will generate only a transient, desensitizing current15. This, in part, might explain the more sustained train of action potentials observed during pH 7.0 applications, compared to the more transient responses to pH 6.5.
To begin to understand the molecular mechanisms underlying the increase in muscle afferent activity associated with incision, we studied labeled muscle afferents by patch-clamp technique. We found that deep tissue incision induced an increase in mean current amplitudes of acid-evoked currents in labeled muscle afferents. We found a higher percentage of isolated muscle afferents in culture (84%) responded to acidic pH compared to our muscle afferent fiber recording in the muscle-nerve preparation, and unlike our muscle-nerve prep data the percentage of responders did not increase after incision. Perhaps, in part, these differences can be explained by the fact that we exposed cultured muscle afferents to a much lower pH to fully characterize their acid responsiveness, compared to the more physiological pH levels that we applied in the muscle-nerve preparation. More importantly, some muscle afferents probably express pH sensitive channels to a degree that allows for the recording of small acid-evoked currents recorded by patch-clamp, however this degree of expression might not be sufficient to generate action potentials. Along this regard, we speculate that deep tissue incision induced an increase in expression of pH sensitive ion channels. This increase in pH receptors would manifest as an increase in mean current amplitudes of acid-evoked currents in isolated DRG innervating muscle, whereas in our muscle-nerve preparation, this increase in channel expression could cause a larger percentage of muscle afferents to reach threshold depolarization with acid exposure.
Although we did not directly quantitate expression of pH receptors, our data is consistent with an increase in ASIC expression. First, the acid-evoked currents we characterized from labeled muscle afferents are consistent with ASIC channels: they possess rapid activation and desensitization kinetics, and they are inhibited by the ASIC blocker amiloride. In addition, the currents were not inhibited by the TRPV1 antagonist AMG9810. Secondly, acid-evoked current amplitudes were increased after deep tissue incision, and a greater proportion of the pH 5-evoked currents were inhibited by amiloride after incision. While we suspect that most of the current evoked at this pH is carried by ASICs, certainly other pH activated channels are also activated including TRPV1. TRPV1 is believed to be a contributor to the sustained currents evoked at more acidic pH values, however we did not see an increase in the sustained currents after incision. Since amiloride blocked more of the current after incision, we conclude that ASICs contribution to the currents is increased after incision. Third, ASIC expression in sensory neurons is known to increase in other models of nociception. ASICs messenger RNA is increased after paw 16, joint 17, and muscle inflammation 18, as well as compression of the lumbar nerve root from spinal disk herniation 19. In addition, previous reports show that ASIC current density is increased in rat DRG neurons in models of nerve injury, stomach ulcers, and after hindpaw inflammation 14,20,21.
Previous studies by Deval et al. 22 demonstrated that local administration of the ASIC3 receptor blocker, APETx2, reduced pain behaviors after plantar incision. In addition, ASIC-3 messenger RNA expression was increased and the percentage of ASIC-3-immunopositive cells was increased in small diameter neurons innervating the hindpaw by incision. However, it is unclear in the study by Deval et al. 22 if the labeled cells in the DRG innervated muscle because they were labeled by dye injection using a blind technique into the hindpaw where spread of the injection out of the muscle and into subcutaneous tissue is possible.
ASICs are members of the epithelial sodium channel/degenerin family. They are present in both the peripheral and central nervous system.23 In the periphery, ASICs are present in some nonneuronal cells and are highly expressed in DRG where they act as receptors for extracellular protons.24 Because of this proton sensitivity that approximates physiologic and pathologic acidosis, ASICs have also been implicated in pain associated with myocardial ischemia,10 pain during inflammation,25,26 and fatigue.27 ASICs also contribute to mechanosensation.28-32 In the periphery, loss of ASICs has been attributed to deficits in hearing33 and reduced visceral sensation29 but increased cutaneous mechanoreceptor function.28
ASIC inhibitors may be analgesic in perioperative pain, however, given their hypothesized roles, this is not completely understood. For incisional pain, blockade of ASICs may increase cutaneous mechanosensitivity, but decrease deep tissue fascia and muscle chemosensitivity. Because we hypothesize postoperative pain is largely driven by deep tissue injury, we propose that ASIC antagonists will reduce postoperative pain, even in the setting of increasing cutaneous mechanosensitivity. Reduced pain after fracture and bone surgery, as well as other deep surgical tissues, may contribute to analgesic effects of ASIC inhibitors in the perioperative period.
Conclusion
More afferent fibers responded to small decreases in pH to 6.5; many also were activated by pH 7.0. The optimal pH activating muscle afferent fibers varied, some had greater responses to pH 7.0 than to pH 6.5. ASIC channels may be upregulated at the sensory afferent terminal within 24 hours after incision and combined with increased lactic acid may be one mechanism whereby sensory afferents in deep tissue may produce ongoing pain. Other symptoms that occur after surgery, like fatigue, may be signaled in part by lactic acid. Finally, in the future, ASIC channel antagonists may be analgesic for postoperative patients.
MS #201211096 – Final Boxed Summary Statement.
What we already know about this topic:
* Afferent nerve fibers serving deep tissues such as muscle and bone have distinct responses to injury.
What this article tells us that is new:
* Using a muscle-nerve preparation from the rat hind paw, the authors were able to demonstrate that incision sensitizes afferent nerve fibers serving deep tissues. Acid-sensing ASIC ion channels may be responsible for this pain-related sensitization.
Acknowledgments
Financial support: National Institutes of Health, Bethesda, Maryland, GM067762 to T.J.B.
Footnotes
The authors declare no competing interests.
Presented, in part, at the Society for Neuroscience Annual Meeting November 13, 2011 in Washington, D.C.
Contributor Information
Kanta Kido, Department of Anesthesia, University of Iowa Hospitals and Clinics, Iowa City, IA.
Mamta Gautam, Department of Internal Medicine, Roy J. and Lucille A. Carver College of Medicine and Veterans Medical Center, University of Iowa, Iowa City, IA
Christopher J. Benson, Department of Internal Medicine, Roy J. and Lucille A. Carver College of Medicine and Veterans Medical Center, University of Iowa, Iowa City, IA
He Gu, Department of Anesthesia, University of Iowa Hospitals and Clinics, Iowa City, IA.
Timothy J. Brennan, Departments of Anesthesia and Pharmacology, University of Iowa Hospitals and Clinics, Iowa City, IA.
References
- 1.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–39. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112:153–64. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Richebe P, Brennan TJ. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. Eur J Pain. 2009;13:820–8. doi: 10.1016/j.ejpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Gu H, Brennan TJ. Increased sensitivity of group III and group IV afferents from incised muscle in vitro. Pain. 2010;151:744–55. doi: 10.1016/j.pain.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiura T, Bielefeldt K, Gebhart GF. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am J Physiol Cell Physiol. 2007;292:C1768–74. doi: 10.1152/ajpcell.00440.2006. [DOI] [PubMed] [Google Scholar]
- 9.Gautam M, Benson CJ, Sluka KA. Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neuroscience. 2010;170:893–900. doi: 10.1016/j.neuroscience.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–8. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 11.Wenk HN, McCleskey EW. A novel mouse skeletal muscle-nerve preparation and in vitro model of ischemia. J Neurosci Methods. 2007;159:244–51. doi: 10.1016/j.jneumeth.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–70. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 13.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 14.Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–34. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–9. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 16.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–33. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain. 2009;10:336–42. doi: 10.1016/j.jpain.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walder RY, Rasmussen LA, Rainer JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in development of hyperalgesia following inflammatory muscle injury. J Pain. 2010;11:210–8. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtori S, Inoue G, Koshi T, Ito T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine. 2006;31:2048–52. doi: 10.1097/01.brs.0000231756.56230.13. [DOI] [PubMed] [Google Scholar]
- 20.Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–55. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–27. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31:6059–66. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–86. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Li WG, Xu TL. ASIC3 channels in multimodal sensory perception. ACS Chem Neurosci. 2011;2:26–37. doi: 10.1021/cn100094b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–12. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain. 2011;152:2348–56. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol. 2008294::R1347–55. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PloS One. 2012;7:e35225. doi: 10.1371/journal.pone.0035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–47. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 30.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–11. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 31.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–83. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 32.Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–9. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand MS, de Silva MG, Klockars T, Rose E, Price M, Smith RJ, McGuirt WT, Christopoulos H, Petit C, Dahl HH. Characterisation of DRASIC in the mouse inner ear. Hear Res. 2004;190:149–60. doi: 10.1016/S0378-5955(04)00015-2. [DOI] [PubMed] [Google Scholar]