Abstract
Background
Sudden death syndrome (SDS) caused by the ascomycete fungus, Fusarium virguliforme, exhibits root necrosis and leaf scorch or foliar SDS. The pathogen has never been identified from the above ground diseased foliar tissues. Foliar SDS is believed to be caused by host selective toxins, including FvTox1, secreted by the fungus. This study investigated if the xylem sap of F. virguliforme-infected soybean plants contains secreted F. virguliforme-proteins, some of which could cause foliar SDS development.
Results
Xylem sap samples were collected from five biological replications of F. virguliforme-infected and uninfected soybean plants under controlled conditions. We identified five F. virguliforme proteins from the xylem sap of the F. virguliforme-infected soybean plants by conducting LC-ESI-MS/MS analysis. These five proteins were also present in the excreted proteome of the pathogen in culture filtrates. One of these proteins showed high sequence identity to cerato-platanin, a phytotoxin produced by Ceratocystis fimbriata f. sp. platani to cause canker stain disease in the plane tree. Of over 500 soybean proteins identified in this study, 112 were present in at least 80% of the sap samples collected from F. virguliforme-infected and -uninfected control plants. We have identified four soybean defense proteins from the xylem sap of F. virguliforme-infected soybean plants. The data have been deposited to the ProteomeXchange with identifier PXD000873.
Conclusion
This study confirms that a few F. virguliforme proteins travel through the xylem, some of which could be involved in foliar SDS development. We have identified five candidate proteinaceous toxins, one of which showed high similarity to a previously characterized phytotoxin. We have also shown the presence of four soybean defense proteins in the xylem sap of F. virguliforme-infected soybean plants. This study laid the foundation for studying the molecular basis of foliar SDS development in soybean and possible defense mechanisms that may be involved in conferring immunity against F. virguliforme and other soybean pathogens.
Introduction
Sudden death syndrome (SDS) is an important soybean (Glycine max (L.) Merr) disease in US, Canada, Argentina, Brazil, Uruguay, Paraguay, and Bolivia [1], [2], [3]. In the United States, it is among the top four yield reducing soybean diseases [4]. In some states, SDS ranks second after soybean cyst nematode (SCN) in terms of yield suppression caused by these diseases in soybean [5]. It has been shown that soybean fields with high population density of SCN have a higher chance of SDS incidence [6]. The estimated soybean yield suppression from SDS in 2010 was 2.1% of the total yield valued at $0.82 billion [7].
Four Fusarium species, Fusarium brasiliense, F. cuneirostrum, F. tucumaniae sp. nov., and F. virguliforme, can cause sudden death syndrome across the world. All four species except F. virguliforme cause SDS in Brazil. F. cuneirostrum and F. virguliforme are causal agents of SDS in Argentina. F. virguliforme (Akoi, O’Donnell, Homma & Lattanzi), formally known as F. solani (Mart.) Sacc. f. sp. glycines, is the only Fusarium species that causes SDS in the U.S. [8], [9].
Fusarium virguliforme is a soil-borne fungus that belongs to the class Sordariomycetes and is known to produce one or more phytotoxins in culture media [10], [11], [12], [13]. Though the pathogen only infects soybean roots, the disease symptoms are seen on both roots and foliar tissues. The pathogen has never been isolated from the diseased foliar tissues. Hence, it is considered that toxin(s) produced by the fungus is responsible for the foliar SDS symptoms. It was suggested that in the presence of light, the phytotoxins secreted by the F. virguliforme to the culture media cause the degradation of the RuBisCo large subunit and the accumulation of free radicals, which presumably trigger programmed cell death leading to foliar SDS symptoms [14].
A purified 17 kDa proteinaceous toxin from the F. virguliforme cultures was shown to cause necrosis on soybean cotyledons and leaves [15]. However, the gene encoding this putative toxin has never been isolated. Recently, FvTox1 toxin was purified from the culture filtrates and the gene, FvTox1, encoding this toxin has been isolated [13]. The FvTox1 protein, expressed in an insect line, was shown to cause foliar SDS-like symptoms in soybean leaf discs, only in the presence of light [13]. FvTox1 is a 13.5 kDa acidic protein. This toxin can rapidly cause foliar SDS-like symptoms in leaf discs of soybean lines, highly susceptible to F. virguliforme [13]. Expression of a single chain variable fragment (scFv) antibody against FvTox1 enhanced foliar SDS resistance in transgenic soybean plants supporting the role of FvTox1 in foliar SDS development [16]. Investigation of fvtox1 mutants suggested that FvTox1 is a major virulence factor involved in foliar SDS. The same study also revealed that additional toxins might play a minor role in foliar SDS development [17].
Proteomic research has gained new heights due to the availability of a wide array of gel-free proteomic technologies such as isobaric tagging for relative and absolute quantification (iTRAQ), multi-dimensional protein identification technology (MudPIT), isotope-coded affinity tag (ICAT), and coupled techniques such as liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). Most of these techniques are faster, allow multiplexing of samples, and have better sensitivity and reproducibility [18]. Even with the currently available proteomic technologies, relatively few proteomic researches have been focused on the variation in the proteomes of host-pathogens interactions. These studies have shown that a variety of proteins, including peroxidases, chitinases, proteases, and pathogenicity related (PR) proteins to be differentially expressed in plants in response to pathogen invasion [19], [20], [21], [22]. Not only that there is variation in the relative abundance of certain proteins, some could also be induced only in response to either the compatible or the incompatible interaction [23], . These proteins could be involved in antifungal activities, signal transduction, anti-oxidation, protein folding, and an array of other plant functions and biological processes.
Recent studies have looked at the xylem sap proteome of several annual plants including soybean in detail [21], [29], [30], [31], [32], [33]. Very few studies have shown differential accumulation of proteins in xylem sap following pathogen infection [32], [24], [26]. Li et al [34] identified a stress-induced soybean protein in the stem exudates of soybean seedlings infected with F. virguliforme. Houterman et al. [24] reported the presence of 33 proteins including 21 tomato proteins and seven F. oxysporum proteins in the xylem sap of F. oxysporum infected tomato plants (Solanum lycopersicum). It is most likely that the host-selective proteinacious toxins produced by F. virguliforme are transported to the leaves via the vascular system to cause foliar SDS. Therefore, study of the xylem sap proteins of both infected and uninfected soybean plants could lead to identification of such F. virguliforme toxin proteins. The main objective of this study was to investigate if the xylem sap of F. virguliforme-infected soybean plants contains any secreted F. virguliforme-peptides/proteins. We applied LC-ESI-MS/MS in analyzing the proteomes of the xylem saps collected from either healthy, F. virguliforme-uninfected or F. virguliforme-infected soybean plants and identified five F. virguliforme proteins, one of which showed similarity to a previously characterized pathogen toxin.
Materials and Methods
Inoculum Preparation
Fusarium virguliforme isolates, Scott and Clinton, were grown on half strength potato dextrose agar (PDA) for about a week. Inoculum was prepared in sorghum meals as described by Hartman et al. [35]. In short, 200 g of sorghum (Sorghum bicolor (L.) Moench) seeds were soaked overnight in water in 1 quart Mason jars and autoclaved twice. Once the autoclaved sorghum seeds were cooled down, they were inoculated with 10 mycelial plugs containing conidial spores from each isolate. The cultures were allowed to grow on sorghum for four weeks; then harvested and air-dried. Fully dried inoculum was ground in a blender into powder. One part of the ground F. virguliforme-infested sorghum was mixed with 10 parts of sterile 1∶2 soil:sand mixture to make up the inoculum for the root infection assay.
Plant Material and Xylem Sap Collection
The soybean variety “Spencer,” highly susceptible to F. virguliforme, was used in this study. Plants were grown using the modified layer method of Hartman and his co-workers [35], [36]. Three seeds were planted in one 8-oz styrofoam cup that were first half (150 mL) filled with the 1∶2 sterile soil:sand mixture and covered with 30 mL of the inoculum prepared on sorghum meals. Un-inoculated sterilized sorghum seeds were ground and mixed to 1∶10 ratio with the sterile 1∶2 soil:sand mixture to serve as a control. Cups were randomly placed in a growth chamber and the plants were grown at 25°C for 16 h under light (200 µ mol photons m–2s–1 light intensity) and at 16°C for 8 h in the dark. Plants were watered once daily. In each experiment, 120 plants were grown in either F. virguliforme inocula-containing soil or sorghum meal-mixed soil.
Xylem sap was collected between 14–21 days, immediately following observation of foliar SDS symptoms. Plants were thoroughly watered in the evening of the day before collecting the xylem sap. A slightly modified method, described earlier by Djordjevic and his co-workers [31], was used to collect the xylem sap. The plants were de-capitated about 3–5 cm above the soil surface with a sterile surgical blade. Cut surface was gently wiped with a fresh lint-free Kimwipe (Kimberly-Clark Corporation; Roswell, GA) to avoid any contamination. The free end of a 5-cm long rubber tube attached to a 1 mL syringe was connected to the cut surface of the hypocotyl to collect the xylem sap. Vaseline was applied to establish a proper seal between the hypocotyl and the rubber tube. Syringe was securely tied to a small stick placed on the middle of the cup (Figure 1). Plunger of the syringe was pulled back to maintain a vacuum and to facilitate the xylem sap accumulation. Xylem sap was collected at a 2-h interval for up to 6 h from the tubing, and stored in pre-cooled 1.5 mL labeled Eppendorf tubes placed on ice. After each collection, Eppendorf tubes were stored at −80°C. Bradford assay (Bio-Rad, Hercules, CA) was conducted to estimate the concentration of proteins in the collected xylem sap. The xylem sap was collected from five independent experiments and stored at −80°C for analyses.
Figure 1. Collection of xylem sap from 14 to 21-day old F. virguliforme-infected or -uninfected soybean plants.

The free end of a rubber tube attached to a 1 mL syringe was securely fasten to the cut soybean hypocotyl and sealed with Vaseline. Low pressure was created by pulling the plunger of the syringe to facilitate xylem sap accumulation.
1D Gel Electrophoresis
Twenty µl of the crude xylem sap samples (containing about 1 µg of proteins) were fractionated in a 12% sodium dodecyl sulfate polyacrylamide gel (wt/vol) at 120 V for 90 min and stained with a mixer of 45% methanol (vol/vol), 10% glacial acetic acid (vol/vol), and 0.5% Coomassie brilliant blue G-250 (wt/vol).
Protein Identification by Nano LC-ESI/MS/MS Analysis
Xylem sap samples were analyzed by nano LC-ESI/MS/MS at the Cornell University Proteomics and Mass Spectrometry Core Facility by Dr. Sheng Zhang and Mr. James McCardle. Ten xylem sap samples and one sample of the cell-free F. virguliforme culture filtrate (CF) were subjected to trypsin digestion followed by solid phase extraction (SPE). The tryptic digests were reconstituted in 2% acetonitrile (ACN) with 0.5% formic aid (FA) for nano LC-ESI-MS/MS analysis, which was carried out using an LTQ-Orbitrap Velos (Thermo-Fisher Scientific, San Jose, CA) mass spectrometer equipped with “Plug and Play” nano ion source device (CorSolutions LLC, Ithaca, NY). The Orbitrap was interfaced with an UltiMate3000 RSLCnano system (Dionex, Sunnyvale, CA). The nanoLC was carried out by a Dionex UltiMate 3000 RSLCnano system (Dionex, Sunnyvale, CA). The reconstituted peptides (2 µL) were injected under “User Defined Program” onto a PepMap C18 trap column (5 µm, 300 µm×5 mm, Dionex) at a 20 µL/min flow rate for on-line desalting and then separated on a PepMap C18 RP nano column (3 µm, 75 µm×15 cm, Dionex) which was installed in the “Plug and Play” device with a 10-µm spray emitter (New Objective, Woburn, MA) mounted in front of the Orbitrap orifice.
The peptides were eluted in a 90 min gradient of 5% to 38% ACN in 0.1% FA at 300 nL/min. The Orbitrap Velos was operated in the positive ion mode with nano spray voltage set at 1.5 kV and source temperature at 275°C. Internal calibration was performed using the background ion signal at m/z 445.120025 as a lock mass. The instrument was operated in data-dependent acquisition (DDA) mode using the FT mass analyzer for one survey MS scan at a resolution of 60,000 for precursor ions followed by MS/MS scans at a resolution of 7,500 on the top 10 most abundant peaks with multiple charged ions above a threshold ion count of 15,000 in the Linear Ion Trap mass analyzer. Dynamic exclusion parameters were set at repeat count 1 with a 30 s repeat duration, exclusion list size at 500, exclusion duration at 13 s, and mass width at ±10 ppm exclusion. Collision induced dissociation (CID) parameters were set at the following values: isolation width at 2.0 m/z, normalized collision energy at 35%, activation Q at 0.25, and activation time of 0.1 ms. All data are acquired under Xcalibur 2.1 operation software (Thermo-Fisher Scientific, San Jose, CA).
Data Analysis
All raw data were searched using Mascot 2.2 (Matrix Science) software against the NCBI public database with taxonomy of Green Plants or the F. virguliforme genome sequence database containing 14,845 predicted F. virguliforme genes (http://fvgbrowse.agron.iastate.edu). The peptide tolerance was set to 10 ppm and MS/MS tolerance was set to 0.8 Da. Fixed carbamidomethyl modification of cysteine, variable modifications of methionine oxidation, deamination of asparagine and glutamine were considered. Data filtering parameters of 0.01 and 0.001 significance thresholds and an ion cut-off score of 32 and 27 k were applied to the results for searches against the Green Plants and F. virguliforme genome sequence databases.
False discovery rates (FDR) were calculated for each of the samples using the formula; FDR = (Ndecoy/Nreal+NDecoy)*100. This is an indication of the percentage of the random or “false” peptide identifications in the raw data. The relative abundance of the proteins identified by LC-ESI-MS/MS was estimated by determining the protein abundance index (PAI) and the exponentially modified protein abundance index (emPAI). Protein abundance index was calculated as follows: number of detected peptides divided by the number of observable peptides per protein normalized by the theoretical number of peptides expected via in silico digestion. The emPAI was calculated as 10PAI-1 [37].
Functional Annotation
SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/) was used to identify N-terminal signal sequences [38]. Protein sequences obtained from NCBI were BLAST analyzed against the Glycine max protein database using Phytozome (www.phytozome.org). Sequences of the annotated soybean proteins were uploaded to Blast2Go V.2.6.2. program (http://www.blast2go.com/b2ghome) to perform functional annotation with gene ontology (GO) terms using default values [39]. Sequence distribution for molecular function was visualized at the ontology level 2 with a cutoff of 5. Interpro scan was also run and the results were merged with the annotations as described in the Blast2Go tutorial [40]. Sequences were also annotated using Kyoto Encyclopedia of genes and Genomes (KEGG) with the aid of Blast2Go to place the proteins into metabolic pathways.
Results
Similar Protein Profiles were Observed between Xylem Saps of F. Virguliforme-infected and -uninfected Plants Following 1D Gel Electrophoresis
In order to identify proteins involved in foliar SDS, the protein profiles of xylem sap samples collected from the F. virguliforme-infected and -uninfected plants were compared by fractionating the sap samples on 12% SDS-PAGE gels. There were no qualitative or obvious quantitative differences between the crude xylem sap samples of the F. virguliforme-infected and -uninfected control plants for protein profiles (Figure 2).
Figure 2. Protein profile of xylem sap collected from either F. virguliforme-infected or F. virguliforme-uninfected soybean plants.
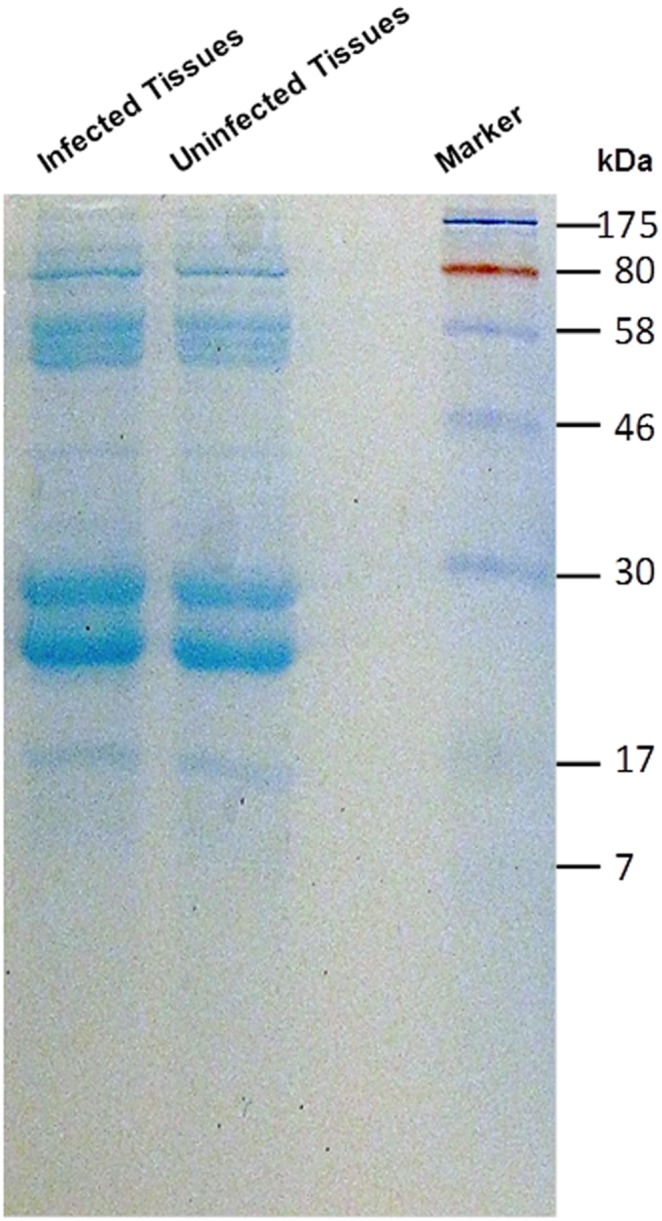
Xylem saps were fractionated by 12% SDS-PAGE gel and stained with Coomassie blue. Sizes of the molecular weight marker in kDa are shown at the right side. Infected, xylem sap from soybean plants infected with F. virguliforme; Uninfected, xylem sap from soybean plants that were not infected with F. virguliforme.
F. virguliforme Proteins were Identified from the Xylem Sap of the Infected Plants
Differences between xylem sap samples of the F. virguliforme-infected and -uninfected plants were detected through LC-ESI-MS/MS analysis. Five F. virguliforme proteins were identified in the xylem sap of the infected soybean plants. All five proteins possessed N-terminal secretory signal peptide sequences (Table 1). One of these proteins showed high sequence similarity to the cerato-platanin toxin, which was found to be the most abundant F. virguliforme protein in the xylem sap collected from the F. virguliforme-infected soybean plants (Table 1). Identified peptides of these five proteins are presented in Table S1.
Table 1. F. virguliforme peptides identified from the xylem sap of F. virguliforme-infected soybean plants.
| Protein IDa | Description | Protein score | Protein mass (kDa) | # of peptides | Times identifiedb | emPAIc | Signal peptide |
| g3913 | Cerato-platanin | 6146 | 15.2 | 2 | 3(422) | 1.2 | Y |
| g12110 | PAN_1 PAN domain | 1008 | 28.2 | 2 | 2(72) | 0.1 | Y |
| g8948 | Unknown | 943 | 20.9 | 2 | 1(19) | 0.6 | Y |
| g11360 | Unknown | 687 | 25 | 2 | 2(45) | 0.3 | Y |
| g10227 | Lipoprotein_15 Secreted repeat of unknown function | 199 | 23.1 | 2 | 2(11) | 1 | Y |
Protein identification numbers are same as the gene IDs of the F. virguliforme genome database (http://fvgbrowse.agron.iastate.edu) [56].
Number of times the peptides were identified from five biological replicates of xylem saps collected from F. virguliforme-infected soybean plants. The total number of times a peptide(s) was identified is presented in parentheses.
Exponentially modified protein abundance index. This equals to 10PAI-1, which is proportional to the protein content in a protein mixture.
Excreted Proteome of the Cell-free F. virguliforme Culture Filtrate Contains All Five Xylem Sap F. virguliforme Proteins
To determine if any of the five proteins (Table 1) identified from the xylem sap of the F. virguliforme-infected plants were also excreted to the culture medium, the proteomes of the cell-free F. virguliforme culture filtrates (CF) were investigated by conducting LC-ESI-MS/MS. Ninety four proteins were identified in the cell-free F. virguliforme culture filtrates (Table S2). Forty-four proteins with more than one peptide are presented in Table 2. A lipoprotein-15 was found to be the most abundant protein in the CF proteome. Of the 44 reported proteins, 37 shown to have predicted secretary signal peptides (Table 2). These proteins included cerato-platanin and FvTox1, with cerato-platanin toxin being the second most abundant protein (Table 2). All five proteins identified from the xylem sap of the F. virguliforme-infected soybean plants were also present in the pathogen CF (Table 2). The CF proteome contained proteins that showed high identity to cutinase, catalase, sporozite P67 surface antigen, eukaryotic type carbonic anhydrase, alpha amylase inhibitor, and laminin. Glycosyl hydrolases belonging to four different families were also identified (Table 2).
Table 2. Details of the F. virguliforme proteins identified in cell-free culture filtrates.
| Protein IDa | Description | Protein score | Proteinmass (kDa) | # ofpeptides | Timesidentifiedb | emPAIc | Signalpeptide |
| g10227d | Lipoprotein_15Secreted repeat ofunknown function | 11495 | 13.06 | 6 | 620 | 24.74 | Y |
| g3913d | Cerato-platanin | 2844 | 15.19 | 8 | 266 | 19.59 | Y |
| g11360d | Unknown | 2716 | 24.96 | 9 | 124 | 6.46 | Y |
| g11634 | Unknown | 1899 | 19.99 | 7 | 76 | 3.74 | Y |
| g12110d | PAN_1 PAN domain | 1808 | 28.21 | 9 | 118 | 5.68 | Y |
| g5600 | Glycolipid anchoredsurface protein (GAS1) | 1169 | 58.57 | 9 | 34 | 0.93 | Y |
| g13851 | Alpha amylase inhibitor | 963 | 17.01 | 4 | 48 | 1.48 | Y |
| g8948d | Unknown | 880 | 20.85 | 2 | 23 | 0.35 | Y |
| g12236 | Unknown | 774 | 15.60 | 8 | 69 | 4.85 | Y |
| g11991 | Glycosyl hydrolases family 16 | 673 | 29.25 | 7 | 35 | 0.91 | Y |
| g7574 | Cutinase | 672 | 15.78 | 2 | 30 | 0.79 | Y |
| g8691 | TIL Trypsin Inhibitorlike cysteine rich domain | 546 | 8.96 | 2 | 16 | 1.68 | Y |
| g2004 | Glycosyl hydrolasesfamily 15 | 543 | 78.02 | 8 | 26 | 0.51 | Y |
| g6599 | Unknown | 506 | 15.87 | 3 | 12 | 1.17 | Y |
| g4858 | CFEM domain | 432 | 23.13 | 3 | 30 | 1.58 | N |
| g1353 | Sporozoite P67surface antigen | 419 | 21.89 | 2 | 9 | 0.33 | N |
| g9768 | Unknown | 366 | 38.18 | 3 | 8 | 0.39 | Y |
| g10536 | CFEM domain | 360 | 94.64 | 2 | 13 | 0.15 | Y |
| g6924 | FvTox1 | 346 | 40.75 | 2 | 20 | 0.37 | Y |
| g13548 | Glycosyl hydrolase family 4 | 312 | 40.11 | 4 | 32 | 0.17 | Y |
| g2624 | Catalase | 263 | 78.29 | 3 | 15 | 0.23 | Y |
| g11346 | PLA2_B Lysophospholipasecatalytic domain | 259 | 72.69 | 5 | 9 | 0.14 | Y |
| g3867 | Glycolipid anchoredsurface protein (GAS1) | 252 | 49.54 | 3 | 6 | 0.21 | Y |
| g10551 | Glycosylhydrolases family 16 | 235 | 46.11 | 4 | 27 | 0.51 | Y |
| g8790 | Unknown | 225 | 15.01 | 3 | 10 | 1.27 | Y |
| g4057 | Unknown | 218 | 26.91 | 6 | 26 | 0.6 | Y |
| g12686 | Laminin_G_2 LamininG domain | 208 | 29.63 | 4 | 9 | 0.53 | Y |
| g4142 | Unknown | 207 | 18.56 | 3 | 17 | 0.95 | Y |
| g7538 | Isochorismatase | 197 | 27.15 | 3 | 15 | 0.41 | N |
| g1131 | Unknown | 189 | 32.68 | 2 | 5 | 0.21 | Y |
| g3481 | Squalene epoxidase | 183 | 86.96 | 2 | 7 | 0.16 | N |
| g7658 | Cerato-platanin | 164 | 23.72 | 2 | 4 | 0.3 | Y |
| g2029 | Glyceraldehyde 3-phosphatedehydrogenase,C-terminal domain | 158 | 36.21 | 2 | 8 | 0.19 | Y |
| g1554 | Beta-glucosidase(SUN family) | 136 | 47.22 | 2 | 3 | 0.14 | Y |
| g7569 | Eukaryotic-typecarbonic anhydrase | 120 | 33.89 | 5 | 10 | 0.6 | Y |
| g6537 | Cis-muconatelactonizing enzyme | 113 | 40.78 | 4 | 8 | 0.37 | Y |
| g6259 | Glycosyl hydrolasefamily 45 | 101 | 30.09 | 2 | 6 | 0.11 | Y |
| g8786 | Unknown | 90 | 146.50 | 2 | 2 | 0.05 | N |
| g5055 | Ubiquitin Ubiquitin family | 84 | 113.49 | 3 | 8 | 0.06 | N |
| g8971 | Chitosanase Fungalchitosanase | 71 | 32.54 | 2 | 2 | 0.1 | Y |
| g2888 | N terminal extensionof bacteriophageendosialidase | 68 | 87.69 | 2 | 3 | 0.08 | Y |
| g2768 | Unknown | 51 | 15.18 | 2 | 2 | 0.5 | Y |
| g8551 | Trypan_PARP(Procyclic acidic repetitive protein) | 50 | 69.16 | 2 | 5 | 0.1 | Y |
| g12211 | Peptidase family M28 | 40 | 38.73 | 2 | 2 | 0.18 | N |
Protein identification numbers are same as the gene IDs of the F. virguliforme genome database (http://fvgbrowse.agron.iastate.edu) [56].
Number of times a peptide(s) was identified in one biological replicate of the culture filtrate.
Exponentially modified protein abundance index.
F. virguliforme proteins that were identified from the xylem sap of F. virguliforme-infected soybean plants (Table 1).
Soybean Proteins Detected in the Xylem Sap Samples Collected from the F. virguliforme Infected or Uninfected Plants
Over 500 soybean proteins were identified from the xylem saps collected from F. virguliforme-infected and -uninfected plants by conducting LC-ESI-MS/MS. The high number of protein detection could be due to the slightly high percentage of false discovery rate (FDR). The FDR percentage ranged from 2–3.3% among the different biological replicates. Details of the Glycine max accession numbers, GO annotations, as well as the biological replications from which these proteins were identified are provided (Table S3). Of these, 129 proteins were identified at least 80% of the time from sap samples of both F. virguliforme-infected and -uninfected plants (Table S4). However, at least two peptides were detected only for 112 soybean proteins (Table 3). Fifty of these proteins possessed predicted N-terminal secretory signal peptides. The most abundant proteins commonly found in the xylem saps of both F. virguliforme-infected and -uninfected plants were protease inhibitor/seed storage/lipid-transfer protein (LTP) family proteins (Table 3). Gamma-glutamyl hydrolase, 50S ribosomal proteins, trypsin and protease inhibitor protein, and peroxidases were some of the soybean proteins commonly found in sap samples of both F. virguliforme-infected and -uninfected plants.
Table 3. Soybean proteins identified from the xylem saps of both F. virguliforme-infected and -uninfected, healthy soybean plants.
| Protein IDa | Description | Proteinscore | Proteinmass (kDa) | # ofpeptides | emPAIb | Signalpeptide |
| Glyma18g41320.1 | Proteaseinhibitor/seed storage/LTP family | 3281 | 13.0 | 7 | 134.77 | Y |
| Glyma08g21410.1 | 50S ribosomal protein | 2005 | 29.4 | 17 | 13.58 | Y |
| Glyma07g01730.1 | 50S ribosomal protein | 1684 | 29.2 | 20 | 13.87 | Y |
| Glyma06g45700.1 | Glycosyl hydrolase family 14 | 1161 | 55.8 | 20 | 2.17 | N |
| Glyma13g34290.1 | Gamma-glutamyl hydrolase | 1073 | 37.8 | 13 | 3.52 | Y |
| Glyma02g07140.1 | Ribonuclease T2 family | 630 | 27.0 | 8 | 2.6 | Y |
| Glyma05g22180.1 | Peroxidase | 571 | 35.9 | 12 | 2.75 | Y |
| Glyma08g45490.1 | Trypsin andprotease inhibitor | 506 | 22.0 | 10 | 5.33 | Y |
| Glyma07g17000.2 | Protease inhibitor/seedstorage/LTP family | 492 | 11.1 | 6 | 4.07 | Y |
| Glyma16g04240.1 | Methionine synthase II(Cobalamine- independent) | 480 | 84.4 | 23 | 0.84 | N |
| Glyma17g17730.1 | Peroxidase | 479 | 36.0 | 11 | 2.75 | Y |
| Glyma03g16620.3 | Protease inhibitor/seed storage/LTP family | 459 | 13.9 | 7 | 10.22 | Y |
| Glyma12g06910.1 | Heat shockprotein 70 kDa | 445 | 75.6 | 11 | 0.67 | N |
| Glyma12g19520.1 | Malate dehydrogenase | 389 | 36.4 | 11 | 1.01 | N |
| Glyma03g32850.1 | 70 kDa heatshock protein | 357 | 71.9 | 11 | 0.64 | N |
| Glyma04g14650.1 | Acyl CoAbinding protein | 348 | 10.1 | 5 | 9.6 | N |
| Glyma06g15030.1 | Peroxidase | 323 | 35.0 | 8 | 0.89 | Y |
| Glyma16g04770.1 | Nucleoside phosphatase | 305 | 50.8 | 9 | 0.55 | Y |
| Glyma19g37520.1 | Enolase | 305 | 48.0 | 12 | 0.82 | N |
| Glyma02g00810.1 | Cytosolic malatedehydrogenase | 297 | 35.9 | 6 | 1.03 | N |
| Glyma09g34770.1 | Acyl CoA binding protein | 293 | 10.1 | 5 | 9.6 | N |
| Glyma18g52610.1 | Cu/Zn superoxide dismutase | 281 | 65.6 | 17 | 0.47 | N |
| Glyma10g40720.1 | Plant Basic Secretory Protein | 280 | 26.9 | 6 | 0.79 | Y |
| Glyma19g30140.1 | Calmodulin | 273 | 15.6 | 3 | 0.8 | N |
| Glyma17g11790.1 | Purple acidphosphatase-like protein | 260 | 59.7 | 4 | 0.31 | N |
| Glyma18g41590.1 | Proteaseinhibitor/seed storage/LTP family | 258 | 11.1 | 3 | 1.95 | Y |
| Glyma05g28490.1 | Glycine/serine hydroxymethyltransferase | 256 | 53.3 | 8 | 0.43 | N |
| Glyma08g11480.1 | S-adenosylhomocysteine hydrolase | 248 | 53.8 | 9 | 0.35 | N |
| Glyma08g18760.1 | TCP-1/cpn60 chaperonin family | 248 | 53.8 | 7 | 0.35 | N |
| Glyma03g04960.1 | Proteaseinhibitor/seed storage/LTP family | 239 | 12.9 | 6 | 4.21 | Y |
| Glyma11g14950.1 | 70 kD heat shock protein | 230 | 23.4 | 4 | 0.95 | N |
| Glyma04g39475.1 | Alginate lyase | 228 | 24.6 | 5 | 0.66 | Y |
| Glyma12g06606.1 | ribulose-bisphosphatecarboxylase large chain | 216 | 50.9 | 23 | 0.47 | N |
| Glyma17g35720.1 | Cysteine proteinase Cathepsin L | 214 | 52.6 | 3 | 0.2 | Y |
| Glyma13g16590.1 | Peroxidase | 213 | 36.1 | 8 | 1.02 | Y |
| Glyma07g13710.1 | Nucleosidediphosphate kinase | 211 | 16.5 | 5 | 1.11 | N |
| Glyma17g03350.1 | Pathogenesis-relatedprotein Bet v I family | 204 | 16.8 | 3 | 0.74 | N |
| Glyma07g39120.1 | Lactoylglutathione Lyase | 196 | 31.7 | 2 | 0.1 | N |
| Glyma02g09780.2 | Proteaseinhibitor/seed storage/LTP family | 195 | 10.4 | 5 | 4.58 | Y |
| Glyma12g07780.3 | Peroxidase | 193 | 27.2 | 5 | 0.78 | N |
| Glyma06g10650.2 | Tyrosine3-monooxygenase/tryptophan 5-monooxygenaseactivation protein | 192 | 29.3 | 8 | 0.91 | N |
| Glyma08g45531.1 | Trypsin and proteaseinhibitor | 187 | 24.4 | 6 | 1.17 | N |
| Glyma06g28890.1 | Peroxidase | 187 | 35.6 | 5 | 0.31 | Y |
| Glyma15g19580.1 | Cysteine proteinaseCathepsin L | 178 | 39.7 | 4 | 0.38 | Y |
| Glyma10g43990.1 | Transketolase precursor | 175 | 18.5 | 4 | 0.96 | N |
| Glyma11g10240.1 | Pollen allergen | 175 | 52.1 | 4 | 0.42 | Y |
| Glyma08g19180.1 | Peroxidase | 170 | 35.1 | 9 | 0.57 | Y |
| Glyma05g38130.1 | Thaumatin family | 166 | 22.4 | 7 | 2.06 | N |
| Glyma13g41960.1 | Fructokinase | 164 | 18.6 | 2 | 0.4 | N |
| Glyma12g07370.1 | Fasciclin domain | 160 | 30.7 | 5 | 0.51 | Y |
| Glyma16g08040.1 | Zinc-binding oxidoreductase | 158 | 34.6 | 8 | 0.9 | N |
| Glyma14g35530.1 | Plastocyanin-like domain | 153 | 21.2 | 3 | 0.55 | Y |
| Glyma07g00900.1 | Lipoxygenase | 151 | 96.9 | 9 | 0.26 | N |
| Glyma01g32750.1 | Proteaseinhibitor/seed storage/LTP family | 144 | 12.2 | 3 | 2.45 | Y |
| Glyma07g04960.1 | Subtilisin/Kexin-RelatedSerine Protease | 142 | 82.3 | 2 | 0.08 | Y |
| Glyma04g04730.1 | Subtilisin/Kexin-Related Serine Protease | 142 | 82.3 | 3 | 0.08 | Y |
| Glyma11g10480.2 | Cyclophilin | 141 | 18.4 | 5 | 0.57 | N |
| Glyma06g47510.2 | Ribosomal protein L16 | 138 | 21.0 | 2 | 0.35 | N |
| Glyma10g13450.1 | Lectin | 134 | 30.2 | 6 | 0.69 | Y |
| Glyma18g53150.1 | Proteaseinhibitor/seed storage/LTP family | 134 | 9.4 | 2 | 0.87 | Y |
| Glyma04g02240.1 | Plastocyanin | 130 | 16.6 | 4 | 1.52 | N |
| Glyma05g37490.1 | Glycosylhydrolases family 28 | 128 | 26.7 | 3 | 0.6 | N |
| Glyma05g35970.1 | Profilin | 125 | 14.1 | 2 | 0.24 | N |
| Glyma03g40110.1 | 40S ribosomal protein S14 | 122 | 16.4 | 3 | 0.37 | N |
| Glyma05g30380.1 | Plastocyanin-like domain | 115 | 13.2 | 4 | 2.98 | Y |
| Glyma02g07150.1 | Ribonuclease T2 family | 115 | 27.1 | 7 | 2.19 | Y |
| Glyma13g42330.1 | Lipoxygenase | 113 | 69.0 | 9 | 0.44 | N |
| Glyma16g00410.1 | Trypsin and protease inhibitor | 112 | 56.0 | 4 | 0.12 | N |
| Glyma12g08310.1 | Mitochondrialchaperonin | 110 | 52.9 | 6 | 0.17 | N |
| Glyma10g07820.1 | Monodehydroascorbate/ferredoxinreductase | 109 | 43.7 | 5 | 0.24 | N |
| Glyma02g05350.1 | ferredoxin–NADP+ reductase [ | 109 | 40.6 | 3 | 0.17 | N |
| Glyma04g14640.4 | 60S ribosomalprotein L13 | 108 | 23.8 | 2 | 0.14 | N |
| Glyma13g17421.1 | Glycosyltransferase | 105 | 79.1 | 10 | 0.24 | N |
| Glyma12g02790.2 | Cyclophilin | 104 | 18.4 | 2 | 0.66 | N |
| Glyma08g41280.1 | 60S ribosomalprotein L34 | 102 | 13.8 | 2 | 0.56 | N |
| Glyma19g42890.1 | Hsp70 protein | 102 | 62.0 | 4 | 0.14 | N |
| Glyma06g02481.1 | Subtilisin/Kexin-Related Serine Protease | 100 | 82.2 | 6 | 0.17 | Y |
| Glyma12g16340.1 | Plastocyanin-likedomain | 99 | 22.6 | 2 | 0.32 | Y |
| Glyma15g04670.1 | 60S ribosomal protein L23A | 98 | 17.3 | 2 | 0.71 | N |
| Glyma15g04805.1 | Histone H4 | 97 | 11.3 | 3 | 1.22 | N |
| Glyma17g06090.1 | Peroxidase | 95 | 36.4 | 6 | 0.55 | Y |
| Glyma02g18090.1 | Lectin | 94 | 22.6 | 6 | 0.74 | Y |
| Glyma05g14330.2 | Proteasomesubunit | 93 | 24.2 | 4 | 0.22 | N |
| Glyma04g01020.1 | Fructose-biphosphatealdolase | 92 | 14.1 | 2 | 0.54 | N |
| Glyma16g17190.1 | Pectin acetylesterase | 91 | 43.9 | 2 | 0.16 | Y |
| Glyma15g09530.1 | GDSL-like Lipase/Acylhydrolase | 91 | 43.1 | 8 | 0.81 | Y |
| Glyma06g03410.1 | Nad DependentEpimerase/Dehydratase | 89 | 34.3 | 7 | 0.74 | N |
| Glyma17g17850.1 | aspartateaminotransferase | 87 | 53.0 | 5 | 0.19 | N |
| Glyma08g43690.1 | 40S ribosomal protein S8 | 86 | 25.1 | 3 | 0.28 | N |
| Glyma10g28890.2 | Calreticulin | 81 | 48.3 | 5 | 0.3 | Y |
| Glyma14g36850.1 | Fructose-biphosphate aldolase | 81 | 38.5 | 9 | 0.78 | N |
| Glyma02g05370.1 | 40S ribosomal protein S4 | 73 | 29.8 | 4 | 0.37 | N |
| Glyma08g21390.1 | 60S ribosomal protein L10 | 72 | 29.0 | 2 | 0.24 | Y |
| Glyma11g21001.1 | Dirigent-like protein | 71 | 21.2 | 4 | 0.34 | Y |
| Glyma11g13580.1 | fructokinase | 71 | 35.5 | 2 | 0.09 | N |
| Glyma11g38220.1 | PLAT/LH2 family protein | 70 | 20.4 | 6 | 0.16 | Y |
| Glyma16g01090.1 | Subtilisin/Kexin-Related Serine Protease | 70 | 86.6 | 2 | 0.08 | N |
| Glyma14g10456.2 | Glycosyl hydrolase family 10 | 70 | 109.9 | 3 | 0.12 | N |
| Glyma17g33050.1 | Demethylmenaquinone methyltransferase | 70 | 59.9 | 5 | 0.08 | Y |
| Glyma16g33710.1 | Trypsin andprotease inhibitor | 69 | 23.9 | 4 | 0.48 | Y |
| Glyma13g39600.1 | Serine carboxypeptidase | 67 | 51.2 | 7 | 0.45 | Y |
| Glyma03g23890.1 | Zinc-binding dehydrogenase | 67 | 38.1 | 6 | 0.09 | N |
| Glyma06g18110.1 | Glyceraldehyde 3-phosphate dehydrogenase | 65 | 36.8 | 13 | 1.58 | N |
| Glyma13g19830.1 | GlutathioneS-transferase | 65 | 27.1 | 2 | 0.26 | N |
| Glyma12g31850.3 | Carboxymethylenebutenolidase | 63 | 21.9 | 2 | 0.23 | N |
| Glyma18g47760.1 | Hsp70 protein | 58 | 28.9 | 2 | 0.17 | N |
| Glyma08g45610.1 | Trypsin andprotease inhibitor | 52 | 26.2 | 3 | 0.43 | Y |
| Glyma03g26060.1 | Plastocyanin-likedomain | 45 | 19.3 | 3 | 0.62 | Y |
| Glyma20g26610.1 | Plant basicsecretory protein | 44 | 25.3 | 9 | 1.7 | Y |
| Glyma18g44810.1 | Cellulase(glycosyl hydrolase family 5) | 42 | 52.8 | 5 | 0.27 | Y |
| Glyma03g22260.1 | Protein ofunknown function(DUF568) | 38 | 25.7 | 2 | 0.28 | Y |
| Glyma15g04290.1 | Triosephosphateisomerase | 33 | 27.4 | 7 | 0.77 | N |
Glycine max protein identification number from Phytozome database (http://www.phytozome.net/search.php).
Exponentially modified protein abundance index.
Soybean Proteins that were Specific to the Xylem Sap Samples Collected from Plants, Either Infected or Uninfected with F. virguliforme
This study identified six soybean proteins that were only detected in xylem sap of F. virguliforme-infected plants; whereas, five soybean proteins only in healthy, F. virguliforme-uninfected plants. These proteins were detected in at least two out of the five biological replications of xylem sap samples collected from either F. virguliforme-infected or F. virguliforme-uninfected plants (Table 4 A, B). Only one infection induced proteins contained a predicted N-terminal secretory signal. Several stress related proteins were detected in the xylem sap of the infected plants but not in the sap of the uninfected plants. Three pathogenicity related proteins, glucan 1-3- β-glucosidase related protein, and NADP+ dependent malic enzyme were among the proteins identified from the infected plants (Table 4A). Pathogenicity related family 5 (PR5) protein was the most abundant of the six proteins identified from sap of the infected plants. Serine carboxypeptidase, disulfide oxidoreductase, and citrate synthase were among the proteins only detected in the sap of the healthy, F. virguliforme-uninfected soybean plants (Table 4B).
Table 4. Soybean proteins differentially accumulated in the F. virguliforme-infected (A) or F. virguliforme-uninfected (B) soybean plants.
| Protein IDa | Description | Protein score | Protein Size (kDa) | # of peptides | Times identifiedb | emPAIc | Signal peptide |
| (A) | |||||||
| Glyma11g10080.1 | Glucan 1,3-beta-glucosidase-related | 64 | 25.64 | 2 | 3 | 0.13 | N |
| Glyma15g02230.1 | NADP+-dependent malic enzyme | 68 | 73.75 | 2 | 2 | 0.09 | N |
| Glyma17g03340.1 | Pathogenesis-related protein Bet v I family | 109 | 17.19 | 3 | 3 | 0.43 | N |
| Glyma17g04040.1 | Plant invertase/pectin methylesterase inhibitor | 66 | 19.88 | 2 | 2 | 0.17 | Y |
| Glyma05g38110.1 | pathogenesis-related thaumatin-like protein (PR5) | 217 | 13.2 | 4 | 3 | 1.51 | N |
| Glyma07g37240.2 | Pathogenesis-related protein Bet v I family | 315 | 1.09 | 4 | 4 | 1.09 | N |
| (B) | |||||||
| Glyma13g39730.1 | Serine carboxypeptidases (lysosomal cathepsin A) | 35 | 33.03 | 2 | 2 | 0.21 | N |
| Glyma05g28480.2 | Adenosylhomocysteinase | 77 | 53.73 | 5 | 2 | 0.2 | N |
| Glyma06g19820.1 | Aldehyde dehydrogenase-related | 50 | 55.39 | 2 | 2 | 0.12 | N |
| Glyma08g02100.1 | Disulfide oxidoreductase | 112 | 52.42 | 2 | 3 | 0.13 | N |
| Glyma15g15020.1 | Citrate synthase | 65 | 66.34 | 3 | 2 | 0.05 | N |
Glycine max protein identification number from Phytozome database.
Number of times the peptides were identified among five biological replications.
Exponentially modified protein abundance index.
GO Annotation and KEGG Pathway Analyses Revealed that Xylem Saps of Soybean Plants are Active in Carbon and Amino Acid Metabolisms
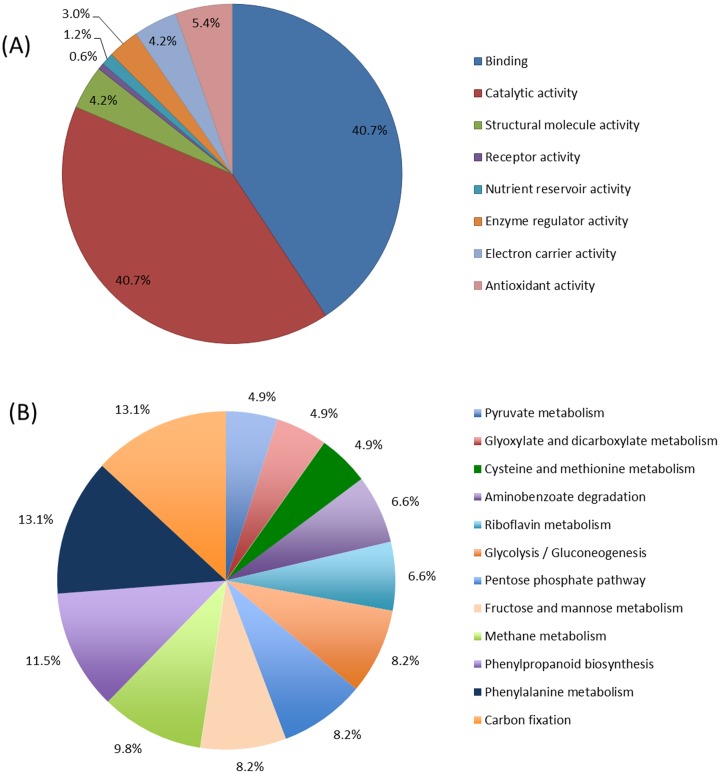
To determine the type metabolic activities in the soybean xylem saps, the sequences of the 112 putative soybean xylem sap proteins detected in xylem sap samples of both F. virguliforme-infected and F. virguliforme-uninfected plants were further subjected to functional annotation in GO terms. Most of these proteins were involved in more than one biological process and had more than one molecular function (Tables S5). Based on the sequence distribution with a cutoff of 5, majority of the 112 common xylem sap proteins showed binding and catalytic activities (Figure 3A). Among the 112 proteins that were assigned with enzyme commissions (EC), 44 metabolic pathways including phenylalanine metabolism, glycolysis, methane metabolism, carbon fixation, and phenylpropanoid biosynthesis were observed (Figure 3B, Table S6). The soybean xylem sap in general shown to be active in both carbon and amino acid metabolisms.
Figure 3. Classification of the 112 most abundant proteins identified from xylem saps of both F. virguliforme-infected and F. virguliforme-uninfected soybean plants based on molecular function.
(A) Percentage of proteins in different functional categories at ontology level 2, with a cutoff of 5. (B) Secondary functional categories based on KEGG pathway. Only the prominent pathways with a sequence cutoff of 3 are reported here.
Discussion
Sudden death syndrome is considered to be caused by one or more toxins released by F. virguliforme to the infected soybean roots because the root pathogen has never been detected in the above ground tissues showing foliar SDS. We have shown that FvTox1 is a major virulence factor that is involved in foliar SDS in soybean [13], [16], [17]. The foliar SDS was not completely absent in fvtox1 infected soybean plants [17] suggesting that additional toxins may be involved in foliar SDS development; and it is conceivable that such toxins may be present in xylem sap of infected soybean plants. Therefore, the main objective of this investigation was to identify those possible F. virguliforme proteins by studying the xylem sap of F. virguliforme-infected soybean seedlings that showed foliar SDS. In addition, we investigated if there are changes in proteomes of xylem sap of soybean plants following F. virguliforme infection.
In this investigation we identified five F. virguliforme secreted proteins, including one with similarity to a known phytotoxin, cerato-platanin, from the xylem sap of infected soybean plants. This observation strengthens the previous speculations that the pathogen uses the vascular system to transport host-selective toxins to the above ground plant parts to cause foliar SDS. We, however, have to establish the functions of these putative proteinacious toxins by generating knockout mutants to establish their roles in generating foliar SDS in soybean.
We failed to detect FvTox1 from the xylem sap suggesting that our system is not sensitive enough to detect all proteins or peptides of the xylem sap. FvTox1 was localized to chloroplasts (H.K. Brar and M.K. Bhattacharyya, unpublished) and shown to be involved in SDS development in foliar tissues [16]. Most likely FvTox1 is produced at much lower concentration which was insufficient for detection in our LC-ESI-MS/MS study. This could also be the reason for not detecting the five F. virguliforme proteins in all five xylem sap replicates of the infected soybean plants. All five xylem sap F. virguliforme proteins were detected in the CF proteome suggesting that they all have functional signal peptides for excretion; and most likely they were excreted by the pathogen into the infected roots for uploading into the xylem vessels.
LC-ESI-MS/MS used in this study does not detect peptides that are smaller than seven amino acids. Therefore, if there were any small nonribosomal phytotoxic peptides in the xylem sap, then those were not identified in our study.
Cerato-platanin is a phytotoxin produced by the ascomycete fungus Ceratocystis fimbriata f. sp. platani that causes canker stain disease in the European plane tree (Platanus acerifolia). This 12.4 kDa proteinaceous toxin, the first member of the cerato-platanin protein family, was identified from the culture filtrate of C. fimbriata f. sp. platani [41], [42]. Cerato-platanin toxin is considered to be a pathogen associated molecular pattern (PAMP) because it can induce defense related responses in the host plant [43]. Proteins belonging to the cerato-platanin protein family can enhance plant defenses by inducing defense related genes, phytoalexins synthesis, and initiating cell death [43], [44], [45], [46]. We identified a cerato-platanin-like protein from both xylem sap and F. virguliforme (Fv) culture filtrates. Investigation of mutants for the gene encoding this cerato-platanin-like protein will assist us in determining the function of this protein in F. virguliforme.
This xylem sap study, in addition to identifying candidate pathogenicity F. virguliforme proteins, detected pathogenicity related soybean protein families (Table 4A). Beta-1,3-glucanase is a pathogenic related family 2 (PR-2) type protein known to be secreted upon pathogen attack and shown to be present in the xylem sap of pathogen infected plants [26], [47],[48]. This protein has the ability to inhibit the fungal growth by degrading β-1,3-glucans of the fungal cell wall [49], [50]. Cytosolic isoforms of NADP malic enzyme are known to be involved in plant defense responses [51], and an NADP-dependent malic enzyme was detected in the xylem sap of F. virguliforme-infected soybean plants (Table 4A). The expression of pathogenicity-related Bet v I family proteins (PR10) are known to be induced during wounding, abiotic stress, or pathogen infection. The possible roles of these proteins in the soybean- F. virguliforme interaction are yet to be established.
Most of the soybean proteins identified in this study were previously reported in the xylem sap of other plant species including soybean [31], [32], [33]. Xylem saps have been shown to contain peroxidases, which may play a role in plugging damaged vascular tissue [52]. We observed peroxidases in the xylem saps of both infected and healthy plants. Usually peroxidases are produced in response to biotic stresses; but some peroxidases are not specific to infection. Even though found in the xylem sap, the origin of these proteins is still questionable. The xylem sap collection method could impose stress on the plants. Detection of stress-induced proteins such as ribonucleases from both diseased and healthy plants could therefore have resulted from the stress associated with the xylem sap collection method.
Ligat et al. [53] has shown that xylem sap proteins can be produced in the root tips and then loaded into the xylem sap. Other studies have also suggested these proteins to be synthesized in the roots [29], [52], [54]. Hence it is possible the xylem sap proteins identified in this study are in fact secreted by the root tissues and loaded into the xylem sap. Some of these sap soybean proteins could have been synthesized to defend F. virguliforme infection. Pathogenicity related (PR) proteins are implicated as plant defense molecules and were identified in the xylem sap of F. virguliforme-infected plants. Further study is needed to determine if PR genes are transported long distance as a defense arsenal and involved in defending soybean against F. virguliforme and other pathogens.
Conclusion
This study identified five secreted F. virguliforme proteins from the xylem sap of soybean plants infected with F. virguliforme. These proteins were also found to be secreted by F. virguliforme into the culture medium. This study provides evidence that F. virguliforme secreted proteins travel through the xylem sap. The presence of a protein with high similarity to the phytotoxin, cerato-platanin in xylem sap of the F. virguliforme-infected plants shows that multiple host-selective toxins are produced by F. virguliforme and could be responsible for the foliar SDS development. We have also detected 112 soybean proteins in xylem saps of at least eight of the 10 replications. Most importantly we have identified four types of pathogenicity-related defense proteins only from the xylem sap of F. virguliforme-infected soybean plants. Thus, this study laid the foundation for studying the molecular basis of foliar SDS development in soybean and possible defense mechanisms that may be involved in conferring immunity against F. virguliforme and other soybean pathogens.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository [55] with the dataset identifier PXD000873.
Supporting Information
Peptides of five F. virguliforme proteins that were detected in the xylem sap of the infected soybean plants.
(XLSX)
Peptides of F. virguliforme proteins that are excreted to the culture medium. First five proteins were identified in xylem sap of the F. virguliforme -infected plants.
(XLSX)
Gene Ontology (GO) annotation of over 500 soybean proteins identified from the xylem sap.
(XLSX)
Detection of over 500 soybean proteins across 10 biological replications of xylem sap: (i) five replications of F. virguliforme -uninfected tissues and (ii) five replications of F. virguliforme -infected tissues. 0, not detected; 1, detected.
(XLSX)
Blast2Go results for the 112 soybean proteins identified in the xylem saps of both F. virguliforme -infected and F. virguliforme -uninfected soybean plants.
(XLSX)
Analyses for KEGG pathway of the 112 most abundant soybean proteins identified from both F. virguliforme -infected and F. virguliforme -uninfected soybean plants.
(XLSX)
Acknowledgments
The authors wish to thank Dr. Sheng Zhang and Mr. James McCardle at Cornell University Proteomics and Mass Spectrometry Core Facility for conducting the nano LC-ESI-MS/MS analysis of the samples. We thank Ms. Katelynn Davis for technical assistance, Dr. Prashant Singh and Ms. Jordan Baumbach for reviewing the manuscript, and the PRIDE support team for assistance in uploading the dataset.
Funding Statement
The authors are thankful to United Soybean Board (USB) for funding this project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ploper D (1993) Síndrome de la muerte súbita: nueva enfermedad de la soja en el noroeste argentino. Avance Agroindustrial Año. 13: 5–9. [Google Scholar]
- 2. Anderson TR, Tenuta AU (1998) First report of Fusarium solani f. sp. glycines causing sudden death syndrome of soybean in Canada. Plant Dis 82: 448. [DOI] [PubMed] [Google Scholar]
- 3. Nakajima T, Mitsueda T, Charchar MJD (1996) First occurrence of sudden death syndrome of soybean in Brazil. Jpn Agric Res Q 30: 31–34. [Google Scholar]
- 4. Wrather JA, Koenning SR (2006) Estimates of disease effects on soybean yields in the United States 2003 to 2005. J Nematol 38: 137–180. [PMC free article] [PubMed] [Google Scholar]
- 5. Butzen S (2010) Sudden death syndrome of soybean. Crop Insights 19: 1–4. [Google Scholar]
- 6.Babadoost M (1997) Sudden death syndrome of soybeans. RPD No. 512. http://ipm.illinois.edu/diseases/series500/rpd512/.
- 7.Wrather JA (2011) Soybean disease loss estimates for the United States, 1996–2010. http://aes.missouri.edu/delta/research/soyloss.stm. [DOI] [PubMed]
- 8. Aoki T, O’Donnell K, Homma Y, Lattanzi AR (2003) Sudden death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex-F. virguliforme in North America and F. tucumaniae in South America. Mycologia 95: 660–684. [PubMed] [Google Scholar]
- 9. Aoki T, O’Donnell K, Scandiani MM (2005) Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp nov, F. cuneirostrum sp nov, F. tucumaniae and F. virguliforme . Mycoscience 46: 162–168. [Google Scholar]
- 10. Li S, Hartman GL, Widholm JM (1999) Viability staining of soybean suspension-cultured cells and a seedling stem cutting assay to evaluate phytotoxicity of Fusarium solani f. sp glycines culture filtrates. Plant Cell Rep 18: 375–380. [Google Scholar]
- 11. Roy KW, Lawrence GW, Hodges HH, McLean KS, Killebrew JF (1989) Sudden death syndrome of soybean: Fusarium solani as incident and relation of Heterodera glycines to disease severity. Phytopathology 79: 191–197. [Google Scholar]
- 12. Rupe JC (1989) Frequency and pathogenecity of Fusarium solani recovered from soybean with sudden death syndrome. Plant Dis 73: 581–584. [Google Scholar]
- 13. Brar HK, Swarminathan S, Bhattacharyya MK (2011) The Fusarium virguliforme toxin FvTox1 causes foliar sudden death syndrome-like symptoms in soybean. Mol Plant Microbe Interact 24: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 14. Ji J, Scott MP, Bhattacharyya MK (2006) Light is essential for degradation of ribulose-15-bisphosphate carboxylase-oxygenase large subunit during sudden death syndrome development in soybean. Plant Biol 8: 597–605. [DOI] [PubMed] [Google Scholar]
- 15. Jin H, Hartman GL, Nickell CD, Widholm JM (1996) Characterization and purification of a phytotoxin produced by Fusarium solani the causal agent of soybean sudden death syndrome. Phytopathology 86: 277–282. [Google Scholar]
- 16. Brar HK, Bhattacharyya MK (2012) Expression of a single-chain variable-fragment antibody against a Fusarium virguliforme toxin peptide enhances tolerance to sudden death syndrome in transgenic soybean plants. Mol Plant Microbe Interact 25: 817–824. [DOI] [PubMed] [Google Scholar]
- 17.Pudake RN, Swaminathan S, Sahu BB, Leandro LF, Bhattacharyya MK (2013) Investigation of the Fusarium virguliforme fvtox1 mutants revealed that the FvTox1 toxin is involved in foliar sudden death syndrome development in soybean. Curr Genet: DOI:10-1007/s00294-013-0392-z. [DOI] [PubMed]
- 18. Kav NNV, Srivastava S, Yajima W, Sharma N (2007) Application of proteomics to investigate plant-microbe interactions. Curr proteomics 4: 28–43. [Google Scholar]
- 19. Mehta A, Brasileiro ACM, Souza DSL, Romano E, Campos MA, et al. (2008) Plant-pathogen interactions: what is proteomics telling us? FEBS J 275: 3731–46. [DOI] [PubMed] [Google Scholar]
- 20. Dafoe NJ, Constabel CP (2009) Proteomic analysis of hybrid poplar xylem sap. Phytochem 70: 856–63. [DOI] [PubMed] [Google Scholar]
- 21. Aki T, Shigyo M, Nakano R, Yoneyama T, Yanagisawa S (2008) Nano scale proteomics revealed the presence of regulatory proteins including three FT-like proteins in phloem and xylem saps from rice. Plant Cell Physiol 49: 767–90. [DOI] [PubMed] [Google Scholar]
- 22. Kehr J, Buhtz A, Giavalisco P (2005) Analysis of xylem sap proteins from Brassica napus . BMC Plant Biol 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou W, Eudes F, Laroche A (2006) Identification of differentially regulated proteins in response to a compatible interaction between the pathogen Fusarium graminearum and its host Triticum aestivum . Proteomics 6: 4599–4609. [DOI] [PubMed] [Google Scholar]
- 24. Houterman PM, Speijer D, Dekker HL, De Koster CG, Cornelissen BJC, et al. (2007) The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol Plant Pathol 8: 215–221. [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Bricker TM, Lefevre M, Pinson SRM, Oard JH (2006) Proteomic and genetic approaches to identifying defence-related proteins in rice challenged with the fungal pathogen Rhizoctonia solani. . Mol Plant Pathol 7: 405–416. [DOI] [PubMed] [Google Scholar]
- 26. Rep M, Dekker HL, Vossen JH, de Boer AD, Houterman PM, et al. (2002) Mass spectrometric identification of isoforms of PR proteins in xylem sap of fungus-infected tomato. Plant Physiol 130: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim ST, Kim SG, Hwang DH, Kang SY, Kim HJ, et al. (2004) Proteomic analysis of pathogen-responsive proteins from rice leaves induced by rice blast fungus Magnaporthe grisea . Proteomics 4: 3569–3578. [DOI] [PubMed] [Google Scholar]
- 28. Pos V, Halasz K, Mesterhazi A, Csosz L, Manninger K, et al. (2005) Proteomic investigation of wheat intercellular washing fluid. Proceedings of the 8th Hungarian congress on plant physiology and the 6th Hungarian conference on photosynthesis. Acta Biol Szegediensis 49: 31–32. [Google Scholar]
- 29. Rep M, Dekker HL, Vossen JH, de Boer AD, Houterman PM, et al. (2003) A tomato xylem sap protein represents a new family of small cysteine-rich proteins with structural similarity to lipid transfer proteins. FEBS Lett 534: 82–86. [DOI] [PubMed] [Google Scholar]
- 30. Alvarez S, Goodger JQD, Marsh EL, Chen MS, Asirvatham VS, et al. (2006) Characterization of the maize xylem sap proteome. J Prot Res 5: 963–972. [DOI] [PubMed] [Google Scholar]
- 31. Djordjevic MA, Oakes M, Li DX, Hwang CH, Hocart CH, et al. (2007) The Glycine max xylem sap and apoplast proteome. J Prot Res 6: 3771–3779. [DOI] [PubMed] [Google Scholar]
- 32. Subramanian S, Cho U, Keyes C, Yu O (2009) Distinct changes in soybean xylem sap proteome in response to pathogenic and symbiotic microbe interactions. BMC Plant Biol 9: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krishnan HB, Natarajan SS, Bennett JO, Sicher RC (2011) Protein and metabolic composition of xylem sap from field-grown soybeans (Glycine max). Planta 233: 921–931. [DOI] [PubMed] [Google Scholar]
- 34. Li S, Hartman GL, Lee B-S, Widholm JW (2000) Identification of a stress-induced protein in stem exudates of soybean seedlings root-infected with Fusarium solani f. sp. glycines . Plant Physiol Biochem 38: 803–809. [Google Scholar]
- 35. Hartman GL, Huang YH, Nelson RL, Noel GR (1997) Germplasm evaluation of Glycine max for resistance to Fusarium solani, the causal organism of sudden death syndrome. Plant Dis 81: 515–518. [DOI] [PubMed] [Google Scholar]
- 36.Lightfoot DA, Gibson PT, Meksem K (2007) Method of determining soybean sudden death syndrome resistance in a soybean plant. U.S. Patent 7,288,386.
- 37. Ishihama Y, Yoda Y, Tabuta T, Sato T, Nagasu T, et al. (2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 4: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 38. Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods 8: 785–786. [DOI] [PubMed] [Google Scholar]
- 39. Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 40.Conesa A, Gotz S (2009) Blast2Go tutorial. www.blast2go.com/data/blast2go/b2g_tutorial_ 23062009.pdf.
- 41. Pazzagli L, Cappugi G, Manao G, Camici G, Santini A, et al. (1999) Purification of cerato-platanin a new phytotoxic protein from Ceratocystis fimbriata f. sp platani. . J Biol Chem 274: 24959–24964. [DOI] [PubMed] [Google Scholar]
- 42. Pazzagli L, Pantera B, Carresi L, Zoppi C, Pertinhez TA, et al. (2006) Cerato-platanin the first member of a new fungal protein family: cloning expression and characterization. Cell Biochem Biophys 44: 512–521. [DOI] [PubMed] [Google Scholar]
- 43. de Oliveira AL, Gallo M, Pazzagli L, Benedetti CE, Cappugi G, et al. (2011) The structure of the elicitor Cerato-platanin (CP), the first member of the CP fungal protein family, reveals a double-barrel fold and carbohydrate binding. J Biol Chem 286: 17560–17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scala A, Pazzagli L, Comparini C, Santini A, Tegli S, et al. (2004) Cerato-platanin, an early-produced protein by Ceratocystis fimbriata f. sp. platani, elicits phytoalexin synthesis in host and non-host plants. J Plant Pathol 86: 23–29. [Google Scholar]
- 45. Fontana F, Santini A, Salvini M, Pazzagli L, Cappugi G, et al. (2008) Cerato-platanin treated plane leaves restrict Ceratocystis platani growth and overexpress defense-related genes. J Plant pathol 90: 295–306. [Google Scholar]
- 46. Bernardi R, Baccelli I, Carresi L, Comparini C, Pazzagli L, et al. (2011) Cerato-platanin elicits transcription of defence-related genes earlier than Ceratocystis platani on Platanus acerifolia. . Forest Pathol 41: 255–261. [Google Scholar]
- 47. Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, et al. (1993) Plant pathogenesis related proteins and their role in defense against pathogens. Biochimie 75: 687–706. [DOI] [PubMed] [Google Scholar]
- 48. Simmons CR (1994) The physiology and molecular biology of plant 1,3-beta-d-glucanases and 1,3;1,4-beta-d-glucanases. Crit Rev Plant Sci 13: 325–87. [Google Scholar]
- 49. Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, van den Elzen PJM, et al. (1993) Only specific tobacco (Nicotiana tabacum) chitinases and β-1,3-glucanases exhibit antifungal activity. Plant Physiol 101: 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, van den Elzen PJM, Cornelissen BJC (1991) Pathogen induced proteins with inhibitory activity towards Phytophthora infestans . The Plant cell 3: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schaaf J, Walter MH, Hess D (1995) Primary metabolism in plant defense (regulation of a bean malic enzyme gene promoter in transgenic tobacco by developmental and environmental cues). Plant Physiol 108: 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biles CL, Abeles FB (1991) Xylem sap proteins. Plant Physiol 96: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ligat L, Lauber E, Albenne C, Clemente HS, Valot B, et al. (2011) Analysis of the xylem sap proteome of Brassica oleracea reveals a high content in secreted proteins. Proteomics 11: 1798–1813. [DOI] [PubMed] [Google Scholar]
- 54. Sakuta C, Satoh S (2000) Vascular tissue-specific gene expression of xylem sap glycine rich proteins in root and their localization in the walls of metaxylem vessels in cucumber. Plant Cell Physiol 41: 627–638. [DOI] [PubMed] [Google Scholar]
- 55. Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O’Kelly G, Schoenegger A, Ovelleiro D, Perez-Riverol Y, Reisinger F, Rios D, Wang R, Hermjakob H (2013) The Proteomics Identifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41(D1): D1063–9 doi:10.1093/nar/gks1262. PubMed PMID: 23203882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Srivastava SK, Huang X, Brar HK, Fakhoury AM, Bluhm BH, Bhattacharyya MK (2014) The genome sequence of the fungal pathogen Fusarium virguliforme that causes sudden death syndrome in soybean. PLoS One 9: e81832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peptides of five F. virguliforme proteins that were detected in the xylem sap of the infected soybean plants.
(XLSX)
Peptides of F. virguliforme proteins that are excreted to the culture medium. First five proteins were identified in xylem sap of the F. virguliforme -infected plants.
(XLSX)
Gene Ontology (GO) annotation of over 500 soybean proteins identified from the xylem sap.
(XLSX)
Detection of over 500 soybean proteins across 10 biological replications of xylem sap: (i) five replications of F. virguliforme -uninfected tissues and (ii) five replications of F. virguliforme -infected tissues. 0, not detected; 1, detected.
(XLSX)
Blast2Go results for the 112 soybean proteins identified in the xylem saps of both F. virguliforme -infected and F. virguliforme -uninfected soybean plants.
(XLSX)
Analyses for KEGG pathway of the 112 most abundant soybean proteins identified from both F. virguliforme -infected and F. virguliforme -uninfected soybean plants.
(XLSX)