Abstract
Human birth weight is subject to stabilizing selection; babies born too small or too large are less likely to survive. Particular combinations of maternal/fetal immune system genes are associated with pregnancies where the babies are ≤5th birth weight centile. Specifically an inhibitory maternal KIR AA genotype with a paternally derived fetal HLA-C2 ligand. We have now analysed maternal KIR and fetal HLA-C combinations at the opposite end of the birth weight spectrum. 1316 mother/baby pairs were genotyped for maternal KIR as well as fetal and maternal HLA-C. Presence of a maternal activating KIR2DS1 gene associated with increased birth weight, in linear or logistic regression analyses of all pregnancies >5th centile (p=0.005, n=1316). Effect of KIR2DS1 was most significant in pregnancies where its ligand, HLA-C2, was paternally but not maternally inherited by a fetus (p=0.005, OR=2.65). Thus maternal KIR are more frequently inhibitory with small babies but activating with big babies. At both extremes of birth weight the KIR associations occur when their HLA-C2 ligand is paternally inherited by a fetus. We conclude that the two polymorphic immune gene systems, KIR and HLA-C, contribute to successful reproduction by maintaining birth weight between two extremes with a clear role for paternal HLA.
Introduction
Birth weight has been considered “perhaps the most clear cut example of a human character that is subject to stabilizing selection” and is pertinent for those studying genetic variants influencing human evolution, disease and natural selection.1 The size of a human infant at the end of gestation determines both the risk of obstetric complications as well as the chances of survival of the baby in the neonatal period.2,3 High maternal and fetal perinatal mortality rates are strongly associated with being too small or too large at birth, with the lowest perinatal mortality occurring very close to the mean of birth weight distribution.1,4,5 Mothers are also at risk at these two extremes: either from pre-eclampsia, a systemic syndrome triggered by poor placental perfusion, or from prolonged obstructed labour with the risk of post-partum haemorrhage and sepsis. Although genetic factors are known to be important, there is no consensus on what is the differential contribution of maternal and paternal genes to birth weight nor which genes are responsible.6–8 Fetal growth in utero partly depends on the development of a maternal blood supply to the placenta that requires structural modifications of the uterine spiral arteries. Arterial transformation is mediated by infiltration of placental trophoblast cells into the maternal decidua and arterial walls.9 A growing body of evidence indicates that this process, which must be carefully balanced between under- and over-trophoblast invasion, is controlled by the maternal uterine Natural Killer cells (uNK) that are a distinctive feature of the decidua during placentation.10
uNK express Killer Immunoglobulin-like Receptors (KIR) that can bind to HLA-C, the only classical HLA molecule expressed by trophoblast.11,12 Both KIR and HLA-C genes are polymorphic meaning that in each pregnancy maternal KIR and fetal HLA-C genetic combinations can differ. KIR are highly variable both in the number of genes on a haplotype as well as allelic polymorphism at individual KIR loci.13 Two main KIR haplotypes are found in all human populations, A and B. KIR A haplotypes carry fewer genes, most of which encode inhibitory receptors including KIR2DL1 and KIR2DL3 which bind HLA-C. KIR B haplotypes have varying numbers of additional, mainly activating receptors but only one, KIR2DS1, can bind to trophoblast HLA-C molecules.12 KIR distinguish between all HLA-C allotypes as two mutually exclusive epitopes, HLA-C1 or HLA-C2 (C1 or C2), defined by a dimorphism at position 80 of the α1 domain of the alpha helix.14 All C2 allotypes are bound specifically by KIR2DL1 and KIR2DS1. In white British the KIR A and KIR2DS1 haplotype frequencies are 60% and 20% respectively and the HLA-C2 allele frequency is nearly 30%.
Immunogenetic studies provide evidence for a co-operative interaction of uNK and trophoblast to regulate placentation. Three disorders of pregnancy - pre-eclampsia, fetal growth restriction (FGR) and recurrent miscarriage share a common primary pathogenesis of defective arterial transformation by trophoblast.12,15,16 These pregnancy disorders all associate with the same combinations of maternal KIR and fetal HLA-C genotypes: two maternal KIR A haplotypes (AA genotype), specifically when C2 is present in the fetus.12,17,18 In this scenario, strong inhibition of uNK will occur, mediated by KIR2DL1 (located on the KIR A haplotype) binding to trophoblast HLA-C2. The association appears particularly when a fetal C2 allele is inherited from the father.12 Conversely women who have the telomeric region of the KIR B haplotype, where the activating receptor for C2 (KIR2DS1) is located, are significantly protected from these pregnancy disorders when a fetal C2 allele is present.12 Thus, these disorders of inadequate arterial transformation show that strong uNK inhibition mediated by binding to C2+ trophoblast is detrimental to placentation and uNK activation counterbalances this effect. We have now analysed KIR and HLA genotypes of pregnancies across the birth weight spectrum. Our new findings indicate a balance of maternal KIR and fetal HLA-C genotype frequencies in the human population allows birth weight to be kept at an optimum for maternal and fetal survival.
Materials and Methods
Study design
This study was designed to test the hypothesis that the polymorphic immune system genes, KIR and HLA-C, influence human birth weight. We have shown that certain maternal KIR and fetal HLA-C genes were associated with pregnancy outcome where the birth weight was ≤5th centile in a UK population.12 To analyse KIR and HLA-C frequencies in pregnancies with birth weights >5th centile, we supplemented our UK dataset with a large number of pregnancies from the Norwegian MoBa cohort where over 90,000 well-documented pregnancies have been collected.19,20
Subjects
Details of the participants in our UK cohort study have been previously described.12,17 Briefly, these comprised 747 pregnancies with pre-eclampsia (defined by new hypertension >140/90 mmHg after week 20 together with new protein urea >300 mg/24 h), 118 pregnancies with fetal growth restriction (birth weight ≤5th centile) and 404 normal pregnancies of primiparous women. From the MoBa cohort 995 normal pregnancies were analysed. These were primigravida who had a single live born, normally formed infant with a gestation length of >259 days and <300 days determined from last menstrual period and confirmed by ultrasonogram. The mothers had no medical conditions including hypertension, renal disease, pre-eclampsia, thyroid disease, gestational diabetes or fetal hydrops. An additional 141 pregnancies with pre-eclampsia from the MoBa cohort were analysed. These were defined by new hypertension >140/90 after 20 weeks gestation combined with proteinuria >+1 dipstick on at least two occasions. Ethical approval for analysis of UK subjects was obtained from the Cambridge Research Ethics Committee (reference nos. 01/197 and 05/Q0108/367; Cambridgeshire, United Kingdom). The Regional Committee for Ethics in Medical Research and the Data Inspectorate have approved the Norwegian Mother and Child Cohort Study (MoBa) study. In both cohorts informed written consent was obtained from all participants.
Genotyping
Typing of 12 KIR genes in the mothers and HLA-C groups C1 and C2 in both mothers and babies was carried out using PCR with sequence specific primers as previously described.17,18
Statistical analysis
The separate analyses of UK and Norwegian subjects, in which pregnancies with pre-eclampsia and FGR were compared to those with median and high birth weights (Suppl Fig. 1, Suppl Tab 1), was performed using the χ2 and 2-tailed Fisher’s exact test (Open Source Epidemiologic Statistics for Public Health, Version 2.3.1). The combined analyses of pregnancies from both the UK and Norwegian cohorts, used all pregnancies where the babies’ birth weight was >5th centile and there were no complications such as pre-eclampsia, to test for effect of KIR on birth weight (Tables 1, 2 and S2). Here linear and logistic regression was performed using R functions lm and glm.21 Effect of KIR2DS1 on birth weight in the presence of different maternal (m) and fetal (f) HLA-C types was tested for the groups of subjects defined in Table 3. Two-sided p values of <0.05 were considered statistically significant throughout.
Table 1.
Presence of maternal KIR2DS1 associates with increased birth weight
| A. Categorical analysis*
| |||||
|---|---|---|---|---|---|
| Maternal KIR2DS1 genotype | Median birth weight (6–89th centile) | High birth weight (≥90th centile) | Effect of KIR2DS1 on birth weight | ||
| OR | 95% CI | P value | |||
| Positive | n=389 (39.1%) | n=148 (46.1%) | 1.38 | 1.07–1.79 | 0.01 |
| Negative | n=606 (60.9%) | n=173 (53.9%) | |||
| B. Continuous analysis**
| |||||
|---|---|---|---|---|---|
| Subjects | Covariates | Effect of presence of KIR2DS1 on birth weight (g) | |||
| n | Mean increase(g) | SE | P value | ||
| UK | Sex of fetus | 404 | 84 | 43 | 0.05 |
| Norway | Sex of fetus | 912 | 93 | 44 | 0.04 |
| UK and Norway | Cohort and sex of fetus | 1316 | 89 | 33 | 0.008 |
| UK and Norway | Cohort, sex of fetus and gestational age | 1316 | 78 | 28 | 0.005 |
KIR2DS1 frequency is significantly increased in high compared to median birth weight pregnancies. Presence versus absence of KIR2DS1 was tested for effect on birth weight as a categorical variable in a logistic regression analysis of 1316 pregnancies. Samples from both the UK and Norway cohorts were combined, with cohort and sex of the baby included as covariates. n = number of pregnancies, OR = odds ratio and CI = confidence interval.
Presence of KIR2DS1 associates significantly with increased birth weight. Presence versus absence of maternal KIR2DS1 was tested for an effect on birth weight as a continuous variable in grams, in linear regression analyses of pregnancies >5th birth weight centile. Sex of the baby was included as a covariate in every analysis. n = number of pregnancies analysed, mean = the average increase in birth weight (in grams) conferred by presence of the test variable and SE = standard error.
Table 2.
Maternal KIR2DS1 significantly increases birth weight when the fetus has more HLA-C2 epitopes than the mother (Table 2A) and when a single fetal C2 is paternally but not maternally derived (Table 2B).
| A. Maternal KIR2DS1 significantly increases birth weight when the fetus has more HLA-C2 epitopes than the mother.
| |||||||
|---|---|---|---|---|---|---|---|
| Categorical analysis* | |||||||
| Subjects | Maternal KIR2DS1 genotype | Median birth weight (6 – 89th centile) | High birth weight (>89th centile) | Effect of KIR2DS1 on birth weight | |||
| OR | 95% CI | p value | |||||
| Less C2 in fetus than mother (n=280) | Positive | n = 76 (35.3%) | n = 25 (38.5%) | 1.25 | 0.70 | 2.26 | 0.45 |
| Negative | n = 139 (64.7%) | n = 40 (61.5%) | |||||
| Equal C2 in fetus and mother (n=725) | Positive | n = 215 (39.6%) | n = 75 (41.2%) | 1.10 | 0.78 | 1.55 | 0.59 |
| Negative | n = 328 (60.4%) | n = 107 (58.8%) | |||||
| More C2 in fetus than mother (n=304) | Positive | n = 95 (40.8%) | n = 47 (66.2%) | 2.93 | 1.66 | 5.18 | 0.0002 |
| Negative | n = 138 (59.2%) | n = 24 (33.8%) | |||||
| Continuous analysis**
| ||||
|---|---|---|---|---|
| Subjects | Effect of KIR2DS1 on birth weight (g) | p | ||
| n | Mean increase (g) | SE | ||
| Less C2 in fetus than mother | 280 | 55 | 70 | 0.43 |
| Equal C2 in fetus and mother | 725 | 43 | 46 | 0.35 |
| More C2 in fetus than mother | 304 | 245 | 66 | 0.0002 |
| B. Maternal KIR2DS1 significantly increases birth weight when a single fetal C2 is paternally but not maternally derived.
| |||||||
|---|---|---|---|---|---|---|---|
| Categorical analysis* | |||||||
| Subjects | Maternal KIR2DS1 genotype | Median birth weight (6 – 89th centile) | High birth weight (≥90th centile) | Effect of KIR2DS1 on birth weight | |||
| OR | 95% CI | p value | |||||
| Single fetal C2 paternal (n=204) | Positive | n = 61 (38.9%) | n = 29 (61.7%) | 2.65 | 1.34 | 5.26 | 0.005 |
| Negative | n = 96 (61.1%) | n = 18 (38.3%) | |||||
| Single fetal C2 maternal (n=102) | Positive | n = 28 (37.3%) | n = 9 (33.3%) | 0.81 | 0.31 | 2.11 | 0.67 |
| Negative | n = 47 (62.7%) | n = 18 (66.7%) | |||||
| Continuous analysis**
| ||||
|---|---|---|---|---|
| Subjects | Effect of KIR2DS1 on birth weight (g) | p | ||
| n | Mean increase (g) | SE | ||
| Single fetal C2 paternal | 204 | 196 | 81 | 0.016 |
| Single fetal C2 maternal | 102 | 42 | 130 | 0.75 |
Presence versus absence of KIR2DS1 was tested for effect on birth weight as a categorical variable by logistic regression. UK and Norway cohorts were combined and then analysed in groups defined by combinations of maternal and fetal HLA-C type (Table 3). Cohort and sex of the baby were included as covariates. n = number of pregnancies, OR = odds ratio and CI = confidence interval.
Presence versus absence of maternal KIR2DS1 was tested for an effect on birth weight as a continuous variable in linear regression models of subsets of pregnancies >5th birth weight centile defined by maternal/fetal HLA-C genotype (Table 3). Cohort and sex of the baby were included as covariates. n = number of pregnancies analysed, mean = the average increase in birth weight (in grams) conferred by presence of KIR2DS1 and SE = standard error.
Table 3.
Groups of subjects defined by maternal and fetal HLA-C genotype used to analyse effect of KIR2DS1 on birth weight.
| Group | HLA-C epitopes mother/fetus |
|---|---|
| Less C2 in fetus than mother | mC1C2/fC1C1 and mC2C2/fC1C2 |
| Equal C2 in fetus and mother | mC1C1/fC1C1 and mC1C2/fC1C2 and mC2C2/fC2C2 |
| More C2 in fetus than mother | mC1C1/fC1C2 and mC1C2/fC2C2 |
| Single fetal C2 paternal | mC1C1/fC1C2 |
| Single fetal C2 maternal | mC2C2/fC1C2 |
Results
Birth weight extremes and neonatal morbidity
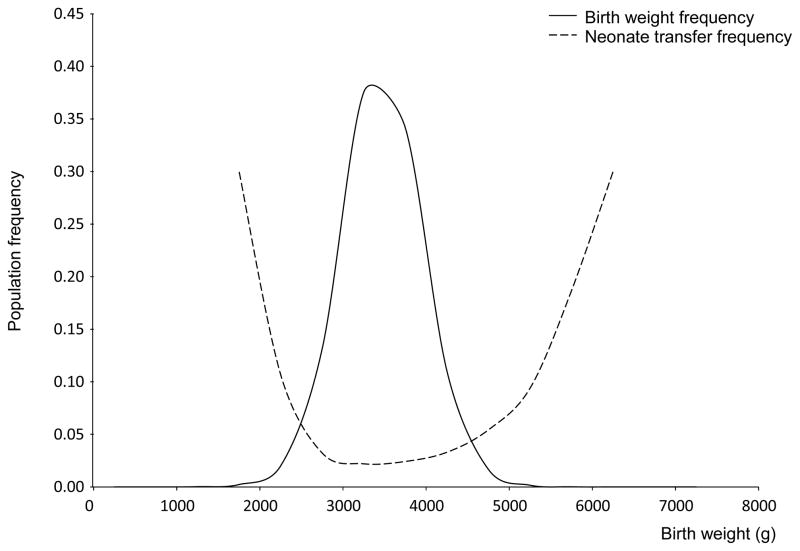
We first confirmed the association of neonatal morbidity with the extremes of birth weight using present-day data from 795,068 Norwegian first pregnancies from 1999 to 2008. We plotted the distribution of birth weights superimposed on the frequency of transfer to a special care neonatal unit (Fig. 1). This recapitulates the original data for UK pregnancies in the 1930s and clearly illustrates the “Obstetric Dilemma”; there are major problems for babies born either too small or too large.1,4
Figure 1.
Distribution of birth weights in the Norwegian population with percentage of babies transferred to the special care baby unit for the years 1999–2008 (n=795,068)
Presence of maternal KIR2DS1 associates with increased birth weight
Our previous studies all focussed on maternal KIR and fetal HLA-C genotypes in pregnancies affected by poor placentation (FGR, pre-eclampsia or recurrent miscarriage), where we find that maternal KIR AA frequency was significantly higher and KIR2DS1 frequency significantly lower than in healthy control pregnancies.12,17,18 A small number of pregnancies with birth weights ≥90th centile (high birth weight, n=66) were available within our UK control cohort. In these pregnancies we see an opposite pattern for maternal KIR genotype with KIR AA decreased and KIR2DS1 at higher frequencies than in controls (Fig S1 in the Supplementary Appendix). To obtain greater numbers of high birth weight pregnancies, we used mother and baby DNA samples from the Norwegian MoBa bank where over 90,000 pregnant women have been recruited.19,20 Detailed clinical data allowed us to choose mother/baby pairs where there was no known medical cause for fetal macrosomia such as gestational diabetes or fetal hydrops. There was a similar trend of high maternal KIR AA, low KIR2DS1 frequency in pregnancies associated with poor placentation (birth weight ≤5th centile and pre-eclampsia) and low KIR AA, high KIR2DS1 frequency in high birth weight pregnancies (Fig S1 in the Supplementary Appendix). Mothers of average (or median) birth weight babies possessed intermediate KIR frequencies. Thus, our previous observations regarding low birth weight pregnancy in the UK cohort replicate in the Norwegian cohort.
Given these preliminary findings that there are distinctive KIR frequencies in women with macrosomic babies, we further analysed all those pregnancies with birth weights >5th centile. UK and Norwegian data were combined and KIR2DS1 tested for an effect on birth weight as either a categorical or continuous variable. The presence of maternal KIR2DS1 was significantly more frequent in pregnancies with high compared to median birth weight in a logistic regression model, with cohort and sex included as covariates (p=0.010, OR 1.38) (Table 1A). We next used raw birth weight in grams as a continuous variable in linear regression analysis. Presence of KIR2DS1 conferred a similar average increase in birth weight in each cohort (84 in UK and 93g in Norway) (Table 1B). Pooling all 1316 pregnancies and including cohort and sex of the baby as covariates in a linear regression model, presence of KIR2DS1 showed a highly significant association with increased birth weight (p=0.008, Table 1B). KIR2DS1 showed the strongest effect on birth weight of all KIR genes that we analysed. Genes in the telomeric B region are in strong linkage disequilibrium but presence of KIR2DS5, the locus adjacent to KIR2DS1, showed an increase in birth weight in the linear regression analysis that was only borderline significant and the KIR AA genotype showed no significant association with birth weight in pregnancies >5th centile (Tables S1 and S2 in the Supplementary Appendix). Birth weight also correlates with gestational age, but the effect of KIR2DS1 on birth weight is independent of both fetal sex and gestational age (Table 1B, p=0.005). These pregnancies are all within a window of 37–40 weeks gestation. Adjusting for these small differences in gestational age, by including estimated gestational age as a covariate, the effect of KIR2DS1 on birth weight became more significant (p=0.005 compared to p=0.008 when gestational age is ignored, Table 1B).
Maternal KIR2DS1 and fetal HLA-C2 combinations affect birth weight
Pre-eclampsia and fetal growth restriction are associated with KIR AA genotypes in combination with a C2 group in the fetus, particularly if the C2 group is inherited from the father and not from the mother.12 We tested if association of increased birth weight with KIR2DS1 was affected by the parental origin of fetal C2. Effect of KIR2DS1 on birth weight was analysed in pregnancies where the fetus (f) has an extra copy of C2 compared to the mother (m). In these pregnancies (mC1C1/fC1C2 and mC1C2/fC2C2) the additional fetal C2 is by default from the father. This was compared with the effect of KIR2DS1 on birth weight in pregnancies where there are fewer copies of C2 in the fetus than the mother or the same number. In both categorical and continuous analyses of all pregnancies >5th centile, presence of maternal KIR2DS1 has a significant effect only when a pregnancy has more C2 in the fetus compared to the mother (categorical: p=0.0002, OR=2.93, continuous: p=0.0002, average increase of 245g when KIR2DS1 present, Table 2A).
As an additional test for the relative contribution of paternal and maternal fetal C2, we also analysed the effect of the presence of KIR2DS1 in pregnancies where the fetus was C1C2. From this group we then chose pregnancies where it was possible to distinguish if the single fetal C2 was paternal (mC1C1/fC1C2) or maternal (mC2C2/fC1C2) in origin. In both categorical and continuous analyses of pregnancies >5th centile we find that the presence of KIR2DS1 only associates with increased birth weight if the C2 group is inherited from the father (categorical: paternal C2 p=0.005, OR=2.65; maternal C2 p=0.67, OR=0.81; continuous: paternal C2 p=0.016; maternal C2 p=0.75, Table 2B). Thus, a combination of maternal KIR2DS1 with a fetal HLA-C2 inherited from the father protects against poor placentation but increases the risk of a macrosomic baby.
Discussion
Maternal inhibitory KIR2DL1 associates with pregnancy disorders linked to inadequate placentation, whereas maternal activating KIR2DS1 associates with increased birth weight. These results suggest that variations in immune system genes, KIR and HLA-C, are under selection as a result of the necessity to keep human birth weight within the limits defined by the harmful consequences of low and high birth weight. A territorial demarcation between the mother and her fetus resulting from the interaction of maternal KIR on uNK and fetal HLA-C expressed by invading trophoblast could be the basis for achieving such a compromise. Both the KIR2DL1 and KIR2DS1 associations, at opposite ends of the birth weight spectrum, occur particularly in pregnancies where the fetus carries an additional HLA-C group 2 allele compared to the mother or the fetus has a single C2 allele that is of paternal but not maternal origin. As C2 is the ligand for KIR2DL1/S1, this argues strongly for a role of the maternal KIR. It further indicates that maternal KIR responses especially to allogeneic C2 inherited from the father affect pregnancy outcome.
Our finding that the combined presence of maternal KIR2DS1 with a fetal C2 of paternal origin results in an average increase of ~250g in birth weight represents a substantial effect, approaching 10% of the average birth weight and twice the difference between males and females at birth.22 This impact of HLA-C alleles of the C2 group when they are inherited from the father provides one possible explanation for the known paternal genetic influences on birth weight.6,7,23 It is also consistent with murine models, where the paternal MHC also influences fetal growth.24 It remains to be seen in humans whether uNK respond to an extra dose of fetal C2 provided by the father or specifically to individual paternal HLA-C allotypes. Dissecting out the relative contributions C1 and C2 groups in the mother as well as the father will require HLA typing down to allele level in large numbers of clinically well-characterised pregnancies, but does indicate some influence of education by maternal HLA-C in the uNK response to placentation.25
How these genetic findings translate into functional consequences is still unresolved. C2 group alleles are much stronger and more specific inhibitors for KIR than C1 and this may explain why fetal C2 is so important.26 When the inhibitory KIR2DL1 receptor on uNK binds to fetal HLA-C, expressed by trophoblast cells, poor placentation results; in the opposite scenario, KIR2DS1 ligation to C2 could stimulate uNK to promote placentation and increase birth weight. We have recently shown that a large proportion of uNK cells do express KIR2DS1 and when they are specifically activated in vitro by binding to HLA-C2 ligands, trophoblast invasion is promoted via NK-derived cytokines such as GM-CSF.27 Hematopoietic stem cell transplantation, a situation analogous to placentation where cells from two individuals are in contact, confirms KIR2DS1 can modulate NK responses to allogeneic C2 in vivo.28
Our findings make sense when considered in an evolutionary context as they reflect the nexus of risks associated with the human birth process.14 The evolution of bipedalism in hominids (~4 million years ago) was accompanied by profound anatomical changes to the pelvis. In addition subsequent acquisition of a large brain and a globular head required the dangerous rotation of the head during its passage through the pelvis at parturition. This situation is unique to humans and often results in obstructed labour. At the same time, the in utero development of this large brain would have required increased energy supplied from the mother via the uterine arteries. These characteristics of specifically human birth correlate with several features of the evolving KIR and MHC gene complexes. The late appearance of MHC-C2 groups in primate evolution (in addition to humans, they are only found in gorillas and chimpanzees) may have been associated with the deep interstitial trophoblast that is characteristic of the great apes.14 The clear distinction between KIR A and B haplotypes is not seen in other primates including chimpanzees. Maternal KIR2DS1 binding to C2 positive trophoblast could promote arterial transformation allowing for a better feto-placental blood supply. Because of the constraints imposed by the pelvis, however, the limit of human brain size has now been reached and KIR B haplotypes will now be selected against because of the complications associated with high birth weight pregnancies.
The prediction of fetal weight has traditionally been by measurement of symphysis fundal height.29 More recently clinicians have used ultrasound to follow fetal growth but this requires specially trained personnel. These methods are both poorly predictive especially as birth weight increases.30,31 Our results raise the possibility of using KIR and HLA-C genotyping to predict pregnancies at risk of fetal growth restriction or macrosomia. Further receptors in addition to KIR can impact uNK function and may also influence birth weight.32 Our findings suggest the variable KIR interactions with HLA-C that govern NK cell responses, could contribute to very different roles in both reproduction and response to infections over the life course of an individual woman.14 These two highly polymorphic human gene systems, KIR and HLA may drive evolution (survival from infection and successful reproduction) by working alongside each other but always maintained in balance.
Supplementary Material
Acknowledgments
1. Grant support
This work was supported by funding from the Wellcome Trust (090108/Z/09/Z, 085992/Z/08/Z), and the British Heart Foundation (PG/09/077/27964). The authors also thank the Centre for Trophoblast Research for generous support. This project has been funded in part with federal funds from the Frederick National Laboratory for Cancer Research, under Contract no. HHSN261200800001E and by the Intramural Research Program of NIH, Frederick National Lab, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
2. Abbreviations
- uNK
uterine Natural Killer cells
- KIR
Killer Immunoglobulin-like Receptors
- HLA
Human Leukocyte Antigen
- FGR
fetal growth restriction
- MoBa
Norwegian Mother and Child Cohort Study
References
- 1.Cavalli-Sforza L, Bodmer WF. The Genetics of Human Populations. WH Freeman and Co; San Francisco, CA: 1971. [Google Scholar]
- 2.Rosenberg M. Birth weights in three Norwegian cities, 1860–1984. Secular trends and influencing factors. Ann Hum Biol. 1988;15:275–288. doi: 10.1080/03014468800009751. [DOI] [PubMed] [Google Scholar]
- 3.Trevathan WR. Human birth: An evolutionary perspective. Transaction Books; Piscataway, NJ: 2011. [Google Scholar]
- 4.Karn MN, Penrose LS. Birth weight and gestation time in relation to maternal age, parity and infant survival. Ann Eugen. 1952;16:147–164. [PubMed] [Google Scholar]
- 5.Steer PJ, Little MP, Kold-Jensen T, Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study. BMJ. 2004;329:1312–1317. doi: 10.1136/bmj.38258.566262.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to birth weight. J Epidemiol Community Health. 2001;55:873–877. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice F, Thapar A. Estimating the relative contributions of maternal genetic, paternal genetic and intrauterine factors to offspring birth weight and head circumference. Early Hum Dev. 2010;86:425–432. doi: 10.1016/j.earlhumdev.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45:76–82. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 10.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 11.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human Leukocyte Antigen (HLA) expression by normal trophoblast cells and placental cell lines using a novel method to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Class FH, Walker JJ, Redman CC, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 14.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–599. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- 16.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Hiby SE, Walker JJ, O’Shaughnessy KM, Redman CWG, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod. 2008;23:972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 19.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 20.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. Version 2.15. [Google Scholar]
- 22.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatrics. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff MA, Mednick BR, Schulsinger C, Secher NJ, Shiono PH. Father’s effect on infant birth weight. Am J Obstet Gynecol. 1998;178:1022–1026. doi: 10.1016/s0002-9378(98)70542-3. [DOI] [PubMed] [Google Scholar]
- 24.Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A. 2011;108:4012–4017. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 26.Hilton HG, Vago L, Older Aguilar AM, Moesta AK, Graef T, Abi-Rached L, Norman PJ, Guethlein LA, Fleischhauer K, Parham P. Mutation at positively selected positions in the binding site for HLA-C shows that KIR2DL1 is a more refined but less adaptable NK cell receptor than KIR2DL3. J Immunol. 2012;189:1418–1430. doi: 10.4049/jimmunol.1100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert Peter J, Ho JJ, Valliapan J, Sivasangari S. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database Syst Rev. 2012;7:CD008136. doi: 10.1002/14651858.CD008136.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Oliver M, McNally G, Leader L. Accuracy of sonographic prediction of birth weight. Aust N Z J Obstet Gynaecol. 2013;53:584–588. doi: 10.1111/ajo.12128. [DOI] [PubMed] [Google Scholar]
- 31.Curti A, Zanello M, De Maggio I, Moro E, Simonazzi G, Rizzo N, Farina A. Multivariable evaluation of term birth weight: a comparison between ultrasound biometry and symphysis-fundal height. J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.858241. [DOI] [PubMed] [Google Scholar]
- 32.King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, McMichael AJ, Loke YW, Braud VM. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.