SUMMARY
The gas nitric oxide (NO), and in some cases its downstream second messenger, cyclic guanosine monophosphate (cGMP) function in different taxa to regulate the timing of life-history transitions. Increased taxonomic sampling is required to foster conclusions about the evolution and function of NO/cGMP signaling during life-history transitions. We report on the function and localization of NO and cGMP signaling during metamorphosis of the nudibranch Phestilla sibogae. Pharmacological manipulation of NO or cGMP production in larvae modulated responses to a natural settlement cue from the coral Porites compressa in a manner that suggest inhibitory function for NO/cGMP signaling. However, these treatments were not sufficient to induce metamorphosis in the absence of cue, a result unique to this animal. We show that induction of metamorphosis in response to the settlement cue is associated with a reduction in NO production. We documented the expression of putative NO synthase (NOS) and the production of cGMP during larval development and observed no larval cells in which NOS and cGMP were both detected. The production of cGMP in a bilaterally symmetrical group of cells fated to occupy the distal tip of rhinophores is correlated with competence to respond to the coral settlement cue. These results suggest that endogenous NO and cGMP are involved in modulating responses of P. sibogae to a natural settlement cue. We discuss these results with respect to habitat selection and larval ecology.
INTRODUCTION
The spectacular diversity of transformations that characterize metamorphosis in marine invertebrates is transcended by three requirements held in common by most, if not all, taxa. (i) Sufficient sensory and juvenile structures must develop in order for larvae to acquire competence to metamorphose. This happens while larvae are metabolizing (whether or not they are feeding) and avoiding predation and requires anywhere from minutes to weeks to complete. (ii) Larvae must encounter a suitable site at which to settle and initiate the irreversible transformations associated with metamorphosis. Typically, this involves sensing a substrate-derived cue that indicates habitat quality or suitability. (iii) If the acquisition of competence and encounter with suitable habitats are asynchronous, the competent larval state must be retained for variable periods. Bishop et al. (2006) analyzed variation in the capacity of larvae to retain the competent larval state and suggested that this capacity is related to the stringency of settlement cues. That is, larvae that require more specific settlement cues tend to refrain from metamorphosing in the absence of their preferred cue. Conversely, larvae with low requirements for cue specificity tend to metamorphose in the absence of that cue. The degree to which the capacity to retain the competent larval state relates to the stringency of environmental cues is of interest because it suggests corresponding differences in the nature of signaling networks that control the timing of metamorphosis among taxa. One approach to assessing the basis of those differences is to compare the function of known signaling molecules among taxa that differ in the specificity of substrate required to induce settlement and metamorphosis.
The nudibranch Phestilla sibogae has long been studied as a model of settlement and metamorphosis in response to specific habitat cues (for a review on coral induced metamorphosis in P. sibogae see Hadfield and Pennington 1990). Juveniles and adults prey on scleractinian corals from the genus Porites (Harris 1970). Competent larvae of P. sibogae can be induced to metamorphose in the presence of Porites compressa, or water conditioned with that coral (Hadfield and Scheuer 1985). In the absence of such cues P. sibogae larvae, if fed, display a very high capacity to retain the competent larval state for protracted periods (reviewed in Hadfield 1998), and very few artificial inducers with known or presumed specificity can likewise induce metamorphosis (Hadfield 1978). These two observations stand in contrast to many other species whose larvae (i) can be induced to metamorphose artificially by applying chemical compounds with demonstrated or presumed specificity, (ii) metamorphose in response to natural cues less specific (at least taxonomically) than that required by P. sibogae, and (iii) undergo increasing levels of metamorphosis in the absence of obvious cues as a function of time in the competent state (also referred to as the “desperate larva hypothesis”; Bishop et al. 2006 for a recent review). Thus, P. sibogae is well suited to investigate a hypothetical relationship between the stringency requirements for environmental signals and the function of particular signaling molecules that transduce environmental signals into an irreversible commitment to initiate settlement and metamorphosis.
Nitric oxide (NO; and in some cases cyclic guanosine monophosphate [cGMP]) signaling controls the timing of life-history transformations in fungi, slime molds, plants, and animals alike (Tao et al. 1992; Ninnemann and Maier 1996; Froggett and Leise 1999; Wilken and Huchzermeyer 1999; Bishop and Brandhorst 2001; Bishop et al. 2001; Golderer et al. 2001; He et al. 2004; Pechenik et al. 2007). NO evidently functions as a component of inhibitory signaling in metamorphosis that, once suppressed (either experimentally, or from endogenous upstream signals), represents a key step in the initiation of the transformation. This designation of a decrease in NO as a “key step” is derived from the observation that a reduction in NO levels is sufficient to induce a transformation in the absence of exogenous cues.
Froggett and Leise (1998) first reported an inhibitory function for NO during metamorphosis of a marine invertebrate and suggested that inhibitory NO signaling conferred upon larvae the capacity to retain the larval state in the absence of cue (requirement [iii] above). This was important because the authors provided a link between a widespread and convergent character (i.e., the capacity to retain the competent larval state in the absence of settlement cues; see Hadfield et al. 2001) and a single signal that, upon pharmacological reduction, was sufficient to initiate metamorphosis. This observation of NO function was extended to ascidians and echinoids (Bishop and Brandhorst 2001; Bishop et al. 2001). Bishop and Brandhorst (2003) suggested that why NO in particular and inhibitory systems in general may be expected to be widely used to integrate environmental and endogenous signals.
Here, we analyze NO and cGMP during metamorphosis in P. sibogae and demonstrate function for these molecules. Our data indicate that NO and cGMP signaling modulates responses of larvae to natural settlement cues and that cGMP production in identified larval cells correlates well with the development of competence. Relative to the irreversible initiation of metamorphosis the function of NO and cGMP differs significantly from all other invertebrates tested so far.
MATERIALS AND METHODS
Maintenance and culture of P. sibogae
Adults were maintained at Kewalo Marine Labs in flowing seawaters tanks. Each day, egg masses were collected from the underside of pieces of P. compressa and incubated in 500–1000ml volumes of filtered seawater (FSW) with air bubbling at 25–26°C in the dark. After 5–6 days, larvae were freed from egg masses with forceps. Newly hatched larvae were harvested by shining a light on a finger bowl. This caused larvae to congregate at the brightest side of the bowl where they were removed by pipetting. Hatched larvae were cultured with air bubbling in a hanging basket system. Hatched larvae were cultured in 73 μm Nitex sieves in 600-ml beakers with water circulation provided by an airlift tube (Miller and Hadfield 1986).
Pharmacological treatments
L-nitroarginine-methyl-ester (L-NAME), D-nitroarginine-methyl-ester (D-NAME), L-arginine, D-arginine (Sigma-Aldrich Inc., St. Louis, MO, USA), and 2-(4-carboxyphenyl)-4,5-dihyrdo-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide (Carboxy-PTIO) (Cayman Chemical Corp, Ann Arbor, MI, USA.) were prepared as 100mM stocks in distilled water. 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (Sigma-Aldrich Inc.) was prepared as a 50mM stock in DMSO. S-Nitroso-N-acetylpenicillamine (SNAP) (Sigma-Aldrich Inc.) and 7-nitroindazole (7-NI) (Cayman Chemical Corp.) were prepared as a 100mM stock in DMSO. 1-Methyl-3-isobutylxanthine (IBMX) (Calbiochem) was prepared as 2mM stock solution in DMSO. All stock solutions were aliquoted and stored at −20°C and thawed and diluted with FSW immediately before use. Coral inducer (CI) was prepared by incubating pieces of coral in FSW overnight with bubbling in the dark at 25°C. After filtering with a coffee filter, the crude extract was either used immediately or stored in the dark at 4°C for up to 1 week. A 10 × dilution of extract was used for assays as a positive control for larval competence.
Experiments were conducted with 10-day postoviposition larvae in 12-well Falcon™ (San Jose, CA, USA) tissue culture plates in 2ml volumes of FSW. After drugs and CI were added, larvae were incubated in the dark at 25°C until the experiment was scored 20–24 h later. With the exception of experiments using the NO generator SNAP, drug and CI were added at the same time. SNAP breaks down and releases NO in aqueous solutions (Askew et al. 1995), so the concentration of NO delivered to larvae gradually decreases (t1/2=37 ± 4 h in water) (Ferrero et al. 1999). We reduced the effect of NO decay by adding 100 μM SNAP together with CI initially and then, after 3 h, supplied another dose of 100 μM SNAP. After 6 h total incubation, larvae were washed and incubated in FSW for an additional 18 h. A 6-h pulse of CI followed by an 18 h FSW chase was found to be sufficient to induce metamorphosis at percentages comparable with 24-h incubations. DMSO (0.1% v:v FSW) was tested as a negative control and it never induced metamorphosis. Each experimental treatment consisted of a single well of 20 larvae. Replicates consisted of larvae derived from eggs oviposited on different days from multiple adults. All larvae were of the same posthatching age at the time of testing. Data were subjected either to a one-way ANOVA and a Tukey’s HSD post hoc test for treatment effect, to a Student’s t-test, or in the SNAP experiments, to a paired t-test.
Gaseous NO detection in Phestilla larvae using electron paramagnetic spin resonance (EPR) and spin-trapping techniques
The application of EPR spectrometry to detection of gaseous NO in opisthobranch mollusks has been reported elsewhere, and here we use the same basic protocols as previously applied for Pleurobranchaea and Aplysia neuronal tissues (Moroz et al. 1998).
Specifically, we used spin trapping and EPR techniques (Archer 1993; Kalyanaraman 1996; Ohnishi 1998) to monitor endogenously produced NO. In the present spin-trapping assay, NO reacts with the spin-trap complex [(MGD)2–Fe2+] to produce a spin adduct (Komarov et al. 1993; Kotake 1996). This spin adduct has a three-line EPR spectrum (aN=12.5G and giso=2.04). Sodium N-methyl-D-glucamine dithiocarbamate (MGD) was synthesized as described (Shinobu et al. 1984). The NO spin trap, [(MGD)2–Fe2+] complex, was prepared by reacting MGD (10mM) with fresh FeSO4 (0.5mM) immediately before each experiment.
EPR spectra were recorded with a Varian (Varian Inc., Palo Alto, CA, USA) X-band spectrometer (at 9.5 GHz, 4G modulation amplitude, 20mW microwave power, 100 kHz frequency). Each spectrum in Fig. 2A represents the mean of 15–30 scans. This method, like many other EPR applications, is considered mainly a qualitative technique (Archer 1993) intended to prove the presence of NO formation in the system investigated. However, spectral signal intensity was a direct reflection of NO concentration. The signal intensity was measured by means of double integration of the first derivative of EPR signals. We used both anoxic NO gas solutions and DEA/NO solutions (Keefer et al. 1996) in our calibrations. Both DEA/NO and NO solution gave comparable results. Calibration with either aqueous NO solutions or DEA/NO was done at the beginning of each experiment. DEA/NO standards (half-life is 2.5min) were prepared immediately before experiments in anoxic 50mM HEPES solution (pH=7.2) and the calibration curves were compared with earlier published data (Keefer et al. 1996). Saturated NO solutions were prepared as described by Archer (1993) under anoxic conditions using argon gas. Various dilutions of this solution also were prepared under anoxic conditions and incubated immediately with [(MGD)2–Fe2+] complex for the NO spin trapping experiments. The actual EPR measurements for a calibration were made in 2–3 h to guarantee the complete saturation of the trap by NO. The detection limit for this assay was 0.5–0.75 μM of NO. All other details of the procedure have been described earlier (Norby et al. 1997; Moroz et al. 1998).
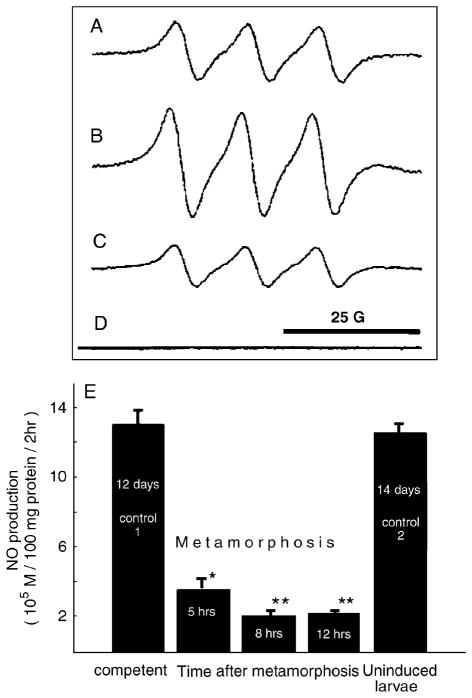
Fig. 2.
Quantification of NO in larvae before and after metamorphic induction with CI using EPR spin-trap spectroscopy. (A–D) Typical EPR spectra of Fe–MGD–NO spin adduct in samples. (A) Calibration solution of 7.5μM of NO. (B) Tissue homogenate of competent larvae, 13 days after hatching. (C) Tissue homogenate of competent 13-day larvae 5 h after induction of metamorphosis by coral conditioned seawater. (D) Coral conditioned filtered seawater sample. (E) Mean NO production in larval homogenates per unit protein (± SEM). Time after metamorphosis refers to the elapsed time after exposure to CI. *P<0.05; **P<0.01. NO, nitric oxide; CI, coral inducer; EPR, electron paramagnetic spin resonance.
Samples were prepared by concentrating 500–800 larvae in 1ml, mechanically homogenizing in ice-cold 0.1M phosphate-buffered saline (PBS) buffer (pH 7.2), and then sonicating for 10 sec. All steps with homogenate preparations were performed at 2–6°C. Homogenate was precipitated by centrifugation (500 g) at a laboratory centrifuge for 5min. Pellets were resuspended in 0.3 ml of ice-cold 0.1M Hepes buffer, pH 7.2, and then again sonicated for 10 sec. Three thousand one-hundred microliters of the homogenate were collected, separated and incubated with 1mM of nicotinamide adenine diphosphate (NADPH) (NOS co-factor) for 10–15 min before the NO-trap was added. NADPH was prepared in 0.1M Hepes, pH 7.2 (100 μl of such solution was added directly to individual homogenate sample to the final concentration). This was mixed with 100 μl of the spin trap complex (10mM MGD and 0.5mM FeSO4) and EPR spectra were obtained within 2–3 h of the sample incubation at room temperature.
As described above for the calibration steps with NO donors, three-line EPR spectra (aN=12.5G and giso=2.04) of NO-spin adduct were detected and the signal intensity was calculated by double integration of the first derivative of EPR signals and converted to NO values using an EPR service software package. Protein assay was performed according to Lowry et al. (1951). Data in Fig. 2E represent mean ± standard error of the mean (± SEM) for at least three independent experiments, with three replicates for each sample. Averages of 30 scans were used to increase signal/ noise of acquired spectra.
Immunohistochemistry
Larvae were prepared for fixation by relaxation in a 1:1 mixture of FSW and 7.5% MgCl2. Most larvae were fixed 30–40 min at −20°C, in a 1:7 mixture of 8% aqueous paraformaldehyde and methanol (Fig. 3, A, C, and D). Some larvae were fixed 50 min at −20°C, in pure methanol (Fig. 3B). After fixation all larvae were washed twice in PBS, decalcified for 30min in 5% EDTA in PBS, washed twice in PBS, and incubated overnight at 4°C in blocking buffer consisting of 5% goat serum in PBS containing 4% Triton X-100 and 0.1%sodium azide (PTA). Larvae were then exposed to primary antibodies (universal anti-NOS rabbit polyclonal antibody: Affinity Bioreagents, Golden, CO, USA; anti-tyrosine hydroxylase monoclonal antibody: Immunostar, Hudson, WI, USA) diluted 1:100 in PTA for 40–45 h at 4°C. Control preparations remained in PTA. Larvae were then washed four to five times over 4–8 h with PTA at 30°C, and transferred to secondary antibodies for 24 h at 4°C. Secondary antibodies were goat anti-mouse Alexafluor 568 (Invitrogen/Molecular Probes, Carlsbad, CA, USA) diluted 1:400 in blocking medium, and goat anti-rabbit Alexafluor 488 (Invitrogen/Molecular Probes) at 1:200. For cGMP immunostaining, we used a polyclonal antibody raised against a thyroglobulin-cGMP conjugate (de Vente et al. 1987). As a negative control, the antiserum was preadsorbed with 10mM cGMP before application to specimens. Larvae were prepared for fixation as above and fixed in 4% formaldehyde in FSW for 1 h at room temperature. Larvae were rinsed with PBS twice and decalcified as above. Larvae were rinsed in PBS and blocked in 2.5% BSA in PBS+0.1% Tx-100 (PBT) for 1 h. Primary antibody was diluted 1:1000 in PBT before addition to larvae. Incubations were carried out overnight at 4°C. After rinsing out the primary antibody, larvae were incubated in a 1:1000 dilution of goat anti-sheep IgG conjugated to the Alexa Fluor 568 (Invitrogen/Molecular Probes Inc.) overnight at 4°C or 2 h at room temperature. Larvae were either dehydrated in an ethanol series, transferred to xylene and then embedded in DPX resin (Sigma-Aldrich), or mounted in 70% glycerol in PBS. Images were collected with a Zeiss LSM510 confocal microscope. Zeiss LSM510 software or Photoshop 8.0 CS were used to construct z-series projections of optical sections and to adjust brightness and contrast settings.
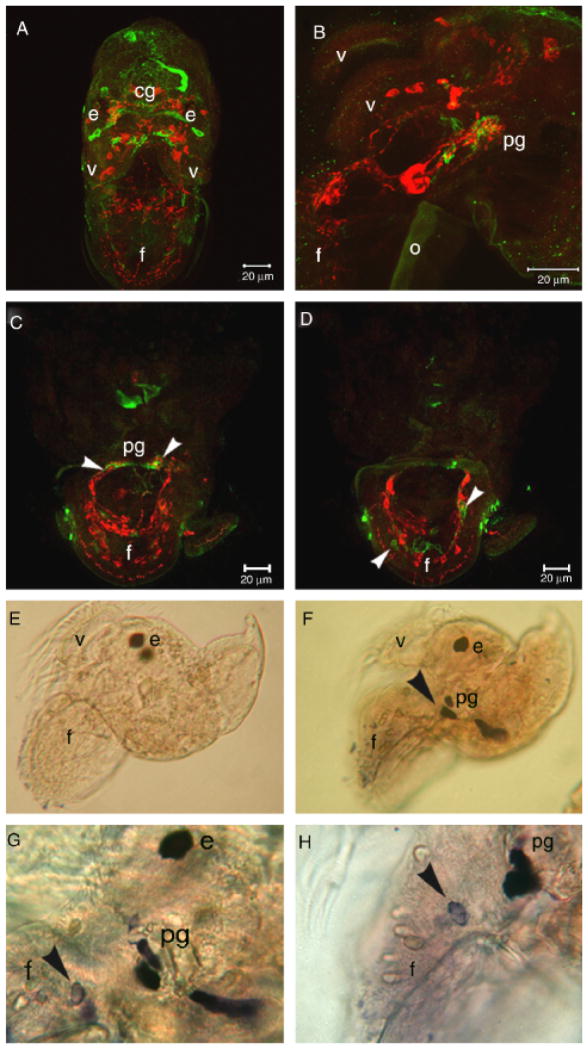
Fig. 3.
Confocal images of nitric oxide synthase-like (NOS-LIR, green) and tyrosine hydroxylase-like (TH-LIR, red) immunoreactivity in veliger larvae of Phestilla sibogae. (A) Frontal aspect of an 11-day competent larva. (B) Lateral aspect of 10-day competent larva. (C, D) Oblique frontal aspect of the foot of a 7-day larva. (C) and (D) were each constructed from two 1 μm optical sections, and represent adjacent regions of the same preparation. (C) is immediately anterior to (D). (C) shows NOS-LIR in neuropil region of pg, near clusters of TH-LIR somata (arrowheads). Note NOS-LIR cells in (D) (arrowheads) adjacent to TH-LIR cells in (C) and (D). (E–H) NADPHd staining of 10-day larvae. (E) Negative control. (F) Lateral aspect of a 10-day larva. Staining is localized to the vicinity of the pedal ganglion (arrowhead). (G) Lateral aspect of a 10-day larva showing a NADPH diaphorase stained cells in the pedal ganglion and the larval foot (arrowhead). (H) Higher magnification view of a similar region as in G. e, eye; f, foot; v, velar lobe; cg, cerebral ganglion; o, operculum. NADPHD, nicotinamide adenine diphosphate.
SNAP/IBMX enhanced immunostaining
Larvae were treated for 2 h at room temperature with 200 μM SNAP and 200 μM IBMX. Before fixation larvae were anesthetized in a 3.75% MgCl2 solution in FSW containing 200 μM SNAP and 200 μM IBMX. Larvae were then transferred to a microcentrifuge tube and gently centrifuged. The treatment solution was removed and replaced with 4% formaldehyde. Fixed larvae were immunostained for cGMP as above.
NADPH diaphorase staining
Larvae were anesthetized as above until they failed to retract the velum and foot upon mechanical perturbation and then fixed in 2% formaldehyde and 1% glutaraldehyde in FSW for 2 h at room temperature. Larvae were rinsed in PBS pH 7.4+0.3% Triton X-100 (PBT) and then incubated in 4mg/ml nitro-blue tetrazolium in PBT-containing NADPH overnight at room temperature. This reaction generates a blue formazan precipitate (Weinberg et al. 1996). The reaction was stopped by rinsing with PBT, and then larvae were cleared in acetone for 3 min, mounted in PBT and examined on a Zeiss compound microscope. Images were collected with a Nikon Coolpix 900 digital camera.
Quantification of cGMP immunoreactivity (cGMP-IR)
Control and treated samples were processed in an identical manner. Brightness and contrast settings were calibrated by averaging brightness values for 10 immunostained treatment and control specimens. These values were generated by the “autobc” function, an algorithm that optimizes brightness and contrast settings before image collection. From these data, mean brightness settings for control and treated larvae were calculated. The lowest mean gain setting was used for all subsequent measurements to avoid saturation of signal. For 10 control and treated specimens, stacks of images of the region to be analyzed were collected and projected as a single image using default settings. Using the “profile” function, we measured pixel values along a region of unstained tissue approximately the same size as the immunostained region. These values, representing background fluorescence, were logged. Then, a second line was drawn along the length of the stained tissues, and again, pixel values were logged. Background pixel values were then subtracted from the pixel values representing immunostained tissue. These subtracted values represented mean pixel values for each immunostained region. The Student’s t-test was performed to test for differences in mean pixel values between 10 control and SNAP/IBMX treated larvae.
Testing for correlation between cGMP accumulation and competence
Under laboratory conditions larvae of P. sibogae become competent to respond to CI between days 7 and 8 postoviposition. From 14 independent clutches 30 larvae aged 7, 8, or 10 days postoviposition were immersed in CI. After 24 h, the percentage of larvae that responded to CI was recorded. At the time that larvae were first exposed to CI, larvae from the same batch were fixed in 4% formaldehyde and immunostained for cGMP. Thirty immunostained larvae were scored for the presence of cGMP-IR in subvelar cells on at least one side of the larva. In cases where the region of interest was not unambiguously identifiable, larvae were not scored. The percentage of larvae having cGMP-IR in subvelar cells was plotted against the percentage of larvae that metamorphosed in response to CI.
RESULTS
NO and cGMP modulate responses to CI
We conducted a series of pharmacological assays to investigate the function of NO and cGMP signaling during metamorphosis of P. sibogae. No treatment induced metamorphosis in the absence of CI but manipulation of both NO and cGMP levels significantly modulated the percentage of larvae that responded to CI in a manner that is consistent with an inhibitory actions for these signaling molecules.
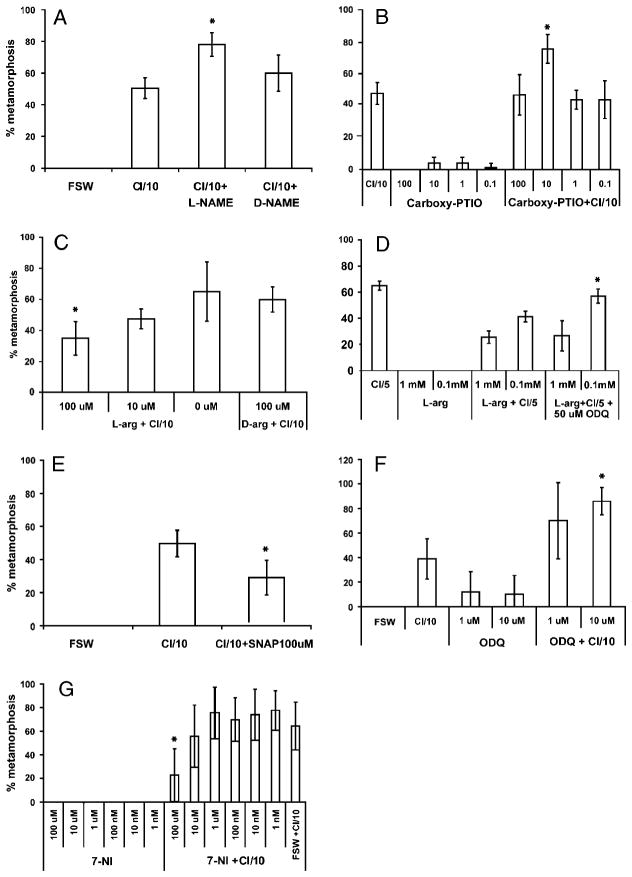
We manipulated NO levels in several ways. L-NAME, a derivative of L-arginine, (the endogenous substrate for NOS) has been used as a competitive inhibitor of NOS activity for vertebrates, opisthobranch mollusks, and sea urchins (Moroz et al. 1996; Bodnarova et al. 2005, Bishop and Brandhorst 2001). Incubation of larvae with L-NAME increased the percentage of larvae that initiated metamorphosis in response to CI, whereas D-NAME, the less active enantiomer of L-NAME, did not (Fig. 1A). Application of Carboxy-PTIO, an NO scavenger, is a nonenzymatic method to reduce endogenous NO levels and has been used in a gastropod to induce metamorphosis (Froggett and Leise 1998). Consistent with L-NAME results, this treatment significantly increased the percentage of larvae that initiated metamorphosis in response to CI (Fig. 1B). These results suggest that an increase in levels of NO attenuates responses of larvae to CI. Indeed, treatment of larvae with L-arginine, which presumably increases NO production, attenuated responses to CI (Fig. 1C). A different method of augmenting endogenous NO levels is to treat larvae with SNAP, a donor of NO. This treatment attenuated responses to CI (Fig. 1D), corroborating the L-arginine treatment. In contrast to all of the above results, 7-NI, a selective inhibitor of the neural isoform of mammalian NOS that acts by inhibiting the binding of molecular oxygen to heme (Mayer et al. 1994; Wolff and Gribin 1994), attenuated larval responses to CI at 100 μM (Fig. 1E).
Fig. 1.
Pharmacological manipulation of NO and cGMP levels. (A) L-NAME but not D-NAME potentiated responses to CI (P=0.02). (B) Carboxy-PTIO potentiated larval responses to CI (P=0.004). (C) L-arginine, but not D-arginine attenuated larval responses to CI (P=0.04). (D) ODQ antagonized the attenuating effects of 0.1mM L-arginine (P=0.004). (E) SNAP attenuated larval responses to CI (P=0.03). (F) 10 μM ODQ but not 1 μM ODQ potentiated larval responses to CI (P<0.001). (G) 7-NI attenuated larval responses to CI at 100 uM (P=0.02). NO, nitric oxide; L-NAME, L-nitroarginine-methyl-ester; D-NAME, D-nitroarginine-methyl-ester; CI, coral inducer; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; SNAP, S-Nitroso-N-acetylpenicillamine; cGMP, cyclic guanosine monophosphate.
cGMP levels were manipulated using ODQ, a selective inhibitor of soluble guanylate cyclase (sGC) (Garthwaite et al. 1995). ODQ enhanced responses to CI, suggesting that cGMP produced by sGC inhibits the initiation of metamorphosis (Fig. 1F). ODQ antagonized the attenuating effects of 0.1mM L-arginine but not 1mM (Fig. 1D). Experiments with either L-NAME or Carboxy-PTIO and ODQ were conducted in a further attempt to induce metamorphosis in the absence of CI. No combination of these treatments did so (data not shown).
NO formation is decreased in after exposure to CI
Our pharmacological data suggest that NO and cGMP act as inhibitors of metamorphosis in P. sibogae. A prediction from these data is that a decrease in NO or cGMP levels is associated with metamorphosis. We quantified NO and cGMP in competent and postmetamorphic larvae to verify this prediction. NO was detected in competent larvae tissue using EPR (Fig. 2, A–C). NO levels per protein mass unit decreased within 12 h of exposure to CI (Fig. 2E). In contrast, NO levels in 14-day larvae that were not exposed to CI remained indistinguishable from those of 12-day larvae, indicating that NO levels decreased specifically after 3–6 h in response to CI (Fig. 2E). However, preliminary data using NO-sensitive electrodes suggest that there is a short-term (<1 h) increase in NO formation immediately after exposure with CI. Unfortunately, this transient change in NO formation is difficult to detect and quantify using EPR-spin trapping techniques, but suggests that NO signaling in response to CI may be biphasic. We also measured cGMP levels in larvae exposed to CI using an ELISA but detected no differences after a 6-h exposure to CI (data not shown).
Cellular distribution of NOS and cGMP
To investigate the distribution of enzymatically produced NO, we relied upon the localization of putative NOS using immunohistochemical and histochemical methods. The anti-NOS polyclonal antibody that we used was generated from a polypeptide that corresponds to an amino acid motif, which is 80% conserved between representatives of mollusks and vertebrates. This indicates a high probability of cross-reactivity to NOS in P sibogae. However, to corroborate our immunostaining results, we also used the NADPH diaphorase (NADPHd) assay. This is a histochemical assay that identifies NOS catalytic activity in aldehyde-fixed tissue (Hope et al. 1991). Both of these methods are in agreement that the three principle regions of NO production are cells in the vicinity of the pedal ganglion, in the larval foot and in the dorsal commisure of the cerebral ganglion (Fig. 3). Other cells were immunoreactive for NOS but these were not corroborated by NADPHd staining so we do not describe them here. NOS-like immunoreactive (NOS-LIR) cells were found diffusely in the cerebral ganglia near clusters of tyrosine hydroxylase-like immunoreactive (TH-LIR) somata (Fig. 3A) as well as in three cells in each pedal ganglion, in close apposition to putative catecholaminergic cells displaying TH-LIR (Fig. 3, B and C). Processes of TH-LIR and NOS-LIR cells are closely associated in the neurophil of the pedal ganglion, but do not appear to be colocalized. Other pedal cells outside the pedal ganglion were also NOS-LIR, both inside and outside of bilateral tracts in the foot containing somata and processes of TH-LIR neurons (Fig. 3D). NADPHd staining was detected in the vicinity of the pedal ganglion and in somata of putative sensory cells in the foot (Fig. 3, F–H).
We examined the distribution of cGMP-IR in developing and competent larvae (Fig. 4). Staining was observed in the apical sensory organ (ASO) (Fig. 4A), cells in the larval foot (Fig. 4B) and in two bilaterally symmetrical superficial regions on the head at the base of the velar lobes (Fig. 4, C–E). These latter cells are uncharacterized but experiments described below indicate that they are fated to reside at the distal tip of adult chemosensory structures called rhinophores. We have named them subvelar cells to reflect their location (Fig. 4, C and D arrows). We also observed weak cGMP-IR at the margin of the velum. cGMP-IR in the ASO was variable between experiments but invariant among larvae within a staining experiment. Experiments in which antiserum was preadsorbed with 10mM cGMP verified the specificity of cGMP-IR (Fig. 4, F and G). Double staining experiments indicate that no cells were observed to contain both NOS and cGMP (data not shown).
Fig. 4.
Confocal images of the distribution of cGMPIR in larvae. (A) Dorsal view of cGMP-IR in the ASO of a 6-day larva. (B) Lateral aspect of a 6-day larva. Anterior is to the right. Arrowhead points to the ASO and arrows point to immunoreactive cells in the foot. (C) Dorsal view of a 10-day larva showing cGMP-IR in the ASO as well as in the subvelar cells. (D) Lateral view of a 10-day larva showing immunoreactivity in subvelar cells. (E) Higher magnification view of the relationship between the ASO and the subvelar cells from the dorsal aspect. (F) Dorsal aspect of a 10-day larva stained with antiserum preadsorbed with 10mM cGMP. Note the absence of staining in the ASO compared with (G), a larva stained with nonpreadsorbed antiserum. This treatment also decreased intensity of, but did not abolish, cGMP-IR in the subvelar cells (data not shown). ASO, apical sensory organ; cGMP, cyclic guanosine monophosphate.
Levels of cGMP in cells are regulated by a combination of guanylate cyclase activity and cGMP hydrolysis. NO, via an interaction with the heme prosthetic group in sGC, is a major stimulator of cGMP production by soluble guanylate cyclase (sGC) (Zhao et al. 1999) whereas phosphodiesterases hydrolyze cGMP. We sought to identify cells that were capable of cGMP production via sGC, but due to the absence of chronic NO stimulation or to the presence of high phosphodiesterase activity (or both), did not contain detectable levels of cGMP under normal physiological conditions. We treated larvae with both SNAP (to increase NO levels) and IBMX (to inhibit phosphodiesterases) in order to, respectively, stimulate cGMP production and attenuate the rate of cGMP degradation. SNAP/IBMX treatment enhanced the intensity of cGMP-IR in sub-velar cells, in the margin of the velum and induced cGMP production in the ASO, validating the variable immunostaining results observed in the ASO under normal physiological conditions (Fig. 5, A and B). This treatment revealed an apparent neural connectivity between the subvelar cells and the ASO (Fig. 5, D and E) and expanded the range of cGMP-IR within the former (Fig. 5, G and H).
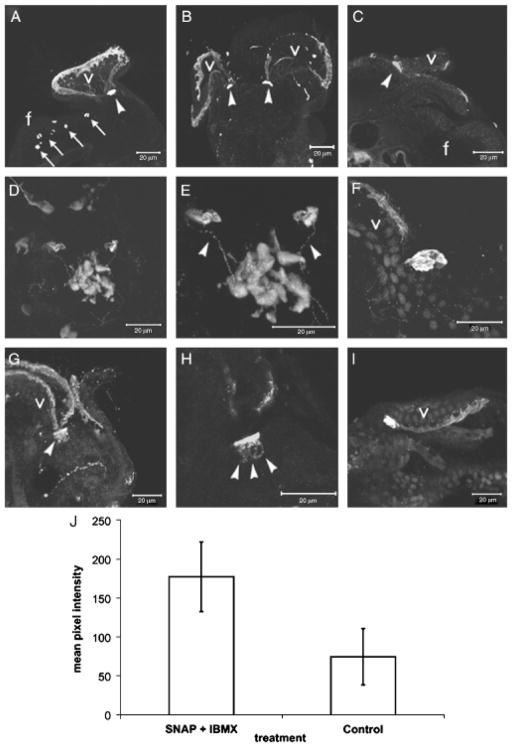
Fig. 5.
Confocal SNAP/IBMX enhanced cGMP immunostaining in 10-day larvae. (A) Lateral aspect. Note the increase in staining intensity of cells at the margin of the velum (v) compared with those in Fig. 4, A–D. Arrows point to cGMP-IR cells in the foot. Arrowheads point to subvelar cells. (B) Dorsal aspect. Anterior is up. (C) Lateral perspective. Anterior is to the right. Arrowhead indicates subvelar cells. (D) Dorsal aspect. Anterior is up. Arrowheads indicate subvelar cells whereas the dashed line encircles the ASO. (E) Higher magnification of (D) showing immunoreactive processes that appear to connect the subvelar cells to the ASO (arrowheads). Anterior is up. (F–H) Dorsal aspect showing closer views of subvelar cells on the left side of the larva, at the base of the velar lobe. Subvelar cells contain an expanded range of cGMP immunoreactivity (arrowheads in G, H; compare with subvelar cells in Fig. 4, C and E). Anterior is up in F–H. (I) Dorsal aspect showing the relationship of subvelar cells to the margin of the velum. Anterior is to the right. Nuclei in (F) and (I) are stained with SYTOX nuclear stain. (J) Mean difference in pixel values of the subvelar cells compared between untreated and SNAP/IBMX treated larvae (P=0.04). ASO, apical sensory organ; SNAP, S-Nitroso-N-acetylpenicillamine; cGMP, cyclic guanosine monophosphate.
To support the qualitative observation that SNAP/IBMX treatment actually increased cGMP levels, we measured levels of cGMP in subvelar cells using an in situ optical approach. Ten-day postoviposition larvae were treated with SNAP/ IBMX and then immunostained for cGMP. The mean difference in pixel values between 10 untreated and 10 treated larvae was assessed (see ‘Materials and Methods’). Consistent with qualitative observations, SNAP/IBMX treatment caused over a 2-fold increase in mean pixel values associated with cGMP-IR in subvelar cells (Fig. 5J). Treatment of larvae with either SNAP or IBMX alone did not elicit this result (not shown). Because the membrane-bound form of guanylate cyclase is not responsive to NO (Garthwaite et al. 1995), this result suggests that the cGMP observed in both the subvelar cells and the ASO is produced by sGC and thus provisionally identifies cells in which the effects of ODQ treatment described above may be localized.
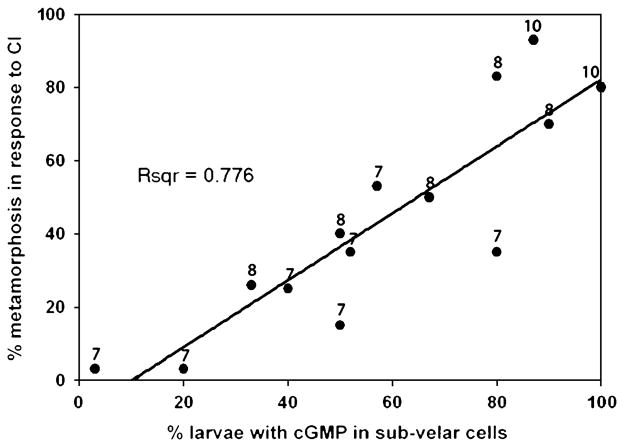
An important aspect of cGMP-IR in the subvelar cells was its apparent correlation with the acquisition of metamorphic competence. We sampled larvae from 14 clutches of larvae on days 7, 8, and 10 postoviposition to test for a correlation between cGMP accumulation in the subvelar cells and metamorphic competence. A strong correlation was found between these two variables (Fig. 6). Linear regression analysis indicates that 78% of variation in the response to CI of larvae of all ages is explained by the percentage of larvae that contain cGMP in subvelar cells.
Fig. 6.
Regression analysis of the percentage of larvae that contain cGMP in subvelar cells and the percentage of larvae that metamorphosed in response to CI. The number beside each data point corresponds to larval age (days postoviposition). CI, coral inducer; cGMP, cyclic guanosine monophosphate.
In order to assess the fate of subvelar cells we immunostained for cGMP during metamorphosis. We fixed larvae 24 and 48 h after exposure to CI. cGMP-IR in subvelar cells is retained during metamorphosis and corresponds to the distal tip of developing rhinophores (Fig. 7).
Fig. 7.

Confocal images of GMP-IR in newly metamorphosed juveniles. (A) Lateral aspect of a juvenile 48 h after exposure to CI. Anterior is to the left. (B) Dorsal aspect of cGMP-IR in a juvenile 24 h after exposure to CI. cGMP-IR is restricted to the tips of the developing rhinophores (arrowheads). Anterior is up. (C) Higher magnification lateral aspect of the tip of a developing rhinophore 48 h after induction. Anterior is to the right. CI, coral inducer; cGMP, cyclic guanosine monophosphate.
DISCUSSION
The function of inhibitory NO signaling in several independently derived life-history transitions suggests important and perhaps unique functionality for NO in this regulatory context (Bishop and Brandhorst 2003; Hodin 2006). Equally informative for our understanding of regulatory mechanisms that control life-history transitions in response to environmental signals are instances in which no function or an altered function for NO is indicated. The veracity of such evidence is more difficult to demonstrate, but correlations (the presence/ absence) of NO signaling to parameters related to life-history transitions (e.g., what environmental variables determine the transition and how variable they are in space and time) may provide opportunities to assess more specifically NO function during life-history transitions. Our data support an inhibitory function for both NO and cGMP during metamorphosis of P. sibogae. In addition to expanding the number of taxa for which NO and cGMP signaling function during metamorphosis, the significance of the present study is that it describes a role for NO and cGMP during metamorphosis that stands in contrast to other marine invertebrates examined to date: pharmacological manipulation of NO or cGMP levels modulated responses to a natural settlement cue but was not sufficient to induce metamorphosis in the absence of inducer.
Of three possible interpretations, the simplest is that our pharmacological treatments did not reduce NO or cGMP levels sufficiently to induce metamorphosis. Because it is not possible to assess what threshold NO or cGMP levels must cross in order to trigger metamorphosis in the absence of cues, and because we did not measure levels of NO in response to pharmacological treatments, we cannot reject this possibility. Still, four considerations counter an interpretation based upon inefficacy of pharmacological effect. (i) Some of the chemicals used here are effective at modulating metamorphosis of larger larvae in the absence of natural cues (Froggett and Leise 1998; Bishop and Brandhorst 2001; Gifondorwa and Leise 2006). Thus, on the basis of larval size, an argument of drug penetration is not valid. (ii) Higher concentrations of ODQ, L-NAME, and Carboxy-PTIO actually decreased responses to inducer with no corresponding increase in mortality. (iii) We used combinations of L-NAME, Carboxy-PTIO and ODQ to lower NO and cGMP levels in concert, but no induction of metamorphosis in the absence of CI was observed. Both (ii) and (iii) suggest that the responses that we observed were approaching the maximum effect of NO or cGMP levels on the inductive event. (iv) The overwhelming majority of pharmacological manipulations designed to artificially induce complete metamorphosis of P. sibogae have failed to do so (Hadfield 1978; Pires and Hadfield 1991; Pires et al. 2000; M. G. Hadfield, unpublished observations). We discuss the significance of this last point in greater detail because it leads us to subsequent interpretations.
Pires et al. (2000) showed that treatment of P. sibogae larvae with L-dopa, a precursor for dopamine synthesis, significantly potentiated metamorphosis in response to CI. However, treatment of larvae with L-dopa in the absence of CI failed to induce metamorphosis. In contrast, L-dopa treatments in the oyster Crassostrea gigas were sufficient to induce complete metamorphosis in the absence of inductive cues (Coon and Bonar 1987; Bonar et al. 1990). Hydrogen peroxide induced complete metamorphosis of larvae of the queen conch Strombus gigas (Boettcher and Target 1998), whereas in P. sibogae, only partial metamorphosis (loss of velum but no evacuation of shell and subsequent elongation of the body) is observed in response to this compound (Pires and Hadfield 1991). Treatment of larvae of the abalone Haliotis rufescens with GABA induced high levels of metamorphosis (Morse et al. 1980) whereas the same compound in P. sibogae did not induce metamorphosis in the absence of cue (M. G. Hadfield,, unpublished observations). Thus, hydrogen peroxide, catecholamine, GABA, and NO signaling are comparable in the sense that, in the absence of natural cues, manipulation of each signal is sufficient to induce complete metamorphosis of at least one mollusk species, but not of P. sibogae. Two possibilities may account for this pattern.
Differences in responses to the same artificial inducers are explained by phylogenetic differences between nudibranchs and other mollusks. Further sampling of nudibranchs can test this possibility.
NO and cGMP signaling during P. sibogae metamorphosis occupies a position in a signaling hierarchy that differs from other species tested and this difference is related to the specificity of cues required to induce metamorphosis. It is well known that marine invertebrate larvae differ in the specificity of habitat-derived cues required to induce settlement and metamorphosis (Hadfield and Paul 2001 for a review). Some larvae will settle readily in clean glass bowls, whereas others require biofilmed surfaces, and still others require contact with certain taxa. Bishop et al. (2006) analyzed variation in the capacity of competent larvae to refrain from metamorphosing in the protracted absence of cues and found a significant correlation between this character and the specificity of cues required to induce metamorphosis. This suggests that as juvenile performance, and ultimately, fitness becomes increasingly contingent upon the settlement location, so should the capacity to refrain from metamorphosing in the absence of appropriate cues. The insufficiency of NO and other signaling molecules to induce metamorphosis of P. sibogae in the absence of cues may indicate a meaningful difference about the system that retains the competent larval state in this species. Importantly, the hypothesis that differences in the capacity to retain the larval state are related to the specificity of inductive habitat cues is testable.
A potentially informative test would be an analysis of NO function during metamorphosis of larvae of the nudibranch Alderia willowi. This species has evolved a bet-hedging strategy during development in which two larval types are produced in one clutch (Krug 2001). One fraction of larvae in the clutch metamorphoses either in the egg capsule or within 2 days of hatching in the absence of a natural settlement cue. The remainder of larvae in the clutch retain the larval state until they die or until they initiate metamorphosis in response to a specific cue derived from the obligate food source of the juvenile (Krug 2001). If a reduction of NO signaling was not sufficient to induce metamorphosis among the more selective larvae, but was among the nonselective larvae, this would strongly support a hypothetical relationship between NO function, the capacity to retain the competent state in the protracted absence of settlement cues and habitat specificity.
The cellular context of metamorphic signaling in P. sibogae
We examined the distribution of NOS and cGMP in larvae to understand the cellular basis of NO-cGMP signaling. The principal results are that (i) the pedal ganglion and cells in the foot are the most likely source of NO formation, (ii) NOS and cGMP do not colocalize in any cells using the methods described herein, (iii) cGMP accumulates in two bilaterally symmetrical groups of subvelar cells on the larval head in strong correlation with competence, and (iv) SNAP/IBMX enhanced cGMP immunohistochemistry reveals neural connectivity of the subvelar cells with the ASO, a sensory organ (Bonar 1978) shown to have an essential function in the metamorphic response pathway (Hadfield et al. 2000).
The apparent connectivity of subvelar cells to the ASO raises the possibility that the former is also involved in reception of signals appropriate to induce metamorphosis. The correlation between accumulation of cGMP in subvelar cells and competence to metamorphose in response to CI supports this possibility. Although competence is operationally defined as the capacity of a larva to complete metamorphosis to a functional juvenile, at the level of cells and tissues the meaning of competence among marine invertebrates is unknown. There are few examples of molecular or cellular correlates of competence (e.g., Eri et al. 1999) and such correlations suggest function during metamorphosis. Intriguingly, subvelar cells are fated to reside at the termini of the juvenile rhinophores. In addition, recent analysis of localization in another opisthobranch mollusc, Aplysia californica, indicates that NO can be associated with chemosensory function by predominant localization of nitrergic systems to primary sensory structures in rhinophores and tentacles (Moroz 2006). Alternately, cGMP accumulation in the subvelar cells may support the intuitive notion that one aspect of metamorphic competence is simply the development of sufficient tissues having a function during the metamorphic transition or during juvenile/adult life.
CONCLUSIONS
We provide evidence for NO and cGMP signaling during metamorphosis of the nudibranch P. sibogae. Differences in the dynamics of these signals relative to metamorphic induction are thus far unique to this specialist coral-eating mollusk. We speculate that this difference may reflect the higher stringency of cue specificity required to induce metamorphosis or phylogenetic differences between nudibranchs and other gastropods. Further sampling efforts are required to falsify the hypothetical relationship between requirements for settlement specificity and the dynamics of NO or cGMP function during settlement and metamorphosis. We have identified regions in the larva that contain putative NOS and soluble guanylyl cyclase. One of these regions is characterized by both the accumulation of cGMP in correlation with competence and by apparent neural connectivity to the ASO, suggesting a function for these cells in establishing competence or sensing settlement cues.
Acknowledgments
We thank the Illinois EPR Research Center and Dr. R.B. Clarkson for the use of EPR instrumentation and facilitating the EPR assay. This work was supported by ONR grant OCE-9907545 to M. G. H.). D. B. was supported by NIH-NIAID research grant AI030464. A. P. was supported by NSF-RUI grant IBN-0110832 and C. B. was supported by a postdoctoral fellowship from NSERC. L. L. M. was supported by grants from the NIH (P50 HG002806), the McKnight Brain Research Foundation, and the University of Florida Opportunity Funds.
References
- Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993;7:349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- Askew SC, Butler AR, Flitney FW, Kemp GD, Megson IL. Chemical mechanisms underlying the vasodilator and platelet anti-aggregating properties of S-nitroso-N-acetyl-DL-penicillamine and S-nitrosoglutathione. Bioorg Med Chem. 1995;3:1–9. doi: 10.1016/0968-0896(94)00139-t. [DOI] [PubMed] [Google Scholar]
- Bishop CD, Bates WR, Brandhorst BP. Regulation of metamorphosis in ascidians involves NO/cGMP signaling and HSP90. J Exp Zool. 2001;289:374–384. doi: 10.1002/jez.1019. [DOI] [PubMed] [Google Scholar]
- Bishop CD, Brandhorst BP. NO/cGMP signaling and HSP90 activity represses metamorphosis in the sea urchin Lytechinus pictus. Biol Bull. 2001;201:294–304. doi: 10.2307/1543617. [DOI] [PubMed] [Google Scholar]
- Bishop CD, Brandhorst BP. On nitric oxide signaling, metamorphosis and the origin of biphasic life cycles. Evol Dev. 2003;5:542–550. doi: 10.1046/j.1525-142x.2003.03059.x. [DOI] [PubMed] [Google Scholar]
- Bishop CD, Huggett MJ, Heyland A, Hodin A, Brandhorst BP. Interspecific variation in metamorphic competence in marine invertebrates: the significance for comparative investigations into the timing of metamorphosis. Int Comp Biol. 2006;46:662–682. doi: 10.1093/icb/icl043. [DOI] [PubMed] [Google Scholar]
- Bodnárová M, Martásek P, Moroz LL. Calcium/calmodulin-dependent nitric oxide synthase activity in the CNS of Aplysia californica: biochemical characterization and link to cGMP pathways. J Inorg Biochem. 2005;99:922–928. doi: 10.1016/j.jinorgbio.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Bonar DB. Ultrastructure of a cephalic sensory organ in larvae of the gastropod Phestilla sibogae (Aeolidacea, Nudibranchia) Tissue Cell. 1978;10:153–165. doi: 10.1016/0040-8166(78)90014-9. [DOI] [PubMed] [Google Scholar]
- Bonar DB, Coon SL, Walch M, Weiner RM, Fitt W. Control of oyster settlement and metamorphosis by endogenous and exogenous chemical cues. Bull Mar Sci. 1990;46:484–498. [Google Scholar]
- Boettcher AA, Target NM. Role of chemical inducers in larval metamorphosis of queen conch, Strombus gigas Linnaeus: relationship to other marine invertebrate systems. Biol Bull. 1998;194:132–142. doi: 10.2307/1543043. [DOI] [PubMed] [Google Scholar]
- Coon SL, Bonar DB. Pharmacological evidence that alpha1—adrenoceptors mediate metamorphosis of the Pacific oyster, Crassostrea gigas. Neuroscience. 1987;23:1169–1174. doi: 10.1016/0306-4522(87)90190-4. [DOI] [PubMed] [Google Scholar]
- Eri R, et al. Hemps, a novel EGF-like protein, plays a central role in ascidian metamorphosis. Development. 1999;126:5809–5818. doi: 10.1242/dev.126.24.5809. [DOI] [PubMed] [Google Scholar]
- Ferrero R, Rodriguez-Pascual F, Miras-Portugal MT, Torres M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production. Br J Pharmacol. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggett S, Leise EM. Metamorphosis in the marine snail Ilyanassa obsolete Yes or NO? Biol Bull. 1999;196:57–62. doi: 10.2307/1543167. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxidesensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharm. 1995;48:184–188. [PubMed] [Google Scholar]
- Gifondorwa DJ, Leise EM. Programmed cell death in the apical ganglion during larval metamorphosis of the marine mollusc Ilyanassa obsoleta. Biol Bull. 2006;210:109–120. doi: 10.2307/4134600. [DOI] [PubMed] [Google Scholar]
- Golderer G, Werner E, Leitner S, Grobner P, Werner-Felmayer G. Nitric oxide synthase is induced in sporulation of Physarum polycepharum. Genes Dev. 2001;15:1299–1309. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield MG. In: Chia F-S, Rice ME, editors. Metamorphosis in marine molluscan larvae: an analysis of stimulus and response; Settlement and Metamorphosis of Marine Invertebrate Larvae: Proceedings of the Symposium on Settlement and Metamorphosis of Marine Invertebrate Larvae: American Zoological Society Meeting; Toronto, Ontario, Canada. December 27–28, 1977; Oxford: Elsevier; 1978. pp. 1–290. [Google Scholar]
- Hadfield MG. The D. P. Wilson Lecture, Research on settlement and metamorphosis of marine invertebrate larvae: past, present and future. Biofouling. 1998;12 (1–3):9–29. [Google Scholar]
- Hadfield MG, Carpizo-Ituarte EJ, del Carmen K, Nedved BT. Metamorphic competence, a major adaptive convergence in marine invertebrate larvae. Am Zool. 2001;41:1123–1131. [Google Scholar]
- Hadfield MG, Meleshkevitch EA, Boudko DY. The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol Bull. 2000;198:67–76. doi: 10.2307/1542804. [DOI] [PubMed] [Google Scholar]
- Hadfield MG, Paul VJ. Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. In: McClintock JB, Baker W, editors. Marine Chemical Ecology. CRC Press; Boca Raton, FL: 2001. pp. 431–461. [Google Scholar]
- Hadfield MG, Pennington JT. Nature of the metamorphic signal and its internal transduction in larvae of the nudibranch Phestilla sibogae. Bull Mar Sci. 1990;46:455–464. [Google Scholar]
- Hadfield MG, Scheuer D. Evidence for a soluble metamorphic inducer in Phestilla: ecological, chemical and biological data. Bull Mar Sci. 1985;37:556–566. [Google Scholar]
- Harris LG. Doctoral Dissertation. University of California; Berkeley: 1970. Studies on the Biology of the aeolid nudibranch Phestilla melanobranchia Bergh 1874. [Google Scholar]
- He Y, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- Hodin J. Expanding networks: Signaling components in and a hypothesis for the evolution of metamorphosis. Integr Comp Biol. 2006;46:719–742. doi: 10.1093/icb/icl038. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B. Detection of nitric oxide by electron spin resonance in chemical, photochemical, cellular, physiological, and pathophysiological systems. Methods Enzymol. 1996;268:168–187. doi: 10.1016/s0076-6879(96)68019-7. [DOI] [PubMed] [Google Scholar]
- Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms methods in enzymology. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- Komarov A, Mattson D, Jones MM, Singh PK, Lai CS. In vivo spin trapping of nitric oxide in mice. Biochem Biophys Res Commun. 1993;195:1191–1198. doi: 10.1006/bbrc.1993.2170. [DOI] [PubMed] [Google Scholar]
- Kotake Y. Continuous and quantitative monitoring of rate of cellular nitric oxide generation. Methods Enzymol. 1996;268:222–229. doi: 10.1016/s0076-6879(96)68024-0. [DOI] [PubMed] [Google Scholar]
- Krug PJ. Bet-hedging dispersal strategy of a specialist marine herbivore: a settlement dimorphism among sibling larvae of Alderia modesta. Mar Ecol Prog Ser. 2001;213:177–192. [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mayer B, Klatt P, Werner ER, Schmidt K. Identification of imidazole as L-arginine-competitive inhibitor of porcine brain nitric oxide synthase. FEBS Lett. 1994;350:199–202. doi: 10.1016/0014-5793(94)00766-7. [DOI] [PubMed] [Google Scholar]
- Miller SE, Hadfield MG. Ontogeny of phototaxis and metamorphic competence in larvae of the nudibranch Phestilla sibogae Bergh (Gastropoda: Opisthobranchia) J Exp Mar Biol Ecol. 1986;97:95–112. [Google Scholar]
- Moroz LL. Localization of putative nitrergic neurons in the peripheral chemosensory areas and the central nervous system of Aplysia californica. J Comp Neur. 2006;495:10–20. doi: 10.1002/cne.20842. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Chen D, Gillette MU, Gillette R. Nitric oxide synthase activity in the molluscan CNS. J Neurochem. 1996;66:873–876. doi: 10.1046/j.1471-4159.1996.66020873.x. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Norby SW, Cruz L, Sweedler JV, Gillette R, Clarkson RB. Non-enzymatic production of nitric oxide (NO) from NO synthase inhibitors. Biochem Biophys Res Commun. 1998;253:571–576. doi: 10.1006/bbrc.1998.9810. [DOI] [PubMed] [Google Scholar]
- Morse DE, Duncan H, Hooker N, Baloun A, Young G. GABA induces behavioral and developmental metamorphosis in planktonic molluscan larvae. Fed Proc. 1980;39:3237–3241. [PubMed] [Google Scholar]
- Ninnemann H, Maier J. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photo-conidiation of Neurospora crassa. Photochem Photobiol. 1996;64:393–398. doi: 10.1111/j.1751-1097.1996.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Norby SW, Weyhenmeyer JA, Clarkson RB. Stimulation and inhibition of nitric oxide production in macrophages and neural cells as observed by spin trapping. Free Radic Biol Med. 1997;22:1–19. doi: 10.1016/s0891-5849(96)00217-1. [DOI] [PubMed] [Google Scholar]
- Ohnishi ST. Measurement of NO using electron paramagnetic resonance. Methods Mol Biol. 1998;100:129–153. doi: 10.1385/1-59259-749-1:129. [DOI] [PubMed] [Google Scholar]
- Pechenik JA, Cochrane DE, Li W, West ET, Pires A, Leppo M. NO inhibits metamorphosis in larvae of Crepidula fornicata, the slippershell snail. Biol Bull. 2007;213:160–171. doi: 10.2307/25066632. [DOI] [PubMed] [Google Scholar]
- Pires A, Croll RP, Hadfield MG. Catecholamines modulate metamorphosis in the opisthobranch gastropod Phestilla sibogae. Biol Bull. 2000;198:319–331. doi: 10.2307/1542688. [DOI] [PubMed] [Google Scholar]
- Pires A, Hadfield MG. Oxidative breakdown products of catecholamines and hydrogen peroxide induce partial metamorphosis in the nudibranch Phestilla sibogae Bergh (Gastropoda: Opisthobranchia) Biol Bull. 1991;180:310–317. doi: 10.2307/1542402. [DOI] [PubMed] [Google Scholar]
- Shinobu LA, Jones SG, Jones MM. Sodium N-methyl-D-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol Toxicol (Copenh) 1984;54:189–194. doi: 10.1111/j.1600-0773.1984.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Howlett A, Klein C. Nitric oxide-releasing compounds inhibit Dictyostelium discoideum aggregation without altering cGMP production. FEBS Lett. 1992;4:49–52. doi: 10.1016/0014-5793(92)81459-y. [DOI] [PubMed] [Google Scholar]
- de Vente J, Steinbusch HW, Schipper J. A new approach to immunocytochemistry of 3′,5′-cyclic guanosine monophosphate: preparation, specificity, and initial application of a new antiserum against formaldehyde-fixed 3′,5′-cyclic guanosine monophosphate. Neuroscience. 1987;22:361–373. doi: 10.1016/0306-4522(87)90226-0. [DOI] [PubMed] [Google Scholar]
- Weinberg RJ, Valtschanoff JG, Schmidt HHW. The NADPH diaphorase histochemical stain. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. Wiley and Son; Chichester, West Sussex: 1996. pp. 237–248. [Google Scholar]
- Wilken M, Huchzermeyer B. Suppression of mycelia formation by NO produced endogenously in Candida tropicalis. Eur J Cell Biol. 1999;78:209–213. doi: 10.1016/S0171-9335(99)80100-9. [DOI] [PubMed] [Google Scholar]
- Wolff DJ, Gribin BJ. The inhibition of the constitutive and inducible nitric oxide synthase isoforms by indazole agents. Arch Biochem Biophys. 1994;311:300–306. doi: 10.1006/abbi.1994.1241. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Brandish PE, Ballou DP, Marletta MA. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. PNAS. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]