Abstract
The retinal pigment epithelium (RPE) is a single layer of nonregenerating cells essential to homeostasis in the retina and the preservation of vision. While the RPE perform a number of important functions, 2 essential processes are phagocytosis, which removes the most distal tips of the photoreceptors to support disk renewal, and the visual cycle, which maintains the supply of chromophore for regeneration of photo-bleached visual pigments. We recently reported that these processes are linked by a noncanonical form of autophagy termed LC3-associated phagocytosis (LAP) in which components of the autophagy pathway are co-opted by phagocytosis to recover vitamin A in support of optimal vision. Here we summarize these findings.
Keywords: autophagy, phagocytosis, vision, visual cycle, 11-cis retinal, vitamin A, all-trans retinol, ATG5, LC3, retinal pigment epithelium, photoreceptors
Phagocytosis and autophagy are 2 ancient systems in which cargos are delivered to the lysosomes for degradation. Cells utilize phagocytosis to ingest extracellular material following engagement of cell surface receptors, whereas autophagy is activated under conditions of cellular stress that isolates portions of the cytoplasm to target cytoplasmic proteins, organelles, and lipids for degradation. Although both processes can be engaged in nutrient recovery, regulation of immunity, and cell survival, they were thought to be distinct, as phagocytosis involves the formation of single-membrane phagosomes, whereas autophagy forms characteristic double-membrane structures termed autophagosomes. Recent studies, however, have shown that there is a functional convergence of autophagy and phagocytosis where components of the autophagy pathway have been co-opted to increase the efficient degradation of the phagocytosed cargo by aiding in lysosome/phagosome fusion and maturation of the phagolysosome. This process, termed LC3-associated phagocytosis (LAP), has been demonstrated in phagocytic cells such as macrophages, plasmacytoid dendritic cells, and mammary epithelial cells that engulf living cells (entosis).
RPE cells phagocytose photoreceptor outer segments (POS) that are shed daily during renewal of photoreceptors, and the phagocytosed disc material is recycled to replenish necessary components of the photoreceptors and remove excess material. The RPE also houses the visual cycle, a series of enzymatic reactions that utilizes vitamin A [all-trans-retinol, ROL)] to maintain the supply of chromophore [11-cis retinal, (RAL)] to regenerate photo-bleached pigments for optimal vision. Defects in either process can have serious consequences for vision.
Recently, we undertook studies to examine the importance of autophagy in the RPE by performing assays commonly used to assess autophagic function. We found that the conversion of microtubule-associated protein 1 light chain 3 (LC3) to its lipidated form (LC3-II) in the RPE is optimal at 7:00 AM (1 h after lights on) and greatly diminishes at 7:00 PM (1 h into the dark cycle). Similarly, the formation of GFP-LC3 puncta in the RPE of GFP-LC3 transgenic mice is also optimal in the morning, coinciding with the optimal time of outer segment disc shedding. We further noted that both ATG5 and LC3 associate with the phagocytosed POS in the RPE cells of normal mice. When the essential autophagy gene Atg5 is deleted specifically in RPE cells using an RPE-specific cre recombinase (Atg5ΔRPE mice) the enzymatic conversion of LC3 and the formation of GFP-LC3 puncta are eliminated, as is the association of LC3 and ATG5 with phagocytosed POS.
We suspected that we might have been observing the formation of the phagosome rather than an autophagosome. This was confirmed by transmission electron microscopy (TEM) where we failed to find double-membrane structures associated with phagocytosed POS, or, indeed, any autophagosomes in the RPE of mice examined in the morning. In sharp contrast the RPE of Atg5ΔRPE mice accumulate phagosomes containing poorly processed POS, further suggesting that ATG5 and LC3 are associated with phagocytosis rather than with conventional autophagy. We confirmed this by using an RPE cell line fed with isolated POS and noted LC3 conversion and the association of LC3 with the POS in phagosomes. Using siRNA silencing, we found that LC3 association with the phagosome is dependent on ATG5 and BECN1, but not on components of the autophagy pre-initiation complex composed of RB1CC1/FIP200, ULK1, and ATG13. Furthermore, silencing of Atg5 (i.e., blocking LAP) prevents colocalization of LAMP1, LAMP2, and the protease CTSD/cathepsin D with the phagosome, indicating that POS degradation is also dependent on LAP. Engulfment of POS, however, is unaffected by the disruption of LAP demonstrating that while LAP is important for degradation of the POS, it is not required for phagocytosis.
Strikingly, Atg5ΔRPE mice display profoundly diminished response to light stimuli, as determined by electroretinogram (ERG) analysis, with defects in both rod and cone photoreceptor function. That these mice have no significant loss of rod or cone photoreceptors suggests that the visual impairment might be more subtle. An interesting possibility is that phagocytosis of POS is linked to the visual cycle, a process that recycles photo-bleached retinoids from the photoreceptors back to the retina for proper visual function. While the quantities of RPE enzymes involved in the visual cycle are not different between wild-type and Atg5ΔRPE RPE cells, Atg5ΔRPE mice have reduced 11-cis, all-trans, and 13-cis retinal suggesting a deficit in intrinsic chromophore synthesis by the visual cycle. Thus, the machinery for chromophore synthesis is in place, but it appears that the supply of raw material (i.e., vitamin A) might be reduced. This was confirmed when vision is restored in Atg5ΔRPE mice by providing an external source of the missing chromophore in the form of 9-cis RAL, a functional analog of the visual chromophore 11-cis RAL.
Our results demonstrate a convergence of the visual cycle and RPE phagocytosis (Fig. 1), 2 vital functions that support vision. Phagocytosis of the POS tips and degradation via LAP supports photoreceptor disk renewal, but also supports the classical visual cycle. These results have a number of implications for the study of vision, phagocytosis, and autophagy. From a practical standpoint, our studies have blurred the distinction between autophagy and phagocytosis in the RPE. It is no longer sufficient to perform western blots for LC3 or quantify GFP-LC3 puncta to measure conventional autophagy in phagocytic RPE cells, as these features are also present during LAP. Second, the possibility exists that defective LAP might account for some of the age-dependent defects in vision often attributed to autophagy. It is often hypothesized that autophagic defects might contribute to age-related eye diseases such as age-related macular degeneration (AMD), and we would suggest that defects in LAP should also be examined as a contributing factor. Thus, it is crucial that we determine ways to distinguish LAP from autophagy before meaningful therapeutic interventions can be designed. Finally, a reduction in vitamin A recovery may be a reason why elderly individuals have difficulties with night vision and supplementation with nutritional vitamin A can improve this condition. Defective LAP with aging might account for these observations.
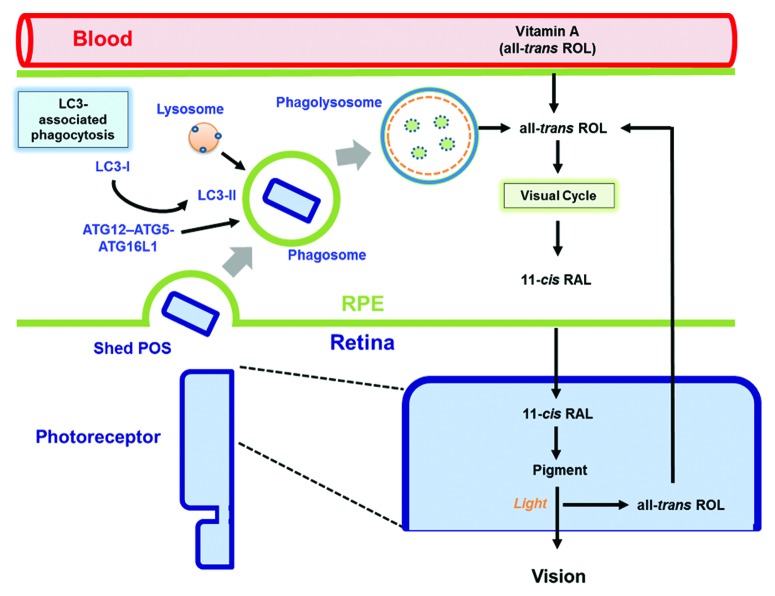
Figure 1. Convergence of phagocytosis, autophagy, and the visual cycle. The RPE performs 2 vital functions that support vision, phagocytosis, and the visual cycle. Phagocytosis and degradation of POS are mediated by a noncanonical form of autophagy termed LC3-associated phagocytosis. Engulfed POS enter the phagosome, and the ATG12–ATG5-ATG16L1 complex is recruited, as is the lipidated form of LC3 (LC3-II). Only then does the lysosome fuse with the phagosome forming the phagolysosome leading to degradation of the POS cargo. The second critical process is the classic retinoid visual cycle that begins following the absorption of light in the photoreceptors by visual pigments containing the chromophore 11-cis RAL. This generates all-trans ROL which is transported to the RPE for conversion back to 11-cis RAL to recharge the pigments. These 2 critical pathways converge as the recovery of all-trans ROL for 11-cis RAL synthesis in the RPE is aided by the POS phagocytosis and degradation pathway.
The interplay between 2 important intracellular lysosomal degradative pathways (phagocytosis and autophagy) to support vision represents a fundamentally new way in which to think about the impact of the autophagy machinery on RPE and photoreceptor cell function. Exploring this exciting process will open new avenues for understanding, and ultimately manipulating, the relationships between the autophagy machinery and disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Institutes of Health Grants EY06765 (TAF), EY015570 (TAF), AI44848 (DRG), and AI40646 (DRG), and a Department of Ophthalmology and Visual Sciences core grant (EY02687). Support was also received from Research to Prevent Blindness (New York, NY), the Carl Marshall Reeves and Mildred Almen Reeves Foundation (Columbus, IN) and the BrightFocus Foundation (Clarksburg, MD). Thanks to all our excellent co-authors for their hard work.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/26735