Abstract
Hematopoietic stem cells (HSCs) are inherently quiescent and self-renewing, yet can differentiate and commit to multiple blood cell types. Intracellular mitochondrial content is dynamic, and there is an increase in mitochondrial content during differentiation and lineage commitment in HSCs. HSCs reside in a hypoxic niche within the bone marrow and rely heavily on glycolysis, while differentiated and committed progenitors rely on oxidative phosphorylation. Increased oxidative phosphorylation during differentiation and commitment is not only due to increased mitochondrial content but also due to changes in mitochondrial cytosolic distribution and efficiency. These changes in the intracellular mitochondrial landscape contribute signals toward regulating differentiation and commitment. Thus, a functional relationship exists between the mitochondria in HSCs and the state of the HSCs (i.e., stemness vs. differentiated). This review focuses on how autophagy-mediated mitochondrial clearance (i.e., mitophagy) may affect HSC mitochondrial content, thereby influencing the fate of HSCs and maintenance of hematopoietic homeostasis.
Keywords: autophagy, mitochondria, mitophagy, hematopoiesis, hematopoietic stem cells, hematopoietic progenitor cells, quiescence, self-renewal, differentiation, commitment
Introduction
Hematopoiesis is the formation of the cellular constituents of blood from a set of highly specialized cells called hematopoietic stem cells. HSCs reside in a hypoxic stromal-rich niche within the bone marrow and replenish blood cells throughout adulthood. HSCs cater to the body’s demands under normal conditions and during stress by fluctuating between quiescent and active states. What governs these states is not fully understood but is an active area of research.
Mitochondria are involved in essential cellular processes, including energy production, calcium homeostasis, regulation of cell death, and reactive oxygen species (ROS) production.1,2 Mitochondrial attributes as well as metabolites affect the state of HSCs. Indeed, mitochondria have been proposed to be one of the determinants of stem-cell fate,3 with mitochondrial quality and quantity undergoing significant changes in HSCs and other stem-cell types during proliferation and differentiation. In the case of HSCs, these alterations of the mitochondrial landscape can be due to the process of hematopoiesis itself; however, there is growing evidence that mitochondria play an active role in regulating hematopoiesis by defining the route and rate of the process.
Mitochondria are dynamic organelles, and they can undergo dramatic changes in morphology, number, subcellular distribution, and energy/metabolite production in response to changes in the intracellular or extracellular milieu. Mitochondrial content and integrity are regulated at many levels (transcriptional, translational, and posttranslational) by de novo synthesis, fusion, fission, and degradation.4,5 Perturbing any of these processes may lead to accumulation of damaged or dysfunctional mitochondria and compromise cellular function. In the context of the hematopoietic system, these outcomes may contribute to a variety of hematopoietic disorders, including cytopenias and malignancies.
Autophagy is among the primary means of eliminating whole organelles, including mitochondria.6 This evolutionarily conserved intracellular catabolic process eliminates superfluous and/or damaged cytosolic components via the lysosomal pathway after engulfing them in a double-membrane structure.7-10 By recycling cellular components, autophagy also provides an alternative source of metabolites under conditions of actual or perceived nutrient deprivation.11 The magnitude of the autophagy response may be regulated by the nature and degree of stress, with excessive autophagy leading to cell death.12 Autophagy is involved in cell survival, immunity, and development, and dysregulation of this cellular process has been implicated in disease states including neurodegeneration, cancer, infection, and autoimmunity.7,12,13
Defects in autophagy and mitochondrial function have been independently linked to myeloproliferative and myelodysplastic disorders in mice,14-18 suggesting that autophagy-mediated degradation of mitochondria (i.e., mitophagy) plays an important role in maintaining HSCs and hematopoietic progenitor cells (HPCs), and may contribute to the pathogenesis of certain hematopoietic disorders. This review highlights the importance of mitochondria in HSC and HPC function, the contribution of autophagy to HSC and HPC maintenance, and possible roles for mitophagy in the context of hematological disorders.
HSCs and Mitochondria
Mitochondrial content
The stromal-rich niche of the bone marrow is maintained with low oxygen tension to preserve HSCs in a quiescent state until they are stimulated to divide. The unequal division leads to two daughter cells with different fates: one destined for self-renewal and the other for differentiation.19 The hypoxic stem-cell niche is ideal for long-term HSCs because they depend primarily on glycolysis and have low mitochondrial activity.20 By contrast, as HSCs proliferate and differentiate, the energy demands of the cell increase, and the overall mitochondrial content of the cell needs to be increased to cope with these changes. Evidence indicates that mitochondrial mass increases with differentiation and lineage commitment in both murine and human hematopoietic systems. For example, human CD34+ HSCs, which are enriched in cells with long-term repopulating ability, have low oxygen consumption rates and low amounts of mitochondrial respiratory chain complexes: The loss of CD34 in these cells correlates with an increase in mitochondrial content.21 Similarly, in mice, an increase in the polarized mitochondrial content within the Lin−ATXN1+C-KIT+ (Lin−Sca1+c-Kit+) compartment occurs that coincides with the loss of long-term repopulating ability.22 Finally, the mitochondrial content in murine HSCs is similar to that in myeloid progenitors but lower than that in erythroid progenitors.23
Mitochondrial ATP production
Because the hypoxic environment of the bone marrow stroma is not conducive to ATP production through oxidative phosphorylation, it is not surprising that HSCs have limited mitochondrial mass and rely on anaerobic glycolysis and fatty acid oxidation to meet their energy demands. STK11/ LKB1 (serine/threonine kinase 11) and AMPK (AMP-activated protein kinase) play important roles in maintaining bioenergetics homeostasis in HSCs. AMPK is activated when intracellular ATP levels decline, and it coordinates the upregulation of catabolic pathways and inhibition of biosynthetic pathways to restore ATP levels.24 STK11 is a tumor suppressor that modulates cellular growth and metabolism by phosphorylating and activating AMPK and 12 AMPK-related kinases.25-28
The absence of STK11 in adult mice results in impaired survival and escape from quiescence of HSCs, exhaustion of the HSC pool followed by a rapid depletion of all hematopoietic subpopulations, and severe pancytopenia, which is not rescued by MTOR (mechanistic target of rapamycin) inhibition or treatment with the anti-oxidant N-acetyl cysteine.29-31 STK11-deficient bone marrow cells show a transient decrease in mitochondrial mass followed by an increase, alterations in lipid and nucleotide metabolism, and depletion of cellular ATP.31 Ampk-deficient HSCs undergo similar changes in mitochondrial function but remain able to reconstitute irradiated mice, indicating that although both AMPK and STK11 help preserve mitochondrial function and ATP levels in HSCs, STK11’s role in HSC maintenance occurs predominantly through an AMPK-independent mechanism that likely involves activation of one or more AMPK-like kinases.29
Mitochondria play an important role in the metabolism of lipids through fatty acid oxidation (FAO).32 The importance of fatty acid metabolism in HSCs is highlighted by the finding that activation of FAO by PPARD (peroxisome proliferator-activated receptor delta), a member of a nuclear receptor superfamily of transcription factors that control nutrient sensing and the transcriptional regulation of fatty-acid transport and FAO,33,34 promotes asymmetric division of HSCs, favoring self-renewal.19 PPARD depletion or chemical inhibition of FAO promotes the symmetric commitment of HSC daughter cells (leading to stem cell exhaustion), whereas PPARD activation increases asymmetric cell division.19
Similarly, depletion of the mitochondrial phosphatase PTPMT1 (protein tyrosine phosphatase, mitochondrial 1), which disrupts the transition from glycolysis and FAO to mitochondrial aerobic metabolism, results in hematopoietic failure associated with accumulation of HSCs that cannot differentiate because of enhanced entry of quiescent stem cells into the cell cycle and a subsequent delay at the G1 phase.35 Mechanistically, PTPMT1 depletion results in increased levels of the phosphatidylinositol phosphates (normally targeted by PTPMT1) and activation of UCP2 [uncoupling protein 2 (mitochondrial, protein carrier)], which inhibits pyruvate from entering the mitochondria and facilitates oxidation of alternative carbon sources.35 PTPMT1-deficient cells also have more activated AMPK than do wild-type controls (likely compensatory), contributing to elevated levels of glycolysis and fatty acid oxidation. Interestingly, PTPMT1 can be depleted from T lymphoid, B lymphoid, or myeloid progenitors in lineage-specific knockout mice without consequence, suggesting a unique and specific requirement for mitochondrial energy metabolism in stem cell/early progenitor differentiation that does not exist in late lineage-restricted progenitors or mature cells.35
Reactive oxygen species
Mitochondria are a major source of ROS, including superoxide, hydrogen peroxide, and hydroxyl radicals. More specifically, during oxidative phosphorylation electrons are transferred from electron transport chain complexes directly to oxygen, generating superoxide radical. Although both oxygen and superoxide are toxic to cells, oxidative phosphorylation yields at least 15-fold more energy than does anaerobic glycolysis, and recent studies have highlighted the role of ROS in HSC signaling.36 Aerobic organisms have developed antioxidant defenses to help protect cells from oxygen and ROS. For HSCs, one such protection strategy may be to limit exposure by lodging in the hypoxic environment of the stromal niche.
The metabolic transition from anaerobic to aerobic metabolism that occurs during HSC differentiation and commitment is associated with increased ROS production. Hematopoietic growth factors such as CSF2/GMCSF [colony stimulating factor 2 (granulocyte-macrophage)], IL3 [interleukin 3 (colony-stimulating factor, multiple)], KITLG/steel factor (KIT ligand), and THPO/TPO (thrombopoietin) induce increases in ROS in quiescent cells,37 partially by activating PtdIns3K (class III phosphatidylinositol 3-kinase)-PTEN (phosphatase and tensin homolog)-AKT (v-akt murine thymoma viral oncogene homolog 1)-MTOR pathway. AKT is a member of a multigene family of serine-threonine kinases (AKT1, AKT2 and AKT3) in which AKT1 and AKT2 are the most widely expressed. AKT1/2 double-deficient HSCs persist in quiescence because of insufficient ROS production.38
AKT inhibits members of the FOXO (forkhead box O) family of transcription factors, which are involved in regulating the cell cycle, apoptosis, and response to oxidative stress.39-41 By contrast, FOXO family member FOXO3 is activated downstream of HIF1A [hypoxia inducible factor 1, α subunit (basic helix-loop-helix transcription factor)] during hypoxic conditions and represses a set of nuclear-encoded mitochondrial genes that mediate the hypoxic suppression of mitochondrial mass, oxygen consumption, and ROS production.42 FOXO-deficient mice (either Foxo1/3/4-null or Foxo3/Foxo3a-null) have elevated levels of ROS, which are associated with defects in HSCs’ reconstitution capability. These defects can be reversed by antioxidant treatment, implicating excessive ROS production as a detrimental signal for HSCs’ function and survival.43-45
MTOR is a nutrient sensor that balances cell growth with nutrient availability by regulating cellular functions, including nutrient uptake, protein synthesis, and autophagy.46 The MTOR pathway also regulates energy metabolism via transcriptional control of mitochondrial oxidative function through the YY1–PPARGC1A transcriptional complex.47 Tuberous sclerosis complex, composed of TSC1 and TSC2, is a major negative regulator of the MTOR pathway, and is negatively and positively regulated by AKT and AMPK, respectively.48 Conditional deletion of Tsc1 in HSCs and the associated MTOR hyperactivation leads to rapid cycling, reduced numbers, and decreased reconstitution capacity of HSCs. The HSCs derived from these TSC1-altered mice also have more mitochondria and higher ROS levels than do their wild-type counterparts.49,50
ROS levels modulate attachment and mobility of HSCs, favoring exit of HSCs from the bone marrow niche.51,52 Although low levels of ROS favor properties of stemness such as quiescence, higher self-renewal potential, maintenance of migratory potential and expansion of primitive and active HSCs in G0/G1 phase, and appropriate amounts of ROS production are required for normal hematopoiesis, excessive ROS levels lead to significant HSC exhaustion, premature senescence, and divergence from stemness attributes, leading to a loss of stem cell function, inhibition of terminal differentiation of HSCs into various lineages, and apoptosis.53-56 In Drosophila, as in mice, lesser than requisite amounts of ROS in multipotent hematopoietic progenitors retard HSC differentiation into mature blood cells, and higher levels of ROS stimulate early differentiation,57 suggesting evolutionary conservation of ROS’ role in determining stem cell fate.
Mitochondrial DNA mutations
The 16.6-kb self-replicating double-stranded DNA mitochondrial genome, which encodes components of the electron transport chain,58 is heavily prone to random mutations because of its proximity to ROS produced during oxidative phosphorylation and the lack of DNA-repair enzymes and protective histones.59 In mice, a defect in the proofreading function of the mitochondrial DNA polymerase POLG (D257A) results in somatic accumulation of mutations in the mitochondrial genome and a progressive decline in mitochondrial function. These mice develop a progeroid phenotype with early onset of several age-related diseases and premature death of mice by the age of 15 mo.16-18 One manifestation of the mitochondrial dysfunction in the hematopoietic compartment is a myelodysplastic syndrome (MDS)-like disorder with macrocytic anemia, lymphopenia, and erythroid dysplasia. The accumulation of mitochondrial mutations (and decreased mitochondrial function) does not affect HSC self-renewal but impairs normal differentiation of erythroid and B lineages.23 These results suggest that hematopoietic progenitors are more severely affected by the accumulation of mitochondrial DNA (mtDNA) mutations than are HSCs, consistent with the increasing reliance of progenitors on intact mitochondrial function.
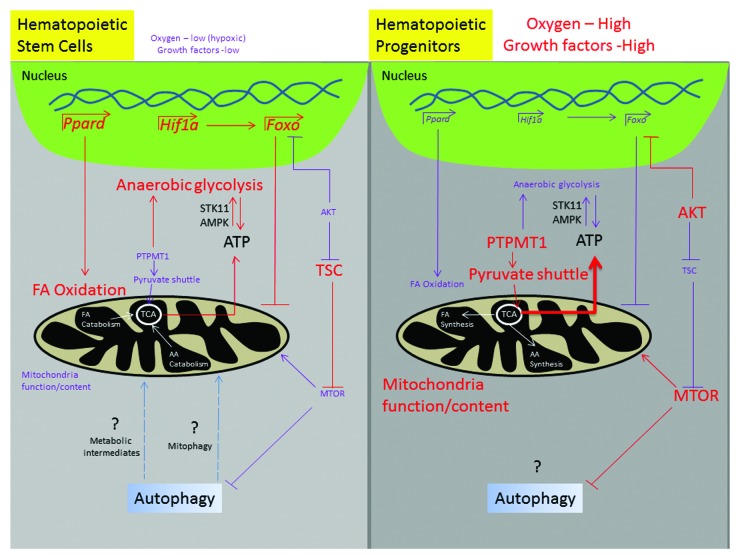
In summary, the hypoxic stem-cell niche maintains quiescence of HSCs in a HIF1A-FOXO-dependent manner, at least in part by limiting cellular mitochondrial content and activity. In the absence of AKT activation, TSC1-inhibition of the MTOR pathway also contributes to HSC quiescence by repressing mitochondrial biogenesis and ROS production. ATP production by anaerobic glycolysis and fatty acid oxidation favors asymmetric division of HSCs (leading to self-renewal) and is at least in part regulated by STK11, AMPK, PPARD, and PTPMT1. Growth factor-induced activation of AKT inhibits TSC1 and FOXO family members: together with the metabolic conversion from anaerobic glycolysis to mitochondrial oxidative phosphorylation, this stimulates increased ROS levels to fuel hematopoietic differentiation (Fig. 1). The increased reliance of hematopoietic progenitor cells, especially erythroid precursors, on functional mitochondria is reflected in the increase in mitochondrial mass/activity in these cells and their sensitivity to mitochondrial dysfunction arising from mtDNA mutations.
Figure 1. Mitochondria and metabolism in hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). HSCs reside in a hypoxic niche with low amounts of growth factors. This environment fosters the activation of transcription factors such as HIF1A, FOXO, and PPARD and the inhibition of AKT kinase. This paradigm facilitates fatty acid oxidation, reduced mitochondrial content (via de-repressing TSC, which inhibits MTOR-mediated mitochondrial biogenesis and FOXO-mediated inhibition of nuclear-encoded mitochondrial biogenesis), lower amounts of ROS, and increased anaerobic glycolysis. Autophagy has been implicated in HSC maintenance, but it is unclear which function of autophagy is critical—clearance of damaged mitochondria or recycling of metabolites. A metabolic shift occurs with commitment of HSCs to differentiate, leading to suppression of fatty acid oxidation and anaerobic glycolysis, increases in mitochondrial content and oxidative phosphorylation, and higher levels of ROS, which are at least partially mediated through AKT and MTOR. The role of autophagy in committed hematopoietic progenitor cells remains unclear. FA, fatty acids; AA, amino acids; TCA, tricarboxylic acid cycle.
HSCs and Mitophagy
Autophagy and mitochondrial function
Autophagy plays an important role in cellular homeostasis by recycling metabolites and eliminating damaged or superfluous organelles. Mouse models lacking key nonredundant autophagy genes, including Atg3, Atg5, Atg7, Atg9, Atg16l1, Ambra1, Rb1cc1/Fip200, and Becn1, show early lethality (embryonal or perinatal).60 These models have implicated autophagy in embryonic development and survival. Accumulation of mitochondria has been documented in several mice models, including tissue-specific gene knockouts of Rb1cc1, Atg5, and Atg7, implicating autophagy in the regulation of mitochondrial mass (Table 1). Among these models, the accumulation of abnormal mitochondria is observed after autophagy impairment by conditional Atg5 or Atg7 deletion in certain tissues but not in others, suggesting a tissue-specific role of autophagy in maintaining mitochondrial integrity. Some of the factors involved in directing the autophagy machinery toward mitochondria are described below.
Table 1. Mitochondrial phenotype in mice with autophagy gene deletions.
| Gene | Promoter/ inducible system | Systemic phenotype | Mitochondrial phenotype | Refs. | |
|---|---|---|---|---|---|
| Technique used | Characteristics | ||||
| Ulk1 | None* | Defect in terminal stages of red blood cell maturation | • EM • FACS (MitoTracker Green, TMRM) • Citrate synthase |
Mitochondrial retention in terminal stages of red blood cell maturation; increased mitochondrial mass in MEFs | 61 |
| Rb1cc1 | Nestin | Cerebellar degeneration; ubiquitinated protein and SQSTM1/p62 accumulation | • EM | Mitochondrial accumulation (smaller, more condensed and fragmented) | 62 |
| Tek/Tie2 | Perinatal lethality; severe anemia; SQSTM1/p62 accumulation | • FACS (MitoTracker Green) • DCF-DA |
Mitochondrial accumulation; increased ROS | 63 | |
| Atg5 | Mertk/ MerCreMer |
Cardiac hypertrophy— left ventricular dilatation; contractile dysfunction; disorganized sarcomere; ubiquitin and SQSTM1/p62 accumulation | • EM | Mitochondrial misalignment and aggregation | 64 |
| Lck (Chimeric mice) | Decreased T cell numbers because of increased cell death and defective proliferation | • FACS (MitoTracker Green) | Increased mitochondrial mass | 65, 66 | |
| Nphs2/ Podocin |
Late-onset glomerulosclerosis; accumulation of oxidized, ubiquitinated proteins and SQSTM1/p62 | • EM | Mitochondrial accumulation (swollen and damaged) | 67 | |
| Atg7 | Mlc1 | Muscle atrophy; sarcoplasmic reticulum distension; disorganized sarcomeres; SQSTM1/p62 and ubiquitin accumulation | • EM • SDH staining |
Mitochondrial accumulation (swollen and damaged) | 68 |
| Mx1 | Hepatomegaly and hepatic cell swelling; ubiquitin-positive inclusions; SQSTM1/p62 accumulation; impaired removal of peroxisomes after DEHP treatment | • EM • WB • SDH activity measurement |
Deformed mitochondria; unchanged CYCS content; higher SDH activity at baseline; no change in SDH activity after fasting | 69–71 | |
| Lck | Decreased T cell numbers because of increased apoptotic cell death | • FACS (MitoTracker Green, TMRE) • WB • Dihydroethidium and CM-H2DCFDA |
Mitochondrial accumulation; increases in surface area, mtDNA, ROS, TOMM20, CYCS, AIFM, and HSPA9 | 65, 72 | |
| Fabp4/Ap2 | Lean animals with decreased white adipose tissue mass; enhanced insulin-sensitivity; increased fatty acid oxidation; accumulation of SQSTM1/p62 | • EM • WB |
Mitochondrial accumulation (number, MT-CO1, and CYCS content increased) | 73, 74 | |
| Rip | Insulin deficiency; hyperglycemia; decreased mass and function of β cells; accumulation of SQSTM1/p62 and ubiquitin | • EM • ATP measurement |
Mitochondrial swelling and accumulation (deformed and branched); low ATP levels | 75, 76 | |
| Vav | Severe anemia; lymphopenia; DNA damage; cell death and increased proliferation in LSK compartment | • EM • FACS (NaO, TMRM, MitoTracker Green, MitoTracker Red, MitoSOX) • Mass spectrometry |
Damaged mitochondrial accumulation and altered membrane potential; increased ROS | 14, 15 | |
Germline mutation. Abbreviations: AIFM/AIF: apoptosis-inducing factor, mitochondrion-associated; CM-H2DCFDA: 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester; CYCS: cytochrome c, somatic; DCF-DA, 2′,7′: dichlorofluorescein diacetate; EM: electron microscopy; FACS: fluorescence-activated cell sorting; HSPA9/mtHSP70: heat shock 70 kDa protein 9 (mortalin); LSK: Lin−ATXN1+C-KIT+ (Lin−Sca1+cKit+); MEFs: murine embryonic fibroblasts; MT-CO1: mitochondrially encoded cytochrome C oxidase I; NaO: N-nonyl-acridine orange; ROS: reactive oxygen species; SDH: succinate dehydrogenase; TMRM: tetramethylrhodamine methyl ester perchlorate; TMRE: tetramethylrhodamine ethyl ester, perchlorate; TOMM20: translocase of outer mitochondrial membrane 20 homolog (yeast); WB: western blot.
Receptors for mitophagy
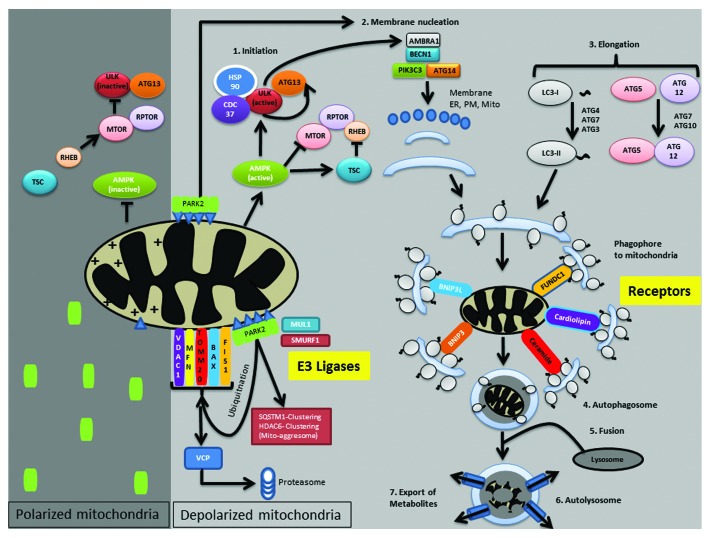
In yeast, mitochondria are targeted for autophagy by a complex formed between Atg32, Atg11, and Atg8. Atg11 functions as a scaffold protein by interacting with the organelle-specific receptor, the outer mitochondrial membrane protein Atg32, and Atg8, a key component of the ubiquitin-like conjugation pathway that regulates autophagosome formation.77,78 Although mammalian homologs of Atg11 or Atg32 have not been identified, there are several molecules that function as receptors and scaffolds, targeting mitochondria to autophagosomes (Fig. 2). Mammalian mitophagy receptor proteins, namely BNIP3L/NIX, BNIP3, and FUNDC1 (FUN14 domain containing 1), interact directly with microtubule-associated protein 1 light chain 3A/B (LC3) or other Atg8 family members via a conserved LC3-interacting region (LIR).79-81 In addition to the aforementioned mitophagy receptor proteins, ceramide, a bioactive sphingolipid, and cardiolipin, an inner mitochondrial membrane phospholipid, also interact directly with LC3 and have been implicated in mitophagy.82,83
Figure 2. Mammalian mitophagy receptors and E3 ligases. In cells with healthy polarized mitochondria, AMPK activity is limited, and the E3 ligase PARK2 remains in the cytoplasm. In the presence of adequate nutrients and growth factors, MTOR is active, inhibiting ULK1 and preventing autophagy induction. By contrast, mitochondrial dysfunction and reduced intracellular ATP levels result in AMPK activation, which not only inhibits MTOR but also directly phosphorylates ULK1 to induce autophagy. Moreover, PINK1 is stabilized on the outer mitochondrial membrane, facilitating PARK2 recruitment. PARK2 subsequently ubiquitinates and promotes degradation of several outer mitochondrial membrane proteins, facilitates mitochondrial aggregation and clustering, and phagophore nucleation. Phagophores are targeted to the mitochondria via receptors such as FUNDC1, BNIP3L, BNIP3, ceramide, and cardiolipin. Sequestered mitochondria are then degraded via fusion to lysosomes, and recycled metabolites are exported back to the cytosol. Other E3 ligases, namely MUL1 and SMURF1, are also implicated in mitophagy. ER, endoplasmic reticulum; PM, plasma membrane; Mito, mitochondria; +, membrane potential; ∆, PINK1; ○, vesicles; ~, phosphatidylethanolamine (PE).
The mitochondria-associated BH3-only protein, BNIP3L, plays an important role in the autophagy-mediated clearance of mitochondria that occurs during the terminal stages of red blood cell (RBC) maturation. BNIP3L is upregulated during erythroid differentiation, and genetic disruption of BNIP3L in mice results in macrocytic anemia and reticulocytosis, with impaired clearance of mitochondria, increased ROS, and decreased RBC life span in vivo.84,85 BNIP3L-deficient reticulocytes contain autophagosomes, but these are devoid of mitochondria, consistent with BNIP3L being a receptor for mitophagy.84
Similar to BNIP3L, the related BH3-only mitochondrial protein BNIP3 also contains an LIR, which promotes binding to Atg8 family members LC3B and GABARAPL2.86 BNIP3 is a pro-apoptotic member of the BCL2 family that is upregulated during heart failure and contributes to loss of myocardial cells during ischemia/reperfusion.87 BNIP3 promotes autophagy and mitochondrial turnover under hypoxic conditions in a HIF1A-dependent manner;88-90 therefore, it may play a role in mitochondrial turnover in HSCs, a possibility that remains to be explored. FUNDC1 is an integral mitochondrial outer membrane protein whose dephosphorylation under hypoxic conditions enhances its interaction with LC3-II, suggesting that FUNDC1 may be another receptor for hypoxia-induced mitophagy.91
Triggers for mitophagy
In yeast, the switch from a nonfermentable carbon source to a fermentable one, and mutations in the ATP synthase subunits of the electron transport chain, trigger selective autophagy-mediated clearance of mitochondria.77,92,93 In mammals, depolarization by using uncouplers such as carbonyl cyanide m-chlorophenylhydrazone (CCCP), photobleaching, or introducing mtDNA mutations can induce mitophagy;94-96 however, drastic clearance of mitochondria from cells seldom occurs except during RBC maturation and early embryogenesis.97-99 The trigger for mitophagy in these specific instances remains unclear.
Executioners of mitophagy
Various E3 ligases have been implicated in mitophagy. PINK1 (PTEN-induced putative kinase 1), a mitochondrial serine threonine kinase,100 is stabilized by loss of mitochondrial membrane potential and helps recruit the E3 ubiquitin ligase PARK2/Parkin to the mitochondria.94,101,102 Recently, the mitochondrial proteins MFN1 (mitofusin 1) and MFN2 have been reported to be substrates of PINK1, whose phosphorylation promotes PARK2 translocation to damaged mitochondria.103 PARK2 E3 activity relies on cysteine-mediated ubiquitin transfer, and trapping ubiquitin abrogates PARK2 translocation to mitochondria, suggesting the possibility that PARK2 catalytic activity plays a role in keeping PARK2 tethered to damaged mitochondria.104 Ultimately, PARK2 translocation to damaged mitochondria results in ubiquitination and proteasome-mediated degradation of several outer mitochondrial membrane proteins that can irreversibly disrupt the integrity of the outer mitochondrial membrane (Fig. 2).105
MFN1 and MFN2, which are involved in mitochondrial fusion, and VDAC1 (voltage-dependent anion channel 1), are among the proteins degraded in a PARK2-dependent manner.105-108 The degradation of mitofusins, and likely other proteins as well, relies on PARK2-mediated recruitment of VCP (valosin containing protein), a type II AAA ubiquitin-selective chaperone ATPase.109 PARK2’s translocation to mitochondria also leads to the recruitment of SQSTM1/p62 and HDAC6 (histone deacetylase 6), both of which promote aggregation of the mitochondria and have been implicated in mitophagy,108,110 although the necessity of SQSTM1/p62 for mitophagy has been debated.111,112
In addition to promoting ubiquitin-mediated recruitment of SQSTM1/p62 and HDAC6, and VCP-dependent degradation of outer mitochondrial membrane proteins, PINK1 and PARK2 interact directly with the BECN1/Beclin1-PtdIns3K complex that promotes autophagosome nucleation. More specifically, PINK1 interacts directly with the BECN1-PtdIns3K complex to activate autophagy,113 and PARK2 interacts with AMBRA1, a BECN1 activator, facilitating the recruitment of AMBRA1 to depolarized mitochondria to activate the PtdIns3K complex.114 Two other E3 ubiquitin ligases are also involved in mitophagy: SMURF1 (SMAD specific E3 ubiquitin protein ligase 1) in heart, brain, and liver tissues, and MUL1 (mitochondrial E3 ubiquitin protein ligase 1) in skeletal muscles (Fig. 2).115,116 The contribution of these and other ubiquitin ligases in mediating mitophagy in HSCs has not yet been explored.
Erythroid maturation model
Terminal RBC maturation is a physiologically relevant model system to study the molecular underpinnings of mitophagy. Genetic approaches have demonstrated the relevance of autophagy in mitochondrial clearance in the RBC maturation model. For example, deletion of Ulk1, the mammalian homolog of yeast ATG1, encoding a serine/threonine kinase involved in autophagy induction,117-119 results in a defect in mitochondrial clearance during erythroid maturation.61 ULK1 is also involved in PINK1-PARK2-induced mitophagy.120 Interestingly, although ATG5 and ATG7 are generally considered to be essential for autophagy, cells lacking ATG5 or ATG7 can still induce a form of autophagy that does not rely on LC3 lipidation and, instead, requires ULK1, BECN1, and RAB9A. This ATG5-ATG7-independent (i.e., noncanonical) autophagy is involved in clearing mitochondria during erythroid development in atg5−/− mice,121 which undergo normal organelle degradation and erythroid differentiation.122 The importance of this noncanonical autophagy pathway in HSCs (and other cell types) remains to be explored.
Even though RBCs and HSCs are widely distinct cell types with different fates, some parallels exist between these two cell types, suggesting that the knowledge gained by studying mitochondrial clearance in RBCs may be relevant for HSCs. In contrast to HSCs, which can self-renew, proliferate, and are destined for lifelong survival in an individual, RBCs are terminally differentiated, nonproliferative, and have a finite life span. Nevertheless, both cell types have limited mitochondrial activity and depend on glycolysis for their energy needs. Mature RBCs are devoid of mitochondria, at least in part to promote survival and limit ROS production in the high oxygen-tension environment of the lung. HSCs also have a relatively low mitochondrial content, limited mitochondrial respiration, and low ROS production, characteristics which, in this case, are related to the reduced oxygen tension of the endosteal niche. HSCs switch to aerobic metabolism for differentiation and commitment, whereas erythroid progenitors switch to anaerobic metabolism, gaining HSC-like attributes, at least in terms of their metabolic profile. Despite significant differences, the existence of certain parallels between RBCs and HSCs encourages the exploitation of the RBC maturation model system to further explore the mitophagy process, which may provide insight pertinent to mitophagy in HSCs.
Autophagy and HSCs
Although specific receptors and triggers for mitophagy have not been elucidated in HSCs or HPCs, analyses of HSC/HPC function in autophagy-deficient knockout models, including those lacking RB1CC1 and ATG7, indicate that autophagy contributes to hematopoietic homeostasis. RB1CC1/FIP200 is a FAK-family interacting protein of 200 kDa123 and a component of the autophagy-initiation complex.124 Conditional deletion of Rb1cc1 in hemo-endothelial precursors in a TEK/Tie2Cre mouse model results in early lethality from severe anemia. Rb1cc1-null mice undergo increased proliferation of HSCs with expansion of the myeloid compartment.63 Moreover, hematopoietic populations from Rb1cc1-null mice enriched in HSCs and HPCs have greater mitochondrial mass and ROS levels than do those isolated from wild-type littermates.
ATG7 is an E1-like activating enzyme that promotes autophagosome membrane elongation by catalyzing the covalent conjugation of ATG12 with ATG5 and that of ATG8 family members (including LC3) with phosphatidylethanolamine (PE).7,11 Conditional deletion of Atg7 from hematopoietic stem cells in a Vav-Cre mouse model leads to a progressive and ultimately lethal anemia accompanied by lymphopenia and an atypical proliferation of histiocytic cells.14 These animals undergo a progressive reduction in HSC and HPC numbers and self-renewal capacity, with a drastic age-dependent decline in the cells’ ability to reconstitute lethally-irradiated mice. Cells within the Lin−ATXN1+C-KIT+ compartment of Atg7-deficient mice, enriched in HSCs and HPCs, have more mitochondria, higher levels of mitochondrial ROS, increased cell proliferation, and more DNA damage than do those from wild-type littermates.15 Together, these studies highlight the essential role of autophagy in maintaining HSC quiescence and proper hematopoietic differentiation.
Factors such as FOXO3 and TSC1 simultaneously regulate mitochondrial function and autophagy. FOXO3, the transcription factor that maintains quiescence of HSCs by limiting cellular mitochondrial content and activity, has also been implicated in regulating stress-induced autophagy in HSCs, which is essential for survival of HSCs during aging and in response to starvation. FOXO3 targets involved in autophagy include Atg4b, Map1lc3b, and Bnip3.125 Similarly, TSC1-mediated inhibition of the MTOR pathway, which contributes to HSC quiescence by repressing mitochondrial biogenesis and ROS production, also promotes autophagy.49,50,126-129
Given the tight regulation of mitochondrial content and activity in the maintenance of HSCs, the role of autophagy in mitochondrial turnover, and the coordinated regulation of mitochondrial function and autophagy, it is tempting to speculate that the defect in HSC/HPC function in autophagy-defective mice reflects a defect in mitochondrial clearance. Alternatively, autophagy may contribute to HSC maintenance by recycling key metabolites critical for survival of HSCs in the stem cell niche. As the pathways involved in selective degradation of mitochondria are elucidated, it will become more feasible to test these hypotheses.
Mitochondrial Dysfunction and Autophagy in the Pathogenesis of Hematopoietic Disorders
Primary mitochondrial disorders
Primary mitochondrial disorders are multisystem diseases that are caused by nuclear or mtDNA mutations resulting in impaired respiratory chain function.130 Hematological manifestations of primary mitochondrial diseases include anemia (aplastic, megaloblastic, or sideroblastic), leukopenia, neutropenia, and thrombocytopenia.131 Syndromic mitochondrial disorders in which hematological manifestations are among the defining features include Pearson marrow-pancreas syndrome, autosomal recessive mitochondrial myopathy with lactic acidosis and sideroblastic anemia (MLASA) syndrome, and Barth syndrome (neutropenia). Additionally, mutations in MT-CO1 (mitochondrially encoded cytochrome c oxidase I) that impair stability of the complex have been identified in nonsyndromic patients with sideroblastic anemia.132
Pearson marrow-pancreas syndrome is a disorder characterized by bone marrow and exocrine pancreas insufficiency.133,134 Patients are heteroplasmic for mitochondrial genomic deletions, with almost half of the patients having a canonical 4,977-bp deletion that involves mtDNA-encoded subunits of respiratory complex I (NADH dehydrogenase), complex IV (cytochrome C oxidase), and complex V (ATP synthase), as well as several mt-tRNA genes. Patients often present as infants with a variety of signs and symptoms, including failure to thrive, lactic acidosis, malabsorption, myopathy, and liver dysfunction. Hematological manifestations include pancytopenia with macrocytic, nonmegaloblastic, erythropoiesis. Bone marrow aspirates contain erythroid precursors with characteristic iron deposits (ringed sideroblasts) and vacuolization of early erythroid and myeloid precursors. The clinical severity of the disease and specific constellation of symptoms in each patient seems to correlate with the relative frequency of abnormal mtDNA in affected and unaffected tissues, which may change over time. Kearns-Sayers develops in a subset of patients with Pearson marrow-pancreas syndrome.
MLASA results from mutations in genes encoding either of two proteins, PUS1 (pseudouridylate synthase 1) or YARS2 (tyrosyl-tRNA synthetase 2, mitochondrial). PUS1 facilitates conversion of uridine to its isomer pseudouridine (ψ), which is thought to stabilize RNA secondary structures.133 The PUS1 mutations decrease the modification of both mitochondrial and cytoplasmic tRNAs, and the disease is associated with decreases in respiratory complexes I and IV; meanwhile, the mutations in YARS2 result in a global decrease in the abundance and synthesis of mitochondrial respiratory complex proteins encoded by the mitochondrial, but not the nuclear, genome. Although the defects in mitochondrial respiration in patients with disease-associated PUS1 and YARS2 mutations are likely to result from impaired mitochondrial protein translation, the mechanism by which these mutations cause accumulation of mitochondrial iron and sideroblastic anemia is not known.
Barth syndrome is an X-linked disease associated with deficits in oxidative phosphorylation resulting from mutations in TAZ1, a gene involved in the metabolism of the mitochondria-specific phospholipid cardiolipin.135 Patients with Barth syndrome have skeletal and cardiac myopathy and cyclic neutropenia, with heart failure and opportunistic infection being major causes of mortality.
Despite the ability of autophagy to degrade mitochondria with reduced mitochondrial potential, the mere presence and accumulation of pathogenic mtDNA mutations in human patients with mitochondrial disorders implies an imbalance between the biogenesis and selective removal of mitochondria containing pathogenic mtDNAs compared with wild-type mtDNAs. It is unclear whether this imbalance is due to a defect in the ability of different cells to recognize and eliminate mitochondria with reduced respiratory function or due to the number of dysfunctional mitochondria exceeding the normal autophagic capacity of the cell. As cardiolipin has just recently been identified as a mitophagy receptor,83 it will be interesting to determine whether mutations in TAZ1 (in patients with Barth syndrome) alter the interaction between cardiolipin and LC3 and interfere with mitophagy. The results of studies using human cytoplasmic hybrid cells indicate that PARK2 overexpression may promote clearance of mitochondria with 1.9-kB deletions in the mtDNA from a patient with Kearns-Sayers or deleterious MT-CO1/COXI mutations,96,136 suggesting that the machinery for recognition and elimination of dysfunctional mitochondria remains intact, at least in certain cell types.
Myelodysplastic syndromes
MDS are a group of clonal stem cell disorders characterized by ineffective hematopoiesis, with dysplastic changes that lead to cytopenias (often including a macrocytic anemia) and increased risk of transformation to acute myeloid leukemia (AML). Many lines of evidence indicate that mitochondrial dysfunction plays a critical role in the pathogenesis of MDS. First, hematopoietic progenitor cells from MDS patients show mitochondrial respiratory chain dysfunction, which contributes to the characteristic sensitivity of MDS bone marrow progenitors to the standard high-oxygen conditions used for culturing cells.137 Second, bone marrow progenitors from patients with MDS or AML with myelodysplasia-related changes have mitochondrial gene expression that is dysregulated compared with that of controls.138 Finally, subsets of patients with MDS have pathogenic mutations in IDH1 and IDH2, which encode isocitrate dehydrogenases and alter metabolism,139 or somatic mitochondrial mutations that affect oxidative phosphorylation.140-143 Mitochondrial dysfunction also appears to be an early event in the development of therapy-related MDS/AML.144,145
Ultrastructural studies show increased numbers of autophagosomes and lysosomes containing mitochondria in early erythroblasts of patients with low-risk MDS and enlarged, abnormal mitochondria with iron accumulation and absence of apoptosis in patients with high-risk disease and transfusion dependency.146,147 These findings suggest that mitochondrial damage may be mitigated by autophagy in low-risk MDS and that disease progression occurs as the ability of cells to eliminate damaged mitochondria is exceeded, leading to increased cell death (and transfusion dependence), and in some cases, to the emergence of clones able to overcome the apoptotic pressure (i.e., AML). Thus, a disruption in the balance between forces promoting mitochondrial damage and the ability of cells to eliminate damaged mitochondria by autophagy may contribute to disease pathogenesis and progression in a subset of patients with MDS. In support of this model, MDS-like features can be elicited in mice either by introducing a homozygous mutation in the mitochondrial proofreading DNA polymerase (POLG/POLGA) that causes an age-dependent accumulation of somatic mitochondrial mutations18 or by disrupting autophagy in hematopoietic stem/progenitor cells by using VavCre+Atg7flox/flox animals.15 However, the MDS-like features are quite different in these two models, with Polg mutation resulting in macrocytic anemia, dysplastic erythropoiesis, and lymphopenia, and Atg7-deletion resulting in bone marrow failure accompanied by an atypical histiocytic proliferation.
Myelodysplastic/myeloproliferative disorders
Juvenile myelomonocytic leukemia (JMML) and chronic myelomonocytic leukemia (CMML) are clonal hematopoietic malignancies that have features of both myeloproliferative and myelodysplastic syndromes.148 These malignancies are characterized by a persistent monocytosis with < 20% blasts. Dysplasia in one or more lineages is a defining feature of CMML. Similarly, although not a diagnostic criteria, erythroid and megakaryocytic dysplasia are frequently present in patients with JMML. Mutations involving genes in the RAS-MAPK pathway are commonly associated with the diseases. Although the role of autophagy (or mitophagy) in development of RAS-associated myeloproliferative/myelodysplastic disorders has not been examined, recent studies have indicated that oncogenic activation of RAS increases autophagy in cultured cells and in tumor samples.149 Interestingly, the tumorigenicity of immortalized baby mouse kidney cells expressing oncogenic RAS mutations in nude mice is impaired by reduced expression of Atg5 or Atg7,150 whereas suppression of autophagy in human cancer cell lines expressing RAS mutations induces cell death that is associated with accumulation of damaged mitochondria, impaired mitochondrial respiration, and ultimately, a shortage of citric acid cycle metabolites and ATP.150,151 Together, these observations suggest that induction of mitophagy may be an important aspect of RAS-driven oncogenesis. It remains to be seen whether or not this pathway contributes to the pathogenesis of hematological disorders associated with RAS mutations.
Concluding Remarks
It is generally accepted that the role of autophagy in the pathogenesis of cancer is complex, with autophagy having different functions at different stages of tumorigenesis and in different cell types.152,153 For example, by preventing the accumulation of damaged/dysfunctional organelles and protein aggregates, autophagy may suppress the formation of tumors (and function as a tumor suppressor);154-157 by contrast, autophagy may enable tumor cell survival under adverse intracellular (i.e., from oncogenes) or extracellular (i.e., tumor microenvironment or in response to chemotherapy) conditions.158,159 A similar picture may emerge for mitophagy as it becomes possible to separate autophagy and mitophagy by using genetic and/or pharmacological tools. The extent to which functions ascribed to autophagy actually stem from elimination of mitochondria by mitophagy in normal HSCs/HPCs and in the context of various hematological diseases will be of particular interest.
Acknowledgments
The authors thank Cherise M. Guess from the Department of Scientific Editing at St. Jude Children’s Research Hospital for editing the manuscript and the National Institutes of Health (HL114697), the Burroughs Wellcome Fund (1006062.01), the American Society of Hematology, and the American Lebanese Syrian Associated Charities (ALSAC) for research funds to MK.
Glossary
Abbreviations:
- AKT
v-akt murine thymoma viral oncogene homolog 1
- AMBRA1
autophagy/Beclin 1 regulator 1
- AMPK
AMP-activated protein kinase
- ATG
autophagy-related
- BNIP3
BCL2/adenovirus E1B 19kD-interacting protein 3
- BNIP3L
BNIP3-like
- FAO
fatty acid oxidation
- FOXO
forkhead box O
- FUNDC1
FUN14 domain containing 1
- HIF1A
hypoxia inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor)
- HPC
hematopoietic progenitor cell
- HSC
hematopoietic stem cell
- LC3
microtubule-associated protein 1 light chain 3 alpha/beta
- MDS
myelodysplastic syndromes
- mtDNA
mitochondrial DNA
- MTOR
mechanistic target of rapamycin
- PARK2
parkinson protein 2, E3 ubiquitin protein ligase (parkin)
- PINK1
PTEN-induced putative kinase 1
- PPARD
peroxisome proliferator-activated receptor delta
- PtdIns3K
class III phosphatidylinositol 3-kinase
- PTPMT1
protein tyrosine phosphatase, mitochondrial 1
- RB1CC1/FIP200
RB1-inducible coiled-coil 1
- RBC
red blood cell
- ROS
reactive oxygen species
- STK11/LKB1
serine/threonine kinase 11
- TSC
tuberous sclerosis
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest and no competing financial interests.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/26681
References
- 1.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–42. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–60. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 3.Parker GC, Acsadi G, Brenner CA. Mitochondria: determinants of stem cell fate? Stem Cells Dev. 2009;18:803–6. doi: 10.1089/scd.2009.1806.edi. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14 Spec No. 2:R283–9. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 5.Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–55. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 8.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–7. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 9.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–67. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A. 2010;107:832–7. doi: 10.1073/pnas.0913170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, Stranks AJ, Glanville J, Knight S, Jacobsen SE, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208:455–67. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 17.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 18.Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA, et al. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045–53. doi: 10.1182/blood-2008-08-169474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, et al. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–8. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–90. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccoli C, Ria R, Scrima R, Cela O, D’Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467–76. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 22.Mantel C, Messina-Graham S, Broxmeyer HE. Upregulation of nascent mitochondrial biogenesis in mouse hematopoietic stem cells parallels upregulation of CD34 and loss of pluripotency: a potential strategy for reducing oxidative risk in stem cells. Cell Cycle. 2010;9:2008–17. doi: 10.4161/cc.9.10.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–8. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–4. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, Tzatsos A, Ozsolak F, Milos P, Ferrari F, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–63. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33:469–77. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi S, Tanaka T, Sakai J. New therapeutic target for metabolic syndrome: PPARdelta. Endocr J. 2007;54:347–57. doi: 10.1507/endocrj.KR-99. [DOI] [PubMed] [Google Scholar]
- 34.Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010;20:124–37. doi: 10.1038/cr.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, Qu CK. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12:62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantel C, Messina-Graham SV, Broxmeyer HE. Superoxide flashes, reactive oxygen species, and the mitochondrial permeability transition pore: potential implications for hematopoietic stem cell function. Curr Opin Hematol. 2011;18:208–13. doi: 10.1097/MOH.0b013e3283475ffe. [DOI] [PubMed] [Google Scholar]
- 37.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, Griffin JD. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93:2928–35. [PubMed] [Google Scholar]
- 38.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–8. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–60. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 40.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–9. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–17. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Jensen KS, Binderup T, Jensen KT, Therkelsen I, Borup R, Nilsson E, Multhaupt H, Bouchard C, Quistorff B, Kjaer A, et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. EMBO J. 2011;30:4554–70. doi: 10.1038/emboj.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Yalcin S, Marinkovic D, Mungamuri SK, Zhang X, Tong W, Sellers R, Ghaffari S. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(-/-) mice. EMBO J. 2010;29:4118–31. doi: 10.1038/emboj.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–40. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 48.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 49.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C, Liu Y, Liu Y, Zheng P. The axis of mTOR-mitochondria-ROS and stemness of the hematopoietic stem cells. Cell Cycle. 2009;8:1158–60. doi: 10.4161/cc.8.8.8139. [DOI] [PubMed] [Google Scholar]
- 51.Hosokawa K, Arai F, Yoshihara H, Nakamura Y, Gomei Y, Iwasaki H, Miyamoto K, Shima H, Ito K, Suda T. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–83. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, Shivtiel S, Kalinkovich A, Caione L, Gammaitoni L, et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011;117:419–28. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- 53.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–63. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao Y, Cheng D, Ma Y, Zhou W, Wang Y. The relationship between oxygen concentration, reactive oxygen species and the biological characteristics of human bone marrow hematopoietic stem cells. Transplant Proc. 2011;43:2755–61. doi: 10.1016/j.transproceed.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 55.Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–50. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 56.Nakata S, Matsumura I, Tanaka H, Ezoe S, Satoh Y, Ishikawa J, Era T, Kanakura Y. NF-kappaB family proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J Biol Chem. 2004;279:55578–86. doi: 10.1074/jbc.M408238200. [DOI] [PubMed] [Google Scholar]
- 57.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–41. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–30. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008;112:1493–502. doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang CC, Wang C, Peng X, Gan B, Guan JL. Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem. 2010;285:3499–509. doi: 10.1074/jbc.M109.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu F, Lee JY, Wei H, Tanabe O, Engel JD, Morrison SJ, Guan JL. FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood. 2010;116:4806–14. doi: 10.1182/blood-2010-06-288589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 65.Stephenson LM, Miller BC, Ng A, Eisenberg J, Zhao Z, Cadwell K, Graham DB, Mizushima NN, Xavier R, Virgin HW, et al. Identification of Atg5-dependent transcriptional changes and increases in mitochondrial mass in Atg5-deficient T lymphocytes. Autophagy. 2009;5:625–35. doi: 10.4161/auto.5.5.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–96. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–15. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 71.Iwata J, Ezaki J, Komatsu M, Yokota S, Ueno T, Tanida I, Chiba T, Tanaka K, Kominami E. Excess peroxisomes are degraded by autophagic machinery in mammals. J Biol Chem. 2006;281:4035–41. doi: 10.1074/jbc.M512283200. [DOI] [PubMed] [Google Scholar]
- 72.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–55. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 73.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–39. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106:19860–5. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–24. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 76.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–32. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Kanki T, Klionsky DJ. Mitophagy in yeast occurs through a selective mechanism. J Biol Chem. 2008;283:32386–93. doi: 10.1074/jbc.M802403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanki T, Wang K, Klionsky DJ. A genomic screen for yeast mutants defective in mitophagy. Autophagy. 2010;6:278–80. doi: 10.4161/auto.6.2.10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Löhr F, Popovic D, Occhipinti A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwarten M, Mohrlüder J, Ma P, Stoldt M, Thielmann Y, Stangler T, Hersch N, Hoffmann B, Merkel R, Willbold D. Nix directly binds to GABARAP: a possible crosstalk between apoptosis and autophagy. Autophagy. 2009;5:690–8. doi: 10.4161/auto.5.5.8494. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Y, Massen S, Terenzio M, Lang V, Chen-Lindner S, Eils R, Novak I, Dikic I, Hamacher-Brady A, Brady NR. Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J Biol Chem. 2013;288:1099–113. doi: 10.1074/jbc.M112.399345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–8. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104:19500–5. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–5. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287:19094–104. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–57. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia J, Sun ZJ, Wang YN, Zhao YF. Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J Cell Physiol. 2012;227:639–48. doi: 10.1002/jcp.22768. [DOI] [PubMed] [Google Scholar]
- 89.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–81. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–85. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 92.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–21. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 93.Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ. 2007;14:1647–56. doi: 10.1038/sj.cdd.4402167. [DOI] [PubMed] [Google Scholar]
- 94.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim I, Lemasters JJ. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid Redox Signal. 2011;14:1919–28. doi: 10.1089/ars.2010.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc Natl Acad Sci U S A. 2010;107:11835–40. doi: 10.1073/pnas.0914569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–4. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- 98.Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–7. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- 99.Sato M, Sato K. Maternal inheritance of mitochondrial DNA: degradation of paternal mitochondria by allogeneic organelle autophagy, allophagy. Autophagy. 2012;8:424–5. doi: 10.4161/auto.19243. [DOI] [PubMed] [Google Scholar]
- 100.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22:320–33. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng X, Hunter T. Parkin mitochondrial translocation is achieved through a novel catalytic activity coupled mechanism. Cell Res. 2013;23:886–97. doi: 10.1038/cr.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–37. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–23. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–70. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 109.Kim NC, Tresse E, Kolaitis RM, Molliex A, Thomas RE, Alami NH, Wang B, Joshi A, Smith RB, Ritson GP, et al. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron. 2013;78:65–80. doi: 10.1016/j.neuron.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–9. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6:1090–106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15:887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michiorri S, Gelmetti V, Giarda E, Lombardi F, Romano F, Marongiu R, Nerini-Molteni S, Sale P, Vago R, Arena G, et al. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 2010;17:962–74. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- 114.Van Humbeeck C, Cornelissen T, Hofkens H, Mandemakers W, Gevaert K, De Strooper B, Vandenberghe W. Parkin interacts with Ambra1 to induce mitophagy. J Neurosci. 2011;31:10249–61. doi: 10.1523/JNEUROSCI.1917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Orvedahl A, Sumpter R, Jr., Xiao G, Ng A, Zou Z, Tang Y, Narimatsu M, Gilpin C, Sun Q, Roth M, et al. Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature. 2011;480:113–7. doi: 10.1038/nature10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lokireddy S, Wijesoma IW, Teng S, Bonala S, Gluckman PD, McFarlane C, Sharma M, Kambadur R. The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab. 2012;16:613–24. doi: 10.1016/j.cmet.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 117.Yan J, Kuroyanagi H, Kuroiwa A, Matsuda Y, Tokumitsu H, Tomoda T, Shirasawa T, Muramatsu M. Identification of mouse ULK1, a novel protein kinase structurally related to C. elegans UNC-51. Biochem Biophys Res Commun. 1998;246:222–7. doi: 10.1006/bbrc.1998.8546. [DOI] [PubMed] [Google Scholar]
- 118.Chan EY, Kir S, Tooze SA. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282:25464–74. doi: 10.1074/jbc.M703663200. [DOI] [PubMed] [Google Scholar]
- 119.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–71. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Joo JH, Dorsey FC, Joshi A, Hennessy-Walters KM, Rose KL, McCastlain K, Zhang J, Iyengar R, Jung CH, Suen DF, et al. Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell. 2011;43:572–85. doi: 10.1016/j.molcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–8. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 122.Matsui M, Yamamoto A, Kuma A, Ohsumi Y, Mizushima N. Organelle degradation during the lens and erythroid differentiation is independent of autophagy. Biochem Biophys Res Commun. 2006;339:485–9. doi: 10.1016/j.bbrc.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 123.Gan B, Guan JL. FIP200, a key signaling node to coordinately regulate various cellular processes. Cell Signal. 2008;20:787–94. doi: 10.1016/j.cellsig.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegué E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–7. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–6. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 129.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–78. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 130.Schapira AH. Mitochondrial diseases. Lancet. 2012;379:1825–34. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- 131.Finsterer J. Hematological manifestations of primary mitochondrial disorders. Acta Haematol. 2007;118:88–98. doi: 10.1159/000105676. [DOI] [PubMed] [Google Scholar]
- 132.Bröker S, Meunier B, Rich P, Gattermann N, Hofhaus G. MtDNA mutations associated with sideroblastic anaemia cause a defect of mitochondrial cytochrome c oxidase. Eur J Biochem. 1998;258:132–8. doi: 10.1046/j.1432-1327.1998.2580132.x. [DOI] [PubMed] [Google Scholar]
- 133.Fleming MD. Congenital sideroblastic anemias: iron and heme lost in mitochondrial translation. Hematology Am Soc Hematol Educ Program. 2011;2011:525–31. doi: 10.1182/asheducation-2011.1.525. [DOI] [PubMed] [Google Scholar]
- 134.DiMauro S, Hirano M. Mitochondrial DNA Deletion Syndromes. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, eds. GeneReviews. Seattle (WA), 1993 [Google Scholar]
- 135.Jefferies JL. Barth syndrome. Am J Med Genet C Semin Med Genet. 2013;163C:198–205. doi: 10.1002/ajmg.c.31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gilkerson RW, De Vries RL, Lebot P, Wikstrom JD, Torgyekes E, Shirihai OS, Przedborski S, Schon EA. Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum Mol Genet. 2012;21:978–90. doi: 10.1093/hmg/ddr529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thompson JE, Conlon JP, Yang X, Sanchez PV, Carroll M. Enhanced growth of myelodysplastic colonies in hypoxic conditions. Exp Hematol. 2007;35:21–31. doi: 10.1016/j.exphem.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schildgen V, Wulfert M, Gattermann N. Impaired mitochondrial gene transcription in myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia-related changes. Exp Hematol. 2011;39:666–75, e1. doi: 10.1016/j.exphem.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 139.Tan PT, Wei AH. The epigenomics revolution in myelodysplasia: a clinico-pathological perspective. Pathology. 2011;43:536–46. doi: 10.1097/PAT.0b013e32834a4061. [DOI] [PubMed] [Google Scholar]
- 140.Wulfert M, Küpper AC, Tapprich C, Bottomley SS, Bowen D, Germing U, Haas R, Gattermann N. Analysis of mitochondrial DNA in 104 patients with myelodysplastic syndromes. Exp Hematol. 2008;36:577–86. doi: 10.1016/j.exphem.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 141.Linnartz B, Anglmayer R, Zanssen S. Comprehensive scanning of somatic mitochondrial DNA alterations in acute leukemia developing from myelodysplastic syndromes. Cancer Res. 2004;64:1966–71. doi: 10.1158/0008-5472.CAN-03-2956. [DOI] [PubMed] [Google Scholar]
- 142.Gattermann N. From sideroblastic anemia to the role of mitochondrial DNA mutations in myelodysplastic syndromes. Leuk Res. 2000;24:141–51. doi: 10.1016/S0145-2126(99)00160-5. [DOI] [PubMed] [Google Scholar]
- 143.Zamzami MA, Duley JA, Price GR, Venter DJ, Yarham JW, Taylor RW, Catley LP, Florin TH, Marinaki AM, Bowling F. Inosine Triphosphate Pyrophosphohydrolase (ITPA) polymorphic sequence variants in adult hematological malignancy patients and possible association with mitochondrial DNA defects. J Hematol Oncol. 2013;6:24. doi: 10.1186/1756-8722-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cano KE, Li L, Bhatia S, Bhatia R, Forman SJ, Chen Y. NMR-based metabolomic analysis of the molecular pathogenesis of therapy-related myelodysplasia/acute myeloid leukemia. J Proteome Res. 2011;10:2873–81. doi: 10.1021/pr200200y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li L, Li M, Sun C, Francisco L, Chakraborty S, Sabado M, McDonald T, Gyorffy J, Chang K, Wang S, et al. Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer Cell. 2011;20:591–605. doi: 10.1016/j.ccr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Houwerzijl EJ, Pol HW, Blom NR, van der Want JJ, de Wolf JT, Vellenga E. Erythroid precursors from patients with low-risk myelodysplasia demonstrate ultrastructural features of enhanced autophagy of mitochondria. Leukemia. 2009;23:886–91. doi: 10.1038/leu.2008.389. [DOI] [PubMed] [Google Scholar]
- 147.van de Loosdrecht AA, Brada SJ, Blom NR, Hendriks DW, Smit JW, van den Berg E, de Wolf JT, Vellenga E. Mitochondrial disruption and limited apoptosis of erythroblasts are associated with high risk myelodysplasia. An ultrastructural analysis. Leuk Res. 2001;25:385–93. doi: 10.1016/S0145-2126(00)00151-X. [DOI] [PubMed] [Google Scholar]
- 148.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. 2008. [Google Scholar]
- 149.McCarthy N. Autophagy: limiting factors. Nat Rev Cancer. 2011;11:313. doi: 10.1038/nrc3054. [DOI] [PubMed] [Google Scholar]
- 150.Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–70. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–51. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 153.Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32:955–63. doi: 10.1093/carcin/bgr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–35. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–82. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]