Abstract
Defective mitochondria exert deleterious effects on host cells. To manage this risk, mitochondria display several lines of quality control mechanisms: mitochondria-specific chaperones and proteases protect against misfolded proteins at the molecular level, and fission/fusion and mitophagy segregate and eliminate damage at the organelle level. An increase in unfolded proteins in mitochondria activates a mitochondrial unfolded protein response (UPRmt) to increase chaperone production, while the mitochondrial kinase PINK1 and the E3 ubiquitin ligase PARK2/Parkin, whose mutations cause familial Parkinson disease, remove depolarized mitochondria through mitophagy. It is unclear, however, if there is a connection between those different levels of quality control (QC). Here, we show that the expression of unfolded proteins in the matrix causes the accumulation of PINK1 on energetically healthy mitochondria, resulting in mitochondrial translocation of PARK2, mitophagy and subsequent reduction of unfolded protein load. Also, PINK1 accumulation is greatly enhanced by the knockdown of the LONP1 protease. We suggest that the accumulation of unfolded proteins in mitochondria is a physiological trigger of mitophagy.
Keywords: unfolded protein response, mitochondria, PINK1, PARK2/Parkin, mitophagy, LONP
Introduction
Thought to mitigate the deleterious effects of reactive oxygen species leaking from the electron transport chain in the mitochondrial inner membrane, eukaryotic cells are equipped with systems to detect and abrogate mitochondrial dysfunction at various levels.1 The first line of defense is composed of molecular chaperones and proteases dedicated to maintain the correct folding and the number of proteins in mitochondria. For example, owing to the complicated architecture of respiratory chain complexes and dual sources of genetic information stemming from mitochondrial and nuclear genomes it is crucial to maintain the correct stoichiometry of individual components. Newly synthesized proteins with mitochondrial targeting signals are guided to mitochondria by cytosolic HSP70 and HSP90 chaperones and imported with the help of HSPA9/mtHsp70.2-4 Then the imported proteins achieve their proper conformations with the aid of HSPA9, HSPD1/HSP60, and HSPE1/CPN10.5 The proteins that cannot fold into the proper tertiary structures are degraded by mitochondrial proteases such as CLPP and LONP1. If the level of misfolded or unfolded proteins overwhelms the capacity of the mitochondrial chaperone systems, mitochondria send a retrograde signal to the nucleus to increase the expression of chaperones and proteases, which is called mitochondrial unfolded protein response.6,7 In mammalian systems, the expression of a deletion mutant of ornithine carbamoyltransferase (ΔOTC) yields Triton X-100 insoluble protein aggregates in the mitochondrial matrix triggering the expression of DNA-damage-inducible transcript 3 (DDIT3/CHOP), a transcription factor responsible for the transcriptional upregulation of HSPD1, DNAJA3 (a mitochondrial DnaJ/Hsp40 homolog) and CLPP.7,8 In C. elegans, treatment with ethidium bromide, which reduces mitochondrial DNA transcription and replication, or knockdown of the mitochondrial protease spg-7/paraplegin or mitochondrial chaperone genes activates UPRmt.9-12 In this process, the short peptides generated by the mitochondrial matrix protease CLPP-1 are exported to the cytosol through the inner membrane ABC transporter HAF-1 to activate the cytosolic transcription factor ATFS-1 (activating transcription factor associated with stress), which is otherwise constitutively imported and degraded in mitochondria.10-12 Activated ATFS-1 is prevented from import into mitochondria by an unknown mechanism diverting it from degradation, thereby allowing it to translocate to the nucleus to activate the transcription of UPRmt-related genes.12
Another level of quality control occurs at the organelle level. The mitochondrial serine/threonine kinase, PINK1 (PTEN-induced putative kinase 1) and the cytosolic E3 ubiquitin ligase PARK2/Parkin, which have been linked to autosomal recessive forms of Parkinson disease,13,14 cause mitophagy of damaged mitochondria.15-20 In healthy mitochondria that maintain their membrane potential, PINK1 levels remain very low or undetectable due to constitutive import into the mitochondrial inner membrane where PINK1 is cleaved by PARL to generate an N-terminal degron motif and subsequently eliminated by N-end rule proteasomal targeting in the cytosol.16,21,22 However, when a mitochondrion loses membrane potential, PINK1 import is blocked and it accumulates on the outer membrane, where it recruits PARK2 specifically to the damaged mitochondrion and activates PARK2 E3 enzyme activity to ubiquitinate mitochondrial substrates and initiate mitophagic clearance.
Pimenta de Castro and colleagues showed that PINK1 mutant Drosophila accumulate misfolded components of respiratory chain complexes and increase UPRmt.23 Also, the accumulation of ΔOTC in the mitochondrial matrix activates AMP-activated protein kinase-dependent autophagy, resulting in a phenotype similar to that of PINK1 and Parkin mutant flies. This suggests a genetic interaction between the UPRmt pathway and the autophagy machinery.
In this study we showed that excess loading of unfolded proteins in the mitochondrial matrix can initiate PINK1/PARK2-mediated mitophagy. We found that the overexpression of ΔOTC induces PINK1 accumulation, PARK2 translocation, mitophagy and the reduction of unfolded proteins. Notably, ΔOTC overexpression-mediated PINK1 accumulation is not accompanied by mitochondrial depolarization, suggesting the presence of another mechanism for PINK1 accumulation, independent of membrane potential loss. We also found that the mitochondrial protease, CLPP, which mediates UPRmt in C. elegans, does not mitigate the unfolded protein-induced PINK1 accumulation, but the matrix protease LONP1 does. Based on the presented results, we propose that the overwhelming QC demands at the protein level can induce QC at the organelle level.
Results
Expression of unfolded proteins in the mitochondrial matrix induces the accumulation of full-length PINK1 on mitochondria that maintain membrane potential
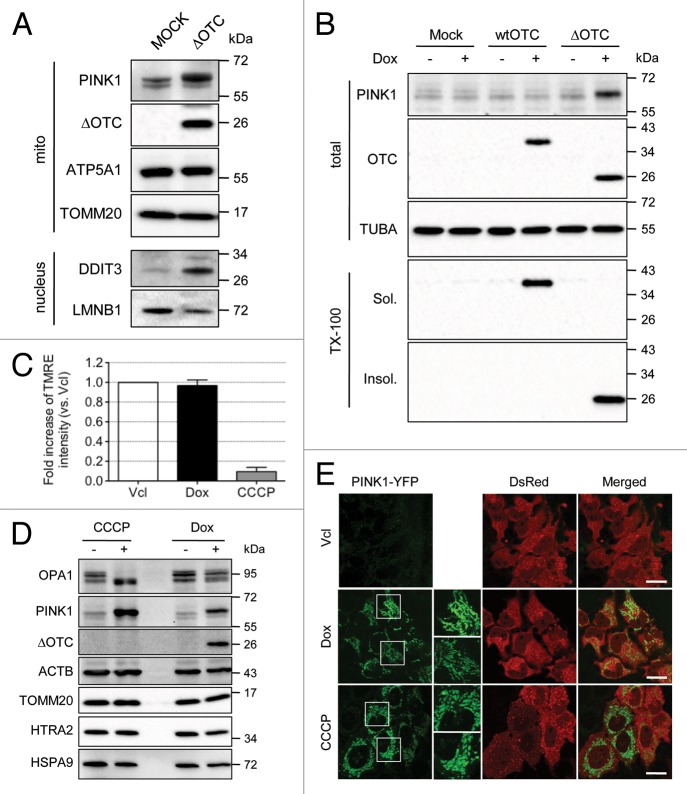
We hypothesized that mitochondria overloaded by unfolded proteins beyond the chaperone capacity might be removed by mitophagy. To test this hypothesis we overexpressed the deletion mutant of OTC (ΔOTC) that is known to accumulate in the mitochondrial matrix and induce UPRmt in mammalian cells and in Drosophila,7,23 and measured the level of PINK1, which accumulates on mitochondria lacking membrane potential and initiates PARK2-mediated mitophagy. Transient expression of ΔOTC greatly increased the level of PINK1 on mitochondria and the level of the UPRmt marker, DDIT3, in the nucleus (Fig. 1A). To confirm this result, we established HeLa-TetOn stable cells lines expressing wild-type (wt) OTC or ΔOTC in a doxycycline (Dox)-inducible manner. The expression of ΔOTC was detected after 24 h of Dox treatment, whereas PINK1 levels gradually increased up to 72 h of Dox treatment (Fig. S1A). The subcellular localization of ΔOTC in the matrix was confirmed by the subcellular fractionation and proteinase K protection assay (Fig. S1B and S1C). To examine if the accumulation of PINK1 was specific for the accumulation of unfolded OTC, we treated vector, wtOTC or ΔOTC-expressing stable HeLa-TetOn cell lines with Dox for 72 h (Fig. 1B). When cells were extracted with 0.5% Triton-X100 (TX-100), wtOTC was detected in the soluble fraction and ΔOTC in the insoluble fraction as shown previously,7 indicating that ΔOTC is misfolded in detergent-insoluble aggregates. Notably, PINK1 accumulation was detected only in ΔOTC-expressing cells. A likely explanation for these results is that the expression of ΔOTC causes depolarization of mitochondria, the only known mechanism to accumulate endogenous PINK1 on mitochondria.16 To assess this possibility, we measured mitochondrial membrane potential by quantifying tetramethylrhodamine, ethyl ester (TMRE) intensity in cells treated with or without Dox using FACS (Fig. 1C). Surprisingly, ΔOTC expression did not lower mitochondrial membrane potential, suggesting that ΔOTC-expressing cells accumulate PINK1 by a mechanism distinct from that of uncouplers such as CCCP. We therefore examined OPA1 isoform proteolysis for independent evidence of mitochondrial membrane potential maintenance in ΔOTC-expressing cells (Fig. 1D). OPA1 expresses five different isoforms by alternative splicing and proteolytic cleavage. The longer forms are constitutively cleaved by YME1L1 and inducibly cleaved by the activated mitochondrial protease OMA1 in depolarized mitochondria.24-26 While CCCP-treated cells displayed a complete loss of long OPA1 isoforms, ΔOTC-expressing cells maintained all isoforms intact confirming the TMRE measurements that the mitochondria are not depolarized. We confirmed the western blot analyses by confocal imaging of PINK1 following transfection of PINK1-YPF into cells expressing ΔOTC (Fig. 1E). In the absence of Dox or CCCP, we could not detect the YFP signal due to the rapid degradation after import as previously shown.21 In Dox- or CCCP-treated cells, however, there was a clear increase in YFP signal localized with mitochondria, confirming that these two treatments induce PINK1 accumulation. Furthermore, while mitochondria in CCCP-treated cells became fragmented as an established consequence of OPA1 degradation by OMA1,26 the mitochondria in Dox-treated cells displayed an elongated network, again corroborating that mitochondria are not depolarized in ΔOTC-expressing cells.
Figure 1. The expression of ΔOTC in the mitochondrial matrix induces the accumulation of full-length PINK1 without loss of membrane potential. (A) HeLa cells were transiently transfected with vector or ΔOTC constructs for 48 h and then fractionated. Mitochondrial and nuclear fractions were directly solubilized in 1× sample buffer and used for western blotting with the indicated antibodies. ATP5A1 (ATP synthase, H+ transporting, mitochondrial F1 complex, α subunit 1, cardiac muscle), TOMM20, and LMNB1 served as loading controls for each fraction. (B) HeLa-TetOn cells stably transduced by retrovirus expressing wtOTC or ΔOTC were treated with doxycycline (Dox; 1 μg/ml) for 72 h. One half of each sample was solubilized in 1x sample buffer and the other half was fractionated into 0.5% Triton X-100 soluble and insoluble fractions. Each fraction was analyzed for expression of PINK1 and/or OTC. TUBA served as a loading control. (C) ΔOTC/HeLa-TetOn cells were incubated for 72 h with or without Dox (1 μg/ml). After 72 h, cells were detached, stained with TMRE, and analyzed for the intensity of TMRE using FACS. As a negative control, untreated cells were stained with TMRE and treated with CCCP (2 μM) before FACS analysis. FACS results were represented as mean ± SEM from three independent experiments. (D) ΔOTC/HeLa-TetOn cells were treated with CCCP (10 μM) for 2 h or Dox (1 μg/ml) for 72 h, and then analyzed by western blotting with the indicated antibodies. ΑCTB, TOMM20, HTRA2, and HSPA9 served as loading controls for total, OMM, IMS, and matrix proteins, respectively. (E) ΔOTC/HeLa-TetOn cells were transiently transfected with a PINK1-YFP/IRES (internal ribosome entry site)/DsRed construct for 24 h and then treated as in (D). The accumulation of PINK1-YFP and mitochondrial morphology in cells expressing DsRed were analyzed by confocal microscopy. Scale bars: 20 μm. White boxes in the left panels are magnified on the right.
PINK1 accumulation by the expression of unfolded protein is fully functional for PARK2-mediated mitophagy to reduce unfolded proteins
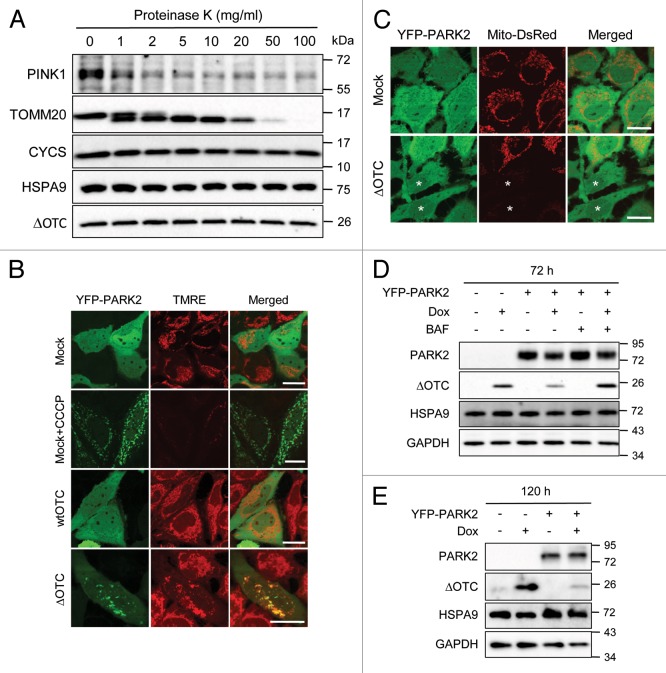
We tested if PINK1 accumulation induced by ΔOTC expression is functional for downstream PARK2 recruitment and mitophagy. The topology of the accumulated PINK1 was assessed by a protease protection assay as described in Materials and Methods (Fig. 2A). Endogenous PINK1 that accumulated upon ΔOTC expression and control outer mitochondrial membrane protein TOMM20/TOM20 were degraded after exposure to very low concentrations of proteinase K. However, cytochrome C (CYCS) in the intermembrane space (IMS) and HSPA9 and ΔOTC in the matrix were stable up to 100 μg/ml of proteinase K, indicating that the PINK1 that accumulated on mitochondria by ΔOTC expression is exposed to the cytosol. We also determined whether ΔOTC expression would induce PARK2 recruitment to mitochondria (Fig. 2B). HeLa cells stably expressing YFP-PARK2 were transiently transfected with vector, wtOTC or ΔOTC. After 48 h of transfection, cells were stained with TMRE to visualize the mitochondrial membrane potential. While PARK2 remained in the cytosol in vector- or wtOTC-expressing cells, CCCP-treated or ΔOTC-expressing cells showed translocation of PARK2 to mitochondria. Notably, ΔOTC-expressing cells showed PARK2 translocation to mitochondria that displayed normal TMRE staining compared with the complete loss of TMRE staining in mitochondria localized with PARK2 in CCCP-treated cells (Fig. 2B). To test whether the observed PARK2 translocation is dependent on PINK1 accumulated by ΔOTC as in CCCP-treated cells, we examined the effect of PINK1 knockdown and found that the ΔOTC-induced PARK2 translocation was completely abolished by PINK1 knockdown, indicating it was completely dependent on PINK1 (Fig. S1F and S1G). Moreover, when HeLa cells stably expressing YFP-PARK2 and mito-DsRed were transiently transfected with vector or ΔOTC, we could detect some cells completely lacking mitochondria, confirming that PARK2 was able to initiate mitophagy upon unfolded protein stress (Fig. 2C). PARK2 translocation and PARK2-induced mitophagy by ΔOTC expression was confirmed in the stable ΔOTC/HeLa-TetOn cell line (Fig. 3B and C; Fig. S1D, S1E, and S1G).
Figure 2. PINK1 accumulated by the expression of ΔOTC is exposed to the cytosol, recruits PARK2 and induces mitophagy. (A) ΔOTC/HeLa-TetOn cells were treated with Dox (1 μg/ml) for 72 h. Mitochondria were fractionated and subjected to a proteinase protection assay with proteinase K at the indicated concentrations as described in Materials and Methods. Protected protein levels were measured by western blotting with the indicated antibodies. (B) HeLa cells stably expressing YFP-PARK2 were transiently transfected with vector, wtOTC or ΔOTC. After 48 h of transfection, cells were stained with TMRE and imaged by confocal microscopy. One replicate transfected with vector was treated with CCCP (10 μM) for 2 h prior to TMRE staining as a control. Scale bars: 20 μm. (C) HeLa cells stably expressing YFP-PARK2 and mito-DsRed were transiently transfected with vector or ΔOTC for 72 h then analyzed for mitophagy by confocal microscopy. *Cells not showing mito-DsRed signal. Scale bars: 20 μm. (D and E) ΔOTC/HeLa-TetOn cells with or without the stable expression of YFP-PARK2 were treated with Dox (1 μg/ml) for 72 h (D) or 120 h (E) in the presence or absence of autophagy inhibitor, bafilomycin A1 (BAF), for the last 24 h (D). Whole cell lysates were analyzed by western blotting with the indicated antibodies. HSPA9 and GAPDH served as loading controls for mitochondrial and total proteins, respectively.
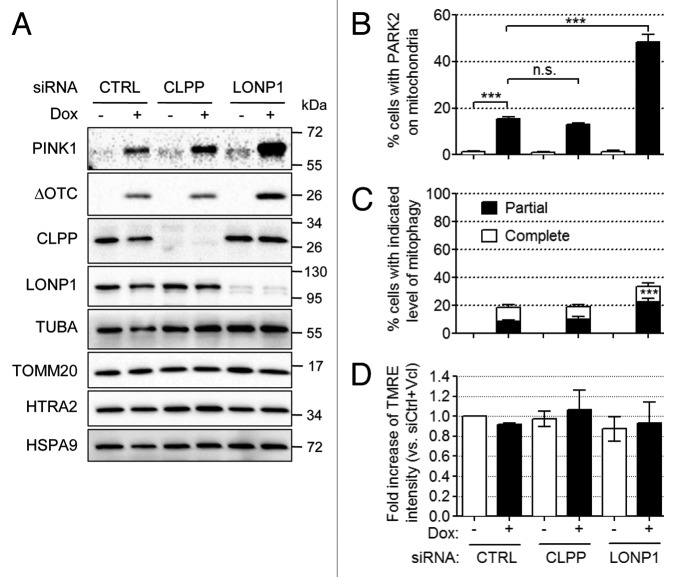
Figure 3. Endogenous LONP1 mitigates ΔOTC and PINK1 accumulation. (A–D) ΔOTC/HeLa-TetOn (A and D) or ΔOTC/YFP-PARK2/HeLa-TetOn (B and C) stable cell lines were transfected with nontargeting control (CTRL), CLPP or LONP1 siRNAs. After 24 h, cells were treated with Dox (1 μg/ml) for 72 h. (A) The level of PINK1 and ΔOTC and the efficiency of knockdown were analyzed by western blotting with the indicated antibodies. TUBA, TOMM20, HTRA2, and HSPA9 served as loading controls for total, OMM, IMS, and matrix proteins, respectively. (B and C) The numbers of cells with YFP-PARK2 on mitochondria (B) or with the indicated amount of mitochondria (C) were counted in each experimental setting. ***P < 0.001; n.s., not significant. Counting results were represented as mean ± SEM from three independent experiments. (D) TMRE intensity of each group was measured as in Figure 1E using FACS analysis. FACS results were represented as mean ± SEM from three independent experiments.
Finally, we tested whether the induced mitophagy can ameliorate the mitochondrial burden of unfolded protein. ΔOTC/HeLa-TetOn cell lines with or without the stable expression of YFP-PARK2 were treated with Dox for 72 h in the presence or absence of the autophagy inhibitor, bafilomycin A1 (BAF) for the last 24 h. Interestingly, the level of accumulated ΔOTC was reduced in the cells expressing YFP-PARK2 and this was blocked by exposure to BAF, indicating that ΔOTC was selectively degraded by PARK2-mediated autophagy (Fig. 2D). When the cells were incubated for a longer period (120 h), the reduction of ΔOTC level was more obvious (Fig. 2E). Furthermore, when YFP-PARK2 was expressed in ΔOTC-expressing cells, mitochondria became fragmented (Fig. 2B), in contrast to cells lacking PARK2 expression (Fig. 1E). PARK2 may induce fragmentation by MFN1 and MFN2 elimination by the UPRmt to facilitate autophagosome engulfment.
UPRmt-induced PINK1 accumulation and PARK2 translocation is mitigated by the mitochondrial matrix protease LONP1
CLPP is required to generate short peptides as a signal from the mitochondrial matrix to the cytosol to induce the UPRmt in C. elegans.10 To test if CLPP is required for unfolded protein-induced PINK1 accumulation, we downregulated CLPP expression using siRNA (Fig. 3A). In contrast to the UPRmt in C. elegans, knockdown of CLPP did not prevent PINK1 accumulation induced by ΔOTC expression (lane 2 vs. 4), indicating that PINK1 accumulation is not mediated by CLPP-generated peptides in mammalian cells. We also tried knockdown of LONP1, which degrades denatured or oxidatively damaged proteins (Fig. 3A).27,28 On the one hand, knockdown of LONP1 strongly increased the amount of ΔOTC and PINK1 accumulated by mitochondria (lane 2 vs. 6). This suggests that LONP1 degrades excess unfolded or misfolded proteins and normally mitigates PINK1 accumulation that is dependent on the residual unfolded or misfolded proteins. On the other hand, the knockdown of either CLPP or LONP1 without the expression of ΔOTC did not induce the accumulation of a significant amount of PINK1 in mitochondria (Fig. 3A, lanes 1, 3, and 5) or DDIT3 in the nucleus (Fig. S2). To confirm the accumulation of PINK1 by LONP1 knockdown in ΔOTC-expressing cells, we quantified PARK2 translocation and mitophagy in cells transfected with nontargeting control, CLPP or LONP1 siRNA (Fig. 3B and C). Consistent with the PINK1 accumulation results, PARK2 translocation and mitophagy were not affected by knockdown of CLPP but were greatly increased by knockdown of LONP1. Also, mitochondrial fragmentation was observed upon induction of ΔOTC expression only in the presence of PARK2, suggesting the fragmentation is dependent on the PARK2-mediated degradation of MFN1 and MFN2 regardless of the level of CLPP or LONP1 (Fig. S3).
As LONP1 plays important roles in the mitochondrial matrix, it is possible that knockdown of LONP1 causes mitochondrial depolarization. However, FACS analysis of cells transfected with the indicated siRNAs revealed TMRE intensities not significantly different from those of cells lacking Dox treatment (Fig. 3D), indicating that the accumulation of PINK1 in cells expressing less LONP1 is not due to the loss of mitochondrial membrane potential.
Discussion
Mitochondrial QC is elaborately organized at the molecular level with chaperones and proteases, at the transcriptional level through the UPRmt, and at the organelle level by mitophagy. It seems reasonable that the failure of QC at one level would initiate QC at the next level. Our results indicate that such a hierarchical link between QC levels indeed exists. We have previously shown that PINK1 imported through the translocase of outer mitochondrial membrane (TOMM) complex is not further imported through the translocase of inner mitochondrial membrane (TIMM) complex if mitochondria are depolarized, but instead accumulates on the outer mitochondrial membrane (OMM) associated with the TOMM complex.16,21,29,30 This appears to stem from the absence of a membrane potential driving force for TIMM complex import. Interestingly, the expression of misfolded protein causes the accumulation of PINK1 on the OMM, despite maintenance of mitochondrial membrane potential. This result suggests that the accumulated unfolded proteins in the matrix inhibits the TIMM complex by an alternative mechanism or facilitates the lateral release of PINK1 to the OMM before the start of import through TIMM complex. PINK1 accumulation by the knockdown of PMPC/MPP encoding peptidase (mitochondrial processing) in the absence of membrane potential loss may be due to the misfolding of the matrix proteins containing the N-terminal targeting signal which should be removed by PMPC in normal mitochondria.31
Regardless of the PINK1 accumulation mechanism, the accumulated PINK1 recruits PARK2 to mitochondria and induces mitophagy. In this situation, a critical point is whether this mitophagy can alleviate the proteinaceous stress in the matrix. As shown in Figure 2D and E, the level of accumulated unfolded ΔOTC is mitigated in an autophagy-dependent manner, supporting our hypothesis for the hierarchical interrelation between the QC at the molecular level and the organellar level. Consistent with this model, a recent paper by Rana et al. reported that overexpressed Parkin reduces proteotoxicity and extends life span in Drosophila.32
When C. elegans are given a proteinaceous stress, peptide fragments generated by ClpP are exported to the cytosol to activate the UPRmt-responsive transcription factor, ATFS-1.10,11 This unique transcription factor has both a mitochondrial targeting signal and a nuclear localization signal. Following stress, a reduced efficiency of mitochondrial import allows the nuclear localization of ATFS-1.12 This general strategy is reminiscent of the mitochondrial import-dependent regulation of PINK1 stability and localization.21 As PINK1 accumulates upon misfolded protein accumulation in mammalian cells, the modulation of import machinery by the unfolded proteins may be a general mode to respond to a proteinaceous stress.
However, in mammalian systems that we used in this report, downregulation of CLPP did not inhibit PINK1 accumulation induced by overexpression of unfolded protein or affect the level of ΔOTC itself, indicating that CLPP is not triggering PINK1 accumulation through peptide generation or ΔOTC degradation. However, the knockdown of another mitochondrial protease, LONP1, robustly increased ΔOTC and PINK1 accumulation, suggesting that ΔOTC is degraded to some extent by LONP1 and that the level of accumulated unfolded protein is important for PINK1 accumulation rather than peptide fragments generated by the protease. This result corresponds to the suggested hypothetical hierarchy of mitochondrial QC. Namely, once the presence of unfolded proteins is detected beyond the capacity of steady-state mitochondrial chaperones and proteases to manage, a retrograde signal is sent to the nucleus to increase mitochondrial chaperone expression. If the capacity of QC through the induction of UPRmt fails to maintain protein homeostasis upon proteinaceous stress, mitophagy is initiated stemming from the accumulation of PINK1. Thus, UPRmt appears to occur upstream of PINK1 accumulation through a mechanism of induction independent of membrane potential.
In conclusion, our results indicate that the accumulation of misfolded proteins in the mitochondrial matrix can initiate mitophagy mediated by PINK1 and PARK2 by a mechanism independent of mitochondrial depolarization. Considering the interrelation between neurodegenerative diseases and aggregate-prone peptides, this situation might have more pathophysiological relevance to disease than complete depolarization of mitochondria that has previously been established to induce mitophagy.
Materials and Methods
cDNAs, siRNAs, and transfection
Wild-type and deletion mutant (Δ30-114) OTCs in pCAGGS were a gift from NJ Hoogenraad (La Trobe University, Melbourne, Australia). The inserts were amplified by PCR and cloned into pRetroX-TRE3G (Clontech, 631188). YFP-PARK2 and PINK1-YFP were cloned in pBMN-LacZ (Allele Biotechnology, ABP-PVL-10011; LacZ was removed) and pRetroX-IRES-DsRedExpress (Clontech, 632521), respectively. For transient expression of OTC constructs, cells were plated in borosilicate chamber slides for imaging, 6-well plates for preparation of whole cell lysates, and 150-mm culture dish for subcellular fractionation. One day after plating, cells were transfected with the indicated constructs using Fugene HD (Promega, E2312) according to the manufacturer’s guidelines. Negative control siRNA and smartpool siRNAs for LONP1 and PINK1 were purchased form Thermo Fisher Scientific (D-001210-02, L-003979-00, and M-004030-02). siRNA for CLPP (5′-GCUCAAGAAG CAGCUCUAUU U-3′) was generated from Qiagen. RNAi MAX (Invitrogen, 56531) was used to transfect the cells with siRNA according to the manufacturer’s guidelines.
Production of retrovirus and generation of stable cell line
To produce retrovirus, 1.5 μg of pRetroX-TRE3G/wtOTC or ΔOTC or pBMN/YFP-PARK2 was tranfected in HEK293T cells together with the plasmids encoding Gag-Pol (1.0 μg) and VSV-G (0.5 μg) using Lipofectamine 2000 (Invitrogen, 52758). Media was replaced at 24 h after transfection, and then, conditioned media containing retroviral particles was collected for another 24 h. Then, the collected media was directly used to infect HeLa-TetOn cells to make a stable cell line expressing wtOTC or ΔOTC in a doxycycline-inducible manner. Transduction mixtures were replaced with fresh medium after 6 h. After 48 h incubation in normal growth media, cells were treated with puromycin (2 μg/ml) to select the transduced cells. The established ΔOTC/HeLa-TetOn cell line was transduced again with YFP-PARK2 retrovirus to make the ΔOTC/YFP-PARK2/HeLa-TetOn cell line.
Cell culture and chemicals
All cell lines were maintained in Dulbecco’s modified Eagle’s medium (31053) containing 10% fetal calf serum (BenchMark, 100-106), 20 mM L-glutamine (25030), 1 mM sodium pyruvate (11360), 1× MEM nonessential amino acids (11140) at 37 °C under an atmosphere of 5% CO2. All chemicals for cell culture except for serum were purchased from Invitrogen.
Western blotting
Cells were washed twice with cold-PBS and then directly lysed with 1× sample buffer for whole cell lysates or lysed with PBS containing 0.5% Triton X-100 and centrifuged at 12,000 × g, 4 °C for 10 min. The supernatant fraction was used as the Triton X-100 soluble fraction and the pellet fraction as the insoluble fraction. For subcellular fractionation, cells were treated as described previously30 and below, and then the pellets were lysed with 1× sample buffer. Twenty μg of proteins were separated by 4–20% Tris-glycine or Bis-Tris SDS-PAGE. The following antibodies were used: anti-PINK1, anti-LONP1 (Novus Biologicals, BC100-494 and NBP1-81734,), anti-OTC, anti-TOMM20/TOM20 (Santa Cruz Biotechnology, Inc., sc-11415), anti-CLPP (Abcam, ab124822), anti-HTRA2/OMI (R&D Systems, AF1458) and anti-GAPDH (Sigma-Aldrich, G-9545) rabbit polyclonal antibodies; anti-LMNB1 (Santa Cruz Biotechnology, Inc., sc-6217) goat polyclonal antibody; anti-tubulin, α (TUBA) (Invitrogen, 32-2500), anti-β-actin (ACTB) (Sigma-Aldrich, A-3853), anti-cytochrome C (CYCS), anti-OPA1 (BD, 556433 and 612607), anti-mitochondrial ATP synthase α subunit (ATP5A1) (Abcam, ab14748), anti-DDIT3 and anti-HSPA9 (Thermo Fisher Scientific, Inc., MA1-250 and MA3-028) mouse monoclonal antibodies.
Subcellular fractionation and proteinase K treatment
For mitochondrial isolation, cells were homogenized using a Teflon pestle (Thomas Scientific, 3431E20) in 20 mM HEPES (pH 7.6), 220 mM mannitol, 70 mM sucrose, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride and 2 mg/ml BSA. Cell homogenates were centrifuged at 800 × g at 4 °C for 10 min to obtain nuclear pellets and then post-nuclear supernatants were centrifuged at 10,000 × g at 4 °C for 20 min to obtain mitochondrial pellets. Both nuclear and mitochondrial fractions were washed with homogenization buffer and solubilized with sample buffer for western blotting.
For proteinase K digestion assays, freshly isolated mitochondria were resuspended in 20 mM HEPES-KOH pH 7.4, 250 mM sucrose, 80 mM KAc, 5 mM MgAc and incubated with various concentrations of proteinase K (Sigma, P6556) for 30 min on ice. A fraction of equally aliquoted mitochondria was sonicated and then incubated with proteinase K as above for the ΔOTC degradation experiment. Digestion was stopped by boiling in sample buffer followed by separation by SDS-PAGE and western blotting.
Live cell imaging and immunocytochemistry
For live cell imaging, cells were pulsed with 600 nM TMRE for 5 min, washed with 150 nM TMRE in normal growth media and imaged using an inverted confocal microscope (LSM510 Meta; Carl Zeiss, Inc.) with 63×/1.4 oil DIC Plan Apo objective. In case of immunocytochemistry, cultured cells were fixed with 4% paraformaldehyde in PBS (USB, 19943) and permeabilized with 0.5% Triton X-100 in PBS. After 30 min blocking with 10% BSA in 0.5% Triton X-100 in PBS, cells were stained with anti-PDHA1 (pyruvate dehydrogenase [lipoamide] α 1) monoclonal antibody (Abcam, ab110334) and then with the goat anti-mouse IgG antibody conjugated with Alexa Fluor 594. Image contrast and brightness were adjusted with Volocity (PerkinElmer).
FACS analysis
For flow analysis, cells were detached and counted. One × 106 cells were aliquoted and washed with modified Ringer’s buffer (127 mM NaCl, 5.5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 0.5 mM KH2PO4, 20 mM HEPES, 10 mM glucose, 1% FBS, pH 7.4). The cells were stained with 75 nM TMRE for 20 min at 37 °C and washed with 7.5 nM TMRE in modified Ringer’s buffer for equilibration. For a negative control, 2 μM CCCP was added to duplicate samples. Samples were immediately analyzed at absorbance/emission = 549/573 nm on Moflo Astrios (Beckman Coulter).
Supplementary Material
Acknowledgments
We thank Dr Nick J Hoogenraad for kindly providing the OTC constructs. This work is supported by the National Institute of Neurological Disorders and Stroke intramural program.
Glossary
- Abbeviations
ATFS-1, activating transcription factor associated with stress
- BAF
bafilomycin A1
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- DDIT3
DNA-damage-inducible transcript 3
- Dox
doxycycline
- IMS
intermembrane space
- OMM
outer mitochondrial membrane
- OTC
ornithine carbamoyltransferase
- PINK1
PTEN-induced putative kinase 1
- QC
quality control
- TIMM
translocase of inner mitochondrial membrane
- TMRE
tetramethylrhodamine, ethyl ester
- TOMM
translocase of outer mitochondrial membrane
- UPRmt
mitochondrial unfolded protein response
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/autophagy/article/26122
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/26122
References
- 1.Baker MJ, Tatsuta T, Langer T. Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol. 2011;3:3. doi: 10.1101/cshperspect.a007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan AC, Bhangoo MK, Young JC. Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J Biol Chem. 2006;281:33313–24. doi: 10.1074/jbc.M605250200. [DOI] [PubMed] [Google Scholar]
- 3.Kang PJ, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–43. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- 4.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/S0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 5.Horwich AL, Weber-Ban EU, Finley D. Chaperone rings in protein folding and degradation. Proc Natl Acad Sci U S A. 1999;96:11033–40. doi: 10.1073/pnas.96.20.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Høj PB, Hoogenraad NJ. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240:98–103. doi: 10.1111/j.1432-1033.1996.0098h.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–9. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldridge JE, Horibe T, Hoogenraad NJ. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One. 2007;2:e874. doi: 10.1371/journal.pone.0000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229–39. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007;13:467–80. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell. 2010;37:529–40. doi: 10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587–90. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 14.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–60. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 15.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–9. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–21. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–83. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–42. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamano K, Youle RJ. PINK1 is degraded through the N-end rule pathway. Autophagy. 2013 doi: 10.4161/auto.24633. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pimenta de Castro I, Costa AC, Lam D, Tufi R, Fedele V, Moisoi N, Dinsdale D, Deas E, Loh SH, Martins LM. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ. 2012;19:1308–16. doi: 10.1038/cdd.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–55. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–36. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–66. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–80. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science. 1994;264:273–6. doi: 10.1126/science.8146662. [DOI] [PubMed] [Google Scholar]
- 29.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125:795–9. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22:320–33. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012;13:378–85. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A. 2013;110:8638–43. doi: 10.1073/pnas.1216197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.