Abstract
The hippocampus creates distinct episodes from highly similar events through a process called pattern separation and can retrieve memories from partial or degraded cues through a process called pattern completion. These processes have been studied in humans using tasks where participants must distinguish studied items from perceptually similar lure items. False alarms to lures (incorrectly reporting a perceptually similar item as previously studied) are thought to reflect pattern completion, a retrieval-based process. However, false alarms to lures could also result from insufficient encoding of studied items, leading to impoverished memory of item details and a failure to correctly reject lures. The current study investigated the source of lure false alarms by comparing eye movements during the initial presentation of items to eye movements made during the later presentation of item repetitions and similar lures in order to assess mnemonic processing at encoding and retrieval, respectively. Relative to other response types, lure false alarms were associated with fewer fixations to the initially studied items, suggesting that false alarms result from impoverished encoding. Additionally, lure correct rejections and lure false alarms garnered more fixations than hits, denoting additional retrieval-related processing. The results suggest that measures of pattern separation and completion in behavioral paradigms are not process-pure.
Keywords: false recognition, gist representation, eye-tracking, encoding, episodic memory
The hippocampus mediates the formation and the retrieval of new memories. The process of pattern separation encodes neural information into distinct episodic memory representations and is critical for preventing older representations from being overwritten by new, similar representations (Yassa & Stark, 2011). The hippocampus can also retrieve a memory representation based on a partial or degraded retrieval cue through a process called pattern completion (Hunsaker & Kesner, 2013; Yassa & Stark, 2011). The neural mechanisms of these operations have been computationally modeled based on rodent (Leutgeb & Leutgeb, 2007) and primate research (Rolls, 2010), and recent behavioral and neuroimaging studies have investigated these processes in humans (Bakker, Kirwan, Miller & Stark, 2008; Yassa & Stark, 2011). Despite this progress and the important clinical implications of this research (Ally, Hussey, Ko & Molitor, 2013), there are outstanding questions regarding whether pattern separation and pattern completion processes are properly measured in human research.
It has been debated whether pattern separation and pattern completion processes compete for output within the hippocampus, resulting in a functional trade-off, or if these processes operate independently (Holden & Gilbert, 2012; Hunsaker & Kesner, 2013; Nakashiba, Cushman, Pelkey, Renaudineau, Buhjl et al., 2013). Human studies have had mixed results in addressing this debate. Some research has shown that changes in the hippocampus due to healthy aging cause a bias towards pattern completion at the expense of pattern separation. Specifically, while healthy older adults exhibit reduced rates of pattern separation relative to younger controls, they also show compensatory increases in pattern completion, suggesting that the two processes trade-off (Yassa, Lacy, Stark, Albert, Gallagher, & Stark, 2011). However, other studies have found that decreased rates of pattern separation do not necessarily lead to increased rates of pattern completion. Research in memory-impaired populations, including patients with hippocampal damage and patients with Alzheimer’s disease, has shown that declines in pattern separation do not coincide with increased pattern completion, suggesting that these processes operate independently (Ally, et al., 2013; Kirwan, Hartshorn, Stark, Goodrich-Hunsaker, Hopkins, & Stark, 2012). These disparate findings may indicate that the tasks used to target pattern separation and pattern completion in humans do not accurately measure these processes, as they are computationally defined, in a process-pure manner.
Human studies have used behavioral recognition tasks that require precise memory discrimination to evoke pattern separation and pattern completion processes (Santoro, 2013). In these tasks, participants study a set of items and are tested with studied items, unstudied but perceptually and/or conceptually similar lures (e.g. category exemplars of studied items), and unstudied novel items. Participants must correctly identify these items as “old,” “similar,” or “new,” respectively. Pattern separation is indexed by correct “similar” responses to lures (lure correct rejections) because the discrimination between the similar lure and the studied item in memory would require highly distinct representations, consistent with predictions from the neurocomputational model of pattern separation (Rolls, 2010; Santoro, 2013). In contrast, pattern completion is operationally defined as incorrect “old” responses to lures (lure false alarms; Kim & Yassa, 2013; Stark, Yassa, Lacy, & Stark, 2013; Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa et al., 2011). It is speculated that the shared perceptual and conceptual information between lures and studied items presumably functions as a noisy or incomplete cue to retrieve studied items via pattern completion (Hunsaker & Kesner, 2013). However, it is not entirely clear why pattern completion would lead to false alarm responses to lures. Previous research has posited that an “old” response to a lure is the result of generalization between the lure and the retrieved studied item (Stark et al., 2013; Yassa, et al., 2011), but the computational definition of pattern completion does not account for generalization (Hunsaker & Kesner, 2013). Because the mechanism underlying lure false alarms cannot be unambiguously attributed to pattern completion alone, the contribution of other cognitive processes must be investigated.
Alternatively, lure false alarms could result from the failure to sufficiently encode study items. Previous research has demonstrated that robustly encoding study items can help create distinct representations that are distinguishable from similar lures, thereby deterring false memory (Dodson, Koutstaal, & Schacter, 2000). One explanation for lure-related responses in studies of pattern separation and pattern completion is the fuzzy-trace account of false memory (Brainerd & Reyna, 2002). Fuzzy-trace theory proposes that an episodic memory is represented with a verbatim trace, which includes sensory details, and a gist trace, which includes abstract meaning. Verbatim traces provide detailed perceptual information that can discriminate between similar representations and reduce the probability of false memory, while gist traces can increase the probability of false memory because conceptual information does not differentiate exemplars from the same basic-level category. Since tasks assessing pattern separation and pattern completion use lures that share gist information with studied items, successful rejection of lures would depend on the availability of verbatim traces in memory. Thus, lure false alarms could occur when the representations of retrieved studied items have degraded verbatim traces due to poor encoding. Because lure false alarms may depend on the quality of encoding, studies of pattern completion must consider both encoding and retrieval processes.
Eye movement patterns recorded at encoding and retrieval can serve as indices of memory-related processing. Foveated areas of a stimulus are input into the visual system with the greatest detail, so a higher quality memory representation of a stimulus is more likely to be formed as the number of fixations during encoding increases (Pertzov, Avidan, & Zohary, 2009). Indeed, increased fixations to an item during its initial encoding are associated with higher memory accuracy for object identity, orientation, and spatial location (Kafkas & Montaldi, 2011; Loftus, 1972; Pertzov et al., 2009). At retrieval, eye movement patterns reflect prior exposure to studied items (Hannula, Baym, Warren, & Cohen, 2012) and have been linked to hippocampal activity (Hannula & Ranganath, 2009). Furthermore, increased fixations to an item during retrieval have been found to correspond with increased reports of recollection compared to familiarity (Kafkas & Montaldi, 2012). Because eye movements manifest important differences in mnemonic processing at encoding and retrieval, they can be used to disentangle whether false alarms to lures occur due to poor encoding or pattern completion.
The current study set out to examine whether false alarms to lures are a consequence of poor encoding or pattern completion. Eye movements were measured while participants viewed a long sequence of pictures that included repetitions, visually similar lures, and novel items. Repetitions and lures were presented at pre-determined lags to measure possible differences in forgetting rates between the different response types (Ally et al., 2013). Repetitions correctly reported as “old” (hits), lures correctly rejected as “similar” (lure correct rejections), and lures erroneously reported as “old” (lure false alarms) were analyzed. Importantly, the fixations made to each of these response types were measured during the presentation of the original studied items (first presentations) and during the presentation of the repetitions or lures used to test them (second presentations) in order to capture encoding and retrieval processes, respectively.
Two accounts of how lure processing contributes to false recognition were compared. The poor encoding hypothesis proposes that responses to lures depend on how well studied items were initially encoded, with lure false alarms resulting from insufficient encoding. Therefore, the poor encoding hypothesis predicts that fewer fixations will be made to the first presentation of items that are later endorsed as lure false alarms compared to those later endorsed as hits and lure correct rejections. In contrast, the pattern completion hypothesis proposes that pattern completion elicits the same neural processing as true recognition (Bakker et al., 2008; Lacy, Yassa, Stark, Muftuler, & Stark, 2011) and that lure false alarms are solely mediated by a retrieval-based pattern completion process. Therefore, the pattern completion hypothesis predicts no difference in the number of fixations between lure false alarms, hits, and lure correct rejections during first presentations. The pattern completion hypothesis also predicts that lure false alarms and hits will receive the same number of fixations during second presentations.
Materials and Methods
Participants
Participants were 24 adult native English speakers with normal or corrected-to-normal vision (12 females) with a mean age of 22.21 years (standard deviation, SD = 1.89) and an average of 14.94 years of education (SD = 1.91). Participants gave informed consent prior to the experiment and were compensated $25/hour. The study was approved by the Behavioral and Social Sciences Committee of the Vanderbilt University Institutional Review Board.
Apparatus
Eye movements were recorded with an EyeLink 1000 tracker (SR Research Ltd., Ontario, Canada) at a sampling rate of 250 Hertz (Hz). Saccade, blink, and fixation data were recorded for each participant. Saccades were defined as any movement of at least 0.1 degree visual angle (°) that exceeded a velocity threshold of 30°/second (s) and an acceleration threshold of 8000°/s2. Blinks were periods of activity with missing pupil data that exceeded 3 consecutive samples. Fixations consisted of all other recordings. Stimuli were displayed on a flat screen monitor (1024×768 pixel resolution, 75 Hz refresh rate) using a computer networked to a host computer running EyeLink 4.51 tracking software. The experiment was programmed using Experiment Builder software (SR Research Ltd.). Responses were collected with an RB-730 response pad (Cedrus, San Pedro, California).
Stimuli and Procedure
The experimental paradigm is depicted in Figure 1. The stimuli and continuous recognition task were the same set used in Ally et al. (2013). Memory items were 192 pairs of full-color pictures of everyday objects fit into a 17.5°×17.5° area. Each pair consisted of exemplars from the same basic level category (e.g. “dogs,” “bicycles”). The stimuli were counterbalanced so that each picture could be seen as a repeated item, a similar lure to a previously viewed item, or a novel item. Repetitions and lures were intermixed with novel items, and their first and second presentations were separated at pseudorandomized lags of 4, 12, or 40 items. There were 88 novel items and 12 repeated items and lures at each lag, for a total of 36 repetitions and 36 lures. Participants completed two experimental blocks that consisted of 116 trials each, totaling 232 trials. Repetitions and lures were distributed evenly across blocks.
Figure 1.
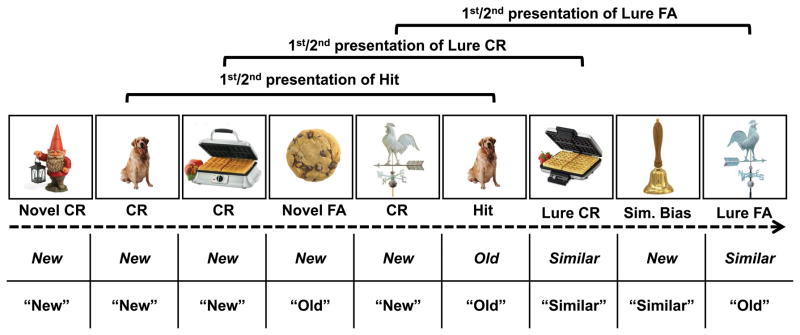
Behavioral paradigm and dependent measures. Participants viewed a series of sequentially presented pictures (left to right in figure) which included repetitions, lures, and novel items. Repetitions and lures were shown at predetermined lags (lag 4 shown). Correct responses and possible responses appear as labels in the two rows below the picture sequence. Italicized labels in the top row represent the correct response to each picture. Quoted labels in the bottom row represent possible participant responses. The relationship between the correct response and actual response determined the response type. Response types used in the analysis are indicated directly above each picture. CR = correct rejection, FA = false alarm.
Participants were seated 57 centimeters in front of the display monitor with their heads secured in a head-rest. Each block began by calibrating eye position with a 3×3 spatial array of fixation points. Eye-tracking accuracy was checked prior to each trial, and eye position was recalibrated for large drifts. The drift checks, which also functioned as the inter-trial interval, lasted about 1000 milliseconds (mean, M = 1075.95, SD = 255.60). Each trial began with the 2500 millisecond presentation of a picture. Immediately following the picture offset, participants viewed a prompt to report the picture as “old,” “similar,” or “new.” Eye movements were recorded during the presentation of each stimulus.
Data Analysis
Behavioral data were corrected for response bias prior to analysis. Responses to repetitions were corrected by subtracting the rates of “old” responses to novel items (novel false alarms) from the rates of “old” responses to repetitions (hits) to calculate Pr (Snodgrass & Corwin, 1988). The rates of “similar” response to lures (lure correct rejections) were bias-corrected by subtracting the rates of “similar” responses to novel items (similar bias), creating behavioral pattern separation (BPS) scores (Stark et al., 2013). Correspondingly, the rates of “old” responses to lures (lure false alarms) were corrected by subtracting the novel false alarm rates, creating behavioral pattern completion (BPC) scores (Ally et al., 2013).
The eye movement data were also filtered and corrected prior to statistical analysis. Fixations and saccades outside the 17.5°×17.5° maximum stimulus bounds were removed, as well as saccades spanning stimulus offset, eliminating approximately 2% of saccades and less than 1% of fixations. Novel items and the first presentations of repetitions and lures were only included in the analysis if they were reported as “new” (correct rejection). In order to measure memory-related eye movement patterns, memory-based fixation counts were isolated by subtracting the number of fixations to novel items from the other item types (first and second presentations for hits, lure correct rejections, and lure false alarms; see Figure 1). These subtractions resulted in difference scores, so that positive values indicated more fixations relative to novel items and negative values indicated fewer fixations relative to novel items. To ensure adequate statistical analysis of the eye movements, a minimum bin size of four trials for each response type was set for the analysis (Hannula et al., 2012). This bin size restriction excluded a subset of participants (n = 4) without a sufficient number of “old” responses to lures after collapsing the responses across the different levels of lag. Importantly, the exclusion of these participants did not disrupt the counterbalancing of the experiment. The following analyses were carried out on the remaining participants (n = 20), who did not differ in age (M = 22.35, SD = 1.81) or education (M = 15.23, SD = 1.85) from the overall group.
Results
Behavioral Data
Behavioral response rates are reported in Table 1. Overall, performance on the recognition task was very accurate. When averaged across Lag, participants correctly identified 90 percent of repeated items as “old” (SD = 0.09) and 69 percent of lures were correctly rejected as “similar” (SD = 0.13). However, participants still made a reliable number of false alarm responses to the lures, with 25 percent of lures erroneously accepted as “old” (SD = 0.11). To determine whether the response rates changed with lag, the corrected behavioral measures, Pr, BPS score, and BPC score, were each submitted to a separate repeated measures analysis of variance (ANOVA) with the within-subjects factor of Lag (lag 4, lag 12, lag 40). The analysis of successful performance on repeated items (Pr) revealed an effect of Lag [F(2,38) = 4.07, p = 0.03, partial eta-squared, ηp2 = 0.18]. Post-hoc paired t-tests showed no difference in Pr from Lag 4 to Lag 12 [t(19) = 1.36, p = 0.19], but showed a decline in performance from Lag 12 to Lag 40 [t(19) = 2.43, p = 0.03] and a marginal decline from Lag 4 and Lag 40 [t(19) = 1.92, p = 0.07]. Next, correct rejection responses to lures (BPS scores) were examined with the same design matrix, resulting in a similar effect of Lag [F(2,38) = 3.76, p = 0.03, ηp2 = 0.17]. Post-hoc paired t-tests showed no difference in BPS scores from Lag 4 to Lag 12 [t(19) = 1.45, p = 0.16] or between Lag 4 and Lag 40 [t(19) = 1.35, p = 0.19], but there was a decline in performance from Lag 12 to Lag 40 [t(19) = 2.68, p = 0.01]. The analysis of false alarm responses to lures (BPC scores) showed an unreliable trend towards an effect of Lag [F(2,38) = 2.58, p = 0.09, ηp2 = 0.12].
Table 1.
Means (standard deviations) for behavioral response rates organized by lag.
| Lag 4 | Lag 12 | Lag 40 | Average | |
|---|---|---|---|---|
| Novel CR | - | - | - | .98 (.03) |
| Novel FA | - | - | - | .003 (.003) |
| Similar Bias | - | - | - | .02 (.03) |
| Hit | .90 (.13) | .94 (.10) | .87 (.12) | .90 (.09) |
| Lure CR | .69 (.19) | .76 (.14) | .63 (.19) | .69 (.13) |
| Lure FA | .25 (.14) | .20 (.13) | .29 (.18) | .25 (.11) |
| Pr | .90 (.13) | .93 (.10) | .86 (.12) | .90 (.09) |
| BPS Score | .67 (.19) | .74 (.15) | .61 (.20) | .67 (.14) |
| BPC Score | .25 (.14) | .20 (.13) | .29 (.18) | .24 (.11) |
CR = correct rejection, FA = false alarm, BPS = behavioral pattern separation, BPC = behavioral pattern completion.
Eye Movement Data
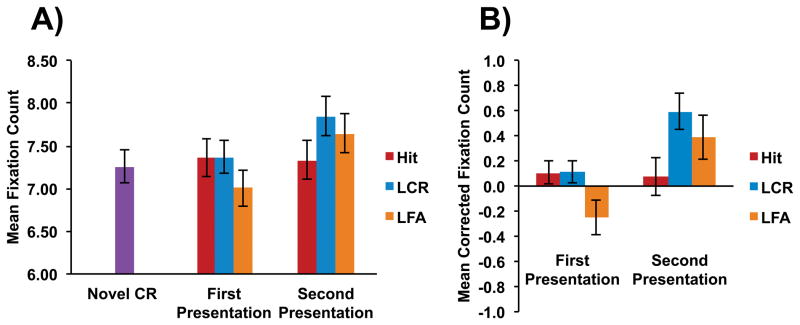
The uncorrected fixation counts and the corrected (memory-based) fixation data are reported in Figure 2. Due to the low rate of “old” responses to lures at each lag, eye movement measures were pooled across Lag to meet the minimum bin size for analysis of lure false alarms (see supplementary material for more information on bin sizes). The mean memory-based fixation counts were submitted to a repeated measures ANOVA using the factors of Presentation (first, second) and Response Type (hits, lure correct rejections, lure false alarms). This analysis revealed an effect of Presentation [F(1,19) = 6.72, p = 0.02, ηp2 = 0.26], an effect of Response Type [F(2,38) = 3.95, p = 0.03, ηp2 = 0.17], and an interaction between Response Type and Presentation [F(2,38) = 9.79, p < 0.001, ηp2 = 0.34]. The interaction was further investigated with paired t-tests. During the first Presentation, there were fewer memory-based fixations for lure false alarms (M = −0.25, SD = 0.63) than hits (M = 0.11, SD = 0.40) [t(19) = 2.33, p = 0.03] and lure correct rejections (M = 0.11, SD = 0.41) [t(19) = 2.23, p = 0.04]. However, there was no difference in fixations between hits and lure correct rejections [t(19) = 0.07, p = 0.94]. During the second Presentation, there were fewer fixations for hits (M = 0.08, SD = 0.69) than lure correct rejections (M = 0.59, SD = 0.64) [t(19) = 3.94, p < 0.001] and lure false alarms (M = 0.39, SD = 0.79) [t(19) = 2.20, p = 0.04], but no difference between lure correct rejections and lure false alarms [t(19) = 1.57, p = 0.13].
Figure 2.
Uncorrected (A) and corrected (B) fixation count for each item type. CR = correct rejection, FA = false alarm. Error bars represent standard error of the mean.
Discussion
The current study investigated recognition memory using eye-tracking measures to test the predictions of two opposing hypotheses about the cognitive processes underlying false alarms to perceptually similar lures. The inclusion of lures has been critical to the study of pattern separation and pattern completion processes in humans (Yassa & Stark, 2011). Similar to previous research (Kirwan & Stark, 2007; Toner et al., 2009; Yassa et al., 2011), the behavioral results in the present study showed that participants were very accurate and predominantly responded “similar” to lures. However, participants still made a reliable number of erroneous “old” false alarm responses to lures. The poor encoding hypothesis claims that lure false alarms result from the insufficient encoding of study items and predicts that fewer fixations would be made to lure false alarms compared to hits and lure correct rejections during the first presentations of items. Conversely, the pattern completion hypothesis states that, because pattern completion is computationally defined as a retrieval-related function (Rolls, 2010; Hunsaker & Kesner, 2013), mnemonic processing during encoding should not differ between lure false alarms and the other response types. However, the eye-tracking data showed that lure false alarms were associated with decreases in memory-based fixations during first presentations when encoding presumably occurred. Based on previous research that has shown a relationship between the number of fixations during encoding and subsequent memory (Loftus 1972; Kafkas & Montaldi, 2011), the relative paucity of fixations during the first presentations of lure false alarms implies that the original studied items were insufficiently encoded, in agreement with the poor encoding hypothesis.
The eye movement data from the first presentations of lure false alarms and lure correct rejections offer an explanation for the apparent trade-off between pattern separation and pattern completion that has been observed in some previous research with humans. These studies have found that decreases in lure correct rejection rates were complemented by increases in lure false alarm rates (e.g. Yassa et al., 2011), which has been attributed to a shift in bias towards pattern completion at the cost of reduced pattern separation. However, the eye movement data in the current study suggest that this trade-off occurs because lure correct rejections and lure false alarms are both contingent on changes during encoding and presumably the strength of the pattern separation process. Therefore, lure correct rejections and lure false alarms may “tradeoff” because they are potentially two ends of pattern separation. Lure correct rejections would exemplify successful pattern separation during the first presentation of items. In contrast, false alarms to lures in the current paradigm are consistent with a failure of pattern separation during the first presentation of items (Schacter, Norman, & Koutstaal, 1998), rather than an isolated pattern completion process during the presentation of the lures.
The eye-tracking results also showed that lure false alarms and lure correct rejections were both associated with a greater number of fixations during second presentations compared to hits. This finding conflicts with previous operational definitions of pattern completion processing when viewing lures, which has been assumed to resemble true recognition during retrieval (Bakker et al., 2008; Lacy et al., 2011). The number of fixations made during retrieval has been shown to be greater for items that are recollected compared to items that only elicit familiarity (Kafkas & Montaldi, 2012), so these additional fixations to lures could reflect the recollection of studied items as part of a recall-to-reject strategy (Gallo, 2004). With this strategy, a participant would retrieve a studied item and mentally compare it to the lure in an effort to reject the lure as a non-studied, similar item (Kirwan & Stark, 2007). The outcome of the recall-to-reject strategy would then depend on the availability of disqualifying verbatim information in memory that could be used to reject the lures as unstudied (Odegard & Lampinen, 2005). In the case of lure false alarms, studied items were encoded with an insufficient number of fixations that resulted in low-quality verbatim representations. Without sufficient verbatim information to disqualify the lures upon comparison with the retrieved representation, participants incorrectly accepted the lures as studied based on the shared gist information between the lures and studied items. Conversely, lure correct rejections demonstrated a successful recall-to-reject process because the studied items were robustly encoded and the stored representations contained verbatim information that distinguished the studied items from the lures upon comparison with the retrieved representations of studied items. This interpretation is consistent with a recent study by Kim and Yassa (2013), which found that both lure false alarms and lure correct rejections are predominantly associated with recollection, rather than familiarity. Hits in the current study likely reflected a mix of familiarity and recollection responses that, on average, attracted fewer fixations than lure false alarms and lure correct rejections.
If participants use the recall-to-reject strategy in this paradigm, then it is likely that lure false alarms and lure correct rejections are not selective measures of pattern completion and pattern separation, respectively. Recall-to-reject states that lures cue the retrieval of studied items, a process that likely requires pattern completion (Kirwan & Stark, 2007). Because all lures potentially trigger pattern completion, both lure false alarms and lure correct rejections would measure pattern completion to some extent. The possibility that lure correct rejections entail pattern completion means that lure correct rejections may not be pure measures of pattern separation, counter to previous conceptualizations of lure correct rejections (Stark et al., 2013). Another critical implication of the recall-to-reject strategy is that the behavioral response following the comparison between the lure and retrieved studied item (i.e. “old” or “similar” response to the lure) depends on the degree of pattern separation when studied items are encoded (Schacter et al., 1998). As previously discussed, lure false alarms likely represent pattern separation failures, suggesting that lure false alarms may be indirect measures of encoding strength and are thus not pure measures of pattern completion. To summarize, the data suggest that lure false alarms and lure correct rejections may be sensitive to both pattern separation and pattern completion to some degree, but they are not pure measures of either process.
Although the eye movement data are consistent with the poor encoding hypothesis, other interpretations of these findings must be considered as well. For example, lure false alarms could reflect the time-dependent decay of studied items, rather than a failure to encode verbatim information. Under these conditions, the recall-to-reject process would fail because the verbatim information has degraded and cannot be used to reject the lures. However, the behavioral data appear to refute this claim. Lure false alarm rates did not vary with lag, suggesting that lure false alarms are not affected by the time between the first and second presentations and that changes in the stored representations have a minimal or negligible effect on these responses. Another possible explanation is that the verbatim traces of studied items were encoded and stored, but could not be retrieved during the presentation of the lures (Guerin, Robbins, Gilmore, & Schacter, 2012). Counter to this suggestion, the eye movement data showed that lure false alarms were distinguished from other response types by encoding impairments (e.g. lower fixation count during the first presentation). Moreover, lure false alarms and lure correct rejections exhibited similar retrieval-related processing, further demonstrating that the behavioral responses to the lures depended on encoding. Thus, both the behavioral and eye-tracking data suggest that lure false alarms stem from poor encoding.
The poor encoding account of lure false alarms can provide support for other studies of false recognition of visual objects. A similar experiment by Yeung, Ryan, Cowell, and Barense (2013) used eye movement recordings to investigate the basis of memory impairments in older adults at risk for mild cognitive impairment. Participants studied a series of pictures and were tested with repeated items, high-similarity lures, and low-similarity lures. An analysis of the fixation data revealed that the at-risk older adults tended to falsely recognize the high-similarity lures, viewing them as if they had been previously seen. These false alarm viewing patterns were thought to result from impoverished object representations that failed to overcome the familiarity of the high-similarity lures. Based on the findings of the current study, the impoverished memories in the at-risk older adults could have resulted from the poor encoding of study items. Together, these two studies demonstrate how false recognition can manifest in eye movement behavior at study (e.g. reduced fixations during encoding) and in sampling behavior at test (Yeung et al., 2013). Although the retrieval-related viewing patterns associated with lure false alarms in the current study did not exhibit the same reprocessing effects elicited by the high-similarity lures in Yeung et al. (2013), this discrepancy may be due to the different tasks used in the experiments. While Yeung et al. (2013) used an implicit recognition task, the current study used an explicit recognition task that is believed to engender recall-to-reject (Bakker et al., 2008; Kirwan & Stark, 2007; Yassa et al., 2011). Given the previously discussed implications of the recall-to-reject strategy for behavioral studies of pattern separation and pattern completion, future studies should directly investigate whether participants indeed use recall-to-reject when responding to lures (cf. Kirwan & Stark, 2007).
In summary, the results of the current experiment suggest that the behavioral paradigms used to study pattern separation and pattern completion in humans do not provide definitive measures of either process. It is clear that a more process-pure measurement of pattern separation and pattern completion must be developed in order to adequately capture these two processes with behavioral responses. Previous studies have discovered lure-related hippocampal activity that is consistent with pattern separation and pattern completion, but the connection between these computationally-defined cellular processes and their consequent behavior still needs to be established (Santoro, 2013). Without this link, human behavioral studies can only offer ostensible evidence of pattern separation and pattern completion. However, the ability to discriminate between lures and studied items is still an important test of memory. Lure discrimination has been found to be a more sensitive test of memory performance than traditional measures of recognition (Stark et al., 2013). Additionally, lure discrimination has been shown to reveal subtle memory impairments in clinical populations (Kirwan et al., 2012) and provide powerful classification between stages of dementia (Ally et al., 2013). Thus, while the cognitive processes underlying these behavioral responses must be more thoroughly investigated, the paradigms can still provide useful information about memory performance.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AG031925 and AG038471 to BAA
References
- Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer’s disease: Evidence of rapid forgetting in patients with amnestic mild cognitive impairment. Hippocampus. 2013;23:1246–1258. doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. Fuzzy-trace theory and false memory. Current Directions in Psychological Science. 2002;11:164–169. [Google Scholar]
- Dodson CS, Koutsaal W, Schacter DL. Escape from illusion: Reducing false memories. Trends in Cognitive Sciences. 4:391–397. doi: 10.1016/s1364-6613(00)01534-5. [DOI] [PubMed] [Google Scholar]
- Gallo DA. Using recall to reduce false recognition: Diagnostic and disqualifying monitoring. Journal of Experimental Psychology. 2004;30:120–128. doi: 10.1037/0278-7393.30.1.120. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Robbins CA, Gilmore AW, Schacter DL. Journal of Memory and Language. 2012;66:68–78. doi: 10.1016/j.jml.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Baym CL, Warren DE, Cohen NJ. The eyes know: Eye movements as a veridical index of memory. Psychological Science. 2012;23:278–287. doi: 10.1177/0956797611429799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Frontiers in Aging Neuroscience. 2012;4:1–6. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and Biobehavioral Reviews. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. Recognition memory strength is predicted by pupillary responses at encoding while fixation patterns distinguish recollection from familiarity. The Quarterly Journal of Experimental Psychology. 2011;64:1971–1989. doi: 10.1080/17470218.2011.588335. [DOI] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. Familiarity and recollection produce distinct eye movement, pupil, and medial temporal lobe responses when memory strength is matched. Neuropsychologia. 2012;50:3080–3093. doi: 10.1016/j.neuropsychologia.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kim J, Yassa MA. Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus. 2013;23:287–294. doi: 10.1002/hipo.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Hartshorn A, Stark SM, Goodrich-Hunsaker NJ, Hopkins RO, Stark CEL. Pattern separation deficits following damage to the hippocampus. Neuropsychologia. 2012;50:2408–2414. doi: 10.1016/j.neuropsychologia.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning & Memory. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & Memory. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning & Memory. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Loftus GR. Eye fixations and recognition memory for pictures. Cognitive Psychology. 1972;3:525–551. [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard TN, Lampinen JM. Recollection rejection: Gist cuing of verbatim memory. Memory & Cognition. 2005;33:1422–1430. doi: 10.3758/bf03193375. [DOI] [PubMed] [Google Scholar]
- Pertzov Y, Avidan G, Zohary E. Accumulation of visual information across multiple fixations. Journal of Vision. 2009;9:1–12. doi: 10.1167/9.10.2. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A computational theory of episodic memory formation in the hippocampus. Behavioural Brain Research. 2010;215:180–196. doi: 10.1016/j.bbr.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Santoro A. Reassessing pattern separation in the dentate gyrus. Frontiers in Behavioral Neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annual Review of Psychology. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung L, Ryan JD, Cowell RA, Barense MD. Recognition memory impairments caused by false recognition of novel objects. Journal of Experimental Psychology: General. 2013;142:1384–1397. doi: 10.1037/a0034021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.