Abstract
Aberrant expression and activation of the cell cycle protein E2F1 in neurons has been implicated in many neurodegenerative diseases. As a transcription factor regulating G1 to S phase progression in proliferative cells, E2F1 is often upregulated and activated in models of neuronal death. However, despite its well studied functions in neuronal death, little is known regarding the role of E2F1 in the mature brain. In the present study, we used a combined approach to study the effect of E2F1 gene disruption on mouse behavior and brain biochemistry. We identified significant age-dependent olfactory and memory related deficits in E2f1 mutant mice. In addition, we found that E2F1 exhibits punctated staining and localizes closely to the synapse. Furthermore, we found a mirroring age-dependent loss of postsynaptic protein-95 in the hippocampus and olfactory bulb as well as a global loss of several other synaptic proteins. Coincidently, E2F1 expression is significantly elevated at the ages in which behavioral and synaptic perturbations were observed. Lastly, we show that deficits in adult neurogenesis persist late in aged E2f1 mutant mice which may partially contribute to the behavior phenotypes. Taken together, our data suggest that the disruption of E2F1 function leads to specific age-dependent behavioral deficits and synaptic perturbations.
Introduction
E2F1 is a highly conserved cell-cycle related transcription factor that regulates the gene targets that are necessary for the transition from G1 to S phase in dividing cells. Additionally, E2F1 also has the capacity to regulate cell death as it can induce p53-dependent and p53-independent apoptosis as well as transcription-dependent and, transcription-independent cell death (Johnson & Degregori, 2006; Iaquinta & Lees, 2007). As a result, aberrant E2F1 expression and activity has been investigated as a contributor to neuronal death in various neurodegenerative diseases (Jordan-Sciutto et al., 2002a; Jordan-Sciutto et al., 2002b; Jordan-Sciutto et al., 2003; Hoglinger et al., 2007; Pelegri et al., 2008). However, studies evaluating its role in various neurotoxicity models in vitro have not conclusively provided a precise mechanism as to how E2F1 mediates death in mature neurons (Park et al., 1997; Giovanni et al., 1999; Giovanni et al., 2000; O’Hare et al., 2000; Park et al., 2000a; Park et al., 2000b).
In the developing brain, E2F1 is expressed abundantly in the ventricular regions during progenitor cell proliferation (Dagnino et al., 1997). As the brain matures, E2F1 mRNA levels are maintained, and protein levels of E2F1 increase into adulthood (Tevosian et al., 1996; Kusek et al., 2001). Disruption to E2f1 in mice can lead to increased cell cycle events as well as reduced expression of neuronal marker calbindin (Wang et al., 2007). Interestingly, in mature neurons, E2F1 is predominantly cytoplasmic, which differs from its nuclear localization in cycling cell types and is inconsistent with its well-characterized role as a transcription factor (Strachan et al., 2005; Wang et al., 2010). This cytoplasmic location of E2F1 has also been observed in other terminally differentiated cells, such as myocytes and keratinocytes (Gill & Hamel, 2000; Ivanova & Dagnino, 2007; Ivanova et al., 2007). While it is logical that terminally differentiated cells would favor a predominantly cytoplasmic localization of E2F1 to ensure that it is not transcriptionally active to trigger cell-cycle re-entry, the exact role of E2F1 in neurons remains poorly understood.
Mice carrying disrupted E2f1 have a substantial reduction in adult neurogenesis at 3 months, even though brain development appears unaffected (Cooperkuhn et al., 2002). To further investigate potential roles for E2f1 in post-mitotic neurons, we assessed the impact of E2f1 disruption on behavior as well as morphologic and biochemical changes in the CNS. In the present study, we demonstrate that mice with E2f1 disruption display age-dependent olfactory and memory related behavioral deficits. In addition, we demonstrate that E2F1 is predominantly cytoplasmic and localizes to synaptic fractions. The disruption of E2f1 results in a significant reduction in the expression of crucial synaptic proteins including postsynaptic density protein-95 (PSD-95), specifically in the hippocampus (HC) and the olfactory bulb (OB). The age in which the synaptic disruptions were observed correlated with that of the olfactory and memory deficits. The synaptic and behavioral effects in the E2f1tm1 mice are likely due to the absence of E2F1 function as the age in which E2F1 expression peaks strongly correlates with the onset of these perturbations. Furthermore, the persistent deficits in adult neurogenesis partially contribute to these age-dependent effects. Taken together, our study provides evidence that the disruption of E2F1 function in mice elicits both synaptic and behavioral deficits in an age-dependent manner.
Materials and Methods
Animal Behavior
E2f1tm1 (B6;129S4-E2F1tm1Meg/J; strain # 002785) and Wildtype F2 hybrid (B6129SF2/J; strain # 101045) mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were described in Field et al. (1996), and are a hybrid of C57BL/6 and SV129 strains (Field et al., 1996). Briefly, mutations in the E2f1tm1 mice were generated by disrupting exon3 with the insertion of the neomycin selection marker and by removing the entire exon4. Disruption of exon3 and the deletion of exon4 of E2f1 lead to, at a minimum, disruption of the E2F1 DNA binding and heterodimerizaton domains. Analysis of RNA from these animals revealed that several E2f1 mRNA species are produced by the disrupted E2f1 locus lacking the coding sequences for the DNA binding region as predicted. The same strain mixture was used for wildtype mice bred for use as controls. All mice were housed at the University of Pennsylvania animal facilities on a 12-hour light/dark cycle and water were provided ad libitum. Only males were used in the behavioral and biochemical studies and all experiments in this study were approved by the Institutional Animal Use and Care Committee and in agreement with the ARRIVE Guidelines.
Odor Habituation
Mice were habituated to a standard size mouse cage for a period of 1 hour before testing (Fletcher & Wilson, 2002; Fadool et al., 2004; Marks et al., 2009). Single odorant alcohols differing by the number of carbon atoms (octanol C9/C10) were diluted 1:100 in mineral oil or water and applied to a cotton swab. The cotton swab was introduced to the mouse through the top of the testing cage and the time of active investigation/smelling of the odor was recorded. Mice were habituated to the first odor of an odor pair combination by repeated stimulation with the odor saturated swab. On the 8th trial, the second odor was presented and the time of exploration was scored. All recorded times were normalized and compared to the animal’s original exploration time prior to habituation to minimize the between-animal variance.
General Anosmia
Naive mice were removed from the home cage and placed in a testing cage (29.2 × 19.1 × 12.7 cm) in which a scented cracker or size-matched marble was hidden from view under the bedding. The item to be retrieved was randomly selected and hidden in a different location on each trial. The retrieval time was recorded from the instant the mouse is released until the item was found. Experiments were terminated at 10 minutes and mice were scored for that time duration if the item was not retrieved (Fadool et al., 2004; Marks et al., 2009)
Novel Object Recognition Memory
Mice were habituated to a testing cage for a period of 1 hour before testing (Jeon et al., 2003; Fadool et al., 2004; Marks et al., 2009). Two non-identical objects were placed in the chamber and mice were allowed to explore them for a 5 minute interval. The time that each mouse was oriented toward each object within one head length was scored as exploratory time. The mice were then removed from the testing cage for either a 1 or 24-hour delay period. Mice were then reintroduced to the testing cage containing the object 1 (familiar object) and object 3 (novel object) in the same original position and scored for a 5 minute interval.
Light/Dark Box
A light/dark chamber resembling the home cage was modified as follows: A black divider was cut to the exact width and height of the cage and a 7×7 cm square opening was cut in the middle on the bottom edge (Crawley & Goodwin, 1980; Marks et al., 2009). The divider was placed in the middle of the cage. One side of the cage was painted black (dark), and the other side painted white (light). Mice were released into the light chamber and the time spent in either chamber was recorded for 300 seconds.
Locomotor Activity
Mice were placed in a testing chamber that was identical to its home cage and set in a photo-beam frame with sensors arranged in an 8-beam array strip (Mackler et al., 2008). Each animal was first habituated to the testing chamber over two 1-hour sessions across two days and the final basal locomotor activity monitored in a 1-hour session. Cumulative number of beam breaks were recorded and quantified into personal computer designed software (Med Associates).
Accelerating Rotarod
Mice were placed on an accelerating rotarod (Med Associates) for four trials a day over four days with a minimum of a 20-minute inter-trial interval. Each trial lasted a maximum of five minutes during which the rotarod accelerated linearly from 3.5 revolutions per minute (RPM) to 35 RPM. The amount of time for the animal to fall from the rod was recorded for each trial and averaged for each day for four days total (Wang et al., 2012).
Tissue Processing
Mice were perfused with phosphate-buffered saline (PBS) (pH 7.4), followed by ice cold 4% paraformaldehyde (PFA) fixation. Tissues were treated of graded cryoprotection in 10%, 20%, and 30% sucrose prepared in PBS. 8–16 μm tissue sections were cut coronally on a Leica CM1850 microtome-cryostat, and sections were stored at −20ºC until use.
Cell Culture and transfection
Primary neuroglial cultures were isolated from the brains of embryonic day 17 Sprague Dawley rats (Wilcox et al, 1994). Dissociated cells in suspensions were plated on poly-L-lysine coated coverslips or plates and the cultures are maintained in neurobasal media with B27 supplement [Invitrogen] at 37°C with 5% CO2. Transfections of primary rat hippocampal neurons were performed using lipofectamine 2000 [Invitrogen] at 10 days in vitro (DIV). Transfection mixture was added to the cells for 2 hours and subsequently replaced with the original, conditioned media. Cells were then fixed 4 days post-transfection using 4% PFA.
Synaptosome fractionation
Synaptic proteins from 21 DIV primary hippocampal cultures were isolated using the Syn-PER extraction buffer according to manufacturer protocol [Pierce]. Synaptoneurosomes were isolated from mouse brains according to Villasana et al, 2006 with minor modifications. Presynaptic and postsynaptic fractions were isolated according to Gurd et al. 1974., Carlin et al, 1980.
Antibodies and Reagents
The following antibodies were purchased from the indicated vendors: E2F1 KH20 (sc-56662); E2F1 KH95 (sc-251); GAPDH (sc-32233) [Santa Cruz], Doublecortin (AB2253); vGluT1 (AB5905); Tyrosine hydroxylase (AB152); PSD-95 (MAB1596) [Millipore], OMP (#544-10001) [Wako]. NMDAR1 (#5704); NMDAR2A (#4205); NMDAR2B (#4212); Synapsin (#5297); GluR2 (#2460); ERK1/2 (#4695); E2F1 (#3742); Lamin A/C (#2032); α-tubulin (#2125) [Cell Signaling], Synaptophysin (ab8049); MAP2 (ab5392) [Abcam], actin (A2066) [Sigma]; SV2 [DSHB]. The following chemical reagents were used from the indicated vendors: DAPI [Molecular Probes]; Coomassie (161-0786); Protein assay dye (500-0005), PVDF membrane [BioRad], Fast Green FCF; protease inhibitor cocktail [Sigma], Pageruler plus protein ladder [Thermos], Luminata Forte Western HRP substrate [Millipore]. All HRP conjugated secondary antibodies were obtained from Pierce and all dye conjugated secondary antibodies were obtained from Jackson Immuno-Research.
Immunoblotting
Tissues were harvested from single animals and not pooled. Cultured cells were homogenized in ice cold, whole cell lysis buffer containing 50 mM Tris, 120 mM NaCl, 0.5% NP-40, 0.4mM sodium orthovanadate and protease inhibitor cocktail. Protein concentrations were determined using the Bradford method. Equal amounts of proteins (2–10 ug from cells and 20–50 ug from tissue) were loaded for immunoblotting and confirmed by staining the gel with Coomassie and the membrane with Fast Green. For densitometric analysis, autographs were scanned and cropped using Adobe Photoshop (Adobe Systems). Pixel intensities of each bands of interest were quantified using Image J software (NIH) and normalized to gel Coomassie stain. Immunoblots shown are representative of three independent biological replicates.
Immunostaining
Glass slides containing frozen tissue sections (~8–16μm per section) were baked rehydrated, and treated with antigen retrieval solution. Tissues were blocked in 10% normal goat serum and incubated with primary antibodies overnight. Slides were washed with PBS containing 0.1% Tween 20 and mounted in Citifluor for subsequent image acquisition. Cells grown on coverslips were fixed, permeabilized, and blocked at room temperature and incubated with primary antibodies overnight at 4°C and appropriate secondary 30 minutes at room temperature. The coverslips were mounted in Aqua-mount [Thermos]. Tyramide Signal Amplification system [Perkin Elmer] was used according to manufacturer instructions for PSD-95 and E2F1 signal amplification in tissue and endogenous E2F1 in cells (Wang et al, 2010).
Image acquisition and analysis
Images from samples were either captured at 400× or 600× on a laser confocal microscope with Biorad Radiance 2100 (Biorad), or 200× or 400× on a standard epifluorescent microscope (Nikon E400). Total E2F1, PSD-95 pixel intensity and MAP2 area in an image were quantified using Metamorph 6.0 (Universal Imaging) while total intensity of PSD-95 puncta and fractions of maximally saturated puncta were quantified using ImageJ.
Statistical analysis
All data were analyzed by either Prism 5.0 software (GraphPad Software). All data are expressed as mean ± SEM with values of p < 0.05 considered significant. Unless otherwise noted, all asterisks denote statistical significance by comparing the means of E2f1tm1 and WT within the same age group using the two-tailed student’s t-test.
Results
1) Age-dependent memory and olfactory deficits and elevated anxiety in the E2f1tm1 animals
To determine if E2F1 has a role in the CNS, we systematically characterized various behavioral functions in the E2f1tm1 mice. We first asked whether the mutant mice have a shorter lifespan since they have been shown to be more susceptible to spontaneous tumor formation. Indeed, E2f1tm1 mice display significantly shorter lifespans than their wildtype counterparts (Fig 1A).
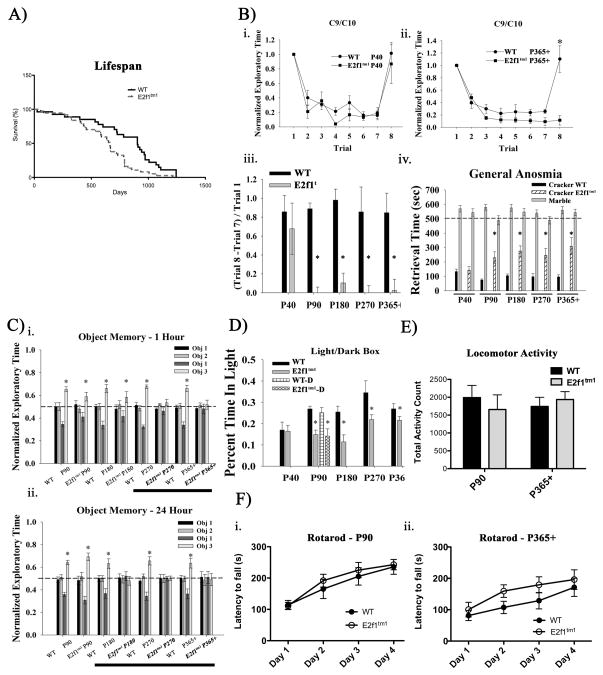
Figure 1.
Behavioral phenotyping of E2f1tm1 animals. A) Survival curve of E2f1tm1 mice compared to WT. The median lifespan of E2f1tm1 mice is approximately 25% less than that of WT (NWT=27, NTM=37, p<.01, log-rank and Gehan-Breslow-Wilcoxon tests). B) E2f1tm1 mice display age-dependent deficits in olfaction. (i–ii.) Odor habituation paradigms using ethyl heptanoate (C9)- ethyl caprylate (C10) ester odorant pairs on (i.) P40 and (iii.) P365+ postnatal age groups (N=7 per group.) Normalized exploratory time is represented as exploratory time in Trial 8/Trial 1. (iii.) Summary of normalized exploratory index of (Trial 8 – Trial 7)/Trial 1 across age groups (N=7 per group). (iv.) General anosmia test on all age groups reveal the similar age-dependent olfactory deficits (N=7 per group.) Black bars represent the WT retrieval time of scented cracker, hatched bars represent that of E2f1tm1, and gray bars represent that of both genotypes of unscented marble. C) E2f1tm1 mice display age-dependent deficits in memory. (i–ii.) Short-term 1-hour delay (i.) and long-term 24 hour delay (ii.) novel object recognition and memory task across all age groups (N=7 per group). * denotes statistical significance in the critical comparison made between the normalized exploratory time of object 3 (light gray bars) and that of object 1 (dark gray bars). Bolded text on the x-axis denotes the age groups of when E2f1tm1 mice failed the task. D) E2f1tm1 mice display age-dependent elevated anxiety. Light/dark box paradigm (N=7–9 per group) show that E2f1tm1 (gray bars) spend less time in the light chamber compared to the WT (black bars) at age P90 and greater. Hatched bars at P90 represent results from mice of both genotypes obtained directly from Jackson laboratory and revealed similar results as mice bred in our colony. E) E2f1tm1 mice have comparable basal activity level as WT. Total beam-break counts in activity chamber of WT (black bars) and E2f1tm1 (gray bars) on two representative age groups P90 and P365+ (P90 N=5, P365+ NWT=6 and NTM=8). F) E2f1tm1 mice do not exhibit any deficits in motor functioning. (i.–ii.) Latency to fall from the accelerating rotarod of WT (closed symbol) and E2f1tm1 (open symbol) on two representative age groups P90 (i.) and P365+ (ii.) (P90 N=5, P365+ NWT=6 and NTM=8 ). All data are represented as mean ± SEM (*, Student’s t-test; α ≤ 0.05).
Apparent aberrant expression of E2F1 has been observed in several neurodegenerative diseases including AD, PD, and HIVE (Jordan-Sciutto et al., 2002a; Jordan-Sciutto et al., 2002b; Hoglinger et al., 2007). Early clinical signs common to these neurodegenerative diseases include pronounced olfactory dysfunctions followed by their respective disease symptoms (Barresi et al., 2012; Doty, 2012). Therefore, we first investigated the olfactory function in the E2f1tm1 mice by subjecting them to an odor discrimination task in which they are exposed to two odorants differing by a single carbon atom (Fig 1B.a–c). We measured scent discrimination by the length of time that the novel scent is explored in the last trial, with longer times indicating that the animal is able to discriminate the novel odor from the habituated odor. When the mice were repeatedly exposed to the initial odor, C9, mice of both genotypes regardless of age were able to habituate to that odor comparably. Mice of both genotypes at age P40 were also able to discriminate between C9 and C10 as indicated by increased exploratory time when presented with the novel odor, C10 (Fig 1Bi.). However, E2f1tm1 mice from age P90 through P365+ lose the ability to discriminate between the two odors while the WT mice retain this ability (Fig 1Bii, 1Biii.). To verify that the olfactory deficit in the E2f1tm1 mice is not specific to ester odors, we subjected the animals to a more general anosmia task. In this task, a scented object (cracker) or unscented object (marble) was buried under the bedding and the time in which the animal retrieved these objects was quantified as a measure of olfactory function. Similar to the odor discrimination task, we observed a marked deficit in olfactory function in the E2f1tm1 mice starting at age P90 and persisting through P365+ (Fig 1Biv.). Both WT and E2f1tm1 mice retrieved the unscented object at comparable rates suggesting that the difference in performance is specific to olfaction and unrelated to basal exploratory activity.
To assess memory function, we subjected WT and E2f1tm1 mice to a novel object recognition task. Briefly, animals were first exposed to two different objects (objects 1 and 2) during the familiarization phase and the mice were subsequently removed. After a one hour (short-term) or 24 hour (long-term) delay, the animals were re-exposed to one of the two previous objects (object 1) and a novel third object (object 3) in the same position. Since mice typically explore objects that they deem as novel, increased exploration of object 3 is indicative of normal recognition memory function as animals retained information regarding object 1 and 2 and thus recognizing object 3 as novel. As expected, we found that E2f1tm1 and WT mice showed no bias towards object 1 or 2. However, while WT of all age groups explored the novel object more in the test phase of both short- and long-term paradigms, E2f1tm1 mice failed the short-term novel paradigm starting at age P270 and the more difficult long-term paradigm starting at age P180 as indicated by their equal exploration time of familiar and novel objects (Fig 1Ci, 2Cii.). Importantly, the deficits of the E2f1tm1 mice in these paradigms are specific to memory since E2f1tm1 mice at P180 can still distinguish between novel object and familiar object after 1 hour delay; however, they fail the task the delay period is increased to 24 hours.
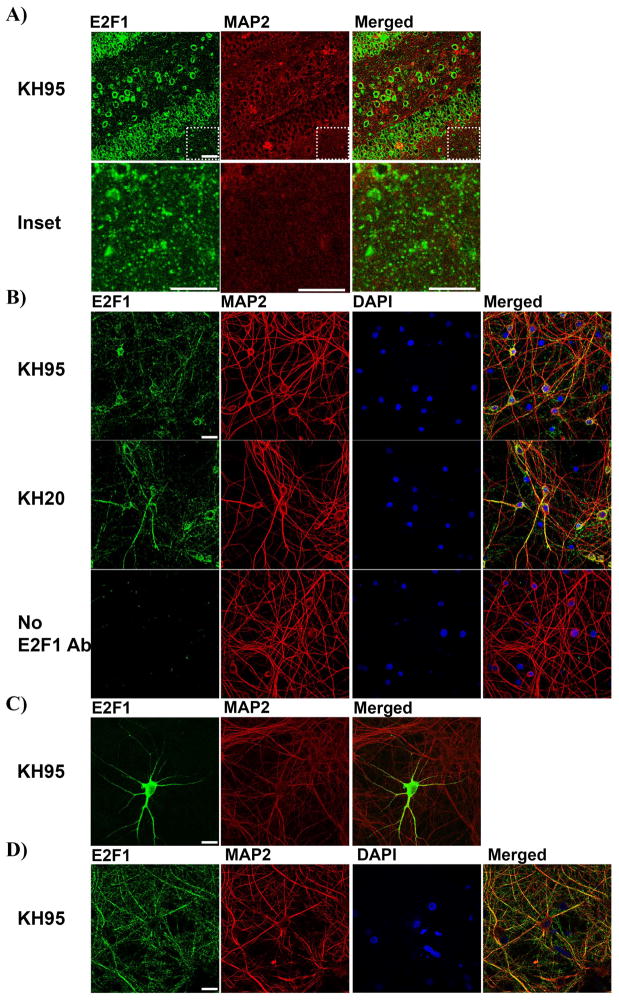
Figure 2.
E2F1 is predominantly cytoplasmic in vivo and in vitro. A) Coronal hippocampal sections from WT P365+ mice were immunolabeled with E2F1 in green and neuronal dendritic marker MAP2 in red (Top). Higher magnification images of the inset areas reveal E2F1 staining can be punctated where neuritic processes are abundant and the cell bodies are absent (Bottom). B) Primary hippocampal neurons at 14 DIV were labeled with E2F1 in green, MAP2 in red, and the nuclei counterstained with DAPI in blue. Two specific E2F1 antibodies KH95 (Top) and KH20 (Middle) were used and produced similar staining patterns. Condition with no E2F1 primary antibodies was included as negative control (Bottom). C) Exogenous E2F1 expression is predominantly cytoplasmic when overexpressed in primary hippocampal neurons. Primary hippocampal neurons were transfected with E2F1 plasmid at 10DIV, fixed at 14 DIV and labeled with E2F1 in green and MAP2 in red. D) Primary cortical neurons at 21 DIV were fixed and labeled with E2F1 in green, MAP2 in red, and nuclei counterstained with DAPI in blue. Scale bar=30μm.
We have also consistently observed that E2f1tm1 mice display increased digging behaviors. Since digging behaviors can be correlated to anxiety, we assayed the anxiety levels of E2f1tm1 mice using the light/dark box paradigm. In this test, reduction in time spent in the chamber with light is an indicator of increased anxiety as mice prefer the dark (Marks et al., 2009). We found that E2f1tm1 mice spent significantly less time in the light chamber of the box compared to the WT starting at age P90 (Fig 1D). To verify that these findings were not due our breeding or housing, we repeated the light/dark box test with P90 WT and E2f1tm1 mice purchased directly from Jackson Laboratories and obtained similar results.
Finally we examined “home cage” activity in the E2f1tm1 mice as a measurement of basal locomotor and the accelerating rotarod test for defects in motor performance. We found that mice of both genotypes from ages P90 and P365+ exhibited a comparable basal locomotor activity level (Fig 1E). Furthermore, we found no significant difference between the genotypes in either the P90 and P365+ age groups on the accelerating rotarod (Fig 1Fi.–ii.). Together, these data suggest that the disruption to E2f1 gene leads to age-dependent reduced performance in olfactory, memory, and anxiety test paradigms but no changes were seen in basal motor activity or a motor related task.
2) E2F1 exhibits cytoplasmic and punctated staining in vivo and in vitro
In order to gain further insight into how the disruption of the E2F1 may lead to these pronounced age-dependent behavioral changes, we examined the localization of E2F1 protein in neurons in the HC. Interestingly, E2F1 expression in hippocampal tissue is predominantly cytoplasmic and exhibits localized puncta where synaptic innervations are formed (Fig 2A). To investigate this localization more closely in vitro, primary rat hippocampal cultures were grown to 21 DIV and immunostained with either a C-terminal E2F1 antibody (KH95) or an N-terminal antibody (KH20) or no primary antibody as a negative control (Fig 2B). Immunostaining with either KH95 or KH20 revealed a cytoplasmic punctated staining for E2F1 that co-localized with neuronal cytoplasmic marker MAP2 but not nuclear counter-stain DAPI, consistent with what we observed in vivo and previous observations in cortical neurons (Strachan et al 2007, Wang 2010, Jordan-sciutto 2002b). Next we examined the subcellular localization of exogenous E2F1 protein by overexpressing E2F1 in hippocampal neurons and immunostained for E2F1. Using the same KH95 E2F1 antibody without signal amplification, we found that exogenously expressed E2F1 is predominantly cytoplasmic as E2F1 signal is found in neuritic processes (Fig 2C). To determine whether the cytoplasmic localization of E2F1 is specific to neuronal subtype, we also investigated E2F1 localization in primary cortical neurons (Fig 2D). As expected, E2F1 was again predominantly cytoplasmic and exhibiting punctated staining throughout the neuritic processes. Furthermore, MAP2 negative E2F1 expression can be found to co-localize with axonal marker staining growth associated protein 43 suggesting that axonal E2F1 is also abundant in these primary cultures. While these findings confirm previous observations that E2F1 is predominantly cytoplasmic in primary neurons, the presence of E2F1 puncta along the MAP2 positive dendrites suggests it may also localize to the synapse.
3) E2F1 is enriched in synaptic fractions
Because of its intriguing punctated staining pattern, we hypothesized that E2F1 may be associated with the synapses in neurons. We first co-immunostained E2F1 and synaptic marker PSD-95 in primary hippocampal cultures and found that E2F1 can be found to co-localize or be adjacent to the PSD-95 puncta (Fig 3A). However, because E2F1 staining is distributed throughout the neuritic processes, E2F1 staining does not exclusively co-localize with the PSD-95 puncta. Therefore, to determine if E2F1 is enriched in the synapses, we isolated synaptosomes from 21 DIV hippocampal cells and immunoblotted for E2F1 expression (Fig 3B). E2F1, along with two synaptic markers PSD-95 and synapsin are significantly enriched in the synaptosomes isolated from these cultures. To determine if E2F1 is also enriched in the synaptic protein-rich fractions in vivo, we isolated synaptoneurosomes from both cortex and HC using size fractionation. E2F1 as well as PSD-95 and vesicular glutamate transporter (vGluT1) were all enriched in the synaptoneurosomes isolated from both cortex and HC of adult mice (Fig. 3C).
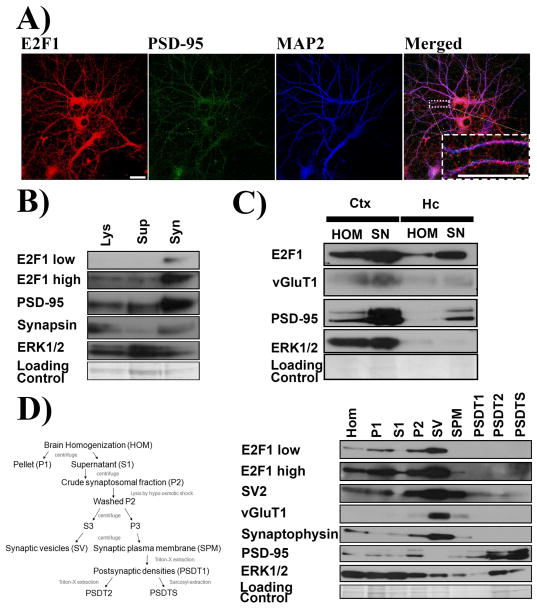
Figure 3.
E2F1 is enriched in the synaptic fractions. A) E2F1 puncta can colocalize with synaptic marker PSD-95. Primary hippocampal neurons at 21 DIV were immunostained with E2F1, PSD-95, and MAP2. Higher magnification of boxed neuritic process is shown as inset. B) E2F1 is enriched in the crude synaptosome isolated from primary hippocampal neuron at 21 DIV. Cell lysates (Lys) were collected using synaptic protein extraction reagent and centrifuged to yield the soluable fraction (Sup) and the synaptosomes (Syn). Enriched synaptic markers PSD-95 and Synapsin were used to validate the isolation of synaptosomes. C) E2F1 is enriched in the synaptoneurosomes isolated from adult mouse cortex and hippocampus. Cortical (Ctx) and hippocampal (Hc) tissues were homogenized (HOM) and the synaptoneurosomes (SN) were isolated. Enriched synaptic markers PSD-95 and vGluT1 were used to validate the isolation of synaptoneurosomes D) E2F1 is enriched in the presynaptic fractions. Pre- and post-synaptic fractions were isolated according to the schematic (Left). Postsynaptic markers PSD-95 was enriched in PSDT1, PSDT2, and PSDT3 whereas presynaptic markers SV2, vGluT1, and synaptophysin were enriched in the SV. All of the synaptic markers were enriched in the crude synaptosomes fraction. Immunoblots for ERK 1/2 are shown in each fractionation experiments as non-synaptic protein loading control. Scale bar=30μm.
Finally, to determine whether E2F1 is enriched presynaptically or postsynaptically, we fractionated brain specimens into synaptic vesicle enriched presynaptic fractions and postsynaptic densities enriched postsynaptic fractions and subsequently immunoblotted for E2F1 expression. As shown in figure 3D, E2F1 was enriched in the synaptosomal P2 fraction along with various synaptic markers. However, E2F1 is noticeably absent in the postsynaptic fractions PSDT1, PSDT2, and PSDTS despite enrichment of PSD-95. On the other hand, E2F1 is significantly enriched in the presynaptic SV fraction along with presynaptic proteins SV2, vGluT1, and synaptophysin. Thus, we observed that E2F1 is closely associated with synapses and particularly abundant in the presynaptic fraction.
4) Age-dependent synaptic protein perturbations in the E2f1tm1 animals
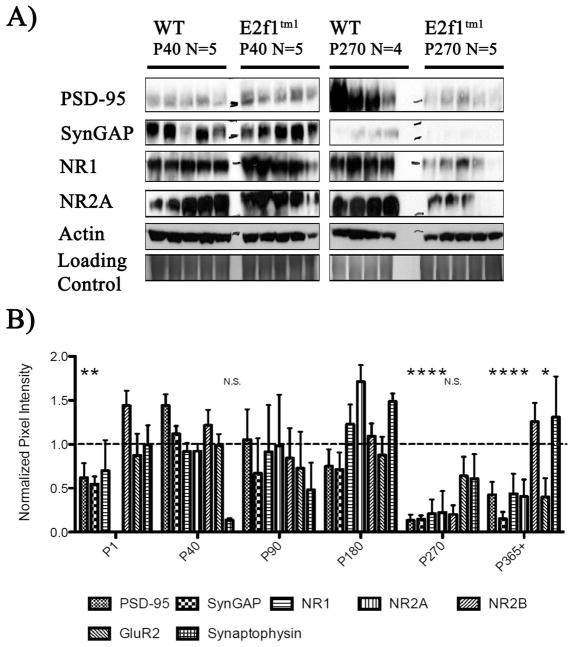
Because E2F1 is closely associated with synapses in vitro and in vivo, we hypothesized that the disruption of the E2f1 gene would disrupt the expression of synaptic proteins. As shown in figure 4A, the expression of a subset of synaptic proteins such as PSD-95, N-methyl-D-aspartate receptor 1(NMDAR1) and NMDA receptor subunit 2A (NR2A), Synaptic Ras GTPase activating protein (SynGAP) were reduced at P270 but not P40 in the E2f1tm1 mice. A closer examination across all age groups revealed that reduced expression of these proteins in the E2f1tm1 mice is age-dependent, manifesting in the animals at P270 and P365+ (Fig 4B). On the other hand, the expression of other synaptic proteins such as synaptophysin and NMDA receptor subunit 2B are unchanged regardless of age. Furthermore, not all components of the synapse are affected similarly across age in the E2f1tm1 mice. While PSD-95 and its interacting protein SynGAP are reduced at P1, when the brain is still developing and rewiring its synaptic circuitry, NMDAR1 and glutamate receptor subunit 2 (GluR2) are unchanged at this time. Thus, mice lacking functional E2F1 exhibit reduced expression of a subset of synaptic proteins during brain maturation as well as a more profound reduction of synaptic proteins in aged brains.
Figure 4.
Age-dependent synaptic protein perturbations in the E2f1tm1 animals. A) Immunoblots of various synaptic proteins PSD-95, synGAP, NR1, and NR2A in the brains of WT and E2f1tm1 mice from two representative age groups P40 and P270. Immunoblots for actin and Coomassie-stained gels are shown as loading controls. B) Quantification of the densitometry analysis of the expression of all tested synaptic proteins displayed as a ratio of the E2f1tm1 to the WT. There were significant reductions of PSD-95 and synGAP expression in P1, P270, and P365+, of NR1 and NR2A in P270 and P365+, and of GluR2 in P365+ in the E2f1tm1 mutants compared to the WT. All data are represented as mean ± SEM (*, Student’s t-test; α ≤ 0.05).
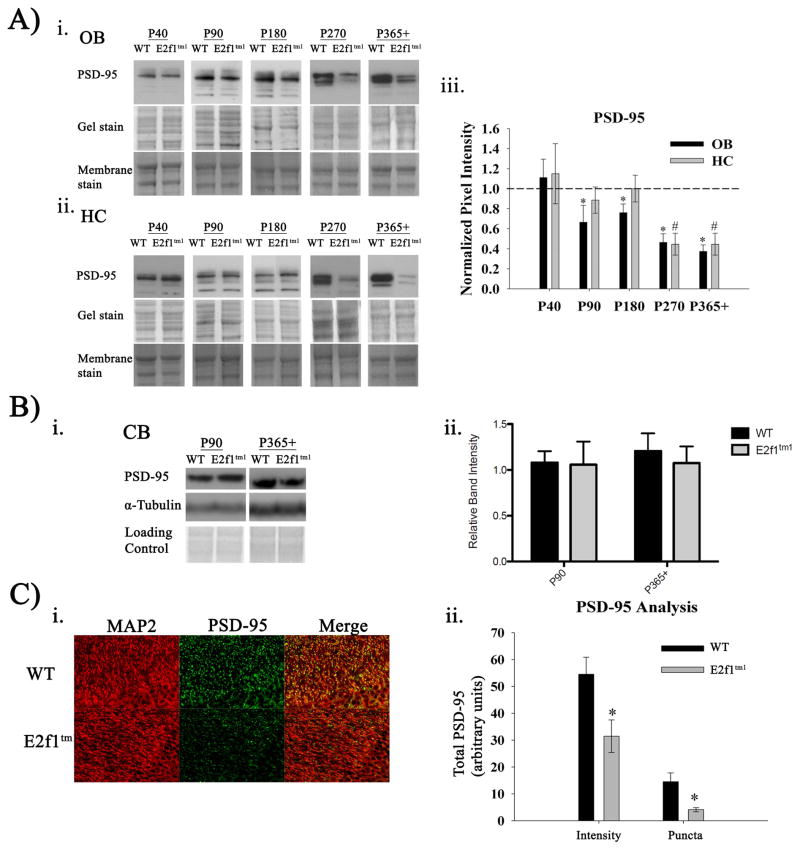
5) Age-dependent reduction of PSD-95 expression in hippocampus and olfactory bulbs
Since PSD-95 has been well documented for its crucial role in synapse maturation and connectivity (Kim & Sheng, 2004), we assessed changes in PSD-95 expression as a marker of synaptic disruption which we hypothesized would correlate with the age-dependent behavioral phenotype observed in the E2f1tm1 mice. Expression of PSD-95 was determined in the brain regions most relevant for the affected behaviors: OB for olfaction and HC for memory. As shown in figure 5A, the expression of PSD-95 in the OBs is significantly reduced in the E2f1tm1 mice when compared to the WT starting at age P90 and persisting through P365+. E2f1tm1 mice can have as much as 60% reduction in PSD-95 expression in the OB when the animals are as old as one year of age compared to the WT (Fig 5Aiii). Furthermore, the age at which the loss of PSD-95 in the OBs in the E2f1tm1 mice corresponds with the age in which the same animals exhibit olfactory deficits: P90–P365+. Interestingly, we observed no differences in the expression of olfactory marker protein or tyrosine hydroxylase in the E2f1tm1 mice suggesting that the reduction in PSD-95 is not due to overall protein loss (data not shown).
Figure 5.
Age-dependent reduction in PSD-95 expression in hippocampus and olfactory bulbs. A) Immunoblots of PSD-95 expression in OB (i.) and HC (ii.) across age groups. Coomassie-stained gels and fast green-stained membranes are shown as loading controls. (iii.) Quantification of the densitometry analysis displayed as a ratio of the E2f1tm1 to the WT. (N=6. *, OB; #, HC) B) (i.) Immunoblots of PSD-95 expression in cerebellum across two representative age groups P90 and P365+. (ii.) Quantification of the densitometry analysis (N=5 P90, N=6 P365+). C) (i.) Representative images of coronal WT and E2f1tm1 P365+ hippocampal sections immunolabeled with MAP2 (red) and PSD-95 (green) captured at 400x. (ii.) Quantification of the total PSD-95 pixel intensity and the intensity-saturated PSD-95 puncta in the WT and E2f1tm1 (N=3 for each genotype, 8 sections each, * Student’s t-test; α ≤ 0.05. All data are represented as mean ± SEM.
Since the hippocampal formation has been shown to be involved in object recognition memory (Broadbent et al., 2010)., we examined the expression of PSD-95 in this brain structure. We observed a reduction of PSD-95 levels starting at P270 and persisting through P365+ (Fig 5Aii.,5Aiii.). As predicted, the age of onset of reduced PSD-95 expression is at P270, which is the same age when the animals fail the 1-hour memory task. Of note, the expression of PSD-95 is unaltered in the cerebellum in P90 and P365+ (Fig 5Bi.–ii.). This lack of change resembles the absence of any significant impairment in the motor function related tasks. To verify the immunoblotting results, we immunostained hippocampal brain sections from WT and E2f1tm1 mice at P365+ for PSD-95 and dendritic marker MAP2 (Fig 5C). Both the total intensity level of PSD-95 staining and total number of PSD-95 puncta in the HC of E2f1tm1 mice are significantly reduced compared to that of the WT. Together, these data show that PSD-95 expression in E2f1tm1 mice is reduced in an age-dependent manner at ages that are coincident with the behavioral deficits.
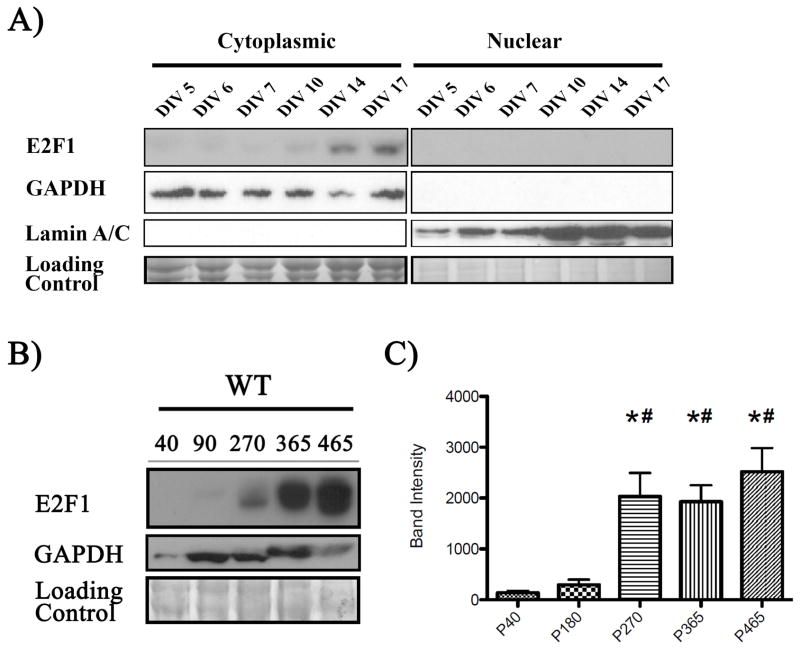
6) E2F1 expression increases with age in vitro and in vivo
Since the synaptic and behavioral phenotypes in the E2f1tm1 mice were age-dependent, it is possible that these effects were not due to E2F1 disruption but instead due to an indirect defect in CNS development. Previous research has characterized the neuroanatomy of the adult E2f1tm1 mice and found no changes in neocortical anatomy (Cooperkuhn et al., 2002). Similarly, we did not detect any overt structural changes in the hippocampus or the olfactory bulbs suggesting that the age-dependent behavioral and biochemical perturbations are not due to abnormal development (data not shown).
Alternatively, we hypothesized that E2F1 is necessary in maturing neurons and adult brains and that its expression increases during neuronal maturation and through adulthood. Though E2F1 has been shown to be increased in adult CNS compared to embryonic brain, a complete age-dependent expression of E2F1 in the adult CNS has not been examined (Kusek et al., 2001). Therefore, we characterized the E2F1 expression profile in maturing neurons in vitro and adult brain in vivo. As shown in figure 6A, neuronal E2F1 expression increases as hippocampal neurons mature from 1 to 14 DIV. The maturation of these hippocampal neurons is evident by the increasing elaboration of neurites marked by GAP43 staining. Furthermore, we also collected cytoplasmic and nuclear lysates by subcellular fractionation and found that E2F1 increases in the cytoplasmic fraction as cortical cells mature (Fig 6B). To determine if E2F1 expression also increases in the adult brain in vivo, we assayed for its expression in brain lysates collected from P40, P180, P270, P365, and P465 WT mice (Fig 6C). Similarly, E2F1 expression increases late into adulthood and peaks at P270 which is the onset of the synaptic disruptions in the E2f1tm1 mice. Taken together, our data show that the E2F1 expression increases in maturing neurons in vitro and in adult brain in vivo correlating with the age of onset of the synaptic and behavioral perturbation in the E2f1tm1 mice.
Figure 6.
E2F1 expression increases with age in vitro and in vivo. A) Cytoplasmic and nuclear lysates were collected from primary cortical cultures at various ages in vitro by subcellular fractionation. E2F1 expression elevates in the cytoplasmic fraction but is undetectable in the nuclear fraction. GAPDH serves as a cytoplasmic fraction marker while Lamin A/C serve as a nuclear fraction marker. B) Representative immunoblot of cortical lysates collected from postnatal age 40, 180, 270, 365 for E2F1 expression (left). Immunoblots for GAPDH and Coomassie-stained gels are shown as loading controls. C) Densitometry analysis of E2F1 reveals significant increase starting at P270 and persist until P465 (right, N=5 per group). *denotes p<.05 cmpared to P40, # denotes p<.05 compared to P180, one way-ANOVA Newman-Keuls post-hoc test.
7) Impairment of adult neurogenesis in the E2f1tm1 persists to 1 year of age
Deficits in adult neurogenesis have been linked to various defects in mouse behaviors (Zhao et al., 2008). Previous work using BrdU labeling to mark proliferating cells have demonstrated that disruption of the E2f1 in mice leads to a significant reduction proliferating cells in the dentate gyrus of the HC and the OB of 3 month old mice (Cooperkuhn et al., 2002). Here, we asked whether the deficits in the adult neurogenesis can persist in aged E2f1tm1 mice and thereby contribute to the synaptic and behavioral defects we observed. To assess adult neurogenesis, we measured the number of cells expressing doublecortin (DCX), a microtubule component used as a marker for newly divided immature neurons, in the OB and the HC of one-year-old mice (Fig 8A) (Rao & Shetty, 2004). In both the OB and the HC, E2f1tm1 mice had significantly reduced number of DCX-positive cells as compared with WT when the animals are one year of age (Fig 8B). The level of reduction of these immature cells is comparable to what was previously reported by BrdU labeling at 3 months. Our results indicate that the deficit in adult neurogenesis in the E2f1tm1 mice in the hippocampus and the olfactory bulbs persists to at least 1 year of age.
Discussion
E2F1 has long been linked to neurodegeneration due to its observed upregulation in post-mortem brains from various neurodegenerative diseases (Jordan-Sciutto et al., 2002a; Jordan-Sciutto et al., 2002b; Hoglinger et al., 2007)). Indeed, experimental manipulations that leads to the overactivity of the E2F1 in post-mitotic neurons results in significant increase of neuronal death in vitro (Giovanni et al., 2000; O’Hare et al., 2000). However, although E2F1 has been studied in the context of neuronal death and neurodegenerative diseases, study focusing on its physiologic role in the developed CNS is lacking. Here we report for the first time the consequence of disrupting E2f1 on the resulting behavior as well as on other biochemical changes that may accompany these behavioral deficits.
In the present study, we characterized several age-dependent behavioral deficits in mice with a disruption in the E2f1 gene. E2f1tm1 mice exhibited significant olfactory deficits and elevated anxiety as early as 3 months of age. Furthermore, memory deficits manifest when the animals were significantly older at 6 to 9 months. The behavioral deficits are not due to general impairment across all behavioral domains as E2f1tm1 mice show no impairment in two motor-related tasks. Additionally, we have corroborated published results that demonstrate that the neuroanatomical development in the E2f1tm1 mice are not disrupted (Cooperkuhn et al., 2002). Given that E2F1 expression is significantly elevated at around the age when the behavioral deficits are most prominent, it is plausible that the behavioral deficits are due to the absence of E2F1 activity in the E2F1tm1 mice instead of other confounds.
Furthermore, we observed that the deficits in adult neurogenesis in the E2F1tm1 mice persist into one year of age. Deficits in adult neurogenesis have long been linked to behavioral abnormalities (Zhao et al., 2008). Hippocampal dependent memory tasks as well as other learning tasks such as eye-blink conditioning, T maze performance, object recognition, and contextual fear conditioning are impaired following the ablation of adult neurogenesis (Leuner et al., 2006; Saxe et al., 2006; Winocur et al., 2006; Jessberger et al., 2009). Similarly, adult neurogenesis has also been shown to be involved in olfactory physiology (Gheusi et al., 2000). Additionally, enriched odor exposure increased OB adult neurogenesis, which is presumably linked to increased olfactory function (Rochefort et al., 2002). Likewise, adult neurogenesis is also strongly involved in anxiety-related behaviors (Vaidya et al., 2007; Revest et al., 2009). Therefore, it is possible that the deficits in adult neurogenesis can partially explain the age-dependent memory and olfactory deficits as well as heightened levels of anxiety that we observed in the E2f1tm1 mice.
The age-dependent synaptic perturbations as evidenced by reduced PSD-95 expression in the E2f1tm1 mice mice can also explain the behavioral deficits. PSD-95 has been shown to be one of the crucial scaffolding proteins and complexes with other synaptic proteins and receptors including SynGAP, NMDAR1, NMDAR2A/B, and GluR2 (Lin et al., 2004; Dosemeci et al., 2007; Sheng & Hoogenraad, 2007). Given its importance in synaptic physiology, behavioral impairments are often accompanied by changes to PSD-95 expression (Sun et al., 2009; Wakade et al., 2010). Training in object-place recognition led to a rapid induction of PSD-95 expression while mice lacking PSD-95 failed to learn simple associations (Soule et al., 2008; Nithianantharajah et al., 2013). Likewise, changes to SynGAP expression also result in significant behavioral impairment as heterozygous deletion of SynGAP leads to deficits in fear conditioning, working memory and reference memory (Guo et al., 2009; Muhia et al., 2010). Consistent with these studies, the age-dependent deficits in memory and olfaction are accompanied by corresponding age-dependent reduction in PSD-95 expression in the HC and OB. The changes to PSD-95 expression as well as other synaptic proteins may have a profound impact on synaptic physiology in the E2f1tm1 mice and thereby contribute to the behavioral deficits.
Although the behavioral deficits E2f1tm1 mice do not manifest until they are at least 3 months, reduction of PSD-95 and SynGAP expression can be as observed as early as P1 when synaptic innervations are formed and pruned (Fig 4B). Interestingly, E2F1 expression in vitro increases as the neurons mature and synaptogenesis takes place (Fig 6). These results may suggest that E2F1 may also be necessary during synaptic development when the synapses form and mature. Additional experiments with more direct temporal control in regulating E2F1 expression are necessary to more precisely define the role of E2F1 in synapse formation.
One of the more intriguing findings of the present study is the subcellular localization of E2F1 in neurons namely, the predominant cytoplasmic localization of E2F1 and its abundance in the synaptic fractions in vitro and in vivo. Using a subcellular fractionation approach, we have found that E2F1 is overwhelmingly associated with the GAPDH-positive, Lamin A/C negative cytoplasmic fraction (Fig 6B). E2F1 is not the only transcription factor operating outside the nucleus in neurons as another transcription factor Elk-1 also localizes to neuritic processes and can bind to the mitochondrial permeability transition pore complex to induce neuronal apoptosis (Barrett et al., 2006). However, we speculate that E2F1 is unlikely to be exclusively found in the cytoplasm as nuclear E2F1/DP1 complexes have been observed in neurons and proposed to be responsible for the unintended activation of cell cycle machinery in post-mitotic neurons (Zhang et al., 2010; Zhang & Herrup, 2011). Using a more sensitive luciferase reporter assay, E2F1 transcriptional activity in neurons has been described in various neuronal death models, though contribution by other E2F family members could not be excluded by this method (Jiang et al., 2007; Shimizu et al., 2007; Hou et al., 2013). Studies that implicate E2F1 in neuronal apoptosis through its transcriptional activity have primarily utilized cerebellar granule neurons, suggesting that E2F1-mediated effects may vary in different neuronal population (O’Hare et al., 2000). Our data from the E2F1tm1 mice similarly suggest that the role of E2F1 may be distinct in different neuronal population as PSD-95 expression in cerebellum is unchanged despite robust reductions of PSD-95 expression in the HC and the OB.
Given that E2F1 is a transcription factor that regulates cell cycle progression, its synaptic enrichment, particularly in the presynaptic terminal, is quite surprising. However, E2F1 is not the only cell cycle protein found in the synapse. Components of the origin recognition complex, known to initiate DNA replication in the nucleus, are also enriched in the synapse and their depletion leads to reduced dendritic branching and dendritic spine morphogenesis (Huang et al., 2005). A neuronal co-activator of CDK5 has also been found in the synapse and its regulation of CDK5 activity has been shown to be crucial for synaptic physiology (Humbert et al., 2000; Morabito et al., 2004). Other transcriptional factors/co-factors such as NF-kappa B, STAT3, and CREB2 can be found in the presynaptic terminal of the synapse and subsequently translocate to the nucleus (Riccio et al., 1997; Meffert et al., 2003; Lai et al., 2008; Ben-Yaakov et al., 2012; Jung et al., 2012). We speculate that E2F1 may also be present in the synapse and upon stimulation, translocate into the nucleus and regulate its target genes. Alternatively, E2F1 function may be mediated through direct protein to protein interaction in the synapse. E2F1 interaction with NPDC-1 which co-localizes with synaptic vesicle markers in the synapse is one potential target (Sansal et al., 2000; Evrard & Rouget, 2005). We have also identified a putative SH3 motif in E2F1 protein sequence that may be important for synaptic protein interactions. Further investigations of the function of E2F1 in the synaptic compartment as well as its potential interacting partners are warranted to understand the role of such localization in neurons.
In the present study, we demonstrate for the first time the behavioral consequence of disrupting the E2F1 gene and report age-dependent deficits in memory and olfaction that correlate with changes in expression of PSD-95 and other components of the synapse. Future investigations to elucidate the role of E2F1 at the synapse and the precise mechanism of regulating the expression of PSD-95 are warranted to gain a broader understanding of the role E2F1 plays in normal neurons and in diseases associated with synaptic damage and loss during aging.
Figure 7.
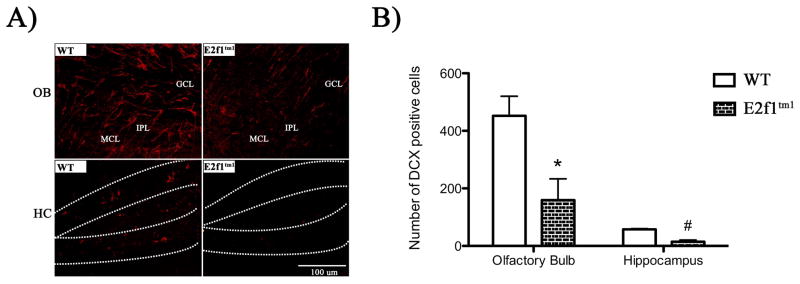
E2f1tm1 mice display a significant reduction in the number of doublecortin-positive cells in the OB and dentate gyrus of HC. A) Coronal OB and HC sections from WT and E2f1tm1 P365+ animals immunolabeled with DCX in red. In HC sections, the dentate gyrus is outlined with dashed lines. B) Quantification reveals a strong reduction in the number of newly generated DCX-positive neurons. All data are represented as mean ± SEM. * Student’s t-test; α ≤ 0.05.
Acknowledgments
We thank Dr. M. Hruska, Dr. S. Scheffler-Colins, Dr. L. Briand, IT. Wang, and M. Spadola in their assistance with several experimental protocols. We also thank Dr. M. Dalva, Dr. J. Eberwine, and Dr. D. Lynch for their vital input for the experimental design and the preparation of the manuscript. We are also grateful to Margaret Maronski for her help in the preparation of primary rat neuronal cultures. This work was supported by National Institute of Health.
This work was funded by National Institute of Health (grant number RO1 NS041202; 1F31NS071787; T32GM007517; T32AI007632)
Abbreviations
- DCX
doublecortin
- DIV
days in vitro GluR2, glutamate receptor subunit 2
- HC
hippocampus
- MAP2
microtubule associated protein-2
- NMDAR1
N-methyl-D-aspartate receptor-1
- NR2A
NMDA receptor subunit 2A
- NR2B
NMDA receptor subunit 2B
- OB
olfactory bulbs
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- PSD-95
postsynaptic density protein 95
- SynGAP
synaptic ras GTPase activating protein
- vGluT1
vesicular glutamate transporter-1
Footnotes
Conflicts of interest:
“The authors have no conflict of interest to declare.”
References
- Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, Bramanti P, Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. Journal of the neurological sciences. 2012;323:16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Barrett LE, Sul JY, Takano H, Van Bockstaele EJ, Haydon PG, Eberwine JH. Region-directed phototransfection reveals the functional significance of a dendritically synthesized transcription factor. Nature methods. 2006;3:455–460. doi: 10.1038/nmeth885. [DOI] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. The EMBO journal. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learning & memory. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperkuhn C, Vroemen M, Brown J, Ye H, Thompson M, Winkler J, Kuhn H. Impaired Adult Neurogenesis in Mice Lacking the Transcription Factor E2F1. Molecular and Cellular Neuroscience. 2002;21:312–323. doi: 10.1006/mcne.2002.1176. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Dagnino L, Fry CJ, Bartley SM, Farnham P, Gallie BL, Phillips RA. Expression patterns of the E2F family of transcription factors during mouse nervous system development. Mechanisms of development. 1997;66:13–25. doi: 10.1016/s0925-4773(97)00083-x. [DOI] [PubMed] [Google Scholar]
- Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP. Composition of the synaptic PSD-95 complex. Molecular & cellular proteomics : MCP. 2007;6:1749–1760. doi: 10.1074/mcp.M700040-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in Parkinson disease. Nature reviews Neurology. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Evrard C, Rouget P. Subcellular localization of neural-specific NPDC-1 protein. Journal of neuroscience research. 2005;79:747–755. doi: 10.1002/jnr.20405. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 2004;41:389–404. doi: 10.1016/s0896-6273(03)00844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RM, Hamel PA. Subcellular compartmentalization of E2F family members is required for maintenance of the postmitotic state in terminally differentiated muscle. The Journal of cell biology. 2000;148:1187–1201. doi: 10.1083/jcb.148.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanni A, Keramaris E, Morris EJ, Hou ST, O’Hare M, Dyson N, Robertson GS, Slack RS, Park DS. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. The Journal of biological chemistry. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- Giovanni A, Wirtz-Brugger F, Keramaris E, Slack R, Park DS. Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F x DP, in B-amyloid-induced neuronal death. The Journal of biological chemistry. 1999;274:19011–19016. doi: 10.1074/jbc.274.27.19011. [DOI] [PubMed] [Google Scholar]
- Guo X, Hamilton PJ, Reish NJ, Sweatt JD, Miller CA, Rumbaugh G. Reduced expression of the NMDA receptor-interacting protein SynGAP causes behavioral abnormalities that model symptoms of Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1659–1672. doi: 10.1038/npp.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Breunig JJ, Depboylu C, Rouaux C, Michel PP, Alvarez-Fischer D, Boutillier AL, Degregori J, Oertel WH, Rakic P, Hirsch EC, Hunot S. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3585–3590. doi: 10.1073/pnas.0611671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou ST, Jiang SX, Aylsworth A, Cooke M, Zhou L. Collapsin response mediator protein 3 deacetylates histone H4 to mediate nuclear condensation and neuronal death. Scientific reports. 2013;3:1350. doi: 10.1038/srep01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zang K, Reichardt LF. The origin recognition core complex regulates dendrite and spine development in postmitotic neurons. The Journal of cell biology. 2005;170:527–535. doi: 10.1083/jcb.200505075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert S, Lanier LM, Tsai LH. Synaptic localization of p39, a neuronal activator of cdk5. Neuroreport. 2000;11:2213–2216. doi: 10.1097/00001756-200007140-00030. [DOI] [PubMed] [Google Scholar]
- Iaquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. Current opinion in cell biology. 2007;19:649–657. doi: 10.1016/j.ceb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova IA, Dagnino L. Activation of p38- and CRM1-dependent nuclear export promotes E2F1 degradation during keratinocyte differentiation. Oncogene. 2007;26:1147–1154. doi: 10.1038/sj.onc.1209894. [DOI] [PubMed] [Google Scholar]
- Ivanova IA, Vespa A, Dagnino L. A novel mechanism of E2F1 regulation via nucleocytoplasmic shuttling: determinants of nuclear import and export. Cell cycle (Georgetown, Tex) 2007;6:2186–2195. doi: 10.4161/cc.6.17.4650. [DOI] [PubMed] [Google Scholar]
- Jeon D, Yang YM, Jeong MJ, Philipson KD, Rhim H, Shin HS. Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron. 2003;38:965–976. doi: 10.1016/s0896-6273(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning & memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SX, Sheldrick M, Desbois A, Slinn J, Hou ST. Neuropilin-1 is a direct target of the transcription factor E2F1 during cerebral ischemia-induced neuronal death in vivo. Mol Cell Biol. 2007;27:1696–1705. doi: 10.1128/MCB.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DG, Degregori J. Putting the Oncogenic and Tumor Suppressive Activities of E2F into Context. Current molecular medicine. 2006;6:731–738. doi: 10.2174/1566524010606070731. [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim CL. Expression patterns of retinoblastoma protein in Parkinson disease. Journal of neuropathology and experimental neurology. 2003;62:68–74. doi: 10.1093/jnen/62.1.68. [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Malaiyandi LM, Bowser R. Altered distribution of cell cycle transcriptional regulators during Alzheimer disease. Journal of neuropathology and experimental neurology. 2002a;61:358–367. doi: 10.1093/jnen/61.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002b;22:2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nature reviews Neuroscience. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nature reviews Neuroscience. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kusek JC, Greene RM, Pisano MM. Expression of the E2F and retinoblastoma families of proteins during neural differentiation. Brain research bulletin. 2001;54:187–198. doi: 10.1016/s0361-9230(00)00447-0. [DOI] [PubMed] [Google Scholar]
- Lai KO, Zhao Y, Ch’ng TH, Martin KC. Importin-mediated retrograde transport of CREB2 from distal processes to the nucleus in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17175–17180. doi: 10.1073/pnas.0803906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler S, Pacchioni A, Degnan R, Homan Y, Conti AC, Kalivas P, Blendy JA. Requirement for the POZ/BTB protein NAC1 in acute but not chronic psychomotor stimulant response. Behavioural brain research. 2008;187:48–55. doi: 10.1016/j.bbr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6734–6751. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nature neuroscience. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhia M, Yee BK, Feldon J, Markopoulos F, Knuesel I. Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. The European journal of neuroscience. 2010;31:529–543. doi: 10.1111/j.1460-9568.2010.07079.x. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Komiyama NH, McKechanie A, Johnstone M, Blackwood DH, St Clair D, Emes RD, van de Lagemaat LN, Saksida LM, Bussey TJ, Grant SG. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nature neuroscience. 2013;16:16–24. doi: 10.1038/nn.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare MJ, Hou ST, Morris EJ, Cregan SP, Xu Q, Slack RS, Park DS. Induction and modulation of cerebellar granule neuron death by E2F-1. The Journal of biological chemistry. 2000;275:25358–25364. doi: 10.1074/jbc.M001725200. [DOI] [PubMed] [Google Scholar]
- Park DS, Morris EJ, Bremner R, Keramaris E, Padmanabhan J, Rosenbaum M, Shelanski ML, Geller HM, Greene LA. Involvement of retinoblastoma family members and E2F/DP complexes in the death of neurons evoked by DNA damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000a;20:3104–3114. doi: 10.1523/JNEUROSCI.20-09-03104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Morris EJ, Greene LA, Geller HM. G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases suppress camptothecin-induced neuronal apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:1256–1270. doi: 10.1523/JNEUROSCI.17-04-01256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Obeidat A, Giovanni A, Greene LA. Cell cycle regulators in neuronal death evoked by excitotoxic stress: implications for neurodegeneration and its treatment. Neurobiology of aging. 2000b;21:771–781. doi: 10.1016/s0197-4580(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Pelegri C, Duran-Vilaregut J, del Valle J, Crespo-Biel N, Ferrer I, Pallas M, Camins A, Vilaplana J. Cell cycle activation in striatal neurons from Huntington’s disease patients and rats treated with 3-nitropropionic acid. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26:665–671. doi: 10.1016/j.ijdevneu.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. The European journal of neuroscience. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Molecular psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansal I, Dupont E, Toru D, Evrard C, Rouget P. NPDC-1, a regulator of neural cell proliferation and differentiation, interacts with E2F-1, reduces its binding to DNA and modulates its transcriptional activity. Oncogene. 2000;19:5000–5009. doi: 10.1038/sj.onc.1203843. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annual review of biochemistry. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Khan MZ, Hippensteel RL, Parkar A, Raghupathi R, Meucci O. Role of the transcription factor E2F1 in CXCR4-mediated neurotoxicity and HIV neuropathology. Neurobiology of disease. 2007;25:17–26. doi: 10.1016/j.nbd.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule J, Penke Z, Kanhema T, Alme MN, Laroche S, Bramham CR. Object-place recognition learning triggers rapid induction of plasticity-related immediate early genes and synaptic proteins in the rat dentate gyrus. Neural plasticity. 2008;2008:269097. doi: 10.1155/2008/269097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan GD, Kopp AS, Koike MA, Morgan KL, Jordan-Sciutto KL. Chemokine- and neurotrophic factor-induced changes in E2F1 localization and phosphorylation of the retinoblastoma susceptibility gene product (pRb) occur by distinct mechanisms in murine cortical cultures. Experimental neurology. 2005;193:455–468. doi: 10.1016/j.expneurol.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Duan RS, Wang AH, Shang W, Zhang T, Zhang XQ, Chi ZF. Alterations of NR2B and PSD-95 expression in hippocampus of kainic acid-exposed rats with behavioural deficits. Behavioural brain research. 2009;201:292–299. doi: 10.1016/j.bbr.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Paulson KE, Bronson R, Yee AS. Expression of the E2F-1/DP-1 transcription factor in murine development. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1996;7:43–52. [PubMed] [Google Scholar]
- Vaidya VA, Fernandes K, Jha S. Regulation of adult hippocampal neurogenesis: relevance to depression. Expert review of neurotherapeutics. 2007;7:853–864. doi: 10.1586/14737175.7.7.853. [DOI] [PubMed] [Google Scholar]
- Villasana LE, Klann E, Tejada-Simon MV. Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. Journal of neuroscience methods. 2006;158:30–36. doi: 10.1016/j.jneumeth.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade C, Sukumari-Ramesh S, Laird MD, Dhandapani KM, Vender JR. Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. Journal of neurosurgery. 2010;113:1195–1201. doi: 10.3171/2010.3.JNS091212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IT, Allen M, Goffin D, Zhu X, Fairless AH, Brodkin ES, Siegel SJ, Marsh ED, Blendy JA, Zhou Z. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21516–21521. doi: 10.1073/pnas.1216988110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang R, Herrup K. E2F1 works as a cell cycle suppressor in mature neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12555–12564. doi: 10.1523/JNEUROSCI.3681-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shyam N, Ting JH, Akay C, Lindl KA, Jordan-Sciutto KL. E2F1 localizes predominantly to neuronal cytoplasm and fails to induce expression of its transcriptional targets in human immunodeficiency virus-induced neuronal damage. Neuroscience letters. 2010;479:97–101. doi: 10.1016/j.neulet.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Zhang J, Herrup K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell cycle (Georgetown, Tex) 2011;10:1208–1214. doi: 10.4161/cc.10.8.15328. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]