Abstract
Background & aims
Chronic liver disease is characterized by fibrosis that may progress to cirrhosis. Nucleotide oligomerization domain 2 (Nod2), a member of the Nod-like receptor (NLR) family of intracellular immune receptors, plays an important role in the defense against bacterial infection through binding to the ligand muramyl dipeptide (MDP). Here, we investigated the role of Nod2 in the development of liver fibrosis.
Methods
We studied experimental cholestatic liver disease induced by bile duct ligation or toxic liver disease induced by carbon tetrachloride in wild type and Nod2−/− mice.
Results
Nod2 deficiency protected mice from cholestatic but not toxin-induced liver injury and fibrosis. Most notably, the hepatic bile acid concentration was lower in Nod2−/− mice than wild type mice following bile duct ligation for 3 weeks. In contrast to wild type mice, Nod2−/− mice had increased urinary excretion of bile acids, including sulfated bile acids, and an upregulation of the bile acid efflux transporters MRP2 and MRP4 in tubular epithelial cells of the kidney. MRP2 and MRP4 were downregulated by IL-1β in a Nod2 dependent fashion.
Conclusions
Our findings indicate that Nod2 deficiency protects mice from cholestatic liver injury and fibrosis through enhancing renal excretion of bile acids that in turn contributes to decreased concentration of bile acids in the hepatocyte.
Keywords: Chronic liver disease, bile acids transporter, IL-1β, renal tubular epithelial cells, bacterial translocation, microbiome
Introduction
Liver fibrosis results from chronic liver injury commonly caused by cholestasis, toxins, alcohol abuse, viral infections or non-alcoholic steatohepatitis (NASH). Hepatic fibrosis can progress to cirrhosis, which is associated with a significant morbidity and mortality. Effective anti-fibrotic therapies are still lacking. Therefore, elucidating the molecular mechanisms initiating and driving liver fibrosis is crucial for the development of anti-fibrotic strategies that should prevent this fatal disease [1–3].
Nucleotide oligomerization domain 2 (Nod2) belongs to the Nod-like receptor (NLR) family which consists of intracellular innate immune receptors for bacterial peptidoglycans. These immune receptors play a crucial role in the host response to bacterial infection [4, 5]. Muramyl dipeptide (MDP), the minimal motif of peptidoglycan from both Gram-positive and Gram-negative bacteria, was identified as the ligand of Nod2 [6]. MDP recognition by Nod2 activates transcription factor NF-κB and induce pro-inflammatory cytokine production by interacting with the RIP-like interacting CLARP kinase (RICK/RIP2) [7]. MDP stimulation also activates Nod2 to process and release mature interleukin (IL)-1β in a caspase-1-dependent fashion [8, 9]. Genetic studies in humans have linked mutations in the Nod2 gene to higher susceptibility to Crohn’s disease in a subset of patients [10]. Additionally, Nod2 plays an important role in intestinal microbial homeostasis [11], intestinal immunity [12] and gut barrier function [13, 14].
The role of Nod2 in liver fibrosis is not known. Using wild type and Nod2 deficient mice, the function of Nod2 in experimental cholestasis- or toxin-induced liver fibrosis was investigated.
Materials and Methods
Material and Methods are described in the Supplementary Data section.
Results
Nod2 deficiency protects mice from cholestasis-induced liver fibrosis
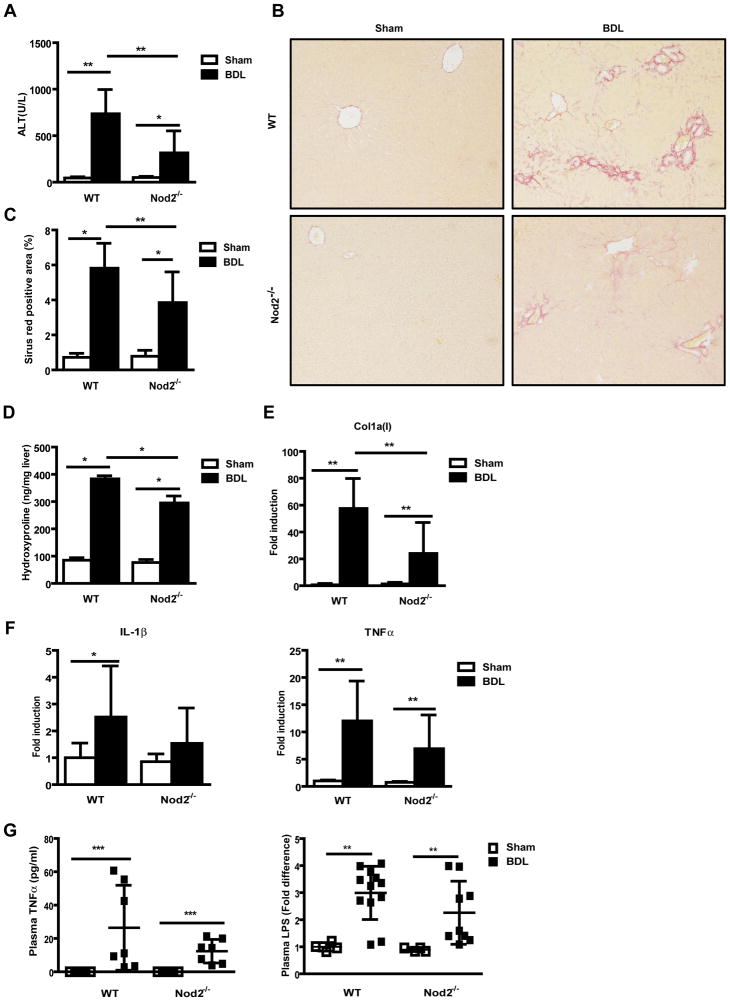
Wild type and Nod2 deficient mice were subjected to cholestatic liver injury induced by bile duct ligation. Following 3 weeks of bile duct ligation, liver injury as determined by ALT levels was dramatically lower in Nod2 deficient mice as compared with wild type mice (Fig. 1A). Plasma alkaline phosphatase (ALP) was also lower in Nod2−/− mice than wild type mice following bile duct ligation (Suppl. Fig. 1A). Liver weight and plasma bilirubin levels were not significantly different between wild type and Nod2−/− mice following bile duct ligation (Suppl. Fig. 1B and C). As a measurement of liver fibrosis, fibrillar collagen deposition was determined by Sirius red staining. Hepatic fibrosis was lower in Nod2−/− mice as compared with wild type mice after bile duct ligation (Fig. 1B). The lower level of Sirius red staining was confirmed by morphometric analysis (Fig. 1C) and hydroxyproline measurement (Fig. 1D). A similar reduction of collagen α1(I) mRNA expression was found in Nod2−/− mice as compared with wild type mice (Fig. 1E).
Fig. 1. Liver fibrosis is prevented in Nod2 deficient mice following 3 weeks of bile duct ligation.
Wild type and Nod2 deficient mice underwent sham operation (n=3–8 for wild type mice; n=3–7 for Nod2 deficient mice) or bile duct ligation (BDL; n=7–13 for wild type mice; n=7–9 for Nod2 deficient mice). (A) Plasma ALT levels. (B and C) Sirius red staining of liver sections are shown and quantitated by image analysis. (D) Hepatic hydroxyproline measurement. (E and F) Hepatic collagen α1(I), IL-1 β and TNFα gene expression. (G) Plasma TNFα and LPS levels. *p < 0.05; **p < 0.01; ***p < 0.001
As Nod2 induces pro-inflammatory cytokines [9], IL-1β and tumor necrosis factor (TNF)-α gene expression were measured. Nod2−/− mice showed a trend towards lower hepatic IL-1β and TNFα mRNA expression following bile duct ligation for 3 weeks compared with wild type mice (Fig. 1F). This trend, however, was not significant suggesting that attenuated fibrosis results from decreased liver damage rather than as a direct consequence of Nod2 deficiency on liver inflammation. Plasma IL-1β levels were below the detection limit of the ELISA in wild type or Nod2−/− mice after sham operation or bile duct ligation for 3 weeks (data not shown), while plasma TNFα was not significantly different between Nod2−/− and wild type mice (Fig. 1G). Since the amount of translocated bacterial products is dependent on the intestinal bacterial burden and since the progression of liver fibrosis is dependent on translocated bacterial products [15], bacterial overgrowth and translocation of microbial products from the gut lumen to the systemic circulation was investigated. There was no significant difference in bacterial overgrowth between wild type and Nod2−/− mice after bile duct ligation (data not shown). Plasma LPS levels were also not significantly different between wild type and Nod2−/− mice following bile duct ligation for 3 weeks (Fig. 1G). Taken together, Nod2 deficient mice are protected from bile duct ligation-induced liver injury and fibrosis which is not explained by a stabilized intestinal mucosal barrier.
Nod2 deficient mice are not protected from toxin-induced liver injury and fibrosis
To determine whether Nod2 deficiency suppresses hepatic fibrosis induced by a different etiology, toxic liver injury was induced by repeated intraperitoneal injections of carbon tetrachloride (CCl4). In contrast to cholestatic liver injury, carbon tetrachloride-induced liver injury was not different in Nod2−/− mice compared with wild type mice as evidenced by plasma ALT levels (Suppl. Fig. 2A). Similarly, hepatic collagen deposition (Suppl. Fig. 2B and C) and collagen α1 (I) mRNA expression (Suppl. Fig. 2D) showed no significant difference between wild type and Nod2−/− mice following carbon tetrachloride injections. Wild type and Nod2−/− mice subjected to carbon tetrachloride injections showed similar hepatic levels of the inflammatory cytokines IL-1β and TNFα as compared with wild type mice (Suppl. Fig. 2D). These and the preceding results indicate that Nod2 deficiency protects from cholestatic, but not toxin-induced liver fibrosis in mice.
Bile acid-induced hepatocyte death is uninfluenced by Nod2
The mode of hepatocyte death is one of the major differences between the cholestatic and toxic model of chronic liver disease. Following cholestasis, an accumulation of bile acids in hepatocytes contributes to cell death and is one of the major pathogenic factors resulting in chronic liver damage and fibrosis. In contrast, carbon tetrachloride is metabolized in hepatocytes to free radicals via the cytochrome p450 enzyme CYP2E1 causing immediate lipid peroxidation and hepatocyte death [16]. To explain the different hepatic phenotype between cholestatic and toxin-induced liver fibrosis in Nod2 deficient mice, we initially investigated whether Nod2 deficiency affects bile acid-induced hepatocyte death. Primary hepatocytes were isolated, treated with MDP control or MDP to stimulate Nod2, and hepatocyte death was induced with glycodeoxycholic acid (GDCA) [17]. Glycodeoxycholic acid induced significant death in cultured wild type and Nod2−/− hepatocytes uninfluenced by the presence of MDP as assessed by propidium iodide (PI) staining (Suppl. Fig. 3A). Quantification of glycodeoxycholic acid-induced cytotoxicity showed non-significant differences between wild type and Nod2 deficient hepatocytes, as evidenced by similar levels of ALT in the supernatant and cell death assessed by lactate dehydrogenase release (LDH) (Suppl. Fig. 3B and C). These results demonstrate that the hepatoprotection is not mediated via Nod2 in hepatocytes during cholestasis.
Hepatic bile acid concentration is lower in Nod2 deficient mice following bile duct ligation
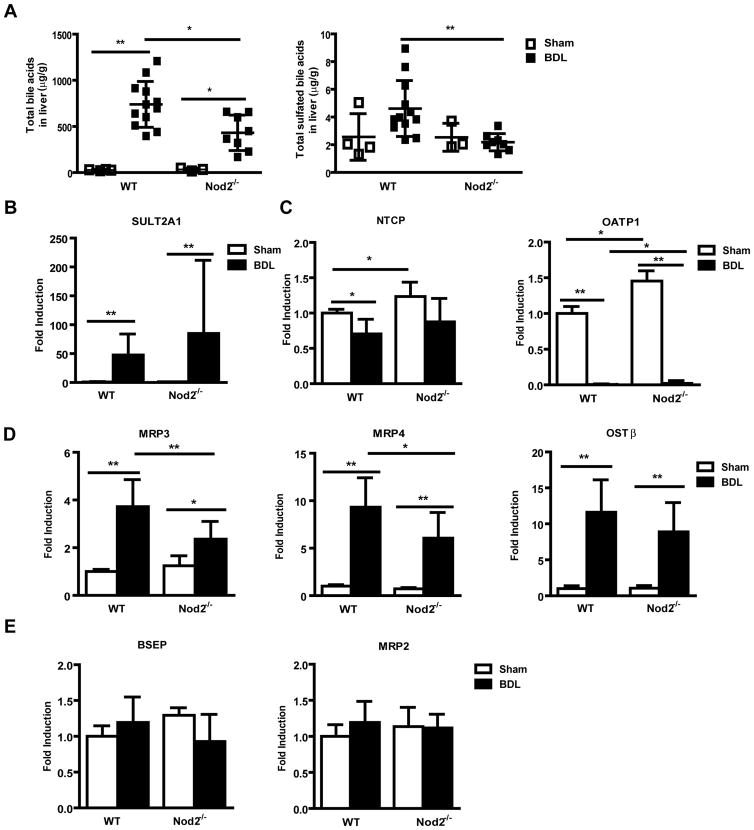
We then focused on bile acid levels and composition as a decreased bile acid pool or change in bile acid composition can modulate hepatoxicity and fibrosis under cholestatic conditions [18, 19]. As expected total liver bile acids were elevated after 3 weeks of bile duct ligation, but hepatic levels were significantly lower in Nod2 deficient mice as compared with wild type mice (Fig. 2A). Additional hydroxylation and sulfation, as well as upregulation of alternative bile acid transporters serve to decrease bile acid-induced injury in cholestatic hepatocytes [20, 21]. Taurine amidated muricholic acid (T-MCA) was the major bile acid accumulating in cholestatic liver. T-MCA was significantly lower in bile duct ligated Nod2 deficient compared with wild type mice (Suppl. Table 1). Total sulfated bile acids were higher in the liver of wild type as compared with Nod2 deficient mice following bile duct ligation for 3 weeks (Fig. 2A). Hepatic gene expression of sulfotransferase (SULT) 2A1, which mediates sulfation of bile acids [20], was induced after bile duct ligation, but was not significantly different between the two groups (Fig. 2B). Thus, the major difference between the Nod2−/− mice and the wild type mice after bile duct ligation was a considerably decreased concentration of bile acids in the liver, providing a possible explanation for the protection of Nod2−/− mice from cholestasis-induced liver injury and disease.
Fig. 2. Bile acid concentration is lower in the liver of Nod2 deficient mice after bile duct ligation.
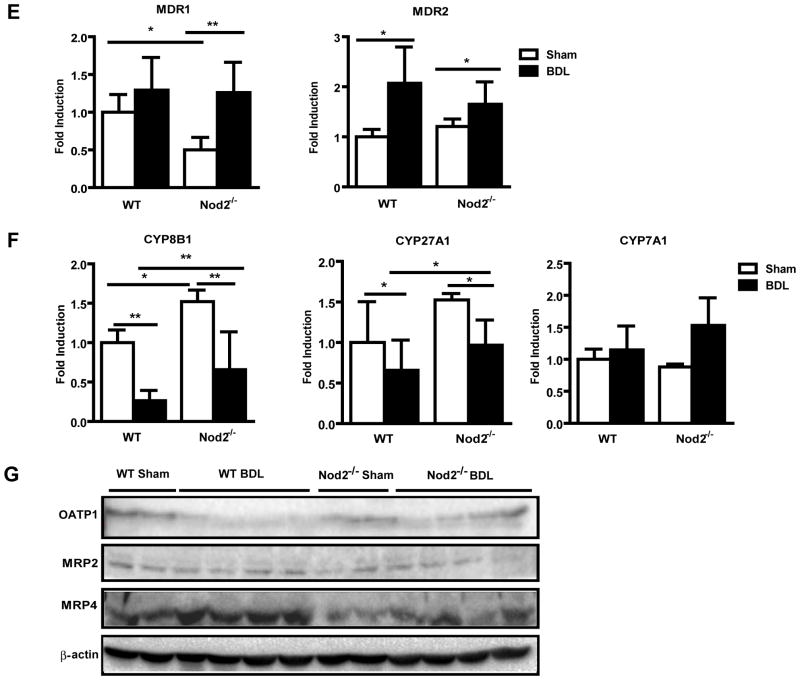
(A) Bile acids were measured in livers of wild type sham (n=4) and bile duct ligated (BDL; n=12), Nod2−/− sham (n=3) and bile duct ligated (n=8) mice. Total bile acid levels and the total amount of sulfated bile acids in the liver. (B–F) Real-time PCR was performed in livers of wild type sham (n=2–4) and bile duct ligated (n=16–17), Nod2−/− sham (n=2–4) and bile duct ligated (n=8–9) mice. (G) Western blot for hepatic OATP1, MRP2 and MRP4; β-actin is shown as loading control. *p < 0.05; **p < 0.01
The concentration of bile acids in the hepatocyte is determined by the balance between uptake mediated by basolateral (sinusoidal) transporters and efflux from the hepatocyte mediated by both canalicular and apical transporters in animals with an intact enterohepatic circulation. In the bile duct ligated animal, the only sources of bile acids are absorption of those bile acids present in the intestine at the time of duct ligation, and those generated by continuing bile acid synthesis. We therefore assessed expression of genes involved in hepatocyte bile acid transport and synthesis [22, 23]. Gene expression of Na-taurocholate cotransporting polypeptide (NTCP; also called solute carrier family 10, member 1 or Slc10a1), the major basolateral bile acid uptake transporter, and organic anion transporting polypeptide (OATP; also called solute carrier organic anion transporter family, member 1a1 or Slco1a1) 1, one of the basolateral (sodium-independent) organic anion transporters was examined. Although NTCP mRNA was not differently expressed in wild type or Nod2−/− mice following bile duct ligation, cholestasis-induced inhibition of OATP1 gene expression was lower in Nod2−/− as compared with wild type mice (Fig. 2C). A similar trend was observed on the protein expression level for OATP1 (Fig. 2G). Bile duct ligation-induced mRNA levels of basolateral bile acid export transporters multidrug resistance-associated protein (MRP; also called ATP-binding cassette, sub-family C, member 3 or Abcc3) 3 and MRP4 (Abcc4) were lower in Nod2 deficient mice as compared with wild type mice (Fig. 2D). A similar trend was observed on the protein expression level for MRP4 (Fig. 2G). The bidirectional transporter organic solute transporter (OST; also called solute carrier family 51, beta subunit or Slc51b) β mRNA was similar in wild type and Nod2 deficient mice 3 weeks after bile duct ligation (Fig. 2D). Gene expression of canalicular export pumps including the bile salt export pump (BSEP; also called ATP-binding cassette, sub-family B member 11 or Abcb11), MRP2 (Abcc2), multidrug resistance protein (MDR; also called ATP-binding cassette, sub-family B, member 1B or Abcb1b)1 and MDR2 (Abcb4) showed no significant difference between wild type and Nod2−/− mice in cholestasis (Fig. 2E). Hepatic MRP2 protein was similarly expressed among the two groups (Fig. 2G).
The rate limiting enzyme in the major pathway of bile acid synthesis, gene CYP7A1 (mediating cholesterol 7α-hydroxylation) showed no significant difference between cholestatic groups (Fig. 2F). Cholestasis-induced suppression of hepatic CYP8B1 (mediating sterol 12-hydroxylation) and CYP27A1 (mediating the initial step in side chain oxidation of cholesterol) mRNA, resulted in these key enzymes for bile acid synthesis being significantly lower in Nod2−/− as compared with wild type counterparts (Fig. 2F). Taken together, Nod2 deficient mice showed a marked decrease in total liver bile acid concentration, despite relatively small differences in the level of mRNA for bile acid synthesis enzymes and hepatocyte bile acid transporters. These changes in expression may be an adaptive and secondary response to a lower total amount of hepatic bile acids in cholestatic Nod2−/− mice. Presumably, the animals are in steady state with newly synthesized bile acids passing into and out of the hepatocyte at the basolateral domains. In addition, there is likely to be continued secretion into the canaliculus followed by an equal amount of ductular reabsorption.
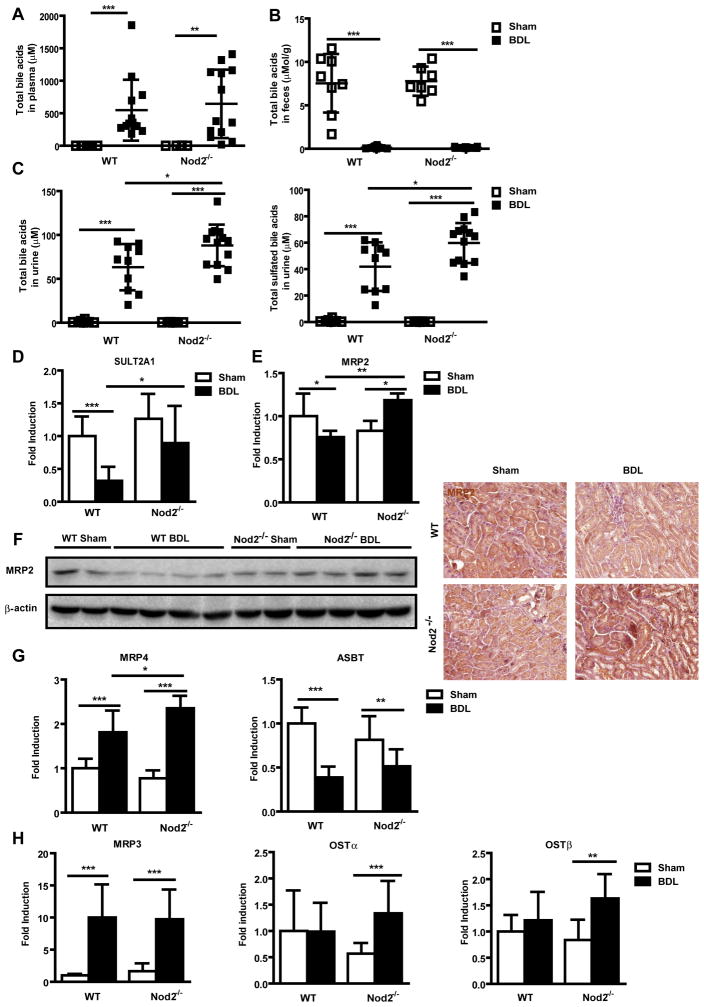
To further delineate the mechanism resulting in a decreased liver bile acid concentration in Nod2 deficient mice following bile duct ligation, bile acid profiles were measured in plasma, feces and urine. Total plasma bile acids were remarkably elevated after bile duct ligation for 3 weeks, but no significant difference was observed between wild type and Nod2−/− mice (Fig. 3A). The composition of bile acids and hence the toxicity profile was similar in cholestatic wild type and Nod2−/− mice (Suppl. Table 2). Total bile acids in feces were reduced to negligible values after bile duct ligation, but no difference in total amount or extent of sulfation or amidation was observed between wild type and Nod2−/− mice during cholestasis (Fig. 3B; Suppl. Table 3).
Fig. 3. Urine bile acid excretion is increased in Nod2 deficient mice following bile duct ligation.
Wild type sham (n=6–8) and bile duct ligated (BDL; n=10–13), Nod2 −/− sham (n=4–7) and bile duct ligated (n=13) mice were used. Total bile acid levels (A) in the plasma and (B) feces. (C) Total bile acids and total sulfated bile acids in the urine. (D, E, G and H) Real-time PCR was performed in kidneys of wild type sham (n=8–11) and bile duct ligated (n=11–16), Nod2−/− sham (n=7) and bile duct ligated (n=11) mice. (F) Western blot and immunohistochemistry for renal MRP2; β-actin is shown as loading control. *p < 0.05; **p < 0.01; ***p < 0.001
FXR is a major regulator of liver injury in cholestasis, and selective intestinal activation of FXR protects the liver from cholestasis in mice [24]. Therefore, FXR and its target gene Fibroblast growth factor (FGF) 15 were determined in the terminal ileum. FXR and FGF15 mRNA expression was similar in wild type and Nod2 deficient mice following bile duct ligation for 3 weeks (Suppl. Fig. 4A and B).
Urinary elimination of bile acids is increased in Nod2 deficient mice following bile duct ligation
Renal excretion is the major route for bile acid elimination during cholestasis [25]. Following bile duct ligation, urinary elimination should eventually become equal to bile acid synthesis. Total bile acids in urine were markedly increased by bile duct ligation (Fig. 3C). A major finding was that urinary bile acid levels were significantly higher in Nod2−/− as compared with wild type mice (Fig. 3C). There was no obvious difference in urine volume between wild type and Nod2−/− mice following bile duct ligation (data not shown). In addition, the total amount of sulfated bile acids was increased in the urine of Nod2−/− as compared with wild type mice after bile duct ligation (Fig. 3C; Suppl. Table 4). Renal gene expression of SULT2A1 was significantly higher in Nod2−/− mice following bile duct ligation (Fig. 3D). Thus, increased elimination of bile acids via the kidney with steady state plasma levels appears to offer an explanation for the lower bile acid concentration in the liver of Nod2 deficient mice.
To determine the underlying mechanism for increased renal elimination of bile acids in Nod2−/− mice, gene expression of bile acid transporters in the kidney was examined. Two important renal apical bile acid efflux transporters are MRP2 (mediating sulfated and some unsulfated bile acid transport) [23] and MRP4 (mediating co-transport of bile acids and glutathione). MRP2 mRNA was suppressed in wild type mice, but induced in Nod2−/− mice after bile duct ligation. The level of MRP2 was significantly higher in Nod2 deficient as compared with wild type mice after 3 weeks of bile duct ligation (Fig. 3E). This was confirmed on the protein level (Fig. 3F). Bile duct ligated Nod2 deficient mice had significantly higher MRP4 gene expression in the kidney compared with wild type mice (Fig. 3G). In contrast, the apical bile acid uptake transporter sodium-dependent bile salt transporter (ASBT; also called Slc10a2) was detected at a similar level in the two cholestatic groups (Fig. 3G). No differences in the expression of the basolateral efflux transporter MRP3 (mediating bile acid glucuronide transport) and the basolateral bi-directional bile acid transporters OSTα and OSTβ were detected in wild type and Nod2−/− mice following bile duct ligation (Fig. 3H). FXR gene expression in the kidney was similar in wild type and Nod2 deficient mice following bile duct ligation for 3 weeks (Suppl. Fig. 4C). These results suggest that a higher expression of the bile acid secreting transporters MRP2 and MRP4 accounts for the increased excretion of bile acids into urine of Nod2 deficient mice. Increased renal sulfation of bile acids might additionally contribute to the process of higher urinary elimination of sulfated bile acids.
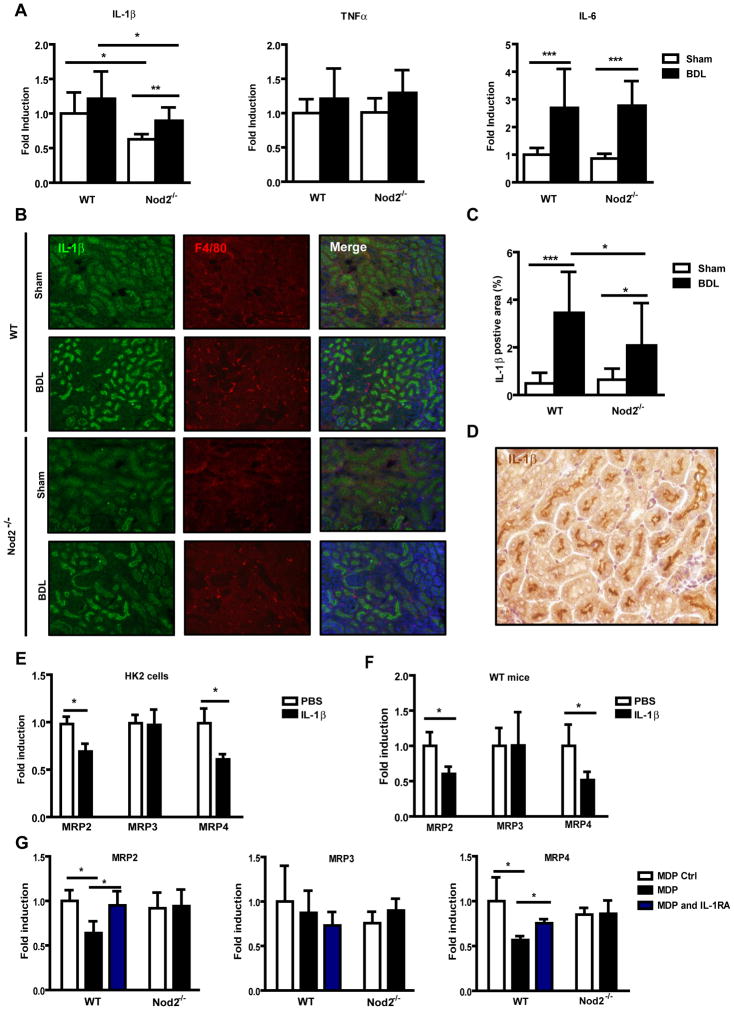
Nod2-dependent IL-1β production suppresses bile acid export transporters in renal tubular epithelial cells
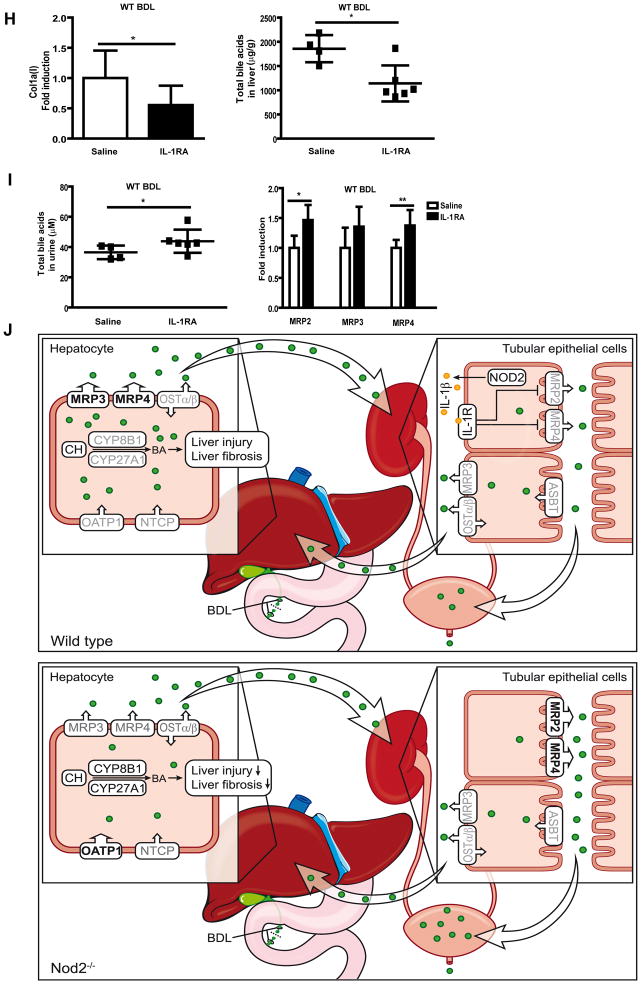
Since Nod2 is an important regulator of inflammation, cytokine expression was examined in the kidney. The mRNA expression of IL-1β was significantly lower in sham operated and bile duct ligated Nod2−/− mice as compared with wild type mice, but there was no difference in TNFα and IL-6 gene expression in wild type or Nod2−/− mice following sham operation or bile duct ligation (Fig. 4A). Immunofluorescence confirmed decreased expression of IL-1β protein in the kidney of Nod2−/− mice following bile duct ligation (Fig. 4B and C). Immunofluorescence double staining with IL-1β and F4/80 as macrophage markers, showed no double positive cells suggesting that macrophages are not the primary source for IL-1β in the kidney (Fig. 4B). Indeed, tubular epithelial cells showed a strong staining for IL-1β (Fig. 4D). We therefore assessed the effect of IL-1β on MRP2/3/4 mRNA expression in HK2 cells, a proximal tubular epithelial cell line from normal human kidney. Consistent with in vivo results, IL-1β treatment resulted in a marked reduction of MRP2 and MRP4 mRNA expression, while MRP3 expression was not affected by IL-1β (Fig. 4E). To further substantiate our data, IL-1β was administered to wild type mice and renal transporters were assessed. IL-1β inhibited gene expression of MRP2 and MRP4, but not of MRP3 (Fig. 4F). Opposite effects were observed in the liver (Suppl. Fig. 5A). Consistent with in vivo data, IL-1β induced MRP2 and MRP4 expression in primary mouse hepatocytes (Suppl. Fig 5B). Furthermore, the effect of MDP on bile acid transporters in the kidney was determined in wild type and Nod2 deficient mice. Following administration of MDP, mRNA levels of MRP2 and MRP4 but not MRP3 were inhibited in wild type mice, while no inhibitory effect was observed in Nod2 deficient mice (Fig. 4G). Blocking IL-1β signaling with the IL-1 receptor antagonist Anakinra abolished MDP-mediated inhibition of MRP2 and MRP4 (Fig. 4G). Blocking IL-1β signaling with Anakinra in bile duct ligated wild type mice significantly reduced collagen α1(I) expression in the liver (Fig. 4H, left panel). Total hepatic bile acids were reduced by approximately 39% after Anakinra treatment (Fig. 4H, right panel), which is similar to a 42% reduction in Nod2−/− mice as compared with wild type mice after bile duct ligation (Fig. 2A). Anakinra increased total (Fig. 4I, left panel), but not sulfated urinary bile acid levels (Suppl. Fig. 6A). A significant induction of renal MRP2 and MRP4 gene expression was observed in bile duct ligated mice following Anakinra treatment (Fig. 4I, right panel), while SULT2A1 expression in the kidney was not significantly altered (Suppl. Fig. 6B). These results suggest that Nod2 dependent IL-1β expression inhibits bile acid efflux transporters in the kidney.
Fig. 4. Nod2-dependent IL-1β release decreases bile acid export transporter expression in renal tubular epithelial cells.
(A) Mice underwent sham (n=7–8) and bile duct ligation (BDL; n=11–13) for 3 weeks. IL-1β, TNFα and IL-6 gene expression in the kidney. (B) Immunofluorescent staining for IL-1β (green) and F4/80 (red), and (C) quantification of IL-1β in kidney sections (n=4 for each group). (D) Immunohistochemistry for IL-1β (brown) was performed in kidney sections of wild type mice following 3 weeks of bile duct ligation. (E) HK2 cells were treated with PBS as control or IL-1β (1 ng/ml) for 16 hrs. MRP2, MRP3 and MRP4 mRNA expression was examined (n = 4 independent experiments performed in duplicate). (F) Wild type mice were injected with PBS (n=5) or IL-1β (n=5) and renal MRP2, MRP3 and MRP4 mRNA expression was examined 24hrs later. (G) Wild type or Nod2−/− mice were injected with MDP control (n=3–4) or MDP (n=4) and treated with or without Anakinra (n=5–9). Gene expression for MRP2, MRP3 and MRP4 in the kidney was assessed 24hrs after the injection. (H and I) Bile duct ligation was performed in wild type mice for a total of 3 weeks. Mice were injected with saline (n=4) or Anakinra (n=6–7) for 4 days prior to harvesting. Collagen α1(I) mRNA expression in the liver (H, left panel). Total bile acids in the liver (H, right panel). Total bile acids in the urine (I, left panel). Gene expression for renal MRP2, MRP3 and MRP4 (I, right panel). (J) Schematic representation of the proposed protective role of Nod2 deficiency in cholestasis. Following cholestatic liver injury, Nod2 dependent IL-1β production in renal tubular epithelial cells inhibits the expression of bile acid efflux transporters MRP2 and MRP4 in an autocrine fashion. Less bile acids are excreted in the urine, and an accumulation of bile acids in the liver results in hepatocyte damage and fibrosis. Bile acids (BA) are indicated as green dots. Grey font color denotes unchanged transporter or enzyme expression between wild type and Nod2−/− mice following bile duct ligation. Up-regulated transporter or enzyme expression between wild type and Nod2−/− mice following bile duct ligation is indicated by a black font color and an increased size of the arrow head. CH, cholesterol; BDL, bile duct ligation.*p < 0.05; **p < 0.01
Discussion
The cytotoxic effects of retained bile acids in cholestasis can be limited by decreasing bile acid synthesis, by decreasing bile acid uptake or increasing bile acid efflux at either pole of the hepatocyte, or by hydroxylation and sulfation of the retained bile acids to decrease their intrinsic hepatotoxicity. And indeed, an increase in renal elimination of bile acids ameliorates cholestatic liver injury and fibrosis [18, 19]. In the OSTα−/− mouse, hepatic toxicity and fibrosis following bile duct ligation is decreased by an increase in urinary bile acid excretion, which is mediated by a reduction in the renal apical bile acid uptake transporter ASBT and an increase in the apical efflux transporters MRP2 and MRP4 [18].
In this study, we investigated the role of Nod2 in experimental models of liver fibrosis. Nod2 deficiency protected from cholestatic, but not from liver toxin-induced fibrosis. This finding could not be explained by a cell protective effect on hepatocytes or a reduction in liver inflammation. We found that Nod2 deficiency results in an increased renal excretion of bile acids, which was mediated by an increased expression of MRP2 and MRP4 in kidney tubular epithelial cells. These changes were associated with lower IL-1β expression in the kidney of Nod2 deficient mice and a lower IL-1β mediated suppression of MRP2 and MRP4. More bile acids were eliminated via the urine with a subsequent decrease of the hepatic bile acid concentration causing less hepatocyte damage and fibrosis (Fig. 4J).
Although macrophages are considered an important cell type for Nod2-induced IL-1β production [7, 8], there was no evidence for IL-1β synthesis by F4/80 positive macrophages in the kidney following bile duct ligation. Most of the IL-1β positive cells were renal tubular epithelial cells. Consistent with our findings, tubular epithelial cells but not infiltrating macrophages are the main source of IL-1β and IL-18 (belonging to the IL-1 superfamily) production in experimental anti-glomerular basement membrane (GBM) disease [26] and renal obstruction [27]. We have no evidence that systemic IL-1β possibly coming from the injured liver reaches the IL-1 receptor on tubular epithelial cells via the bloodstream, because IL-1β was undetectable in plasma 3 weeks after bile duct ligation. We propose that IL-1β expression is Nod2 dependent in tubular epithelial cells of the kidney during cholestasis. And indeed, Nod1 and Nod2 are highly expressed in human and mouse renal tubular epithelial cells and play a crucial role in the pathogenesis of renal ischemia reperfusion injury in which Nod1/2 dependent TNF-α, IL-6 and IL-8 production contribute to disease pathogenesis [28]. Thus, the absence of Nod2 reduces IL-1β expression in tubular epithelial cells of the kidney during experimental cholestatic liver disease.
Hepatic and intestinal MRP2 expression was down-regulated in obstructive cholestasis of rats which was associated with IL-1β upregulation [29, 30]. These studies only revealed an association but not a causative relation between IL-1β and MRP2 expression. IL-1β treatment decreased MRP2 expression in rat and human hepatocytes [31]. In contrast with these studies, MRP2 expression increased following IL-1β injection in wild type mice or following IL-1β treatment of primary mouse hepatocytes. Thus, species differences might account for these obvious different results. Bile duct ligation did not increase hepatic MRP2 expression in our study suggesting that other factors than IL-1β contribute to gene regulation during cholestasis. On the other hand, hepatic MRP4 expression increased after IL-1β injection and following bile duct ligation in wild type mice. Similarly, IL-1β induced MRP4 in primary mouse hepatocytes.
MRP2 and 4 expression in the kidney in response to IL-1β has not been investigated. In this study, renal MRP2 and MRP4 expression were higher in bile duct ligated Nod2 deficient mice, which, in turn, was associated with decreased IL-1β levels in the kidney. Using in vivo experiments, Nod2 stimulation induced IL-1β, which in turn reduced the expression of MRP2 and MRP4 in an autocrine fashion. In health, the renal tubule absorbs filtered bile acids, whereas in cholestasis, the polarity of the proximal renal tubular cell is completely reversed, with the renal tubules secreting rather than absorbing bile acids. The renal apical transporter MRP2 mediates the efflux of sulfated bile acids [23]. And indeed, the total amount of sulfated bile acids was higher in the urine of Nod2 deficient mice as compared with wild type mice following 3 weeks of bile duct ligation. Although sulfated bile acids were detected in our study as opposed to others using one week of bile duct ligation [21], sulfation might be a time-dependent slow adaptation to cholestasis in the mouse. Thus, we propose a concept in which Nod2-dependent IL-1β production suppresses the bile acid efflux transporters MRP2 and MRP4 in tubular epithelial cells during cholestasis. More bile acids are excreted via the urine during cholestasis, which eventually limits cholestatic liver disease. However, at baseline other mediators than IL-1β might be important for the regulation of renal MRP2 and MRP4, because their expression in sham operated Nod2 deficient mice was not different compared with sham operated wild type mice despite lower renal levels of IL-1β.
Our study demonstrates that deficiency of Nod2 protects from cholestatic liver injury and fibrosis. Our findings imply that Nod2-mediated inflammation plays an important role in the regulation of bile acid transporters in the kidney and this regulation decreases the hepatic bile acid concentration in the bile duct ligated mouse. This in turn significantly affects hepatocyte death and liver fibrosis. There are currently no data available about the clinical course of patients with primary sclerosing cholangitis (PSC) carrying a NOD2/CARD15 mutation. It will be of great interest to specifically determine the NOD2 haplotype in patients with PSC, determine bile acid metabolism and compare their clinical course in future studies. Taken together, our study confirms and extends the concept of greatly enhanced renal elimination of bile acids as a major renal adaptation during cholestasis. Treatments (e.g. through IL-1R antagonism) that enhance renal elimination of bile acids should, in principle, be beneficial in cholestatic liver disease.
Supplementary Material
Acknowledgments
Financial support: This study was supported in part by NIH grants K08 DK081830, U01 AA021856 and R01 AA020703, and by ABMRF/The Foundation for Alcohol Research (all to BS).
We thank David A. Brenner for helpful discussion and Diantha La Vine for help with the illustration.
Abbreviations
- NASH
non-alcoholic steatohepatitis
- Nod
nucleotide oligomerization domain
- MDP
muramyl dipeptide
- IL
interleukin
- BDL
bile duct ligation
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- CCl4
carbon tetrachloride
- GDCA
glycodeoxycholic acid
- PI
propidium iodide
- LDH
lactate dehydrogenase
- MCA
muricholic acid
- NTCP
Na-taurocholate cotransporting polypeptide
- Slc
solute carrier family
- OATP
organic anion transporting polypeptide
- Slco
solute carrier organic anion transporter family
- MRP
multidrug resistance-associated protein
- Abc
ATP-binding cassette
- OST
organic solute transporter
- BSEP
bile salt export pump
- MDR
multidrug resistance protein
- CYP
Cytochrome P450
- ASBT
sodium-dependent bile salt transporter
Footnotes
None of the authors has a financial, personal or professional conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12:939–962. xi. doi: 10.1016/j.cld.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torok NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol. 2008;43:315–321. doi: 10.1007/s00535-008-2181-x. [DOI] [PubMed] [Google Scholar]
- 3.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–231. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodsky IE, Monack D. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Seminars in immunology. 2009;21:199–207. doi: 10.1016/j.smim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. The Journal of clinical investigation. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. The Journal of biological chemistry. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 7.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. The Journal of biological chemistry. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 8.Hedl M, Abraham C. Distinct roles for Nod2 protein and autocrine interleukin-1beta in muramyl dipeptide-induced mitogen-activated protein kinase activation and cytokine secretion in human macrophages. The Journal of biological chemistry. 2011;286:26440–26449. doi: 10.1074/jbc.M111.237495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Q, Mathison J, Fearns C, Kravchenko VV, Da Silva Correia J, Hoffman HM, et al. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. Journal of leukocyte biology. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 11.Rehman A, Sina C, Gavrilova O, Hasler R, Ott S, Baines JF, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 12.Biswas A, Petnicki-Ocwieja T, Kobayashi KS. Nod2: a key regulator linking microbiota to intestinal mucosal immunity. Journal of molecular medicine. 2012;90:15–24. doi: 10.1007/s00109-011-0802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, et al. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PloS one. 2007;2:e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barreau F, Madre C, Meinzer U, Berrebi D, Dussaillant M, Merlin F, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut. 2010;59:207–217. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–1340. e1331. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi H, Yoon JH, Grambihler A, Werneburg N, Bronk SF, Gores GJ. Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. The Journal of biological chemistry. 2003;278:454–461. doi: 10.1074/jbc.M209387200. [DOI] [PubMed] [Google Scholar]
- 18.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang JH, Rickenbacher A, Humar B, Weber A, Raptis DA, Lehmann K, et al. Serotonin protects mouse liver from cholestatic injury by decreasing bile salt pool after bile duct ligation. Hepatology. 2012;56:209–218. doi: 10.1002/hep.25626. [DOI] [PubMed] [Google Scholar]
- 20.Alnouti Y. Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicological sciences : an official journal of the Society of Toxicology. 2009;108:225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 21.Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, et al. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Chiang JY. Bile acids: regulation of synthesis. Journal of lipid research. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. Journal of hepatology. 2013;58:155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–365. e351–354. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiological reviews. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 26.Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, et al. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1997;12:1109–1115. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- 27.VanderBrink BA, Asanuma H, Hile K, Zhang H, Rink RC, Meldrum KK. Interleukin-18 stimulates a positive feedback loop during renal obstruction via interleukin-18 receptor. The Journal of urology. 2011;186:1502–1508. doi: 10.1016/j.juro.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigeoka AA, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J, et al. Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. Journal of immunology. 2010;184:2297–2304. doi: 10.4049/jimmunol.0903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denson LA, Bohan A, Held MA, Boyer JL. Organ-specific alterations in RAR alpha:RXR alpha abundance regulate rat Mrp2 (Abcc2) expression in obstructive cholestasis. Gastroenterology. 2002;123:599–607. doi: 10.1053/gast.2002.34758. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich CG, Geier A, Salein N, Lammert F, Roeb E, Oude Elferink RP, et al. Consequences of bile duct obstruction on intestinal expression and function of multidrug resistance-associated protein 2. Gastroenterology. 2004;126:1044–1053. doi: 10.1053/j.gastro.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 31.Diao L, Li N, Brayman TG, Hotz KJ, Lai Y. Regulation of MRP2/ABCC2 and BSEP/ABCB11 expression in sandwich cultured human and rat hepatocytes exposed to inflammatory cytokines TNF-{alpha}, IL-6, and IL-1{beta} The Journal of biological chemistry. 2010;285:31185–31192. doi: 10.1074/jbc.M110.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.