Abstract
A major goal of predictive genetic testing is to alert people to their risk before illness onset; however, little is known about how risk perceptions change following genetic testing and whether information is recalled accurately over time. In the United States, a CDKN2A/p16 mutation confers 76% lifetime risk of melanoma. Following genetic counseling and test reporting, subjective risk estimates and recall of counselor-provided risk estimates were assessed 5 times over the next 2 years among 60 adult members of 2 extended CDKN2A/p16 kindreds. No sustained changes from baseline in risk perceptions were reported. Unaffected carriers (n=15) consistently reported significantly lower subjective risk estimates (46%) than they were actually given (76%, p < .001) or recalled having been given (60%, p < .001). Noncarriers’ (n=27) risk estimates decreased following results disclosure, but rebounded, with both subjective and recalled estimates subsequently exceeding what they were told by the counselor (both ps < .001). Affected carriers’ (n=18) risk estimates for developing a new melanoma corresponded well to counselor-provided information (p =.362). For all 3 patient groups, results were consistent across multiple risk measures and remained similar when demographic, phenotypic, and baseline behavioral contributors to melanoma risk were statistically controlled. These findings are consistent with other studies of risk perception, but additional studies of more diverse populations are needed to understand the reasons behind both the persistence of initial risk estimates and their divergence from information provided by the counselor during genetic counseling. Additionally, determining whether holding subjective risk perceptions that differ from counselor-provided information ultimately affects adherence to management recommendations will help guide the presentation of risk information in genetic counseling practice.
Keywords: melanoma, perceived risk, genetic testing, CDKN2A/p16, genetic counseling
A major goal of predictive genetic testing for familial cancer is to alert people to their elevated risk prior to the development of illness, when early detection and even prevention may be possible. The underlying assumption is that an accurate understanding of the risk associated with mutation carriage will precipitate better compliance with prevention behaviors. In the case of familial melanoma, a pathogenic CDKN2A/p16 (or simply, p16) mutation confers a 35- to 70-fold increase in risk of this aggressive and potentially fatal cancer, corresponding to 76% lifetime risk in US residents (Bishop et al., 2002). Melanoma genetic testing is just beginning to transition to routine clinical use (Leachman et al., 2009), though researchers and clinicians have previously questioned its clinical utility. Concerns about testing have included the low percentage of high-risk melanoma families that could be attributed to p16 mutations, variability of penetrance estimates, the potential for individuals testing negative for a familial mutation to still have increased risk due to the presence of other factors in the family (e.g., phenotype, sun exposure), and the lack of empirical data to determine whether benefits outweigh costs (Kefford et al., 2002; Kefford & Mann, 2003), Recent data, however, support a role for melanoma genetic testing in promoting improved screening among unaffected carrier family members (Aspinwall et al., 2008, 2013b; Glanz et al., 2013; Kasparian et al., 2009). In particular, unaffected carriers in our study of two extended p16 kindreds reported improvements in the thoroughness of skin self-examinations and improved adherence to annual total body skin examinations 2 years following melanoma genetic testing (Aspinwall et al., 2013b). The present study examines one mechanism—changes in perceived risk following counseling and test reporting—that could account for previously published behavior change demonstrated in this cohort.
Little is known about the impact of melanoma genetic test reporting and counseling on subjective risk perceptions. Providing an objective genetic test report and/or a more personally tailored counseling message based on an available report may alter the perception of risk differently than counseling based on family history alone. Additionally, in the specific case of melanoma, both phenotypic (skin tone, hair and eye color, moles, freckling) and behavioral factors (prior sun exposure, sunburns) may influence risk perceptions, and it is unknown how patients’ risk estimates following genetic test reporting may be influenced by these factors. Thus, the present study prospectively examines the impact of melanoma genetic testing on perceived risk over time, taking into account multiple demographic, phenotypic, and behavioral factors that may contribute to melanoma risk.
Understanding the Impact of Individualized Cancer Genetic Risk Information on Perceived Risk
Several straightforward assumptions guide the logic of presenting individualized risk information to members of high-risk families: 1) participants’ risk perceptions will change to be closer to the statistical estimates provided, 2) such interventions will have a long-term impact on risk perceptions, and 3) more accurate risk perceptions will motivate an appropriate level of adherence to recommended health behaviors. However, as will be reviewed, patients do not always accept or agree with risk information that is provided to them during a counseling session, despite the provision of data to support the level of risk.
Effects of Individualized Risk Assessment and Genetic Testing on Perceived Risk
Regarding the first assumption, individuals who undergo genetic counseling and receive risk estimates based on the identification of a gene mutation and/or family history-based risk estimates generally report subsequent risk estimates that correspond more closely to the counseled risk estimates. Most research has focused on genetic counseling for breast cancer risk in women without a personal history of breast cancer. Such patients often overestimate their cancer risk prior to counseling, and adjust their risk perceptions downward following counseling, although these adjusted estimates often remain higher than counselor-provided risk estimates up to 1 year later (for reviews, see Sivell et al., 2008; Croyle & Lerman, 1999; Lerman et al., 1995; Smerecnik, Mesters, Verweij, de Vries, & de Vries, 2009). A meta-analysis found that 5 of 6 prospective studies reported improvements in the accuracy of risk perceptions following family history based genetic counseling for breast, ovarian, or colorectal cancer (Braithwaite, Emery, Walter, Prevost, & Sutton, 2004).
Studies of individuals receiving genetic test results have found that mutation carriers accurately reported elevated levels of risk following genetic test reporting on some items, but seemed to show relatively low risk perceptions on others. For example, following a positive test result, 90% of individuals who tested positive for a mutation conferring elevated colorectal cancer risk accurately stated that their risk was “high” or “very high” (Grover et al., 2009). However, among BRCA1/2 carriers asked how they felt about their risk “independent from their actual risk,” 53.1% underestimated their risk by believing that their “risk of developing breast cancer [was] as high as [their] risk of not developing breast cancer” or believing that they would “(probably) not develop breast cancer” (Claes et al., 2005, p. 354).
We suggest two possible explanations for this apparent risk minimization. First, some of the minimization of risk may be due to the choice of management decisions in mutation carriers. Women in this study who had prophylactic oophorectomy had significantly lower perceived cancer risk than women who had not had risk-reducing surgery. Therefore, long-term subjective risk estimates may differ from estimates given during counseling if individuals believe their prior actions have reduced their subsequent cancer risk. Second, literature from the fields of social and clinical psychology has shown that minimization of risk is a common defensive processing strategy after receiving health-risk information (for reviews, see Ditto & Croyle, 1995; Wiebe & Korbel, 2003; see also Lipkus, McBride, Pollak, Lyna, & Bepler, 2004). For example, participants receiving cholesterol test results systematically recalled their cholesterol levels as lower than they actually were up to 6 months following risk disclosure, and this recall bias was exacerbated among participants with higher cholesterol levels (Croyle et al., 2006). It is unknown whether similar discrepancies between patients’ risk perceptions and actual counselor-provided risk information will occur for genetic testing for familial melanoma, a disease with which participants have greater personal experience (through their own diagnoses or those of family members).
Overestimation of Risk among Family Members Who Receive Negative Genetic Test Results
A quite different set of psychological processes may operate among members of high-risk families who receive negative genetic test results. Noncarrier family members may persist in believing they are at elevated risk following a negative genetic test result as this result may contradict a lifetime of belief about vulnerability to serious illness (Bergenmar, Hansson, & Brandberg, 2009; Kelly et al., 2005; Michie et al., 2002). In one study, unaffected BRCA1/2 mutation noncarriers reported significantly lower subjective risk perceptions one year following receipt of a negative test result than they did prior to receipt of their test result; however, when asked how they felt about their risk “independent from their actual risk,” nearly half reported that they had equal chances of developing and not developing breast cancer (Claes et al., 2005). This overestimation of risk following genetic test reporting has been dubbed “unrealistic pessimism” (Kelly et al., 2005).
Lack of Evidence concerning Long-Term Changes in Risk Perceptions Following Genetic Testing
With respect to the duration of changes in risk perceptions following genetic test reporting, additional evidence suggests that neither carriers nor noncarriers report long-term changes in perceived risk following genetic test reporting for hereditary breast/ovarian cancer (HBOC), hereditary nonpolyposis colorectal cancer (HNPCC), or Alzheimer’s Disease (Heshka, Palleschi, Howley, Wilson, & Wells, 2008). While these findings suggest that previously-tested individuals’ risk estimates do not change from baseline risk estimates, this meta-analysis did not assess how participants’ resulting risk estimates corresponded to the risk estimates provided during genetic counseling.
Do Differences in Subjective Risk Estimates Represent Recall Bias or a Difference of Opinion?
To date, there are limited data regarding the reasons for changes or lack of changes in risk perception following genetic testing, and studies are typically unable to differentiate between purposeful disagreement with the information provided and defensive processing of the information over time. For example, one could accurately recall being counseled about a 76% lifetime risk following a positive melanoma genetic test result, but believe that one’s own risk was considerably lower due to adherence to sun protection practices. Alternatively, one could generate and/or recall a lower risk estimate to reduce feelings of vulnerability and distress associated with high familial cancer risk. To date, only one published study has asked participants to report both their opinion regarding perceived risk and their recall of risk estimates (Gurmankin, Domchek, Stopfer, Fels, & Armstrong, 2005). In this study, individuals’ subjective risk perceptions were significantly higher than their recalled risk perceptions following genetic counseling for breast cancer, while recalled risk perceptions were higher than the actual risk communicated. These findings suggest that both processes -- potentially meaningful differences between subjective risk perceptions and the risk information provided as well as systematic biases in recall for risk information -- may be at work. To examine these possibilities, the current study evaluated both subjective and recalled risk estimates and compared them to the information provided during the genetic testing session.
Multiple Phenotypic and Behavioral Factors Contribute to Melanoma Risk
The multifactorial nature of melanoma risk presents a unique model for evaluating the impact of genetic counseling and testing on risk perception. Members of high-risk families may (correctly) believe that multiple factors contribute to risk. Multiple phenotypic (fair skin, red hair, light eyes, large numbers of moles, freckling) and behavioral (history of sunburns and sun exposure) confer additional increases in melanoma risk independent of the p16 mutation (Hansen, Wadge, Lowstuter, Boucher, & Leachman, 2004). To date, there are few data regarding the influence of such factors on risk perceptions following melanoma genetic testing. A small prospective study that included four individuals who had tested negative for the p16 genetic mutation found that two participants cited behavioral factors as reasons for elevated risk (Bergenmar et al., 2009). The present study examines the impact of melanoma genetic testing and counseling on perceived risk, taking into account multiple demographic, phenotypic, and behavioral factors that may contribute to risk perceptions.
Overview of the Present Study
We examined prospective changes from baseline in subjective risk estimates following the receipt of melanoma genetic test results over a 2-year period. We examined subjective estimates and recall for counselor-provided information in three distinct patient groups, with the following hypotheses:
Patients who receive positive genetic test results but have no history of melanoma (unaffected carriers) may underestimate their risk, consistent with theories suggesting that individuals are resistant to negative health information (Croyle et al., 2006).
Patients who receive negative genetic test results (unaffected noncarriers) may persist in believing they are at elevated risk following provision of genetic test results, as their test results contradict a lifetime of belief about vulnerability to serious illness (Kelly et al., 2005; Michie et al., 2002).
Patients with a history of melanoma who receive positive test results (affected carriers) will be less likely to underestimate their risk of developing a new melanoma, as individuals with personal experience with a disease are less likely to engage in biased processing of information about it (Weinstein, 1980).
Last, to increase the generalizability of the findings and because participants may draw on different sources of information in making different kinds of risk judgments (Klein & Weinstein, 1997; Ranby, Aiken, Gerend & Erchull, 2010), we assessed perceived melanoma risk with two different measures (lifetime percent risk and risk compared to others).
Methods
Participants and Procedures
Companion test-reporting and follow-up studies were approved by the Institutional Review Board at the University of Utah (IRB #s 7916 and 13816) and all participants provided informed consent after the nature of the procedure had been fully explained to them. All participants were members of two large families with multiple cases of melanoma who were enrolled in previous p16 identification research (Cannon-Albright et al. 1992; Kamb et al., 1994; see Aspinwall et al., 2008, 2013a, for additional details). Seventy-seven adult research participants from these families were recontacted and offered the opportunity to receive clinically confirmed genetic test results. Although all participants had received extensive prior counseling regarding their elevated melanoma risk based on family history during their previous participation in the p16 identification research, none were aware of their genetic status or the presence of the p16 mutation in their family prior to disclosure through the test-reporting study. The two families included in this study were each segregating a deleterious mutation in p16, V126D and 5′UTR-34G>T. For the purposes of the present study, each of the DNA samples was submitted for appropriate mutation-specific genetic testing through a Clinical Laboratory Improvement Amendments-certified laboratory. The pre-disclosure counseling and genetic test reporting sessions were offered free of charge, and participants received modest non-monetary thank-you gifts (e.g., water bottles and tote bags) for completion of follow-up questionnaires.
Recruitment and retention
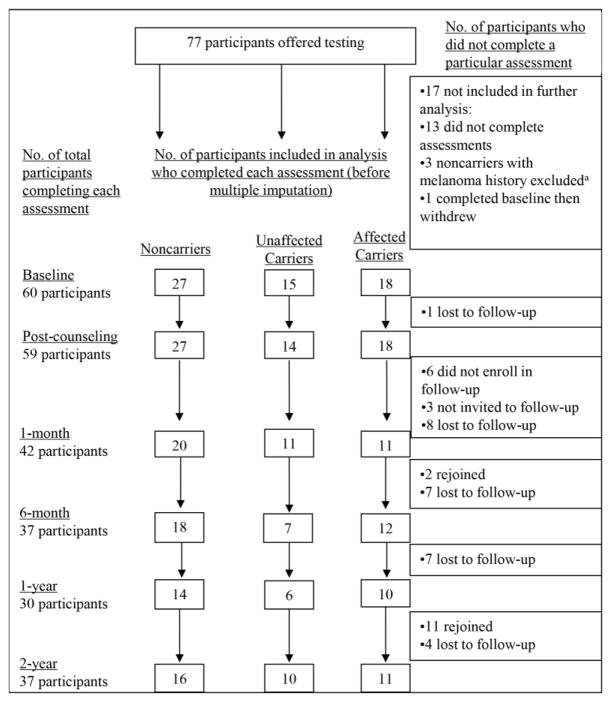
Recruitment into the genetic test reporting and follow-up studies and retention through the 2-year follow-up are summarized in Figure 1. From May through November 2005, 64 (83.1%) participants completed a baseline questionnaire and a test reporting and counseling session. All 64 participants elected to receive results, and 62 completed the immediate post-counseling questionnaire. Fifty-nine of these participants were then invited to enroll in a follow-up study of psychological and behavioral responses to p16 test reporting; of these, 53 (89.8%) enrolled and 45 (76.3%) completed the 1-month, 39 (66.1%) completed the 6-month, 30 (50.9%) completed the 1-year, and 40 (67.8%) completed the 2-year questionnaire. Three participants were not offered participation. One participant was excluded because she received results outside of the seasonal time frame of the study. The other two were excluded because one was adopted and one was from a kindred consisting of a single member. Because a large portion of the study focused on family communication, it was determined that their experience would be different from the other participants who were members of two large kindreds.
Figure 1.
Recruitment, retention, and attrition of noncarriers, unaffected carriers, and affected carriers in the genetic test reporting and follow-up studies.
aAffected noncarriers were excluded from analysis because there were too few participants to permit statistical analyses from which meaningful inferences or comparisons to other patient groups could be made.
Participants were retained for analysis if they completed at least one post-counseling assessment and if they were a member of one of the three participant groups defined by melanoma history and mutation status (unaffected noncarriers, n=27; unaffected carriers, n=15; affected carriers, n=18). As shown in Figure 1, these criteria resulted in the exclusion of three noncarriers with a documented melanoma history and the exclusion of one affected carrier who did not complete any post-counseling assessments. Thus, the total sample consisted of 60 participants. Of these, the proportion who completed each follow-up assessment was 96.7% at immediate post-counseling, 68.3% at 1month, 65% at 6 months, 50% at 1 year, and 61.7% at 2 years.
Melanoma genetics education and risk communication
Participants were provided with a single, individual counseling session in which they were provided with pre-test disclosure information, their personal result, and tailored risk and management information (complete protocol in Aspinwall et al., 2008, supplementary materials). All sessions were conducted by a single licensed genetic counselor. A standardized counseling protocol using a book of visual aids ensured consistency across counseling sessions. The risk associated with p16 mutations was presented in three different ways: 1) as a relative risk of 35-to 70-fold increase above the general population risk; 2) as a bar graph illustrating the 76% lifetime risk of melanoma, and 17% lifetime risk of pancreatic cancer; and 3) as a bar graph illustrating the melanoma risk to be 50% by age 50 and 76% by age 80 (Bishop et al., 2002). Participants were also shown a table with the magnitude of risk associated with having one affected family member (2–3x relative risk), phenotypic features, such as freckling (2–3x relative risk), red hair (2.4–4x relative risk), number of atypical nevi (>6 atypical nevi confer 6.3x relative risk) and number of moles (100 plus moles confer 3.1–16.5x relative risk), skin tone (Type I, 1.4x), and behavioral factors, such as history of blistering sunburn (2–3x relative risk). Those testing negative for the familial mutation were informed that they still had a minimum 1.7-fold increased risk due to other factors in the family such as a melanoma-prone phenotype or history of UVR exposure. Participants receiving positive or negative test results were given the same recommendations for photoprotection and screening. All participants received a follow-up letter approximately one month later that reiterated their test results and management recommendations.
Measures
Demographics, phenotypic factors, and melanoma history
Participants completed standard demographic questions assessing age, gender, marital status, education, and household income. All but two participants were recruited from two large families (Kindreds A and B). Phenotypic factors (skin tone, eye color, hair color, presence of moles, and freckling) were obtained from medical chart review, and complete data were available for all but 3 participants. Table 1 presents the coding categories for each of the demographic and phenotypic factors. We confirmed the melanoma history of each participant through pathology reports, the Utah Cancer Registry (a SEER Registry), and the Utah Population Database.
Table 1.
Demographic, phenotypic, and baseline behavioral characteristics of the sample and their correlations with perceived melanoma risk at baseline controlling for personal history of melanoma.
| Correlation with baseline perceived risk of developing a future melanoma (partial correlations control for personal melanoma history at baseline) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Patient Characteristics | Coding | Mean (SD) | Lifetime percent chance | Comparative | Likelihood | |||
|
| ||||||||
| Zero-order | Partial | Zero-order | Partial | Zero-order | Partial | |||
| Melanoma history | No=0, yes=1 | 30% | 0.53** | 0.37** | 0.46** | |||
| Demographic factors | ||||||||
| Age | 49.56 (14.05) | −.16 | −0.34** | −.21 | −0.33* | −.26 | −0.41** | |
| % Male | Female=0, Male=1 | 50 | .14 | 0.20 | .16 | 0.19 | .01 | 0.03 |
| Kindred | Kindred A=0, Kindred B=1 | 0.54 | .08 | −0.16 | −.22 | −0.28* | −.18 | −0.25 |
| Education | “11th grade or less” =1 to “doctoral degree” =7 | 4.48 (1.17) | .01 | −0.04 | .04 | 0.03 | .06 | 0.05 |
| Income | “Less than $9999”=1 to “$100,000+”=11 | 6.45 (2.50) | .01 | 0.06 | −.08 | −0.05 | −.04 | 0.01 |
| Phenotypic factors | ||||||||
| Freckling | Absent=0, mild=1, moderate=2, severe=3 | 1.44 (0.75) | −.04 | −0.10 | .04 | 0.02 | .11 | 0.09 |
| % with moles | No=0, yes=1 a | 61 | .21 | 0.27* | .28* | 0.31* | .09 | 0.12 |
| Hair color | Red=1, blonde=2, medium brown or brown=3, dark brown=4 | 2.29 (0.95) | −.13 | 0.05 | −.10 | 0.02 | −.19 | −0.05 |
| Eye color | Blue=1, grey, green or hazel=2, light brown, brown, or dark brown=3 | 1.77 (0.78) | −.17 | −0.29* | −.14 | −0.20 | −.21 | −0.31 |
| Skin tone | Skin type: I=1, I or II=1.5, II=2, III=3, IV=4. | 1.89 (0.78) | −.04 | −0.02 | −.05 | −0.03 | −.13 | 10.12 |
| Baseline behavioral factors | ||||||||
| Sunscreen, % time in past 6 mos. | 45.42 (31.26) | .12 | 0.01 | .14 | 0.06 | .25 | 0.18 | |
| Protective clothing, % time in past 6 mos. | 57.07 (33.49) | .21 | 0.10 | .10 | 0.01 | .13 | 0.03 | |
| UVR avoidance, % time in past 6 mos. | 62.65 (31.14) | .12 | −0.03 | .22 | 0.14 | .14 | 0.12 | |
| % with 1 or more sunburns in past winter/spring/summer | No=0, yes=1 | 51 (0.50) | .30 | 0.35** | .23 | 0.27* | .24 | 0.32* |
| Baseline melanoma risk perceptions | ||||||||
| Lifetime percent chance, 0 to 100% | 49.8 (31.91) | -- | ||||||
| Comparative risk, 1–5 | 3.85 (1.26) | .68** | -- | |||||
| Likelihood, 1–5 | 3.22 (1.29) | .79** | .76** | -- | ||||
The number of moles was measured according to categories used in published risk models (Fears et al., 2006) for calculating melanoma risk, which differ according to gender. Women received a ‘0’ if they had less than 5 moles less than 5mm on their back and a ‘1’ if they had more than 5 moles of this size on their back. Men received a ‘0’ if they had less than 2 moles greater than 5mm on their back and less than 7 moles less than 5mm on their back, and a ‘1’ if they had either more than 2 moles greater than 5mm on their back or more than 7 moles less than 5mm on their back.
p<.05
p<.01
Behavioral measures of baseline photoprotection and sunburns
Percentage of time using sunscreen, wearing protective clothing (long pants and sleeves), and avoiding peak UVR exposure from 10 AM to 4 PM were assessed by baseline reports of each behavior over the past 6 months from 1 [about 0% of the time (never used)] to 7 [(about 100% of the time (all the time)]. Sunburns were assessed at baseline by two items asking participants how many sunburns they received last winter and “so far this spring and summer.” Responses were coded as to whether participants reported no sunburns or one or more sunburns during the past winter, spring, and summer.
Subjective risk estimates
Participants completed three items assessing subjective risk perceptions at each assessment. These items, which are widely used to study health risks (see Weinstein & Klein, 1995, for review), were clearly labeled, “Personal Beliefs about Melanoma.” Participants were instructed to “answer these questions based on your own opinions, which may or may not be the same as the information provided in your genetic counseling session.” Participants first estimated their lifetime percent chance of developing a future melanoma: “On a scale from 0 to 100, where 0 is no chance at all and 100 is absolutely certain, what do you think are the chances that you will get a future melanoma during your lifetime?” Next, participants rated their comparative risk and absolute likelihood of developing melanoma (Weinstein & Klein, 1995) in the following ways: comparative risk, “Compared to other people of your gender, age, and skin color, what is the likelihood that you will develop melanoma in your lifetime (another melanoma if you have already had one)?” from 1 (much below average) to 5 (much above average), and absolute likelihood, “Considering all the different factors that may contribute to melanoma, including your own past and present behavior, what would you say your chances are of getting a melanoma in the future (another melanoma if you have already had one)?” from 1 (very unlikely) to 5 (very likely).1
Recall for risk information provided during counseling session
Participants then answered a parallel set of questions concerning their lifetime percent chance, comparative risk, and absolute likelihood “based on what you were told in your counseling session.” These items were clearly labeled, “Information You Were Given in Your Genetic Counseling Session,” and were prefaced by the following instructions: “The next set of questions asks about information you were given in your genetic counseling session. These items assess your understanding of what the genetic counselor told you. Please answer based on that information.” Thus, participants were asked at each follow-up assessment to recall the lifetime and comparative risk estimates given to them by the genetic counselor. To reduce participant burden, recalled lifetime or comparative risk was not assessed at the 6-month follow-up.
Ratings of own melanoma risk as greater than, less than, or equal to risk information provided by counselor
At one month following test reporting, participants completed the following forced-choice item: “We asked you to report both your personal opinions about your risk for developing melanoma and the risk information you were given by the genetic counselor. It is possible that you believe your chances of developing melanoma to be higher, lower, or the same as the information given to you by the genetic counselor. Compared to what the genetic counselor told me, I believe my risk of developing melanoma to be…” with the response options: higher than what the genetic counselor told me, lower than what the genetic counselor told me, or the same as what the genetic counselor told me. Participants were also asked to list reasons for their choice. These responses were coded by two raters with 100% agreement.
Multiple Imputation Procedure
Because a listwise deletion procedure across the multiple assessments in the repeated-measures analyses (see “Overview of Analyses” section) would have resulted in an artificially small sample size of complete cases that may not be representative of the full sample, multiple imputation was performed to generate complete data for all 60 participants (Graham, 2009; Schafer & Graham, 2002). Multiple imputation uses available nonmissing data, including data from completed assessments and “auxiliary variables,” such as demographic, psychosocial, and other baseline values that might either predict noncompletion of subsequent assessments or be useful in predicting subsequent assessments in multiple regression models to estimate values for missing assessments. Following standard practice, we completed 10 imputations sets using NORM (Shafer, 1999) and then transferred the data to PASW18 for analysis. By generating multiple assessments of the missing values, multiple imputation approximates the type of measurement error that is present in real but not singly imputed data (Graham, 2009) and performs well with small sample sizes and large amounts of missing data, as well as when data are missing at random and often when missing not at random. For this reason, multiple imputation is preferred over listwise deletion, reweighting, or mean substitution (Shafer & Graham, 2002). Because the imputed values have greater variability than actual data, these values are underweighted in the analyses compared with actual data. Following standard practice, analyses were repeated with each data set, and all reported results were computed from the average of the coefficients yielded by the 10 separate data sets. The results concerning subjective risk estimates and recall for counselor-provided information in the 3 patient groups were highly similar with or without the multiple imputation procedure. Respondents who completed versus did not complete the 2-year follow-up did not differ significantly on any of the demographic, phenotypic, or behavioral characteristics reported in Table 1.
Data Analysis
We first examined demographic (including kindred membership), phenotypic, and behavioral differences at baseline among the 3 patient groups and then evaluated the correlation of these factors with baseline melanoma risk perceptions. Second, to test whether participants in the 3 patient groups reported changes from baseline in subjective risk estimates over the course of the study, we conducted repeated-measures analyses of variance (ANOVAS) with Patient Group as a between-subjects factor and Time of Assessment (baseline, immediately following melanoma genetic test reporting and 1 month, 6 months, 1 year, and 2 years later) as a within-subjects factor. These analyses were repeated, statistically controlling for any phenotypic, demographic, and baseline behavioral characteristics that were related to baseline risk perceptions. Third, to evaluate the correspondence between subjective risk estimates and recall for counselor-provided risk information at each assessment in the 3 patient groups, we conducted repeated-measures ANOVAs with Patient Group as a between-subjects factor and Type of Risk Estimate (subjective opinion, recall of counselor-provided estimate) and Time of Assessment as within-subjects factors. We then compared each subjective and recalled estimate to the lifetime risk estimates actually provided to patients.
Results
Participant Characteristics and Demographics
Table 1 presents the demographic, phenotypic, and behavioral characteristics of the sample. Participants with a melanoma history had an average of 2.2 melanomas (SD=1.26, range 1–9), that were diagnosed on average 11.06 years prior to the baseline assessment (SD=11.52). Twenty-seven individuals without a history of melanoma received negative test results, while 33 individuals (15 without a history of melanoma, 18 with a history of melanoma) received positive test results. There was no difference among the three patient groups (affected carriers, unaffected carriers, noncarriers) in the proportion of participants completing any of the follow-up assessments [(1-month: X2(2)=0.97, p=.62; 6-months: X2(2)=1.90, p=.39; 1-year: X2(2)=1.86, p<.39; 2-years: X2(2)=0.23, p=.89].
No significant difference was found among the three patient groups on any demographic measure; however, there were some group differences in the phenotypic and baseline behavioral factors. Specifically, unaffected carriers were more likely to have moles (87%) than noncarriers (48%, p=.015), while affected carriers (59%) did not differ significantly from either group, F(2,56)=3.21, p=.048. Unaffected carriers had darker skin tone (M=2.3) than noncarriers (M=1.7, p=.017), and somewhat darker skin tone than affected carriers (M=1.8, p=.081), F(2,56)=3.09, p=.053); as well as darker hair color (M=2.87) than noncarriers (M=2.26, p=.036) and affected carriers (M=1.82, p=.001), F(2,56)=5.62, p=.006. Behaviorally, unaffected carriers sought shade less often (M=46.0% of the time) than affected carriers (M=75.28%, p=.007) and somewhat less often than noncarriers (M=63.48%, p=.072), F(2,57)=4.01, p=.024. Thus, unaffected carriers tended to have more moles and darker skin and hair than members of other patient groups and reported less baseline UVR avoidance.
Because family factors may contribute to perceived risk, we examined differences between the two kindreds from which participants were recruited. The kindreds did not differ in age, gender composition, marital status, education, or income, nor did they differ in the proportion of respondents with a melanoma history or who tested positive for the p16 mutation or the average age of melanoma onset. However, the average number of years between participants’ most recent melanoma and the time of the genetic counseling session was significantly shorter for affected members of Kindred A than it was for affected members of Kindred B [MA=5.86, MB=17.0; t(16) = 2.45, p=.030].
Identification of Demographic, Phenotypic, and Behavioral Factors Associated with Baseline Risk Perceptions
As shown in Table 1, we first examined the correlations of the demographic, phenotypic, and behavioral factors with the measures of baseline risk perceptions. These correlations were statistically controlled for melanoma history, as history was the biggest predictor of risk perceptions. As shown in Table 1, greater age, being a member of kindred A, the presence of a large number of moles greater than 5 mm, lighter eye color, and reporting one or more sunburns in the past winter/spring/summer were significantly associated with greater perceived risk on at least one of the risk items. These 5 variables were accordingly selected for inclusion as covariates in the analyses of changes in risk perceptions over time in the 3 patient groups.
Changes in Subjective Lifetime Risk Estimates Following Genetic Test Reporting
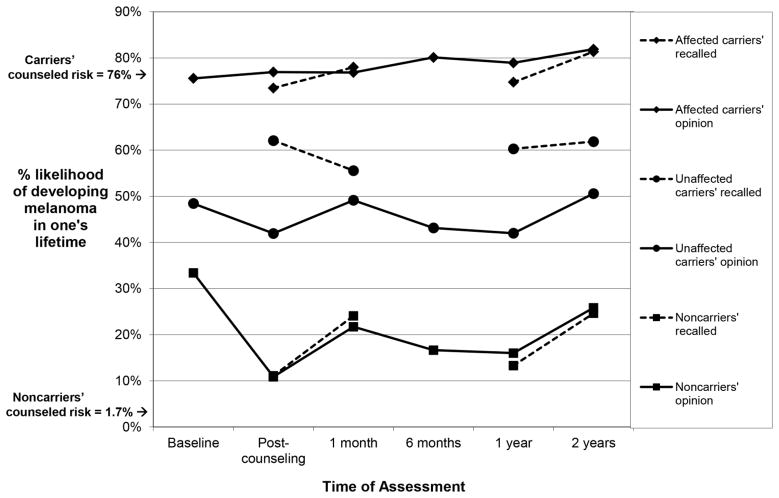
We next examined whether subjective risk perceptions increased among those who received positive melanoma genetic test results, and decreased among those who received negative test results. Analysis of subjective lifetime risk perceptions yielded significant differences between the groups and different patterns of change over time [Group, F(2,57)=55.14, p<0.001; Time, F(5,53)=3.86, p=0.005; Group x Time interaction, F(10,106)=2.32, p=0.016]. As shown in Table 2 and Figure 2 (solid lines), affected family members entered the study with high lifetime risk perceptions (76% at baseline) that persisted through all follow-up assessments, with no significant change over time, F(5,53)=0.72, p=.609. Both groups of unaffected participants reported baseline risk estimates that were lower than those provided by affected family members, but fairly high in an absolute sense (48% for unaffected carriers, 33% for noncarriers). Notably, unaffected carriers’ risk estimates did not change over time following a positive test report, F(5,53)=1.82, p=.124, averaging 45.88% across the 2-year period. Noncarriers’ risk estimates did, however, show significant change over time, F(5,53)=6.35, p<.001. Specifically, as shown in Figure 2, noncarriers’ subjective lifetime risk estimates decreased significantly from baseline to 10.86% immediately following the receipt of a negative genetic test result, t(52)=3.76, p<.001, but rebounded at subsequent assessments, such that estimates at 1 month [21.72%, t(52)=1.50, p=0.139] and 2 years [25.83%, t(52)=0.92, p=0.363] did not differ significantly from baseline.
Table 2.
Comparisons of subjective and recalled lifetime melanoma risk estimates to counselor-provided lifetime risk estimates among noncarriers, unaffected carriers, and affected carriers at each follow-up assessment.
| Counseled risk | Subjective lifetime risk | Subjective risk compared to counseled risk | Recalled lifetime risk | Recalled risk compared to counseled risk | Subjective risk compared to recalled riskc | |
|---|---|---|---|---|---|---|
|
| ||||||
| Participant Group | Comparison value (%) | Mean, % (SD) | t-value | Mean, % (SD) | t-value | t-value |
| Noncarriersa | ||||||
| Baseline | 1.7 | 33.39 (26.49) | 6.22** | |||
| Post-counseling | 1.7 | 10.86 (10.86) | 3.96** | 11.09 (11.52) | 4.24** | −0.17 |
| 1-month | 1.7 | 21.72 (20.24) | 4.85** | 24.08 (23.33) | 5.13** | −0.64 |
| 6-month | 1.7 | 16.65 (18.85) | 4.32** | |||
| 1-year | 1.7 | 16.01 (23.93) | 3.22** | 13.30 (29.77) | 2.24*d | 0.54 |
| 2-years | 1.7 | 25.83 (27.96) | 4.62** | 24.64 (23.80) | 5.48** | 0.24 |
| Unaffected carriersa | ||||||
| Baseline | 76 | 48.43 (28.42) | −3.76** | |||
| Post-counseling | 76 | 41.95 (26.67) | −4.96** | 62.08 (20.93) | −2.58* | −3.17** |
| 1-month | 76 | 49.14 (25.66) | −4.09** | 55.57 (29.09) | −2.76* | −1.57 |
| 6-month | 76 | 43.15 (27.27) | −4.77** | |||
| 1-year | 76 | 42.00 (28.32) | −4.82** | 60.29 (36.73) | −1.76*d | −2.21*d |
| 2-years | 76 | 50.59 (33.51) | −2.98** | 61.85 (26.72) | −2.14*d | −1.51 |
| Affected carriersb | ||||||
| Baseline | 76 | 75.56 (25.78) | −0.07 | |||
| Post-counseling | 76 | 76.94 (20.94) | 0.19 | 73.47 (17.69) | 0.60 | 0.81 |
| 1-month | 76 | 76.83 (31.81) | 0.08 | 77.99 (31.10) | 0.41 | −0.47 |
| 6-month | 76 | 80.11 (26.54) | 0.68 | |||
| 1-year | 76 | 78.93 (30.82) | 0.34 | 74.73 (36.93) | −0.12 | 0.64 |
| 2-years | 76 | 81.89 (28.28) | 0.93 | 81.36 (25.67) | 0.95 | 0.16 |
p <.05
p <.01.
Comparisons of subjective and recalled risk to counseled risk are 1-tailed because we had specific a priori hypotheses about risk over- and underestimations in the 2 groups of unaffected family members.
Comparisons of subjective and recalled risk to counseled risk are 2-tailed.
Comparisons are 2-tailed.
Comparisons did not meet the more stringent criterion (p<.0125) used to control for familywise Type I error within each patient group. Unless otherwise noted, comparisons met the more stringent criterion used to control for familywise Type I error within each patient group (p<.01 for comparisons conducted across 5 follow-up assessments, p<.0125 for comparisons conducted across 4 follow-up assessments).
Figure 2.
Subjective lifetime melanoma risk estimates (solid lines) and recalled counselor-provided lifetime risk estimates (dashed lines) among affected carriers, unaffected carriers, and noncarriers over a two-year period following genetic test reporting and counseling.
We next repeated the analysis controlling for the 5 covariates previously identified as predictors of baseline risk perceptions (age, kindred membership, moles, eye color, sunburns).2 The effects described above remained significant and yielded a virtually identical pattern of risk perceptions, with the adjusted means among unaffected carriers indicating even lower lifetime risk perceptions (starting at 36.67% at baseline and remaining at approximately 40% throughout the 2-year period). This analysis also revealed a significant main effect of age, F(1,46)=5.35, p=.025, such that greater age was associated with lower risk perceptions.
Correspondence between Subjective Lifetime Risk Estimates and Recall for Counselor-Provided Risk Estimates
Next, to examine whether participants may have purposefully disagreed with the estimates provided by the genetic counselor, we examined the correspondence between subjective lifetime risk estimates and participants’ recall for counselor-provided risk estimates over time. Results suggested that unaffected carriers purposefully disagreed with the information provided by the genetic counselor, while noncarriers and unaffected carriers did not. The repeated-measures analysis yielded significant differences among the 3 patient groups in whether their subjective risk estimates diverged from what they recalled having been told by the counselor [Group, F(2,57)=72.33, p<.001; Type of Risk Estimate, F(1,57)=4.82, p=.032; Time, F(3,55)=4.14, p=.010; Group x Type of Risk Estimate interaction, F(2,57)=6.98, p=.002; Group x Type of Risk Estimate x Time interaction, F(6,110)=2.14, p=.055]. As shown in Figure 2 and Table 2, among unaffected carriers, subjective risk estimates were significantly lower -- by an average of 14% -- than recalled estimates [46% vs. 60%, F(1,57)=15.249, p=0.0003], suggesting purposeful disagreement with their recollections of the given risk. For both noncarriers and affected carriers, subjective risk estimates (solid lines) corresponded closely to and were not significantly different from recall for counselor-provided information (dashed lines) at any assessment.
How Did Subjective and Recalled Risk Estimates Compare to Actual Counselor-Provided Information about Lifetime Risk?
We hypothesized that unaffected carriers would underestimate their risk, that unaffected noncarriers would overestimate their risk, and that affected carriers would report accurate risk perceptions compared to the information provided by the genetic counselor. We further examined whether unaffected carriers might recall a lower number than was actually provided, perhaps reflecting efforts to minimize personal risk. These predictions were largely confirmed. As shown in Table 2, a series of one-sample t-tests compared the average subjective and recalled risk estimates in each of the three participant groups to the information provided by the counselor. Among unaffected carriers, both subjective and recalled lifetime risk estimates at all follow-up assessments were significantly lower than the 76% estimate given in the counseling sessions, consistent with the hypotheses concerning defensive processing. In contrast, among noncarriers, subjective lifetime risk estimates and recalled risk at all follow-up assessments were significantly higher than the information provided by the counselor. This finding is consistent with the study hypothesis, but whether unrealistic pessimism or other factors are driving this consistent overestimation of risk is difficult to determine. Finally, among affected carriers, subjective lifetime risk estimates and recalled risk did not differ from counselor-provided estimates at any assessment.
Subjective Comparative Risk Perceptions Following Genetic Test Reporting
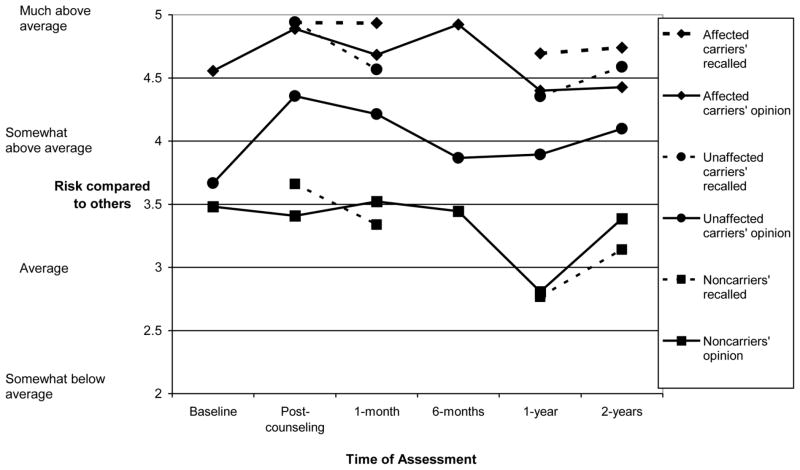
To examine whether the findings for lifetime percent risk generalized to another widely used risk perception measure, we next examined whether patients’ subjective estimates of their risk compared to other people showed similar patterns of change over time. This analysis yielded significant differences among patient groups [Group, F(2,57)=16.63, p<.001; Time, F(5,53)=4.02, p=.004; although the Group x Time interaction was not significant, F(10,106)=1.33, p=.223, the main effect of time had a significant cubic pattern, F(1,57)=6.37, p=0.014, so we examined patterns of change in each group]. As shown in the solid lines of Figure 3, unaffected carriers reported a marginally significant increase in comparative risk estimates after receiving positive test results [Mbaseline=3.67, Mpost=4.36; t(15)=1.89, p=.070]; however, comparative risk perceptions subsequently decreased, with no risk perception significantly exceeding baseline at 1 month, 1 year, or 2 years (ts=0.47–1.12, ps=.271–.639). Thus, as was the case for lifetime risk perceptions, unaffected carriers did not report sustained changes in comparative risk estimates following a positive test result. Affected carriers reported no changes in their subjective comparative risk estimates at any follow-up assessment (ts=0.29–0.97; ps=.338–.776). Finally, noncarriers’ subjective comparative risk estimates did not differ from baseline, except for a marginally significant decline at 1 year, t(52)=1.89, p=.065. Interestingly, the relative ordering of the three groups corresponded well to their respective comparative melanoma risk, such that affected carriers rated themselves as “much above average” compared to others (M=4.65), unaffected carriers rated themselves as “somewhat above average” (M=4.02), and noncarriers rated their risk as closer to “average” (M=3.34).
Figure 3.
Subjective comparative melanoma risk estimates (solid lines) and recalled comparative risk estimates (dashed lines) among affected carriers, unaffected carriers, and noncarriers over a two-year period.
As was the case for the subjective lifetime risk estimates, when we repeated the analysis with the 5 covariates previously described3, results remained similar, with unaffected carriers’ adjusted mean risk estimates shifting down. This analysis also revealed a significant main effect of kindred, F(1,46)=5.72, p=.021, such that being a member of Kindred A was associated with greater comparative risk perceptions.
Correspondence between Subjective Comparative Risk Estimates and Recall for Counselor-Provided Risk Estimates
When we examined the correspondence of subjective comparative risk perceptions to participants’ recall for counselor-provided risk estimates over time, the analysis yielded the same pattern of results as obtained for lifetime percent risk [Group, F(2,57)=34.18, p<.001; Type of Risk Estimate, F(1,57)=7.66, p=.008; Time, F(3,55)=8.49, p<.001; Group x Type of Risk Estimate interaction, F(2,57)=4.39, p=.017]. As shown in Figure 3, both noncarriers and affected carriers reported comparative risk estimates that corresponded well to what they recalled being told by the counselor, but unaffected carriers reported lower subjective comparative risk estimates (Mopinion=4.14) than what they recalled having been told by the counselor [Mrecalled=4.61; F(1,57)=10.013, p=0.002]. Unaffected carriers’ subjective estimates were significantly lower than recalled estimates at all assessments except 1 month [post-counseling, t(14)= −2.10, p<.03; 1 month, t(14)= −1.24, p=.117; 1 year, t(14)= −1.83, p=.044; 2 years, t(14)= −2.45, p=.014].
Qualitative Data Concerning Reasons for Lower Risk Perceptions at 1 Month
Having demonstrated that unaffected carriers reported lower, and noncarriers higher, risk perceptions than they were told, we next examined participants’ individual qualitative accounts of their reasons for holding divergent beliefs about their risk. At one month, 42 participants indicated on a forced-choice item whether they believed their risk to be lower than, equal to, or higher than what the counselor had told them (we do not reported imputed data for these results). The majority of participants (71.4%) believed their risk to be the same as what the counselor told them. However, consistent with the results presented for the quantitative risk perceptions, 45.5% of unaffected carriers, 5% of noncarriers, and 9.1% of affected carriers believed their risk to be lower than what the counselor told them. Unaffected carriers more frequently indicated that they believed their risk to be lower than what the counselor told them than noncarriers and affected carriers combined (Fisher’s exact, p=.009). Several respondents -- 15.0% of noncarriers, 18.2% of affected carriers, but no unaffected carriers -- reported that their risk was higher than what the counselor told them; however, these reports were not more likely among noncarriers than unaffected and affected carriers combined (Fisher’s exact, p=.453).
Respondents who carried a p16 mutation (5 unaffected and 1 affected) who believed their risk was lower than what they were told reported a variety of reasons. The lone affected carrier cited low current levels of UVR exposure and advanced age. Of the unaffected carriers, one each cited low current levels of UVR exposure, being educated about melanoma, believing that genetics are not the only factor causing melanoma, taking behavioral precautions, low family history of melanoma, feeling lucky, and ability to detect a melanoma in early stages. Respondents who believed their risk was higher than what they were told cited their elevated family history of melanoma (2 noncarriers and 1 affected carrier), their own history of melanoma (1 affected carrier) or pre-malignant skin lesion (1 noncarrier), and a high level of prior UVR exposure and blistering sunburns (1 noncarrier).
Discussion
Despite persistent improvement in screening compliance following melanoma genetic testing among unaffected carriers from the same sample (Aspinwall et al., 2013b), there appeared to be no change in perceived risk of getting melanoma over the same 2-year period. These findings suggest that the behavioral changes previously reported in this cohort were not due to a more accurate understanding of their risk of getting melanoma. Further, the effect of melanoma genetic risk counseling and test reporting on risk perceptions differed in several ways from the assumptions underlying such interventions. First, the receipt of melanoma genetic test results did not have a long-term impact on risk perceptions in any patient group. Specifically, across two kinds of risk estimates (lifetime percent, comparative) and multiple follow-up assessments over a 2-year period, the receipt of p16 results did not change patients’ subjective risk estimates to be closer to the information they were given by the genetic counselor. Second, among both unaffected carriers and noncarriers, participants’ recall for counselor-provided information was significantly and consistently different from the actual information provided. Third, on both quantitative and qualitative measures, unaffected carrier family members reported considerable differences between subjective risk perceptions and what they recalled being told by the counselor, suggesting a belief that their personal risk was lower than the statistics provided by the counselor. As reviewed earlier, these findings are consistent with studies of genetic testing for other hereditary syndromes in which mutation carriers tend to underestimate risk and noncarriers persist in having elevated risk estimates (e.g., Claes et al., 2005; Kelly et al., 2005; Michie et al., 2002).
It is unlikely that the observed disagreement between patients’ beliefs and counselor-provided information is due to misunderstanding or misinterpretation of any single risk estimate, such as lifetime percent, because multiple risk measures showed the same pattern. Additionally, using medical chart data and self-reports of sunburns, we established that the findings concerning lower risk perceptions among unaffected carriers and elevated risk perceptions among noncarriers were not due to documented demographic, phenotypic, or baseline behavioral factors that may confer lower or higher melanoma risk. However, the qualitative data indicated that some of these demographic and behavioral factors like greater age and good sun-protection practices were mentioned by participants as reasons for their lower risk perceptions and personal history of nevi and sunburn were cited by noncarriers as reasons for higher estimates. These results suggest that personal interpretations and understanding of risk are complicated and not universally determined by p16 mutation status or melanoma history.
Unaffected Carriers Display Persistent Underestimation of both Subjective and Recalled Risk
Among unaffected carriers, for whom genetic test reporting is intended to be most impactful, subjective risk estimates were consistently significantly below both what participants recalled being told and what they actually were told, and recalled estimates were lower than what they were actually told. These relatively low subjective risk perceptions were not explained by differences in demographic, phenotypic, or baseline behavioral factors. The persistent difference between recall for lifetime risk estimates and the information actually provided by the counselor suggests that unaffected carriers may have engaged in some degree of defensive processing (Ditto & Croyle, 1995; Wiebe & Korbel, 2003) to blunt the negative impact of the information. This conclusion is supported by the fact that discrepancies between recalled and actual risk were not seen in the other two groups. Thus, this pattern of results was not a general trend towards forgetting information, but was unique to the group that received information about an elevated health risk. These findings also extend prior work by Croyle et al. (2006) by demonstrating the stability of biased patterns of recall for risk information over a 2-year period. Although Wiebe and Korbel (2003) suggested that minimization of health threats is likely to be immediate and short-term, rather than prolonged, unaffected carriers’ subjective lifetime risk perceptions and recall for counselor-provided risk estimates remained lower than what participants were told at the time of the intervention over the following two years. The apparent lack of increased risk perceptions is striking, given the magnitude of the intervention and the consequential nature of the risk perception.
Nonetheless, the comparative risk estimates provided by unaffected carriers corresponded well to their relative risk compared to others, consistent with the findings of Croyle et al. (2006). Importantly, these comparative risk judgments, coupled with relatively high lifetime risk perceptions, suggest that unaffected carriers did not completely deny their elevated risk and understood it to be greater than other people’s. It will be important for future research to examine how these relatively accurate judgments of comparative risk may coexist with seemingly defensive underestimation of lifetime risk estimates among unaffected carriers.
Noncarriers Display Persistent Overestimation of both Subjective and Recalled Risk
An early concern in the field of genetic testing was that a false sense of security would develop among noncarriers that might negatively impact compliance with routine cancer preventive strategies such as colonoscopy or mammography. Noncarriers in the present sample reported relief from worry about developing melanoma as a major benefit of counseling and test reporting (Aspinwall et al., 2013a). However, consistent with prior studies of individualized cancer risk communications (Bergenmar et al., 2009; Kelly et al., 2005; Michie et al., 2002), noncarriers also persistently overestimated their risk and also overestimated their recall for the risk estimates provided by the counselor. These findings suggest that a false sense of security, at least from underestimation of risk, is unlikely in melanoma family noncarriers.
The reason(s) for and significance of noncarriers’ overestimation of risk are unclear. Although the persistence of elevated subjective risk perceptions in the present study was not explained by demographic, phenotypic, or baseline behavioral factors in the quantitative analysis, qualitative comments indicated that phenotypic factors and sun exposure may still have been important factors in individuals’ personal assessments of their risk. The counseling included presentation of the relative risks associated with a melanoma-prone phenotype and prior sunburns. Therefore, noncarriers may have still perceived themselves to be at higher risk if they noted those features in themselves. Additionally, as melanoma does have phenotypic risk factors, individuals may have felt at greater risk if they physically resembled affected family members.
Additional research is needed to understand these potential contributors to perceived risk and their implications for adherence to management recommendations. Behaviorally, noncarriers reported somewhat improved thoroughness of skin self-examinations, but decreased uptake of annual total body skin examinations at the 2-year follow-up (Aspinwall et al., 2013b). While noncarriers were counseled that annual total body skin exams and monthly self-skin exams are still beneficial due to the potential residual risk conferred by other melanoma risk factors, standard recommendations for melanoma screening among individuals at this magnitude of risk do not exist, and it is unclear whether their decreases in screening are clinically meaningful. An important target for future research is to understand how noncarriers manage the psychological and behavioral implications of learning that they are at greater than population risk, although at considerably less risk than other family members.
Affected Carriers Display Accuracy in both Subjective and Recalled Risk Estimates
Family members with a history of melanoma showed a consistently high degree of accuracy in both their subjective and recalled estimates for their risk of developing a new melanoma. These participants entered the study with highly elevated and accurate risk perceptions that did not change over the 2-year period. These findings are consistent with others suggesting that participants with personal experience with an illness are unlikely to provide optimistically biased risk estimates or to do so to the same degree as unaffected participants (Weinstein, 1980). Nonetheless, understanding the subjective risk perceptions of participants with a personal history of melanoma is important because melanoma may develop at multiple different primary sites, and there is no prophylactic surgery that would completely reduce one’s risk. Affected participants are frequently excluded from studies of breast and colorectal cancer genetic testing (cf. Claes, Denayer, Evers-Kiebooms, Boogaerts, & Legius, 2004; Lerman et al., 1995; McQueen, Swank, Bastian, & Vernon, 2008), because the perceived risk for these individuals is conceptualized as 100% without considering risk for a future or additional cancer diagnosis. Although a personal history of melanoma seems sufficient to convince individuals of their elevated risk for a new melanoma, as well as to motivate a high level of adherence to photoprotection and screening recommendations (Aspinwall et al., 2008, 2009, 2013b), information about one’s p16 status may be useful for other reasons, such as decisions about genetic testing and risk management for one’s children (Taber, Aspinwall, Kohlmann, Dow, & Leachman, 2010).
Study Limitations
Although the present sample is the largest to date for studies of the outcomes of melanoma genetic testing, stratification by mutation status and melanoma history resulted in relatively modest sample sizes in each group. The resulting sample sizes did not allow us to examine the relationship of subjective risk perceptions to behavioral changes following melanoma genetic testing in specific patient groups. However, we do note that the repeated-measures design was adequately powered to test the primary hypotheses concerning changes in risk estimates following melanoma genetic testing in the 3 patient groups (power to detect a medium effect size at p < .05 = .92 for subjective lifetime estimates and .74 for comparative risk; power = .70 for analyses testing differences between subjective and recalled lifetime risk estimates over time in the 3 groups). Power to detect significant demographic, phenotypic, and behavioral predictors of risk estimates was considerably lower, however, suggesting that the findings concerning relatively few such significant predictors should be interpreted with caution.
A second issue concerns the number of statistical tests conducted. The two risk measures were analyzed separately because an index resulting from the combination of both assessments would not have been easily comparable to the lifetime percentage risk information provided by the genetic counselor. Further, a unique aspect of our design involved the comparison of patients’ subjective and recalled risk estimates to those provided by the genetic counselor at multiple time points, resulting in multiple comparisons in each group. Although the use of multiple risk perception measures and multiple follow-up assessments strengthened our confidence in the findings, it did result in an increased number of statistical tests, thereby increasing the risk of Type 1 error, or an increased likelihood of finding chance results. However, these comparisons generally served to test directional hypotheses concerning under- or overestimation of risk. Further, the high proportion of planned comparisons that were significant (e.g., 10 of 10 comparisons of unaffected carriers’ and noncarriers’ lifetime risk estimates to counselor-provided information; 8 of 8 such comparisons of recalled risk to counselor-provided information) argues against the likelihood of chance findings. When we set familywise Type I error to .05 using a Bonferroni correction (dividing the alpha of .05 by the number of follow-up assessments tested in each patient group), all of the comparisons indicating unaffected carriers’ underestimations of subjective lifetime risk compared to counselor-provided information and noncarriers’ overestimation of such risk met the more stringent criterion for statistical significance, as did 5 out of 8 comparisons of recalled risk to counseled risk. Less robust to corrections for familywise Type I error were the point-by-point comparisons of subjective to recalled risk at each follow-up assessment. In retrospect, this is not surprising, as the ANOVA findings indicating a significant difference between subjective risk estimates and recalled risk in the unaffected carrier group were not qualified by time of assessment for either risk measure. Thus, while the primary finding of a significant difference between subjective and recalled estimates among unaffected carriers seems to be robust, we consider the post hoc comparisons of subjective compared to recalled risk at each follow-up assessment to be exploratory for both risk measures and to await replication. Finally, the inclusion of analyses of covariance statistically controlling for demographic, phenotypic, and baseline behavioral factors also increased the number of tests conducted, though false positives do not appear to be an issue as few of the covariates were significant predictors of risk perceptions over time.
A further limitation is that almost all participants were members of two large kindreds, raising the possibility that kindred size or other features unique to these two kindreds (relative to other families with the p16 mutation) may have influenced results. In order to address this possibility, we performed an extensive comparison between the two kindreds. However, there were only two reliable differences between the kindreds – members of one kindred had more recent melanoma diagnoses on average and reported greater comparative risk assessments at baseline than the other kindred. Importantly, none of the primary findings concerning differences between subjective and recalled risk estimates were qualified by kindred. Additionally, all participants in the present study had received extensive prior counseling as part of their previous involvement in melanoma research, and most participants had multiple relatives participating in the study. The role of family communication and other potential sources of familial influence on beliefs and behaviors cannot be determined from the analyses presented here. Thus, it is unknown whether unaffected members of melanoma-prone families without prior research participation, with differing numbers of affected family members or recency of melanoma diagnoses in the family, or with different communication patterns would provide similarly elevated risk estimates at baseline and whether these estimates would persist following counseling and test reporting. As with any study based on a limited number of families from a specific region, it will be important to examine risk perceptions and other outcomes of melanoma genetic testing in additional samples and settings to determine the generalizability of these findings.
Additionally, the present data cannot differentiate between the impact of genetic counseling and the impact of test reporting accompanied by genetic counseling. Thus, it remains unknown whether similar patterns of subjective risk estimates and recall for counselor-provided estimates would be obtained following counseling based on family history alone. If anything, the greater potential certainty provided by a definitive genetic test result should promote greater recall for the specifics of the information provided by the counselor; however, unaffected carriers in the present study nonetheless recalled being told a lower figure than was actually provided, while unaffected noncarriers recalled being told their risk was higher. Future research directed to understanding how participants represent and recall these different types of risk estimates is needed.
Finally, although we statistically controlled for recent sunburn history at baseline, it is possible that patients’ subjective risk estimates also reflected other aspects of cumulative behavioral risk, such as childhood or later sun behavior (i.e., prior sunburns or tanning), factors that were not assessed in the present study. As noted earlier, it will be important in future research to continue to examine multiple potential contributors to perceived risk.
Practice Implications
Participation in genetic testing and counseling resulted in minimal changes from baseline in perceived risk of getting melanoma. However, before seeking to intervene to improve the correspondence between patients’ risk perceptions and counselor-provided information, it will be important to understand how subjective risk perceptions, especially those that seem to be reliably lower than counselor-provided information, are related to adherence. Accumulating evidence concerning behavioral outcomes in both the present sample and other research programs indicates that unaffected family members who undergo melanoma genetic testing nevertheless report improvements in screening following a positive test result. These studies indicate that in the context of genetic counseling, there is a role for genetic test reporting in clinical practice (Aspinwall et al., 2008, 2009, 2013b; Glanz et al., 2013; Kasparian et al., 2009).
Because improved adherence occurs in the absence of accurate risk perceptions, the relationship between risk perceptions and behavior following genetic testing is complex and requires further study. While presentation of accurate risk information is a key component of genetic counseling, patients will process that information in the context of their attitudes and experience, including beliefs about prevention options. For example, models of behavior change find that when individuals believe the available management options are insufficient to reduce risk or prevent disease, they may be more likely to process fear through avoidance and denial (Witte, 1994). Therefore, psychological techniques such as defensive processing may assist patients in conceptualizing risk in a way that is manageable and actionable. By discussing patients’ perceived risk, genetic counselors can explore patients’ personal beliefs about their risks, identify misconceptions that may interfere with adherence, and support beliefs that may increase beneficial behaviors.
Research Recommendations
Future research will be necessary to elucidate the processes underlying the persistent underestimation and overestimation of subjective risk in the different groups of unaffected patients, as well as to examine whether these results extend to other methods of conveying risk information (e.g., visual versus statistical). In particular, we offer several suggestions for future research directed toward understanding the persistence of relatively low subjective risk estimates among unaffected carriers. First, because risk perceptions did not change following melanoma genetic testing, increases in risk perceptions are unlikely to be the mechanism responsible for the improved compliance with screening recommendations among unaffected carriers identified in prior research (Aspinwall et al., 2008, 2013b). Instead, changes in other health cognitions, such as perceived response efficacy for photoprotection and screening, may account for improvements. Specifically, learning that reducing UVR exposure may reduce melanoma risk even for patients with p16 mutations may promote improved photoprotection behavior (see Aspinwall et al., 2009). Likewise, learning about improved survival rates associated with early detection of thinner melanomas may promote improved adherence to screening. A second possibility is that unaffected carriers’ lower subjective risk estimates may reflect intentions to undertake preventive behaviors (Thompson & Schlehofer, 2008; Weinstein & Nicolich, 1993). Third, a related possibility noted in our discussion of practice implications is that seemingly defensive or optimistic risk estimates may play a constructive role in attention to and management of health risks (Armor & Taylor, 1998; Aspinwall, Richter, & Hoffman, 2001). Specifically, perceiving one’s risk to be objectively high, but lower than the highly elevated risk conveyed in the counseling session, may help people manage emotional challenges posed by the test result. Such decreased risk perceptions may allow for or preserve beliefs that personal actions may prevent melanoma, thus promoting improved adherence to risk-reduction behaviors. Longitudinal studies in a sample sufficiently large to permit analysis of the reciprocal relations among risk perceptions, other health cognitions, and adherence to management recommendations over time in different patient groups are needed to test these hypotheses.
Conclusions
Risk perceptions did not change following melanoma genetic testing in any patient group, despite reported changes in behavioral compliance by these same individuals (Aspinwall et al., 2013b). Consistent with hypotheses, 1) unaffected carriers consistently reported lower melanoma risk estimates than those provided during genetic counseling and test reporting, 2) unaffected noncarriers consistently reported elevated estimates compared to what they were told, and 3) affected carriers reported high risk estimates at all assessments that corresponded closely to counselor-provided estimates. Future research should elucidate the adaptiveness of holding subjective melanoma risk estimates that are lower or higher than the information provided during genetic counseling by examining the basis for such perceptions and the extent to which they may help or hinder patients in managing the emotional and behavioral challenges of elevated familial cancer risk.
Acknowledgments
The authors gratefully acknowledge the generous participation of all the family members in this study without whom this project would not have been possible; Marybeth Hart, Erin Dola, and Lisa Wadge for their contributions to the development of the study; Amber Kostial, Emily Bullough, Michelle Welch, Hoda Wali, Candace Larson, and Taylor Haskell for their service as Study and/or Clinic Coordinators; and Angela Newman for assistance with preparation of the manuscript. This work was supported by a Funding Incentive Seed Grant, Office of the Vice President for Research, University of Utah, and a Cancer Control and Population Sciences Pilot Project Award from the Huntsman Cancer Institute awarded to Lisa G. Aspinwall and Sancy A. Leachman. Support was also received from the Huntsman Cancer Foundation (HCF), the Tom C. Mathews, Jr. Familial Melanoma Research Clinic endowment, the Pedigree and Population Resource of Huntsman Cancer Institute, and the Utah Population Database. This research was supported by the Utah Cancer Registry, which is funded by contract N01-PC-35141 from the National Cancer Institute SEER Program with additional support from the Utah State Department of Health and the University of Utah. The authors acknowledge the use of core facilities supported by the National Institutes of Health through National Cancer Institute (NCI) Cancer Center Support Grant 5P30CA420-14 awarded to Huntsman Cancer Institute, the genetic counseling core facility supported by the Huntsman Cancer Foundation, and National Center for Research Resources grant 1KL2RR025763-01 awarded to the University of Utah by the National Institutes of Health Office of the Director. The authors were supported in part in the preparation of this article by Award Number R01 CA158322-01 from the National Cancer Institute.
Footnotes
Because the absolute likelihood measure was highly correlated with the other 2 risk assessments and yielded highly similar results, complete results have been omitted from the manuscript but may be obtained by writing to the authors.
The ANCOVA also controlled for the interaction of each covariate with Time of Assessment. For lifetime percent risk, this yielded an interaction of Eye Color X Time, which indicated that darker eye color predicted marginally lower risk at baseline (p<.096) but not at any subsequent assessment.
For comparative risk, the main effect of Group remained significant, F(2,46)=18.53, p<.001), but the main effect of Time was no longer significant, F(5,42)=1.44, p=.232). Furthermore, the Group x Time interaction was marginally significant, F(10,84)=1.74, p=.085). The relative ordering of the three groups was similar to that shown in Figure 3, such that affected carriers rated themselves as having the greatest risk (adjusted M=4.74), followed by unaffected carriers (adjusted M=3.76), and noncarriers (adjusted M=3.35). Additionally, there were significant covariate by time interactions for age, eye color, and moles. For age and eye color, there were no significant predictive relationships for either covariate at any assessment; however, the direction of the coefficients depended on time of assessment. Specifically, greater age predicted lower risk at baseline but not subsequent assessments, while darker eye color predicted lower risk at baseline, but greater risk at 6 months and 1 year. The presence of moles showed a strong and significant relationship to increased risk perceptions at baseline and post-counseling, and marginally significant relationships at 1 month and 1 year, but no relationship to risk perceptions at 2 years.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Lisa G. Aspinwall, Jennifer M. Taber, Wendy Kohlmann, Samantha L. Leaf, and Sancy A. Leachman declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Armor DA, Taylor SE. Situated optimism: Specific outcome expectancies and self-regulation. In: Zanna MP, editor. Advances in Experimental Social Psychology. Vol. 30. 1998. pp. 309–379. [Google Scholar]
- Aspinwall LG, Leaf S, Dola E, Kohlmann W, Leachman S. CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(6):1510–1519. doi: 10.1158/1055-9965.EPI-08-0010. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Leaf S, Kohlmann W, Dola E, Leachman S. Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. Journal of The American Academy of Dermatology. 2009;60(5):745–757. doi: 10.1016/j.jaad.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Richter L, Hoffman RR. Understanding how optimism “works”: An examination of optimists’ adaptive moderation of belief and behavior. In: Chang EC, editor. Optimism and pessimism: Theory, research, and practice. Washington: American Psychological Association; 2001. pp. 217–238. [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Genetic testing for hereditary melanoma and pancreatic cancer: A longitudinal study of psychological outcome. Psychooncology. 2013a;22(2):276–289. doi: 10.1002/pon.2080. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiology, Biomarkers, & Prevention. 2013b;22(10):1687–1697. doi: 10.1158/1055-9965.EPI-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenmar M, Hansson J, Brandberg Y. Family members’ perceptions of genetic testing for malignant melanoma--a prospective interview study. European Journal of Oncology Nursing: The Official Journal of European Oncology Nursing Society. 2009;13(2):74–80. doi: 10.1016/j.ejon.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Bishop D, Demenais F, Goldstein A, Bergman W, Bishop J, Bressac-de Paillerets B, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. Journal of The National Cancer Institute. 2002;94(12):894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- Braithwaite D, Emery J, Walter F, Prevost A, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. Journal of The National Cancer Institute. 2004;96(2):122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright LA, Goldgar DE, Meyer LJ, Lewis CM, Anderson DE, Fountain JW, et al. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13–022. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Claes E, Denayer L, Evers-Kiebooms G, Boogaerts A, Legius E. Predictive testing for hereditary non-polyposis colorectal cancer: Motivation, illness representations and short-term psychological impact. Patient Education and Counseling. 2004;55(2):265–274. doi: 10.1016/j.pec.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Claes E, Evers-Kiebooms G, Denayer L, Decruyenaere M, Boogaerts A, Philippe K, et al. Predictive genetic testing for hereditary breast and ovarian cancer: Psychological distress and illness representations 1 year following disclosure. Journal of Genetic Counseling. 2005;14(5):349–363. doi: 10.1007/s10897-005-1371-4. [DOI] [PubMed] [Google Scholar]
- Croyle R, Loftus E, Barger S, Sun Y, Hart M, Gettig J. How well do people recall risk factor test results? Accuracy and bias among cholesterol screening participants. Health Psychology. 2006;25(3):425–432. doi: 10.1037/0278-6133.25.3.425. [DOI] [PubMed] [Google Scholar]
- Croyle RT, Lerman C. Risk communication in genetic testing for cancer susceptibility. Journal of the National Cancer Institute Monographs. 1999;25:59–66. doi: 10.1093/oxfordjournals.jncimonographs.a024210. [DOI] [PubMed] [Google Scholar]
- Ditto PH, Croyle RT. Understanding the impact of risk factor test results: Insights from a basic research program. In: Croyle RT, editor. Psychosocial effects of screening for disease prevention and detection. New York: Oxford University Press; 1995. pp. 144–181. [Google Scholar]
- Fears TR, Guerry D, Pfeiffer RM, Sagebiel RW, Elder DE, Halpern A, et al. Identifying individuals at high risk of melanoma: a practical predictor of absolute risk. Journal of Clinical Oncology. 2006;24:3590–3596. doi: 10.1200/JCO.2005.04.1277. [DOI] [PubMed] [Google Scholar]
- Glanz K, Volpicelli K, Kanetsky PA, Ming ME, Schuchter LM, Jepson C, et al. Melanoma genetic testing, counseling, and adherence to skin cancer prevention and detection behaviors. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:607–614. doi: 10.1158/1055-9965.EPI-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Grover S, Stoffel EM, Mercado RC, Ford BM, Kohlman WK, Shannon KM, et al. Colorectal cancer risk perception on the basis of genetic test results in individuals at risk for lynch syndrome. Journal of Clinical Oncology. 2009;27(24):3981–3986. doi: 10.1200/JCO.2008.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurmankin A, Domchek S, Stopfer J, Fels C, Armstrong K. Patients’ resistance to risk information in genetic counseling for BRCA1/2. Archives of Internal Medicine. 2005;165(5):523–529. doi: 10.1001/archinte.165.5.523. [DOI] [PubMed] [Google Scholar]
- Hansen CB, Wadge LM, Lowstuter K, Boucher K, Leachman SA. Clinical germline genetic testing for melanoma. Lancet Oncology. 2004;5(5):314–319. doi: 10.1016/S1470-2045(04)01469-X. [DOI] [PubMed] [Google Scholar]
- Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genetics in Medicine. 2008;10(1):19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- Kamb A, Shattuck-Eidens D, Eeles R, Liu Q, Gruis NA, Ding W, et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nature Genetics. 1994;1:23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Kasparian N, Meiser B, Butow P, Simpson J, Mann G. Genetic testing for melanoma risk: a prospective cohort study of uptake and outcomes among Australian families. Genetics in Medicine. 2009;11(4):265–278. doi: 10.1097/GIM.0b013e3181993175. [DOI] [PubMed] [Google Scholar]
- Kefford RF, Mann GJ. Is there a role for genetic testing in patients with melanoma? Current Opinion in Oncology. 2003;15:157–161. doi: 10.1097/00001622-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Kefford RF, Newton Bishop JA, Tucker M, Bressac-de Paillerets B, Bianchi-Scarra G, Bergman W, et al. Genetic testing for melanoma. Lancet Oncology. 2002;3(11):653–654. doi: 10.1016/s1470-2045(02)00894-x. [DOI] [PubMed] [Google Scholar]
- Kelly K, Leventhal H, Andrykowski M, Toppmeyer D, Much J, Dermody J, et al. Using the common sense model to understand perceived cancer risk in individuals testing for BRCA1/2 mutations. Psycho-Oncology. 2005;14(1):34–48. doi: 10.1002/pon.805. [DOI] [PubMed] [Google Scholar]
- Klein WM, Weinstein ND. Social comparison and unrealistic optimism about personal risk. In: Buunk BP, Gibbons FX, editors. Health, coping, and well-being: Perspectives from social comparison theory. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 25–61. [Google Scholar]
- Leachman S, Carucci J, Kohlmann W, Banks K, Asgari M, Bergman W, et al. Selection criteria for genetic assessment of patients with familial melanoma. Journal of The American Academy of Dermatology. 2009;61(4):677. e1–14. doi: 10.1016/j.jaad.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Lustbader E, Rimer B, Daly M, Miller S, Sands C, et al. Effects of individualized breast cancer risk counseling: a randomized trial. Journal of The National Cancer Institute. 1995;87(4):286–292. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- Lipkus I, McBride C, Pollak K, Lyna P, Bepler G. Interpretation of genetic risk feedback among African American smokers with low socioeconomic status. Health Psychology. 2004;23(2):178–188. doi: 10.1037/0278-6133.23.2.178. [DOI] [PubMed] [Google Scholar]
- McQueen A, Swank P, Bastian L, Vernon S. Predictors of perceived susceptibility of breast cancer and changes over time: A mixed modeling approach. Health Psychology. 2008;27(1):68–77. doi: 10.1037/0278-6133.27.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, Weinman J, Miller J, Collins V, Halliday J, Marteau T. Predictive genetic testing: High risk expectations in the face of low risk information. Journal of Behavioral Medicine. 2002;25(1):33–50. doi: 10.1023/a:1013537701374. [DOI] [PubMed] [Google Scholar]
- Ranby KW, Aiken LS, Gerend MA, Erchull MJ. Perceived susceptibility measures are not interchangeable: Absolute, direct comparative, and indirect comparative risk. Health Psychology. 2010;29:20–28. doi: 10.1037/a0016623. [DOI] [PubMed] [Google Scholar]
- Schafer JL. NORM: multiple imputation of incomplete multivariate data under a normal model (for Windows 95/98/NT)[computer program] 1999 Version 2. Available from http://www.stat.psu.edu/~jls/misoftwa.html.
- Schafer J, Graham J. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Sivell S, Elwyn G, Gaff C, Clarke A, Iredale R, Shaw C, et al. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: Systematic review. Journal of Genetic Counseling. 2008;17(1):30–63. doi: 10.1007/s10897-007-9132-1. [DOI] [PubMed] [Google Scholar]
- Smerecnik C, Mesters I, Verweij E, de Vries N, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. Journal of Genetic Counseling. 2009;18(3):217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber JM, Aspinwall LG, Kohlmann W, Dow R, Leachman SA. Parental preferences for CDKN2A/p16 genetic testing of minors. Genetics in Medicine. 2010;13:823–838. doi: 10.1097/GIM.0b013e3181f87278. [DOI] [PubMed] [Google Scholar]
- Thompson SC, Schlehofer MM. Control, denial, and heightened sensitivity reactions to personal threat: Testing the generalizability of the threat orientation approach. Personality and Social Psychology Bulletin. 2008;34:1070–1083. doi: 10.1177/0146167208318403. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Unrealistic optimism about future life events. Journal of Personality and Social Psychology. 1980;39(5):806–820. [Google Scholar]
- Weinstein ND, Klein W. Resistance of personal risk perceptions to debiasing interventions. Health Psychology. 1995;14(2):132–140. doi: 10.1037//0278-6133.14.2.132. [DOI] [PubMed] [Google Scholar]
- Weinstein ND, Nicolich M. Correct and incorrect interpretations of correlations between risk perceptions and risk behaviors. Health Psychology. 1993;12:235–245. doi: 10.1037//0278-6133.12.3.235. [DOI] [PubMed] [Google Scholar]
- Wiebe DJ, Korbel C. Defensive denial, affect, and the self-regulation of health threats. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behavior. London: Routledge; 2003. pp. 184–203. [Google Scholar]
- Witte K. Fear control and danger control: a test of the extended parallel process model (EPPM) Communication Monographs. 1994;61:113–134. [Google Scholar]