Abstract
Hepatic mitochondrial adaptations to exercise are largely unknown.
PURPOSE
Here we sought to determine the effects of various exercise modalities on measures of hepatic mitochondrial function and metabolism.
METHODS
Male Sprague Dawley rats were randomly assigned (n=8-10 per group) into sedentary (SED), voluntary wheel running (VWR), VWR with food pulled during the dark cycle (VMR-OF), treadmill endurance exercise (TM-END; 30 m/min, 12% gradient, 60 min/d, 5 d/wk), or treadmill interval sprint training (TM-IST; 50 m/min, 12% gradient, 6×2.5 min bouts, 5 d/wk) groups for a 4 week intervention.
RESULTS
Hepatic mitochondrial state 3 and maximal uncoupled respiration were significantly (p<0.05) increased in all 4 exercise groups compared with SED animals. In addition, hepatic mitochondrial [1-14C] pyruvate oxidation to CO2, an index of pyruvate dehydrogenase (PDH) activity was significantly increased in VWR-OF, TM-END, and TM-IST rats (p<0.05); whereas, exercise-induced increases in [2-14C] pyruvate oxidation and [1-14C] palmitate oxidation to CO2 did not reach statistical significance. Hepatic mitochondrial sirtuin 3 (SIRT3) protein content, which putatively increases activity of mitochondrial proteins, was elevated in the VWR, VWR-OF and TM-END groups (p<0.05). Additionally, only VWR-OF animals experienced increases in hepatic cytochrome c protein content and PEPCK mRNA, while PGC-1α mRNA expression and phospho-CREB protein content was increased in VWR-OF and TM-END groups.
CONCLUSION
Four weeks of exercise training, regardless of exercise modality, significantly increased hepatic mitochondrial respiration and evoked other unique improvements in mitochondrial metabolism that do not appear to be dependent on increases in mitochondrial content.
Keywords: exercise, hepatic mitochondria, fatty acid oxidation, mitochondrial function
INTRODUCTION
Exercise is a stimulus capable of challenging mitochondria, causing adaptation to meet the demand for adenosine triphosphate (ATP) production (8). It is well known that exercise training can elicit increases in skeletal muscle mitochondrial content and function to maximize oxidative capacity and substrate utilization for ATP synthesis (10, 11, 18, 21). However, much less is known about the adaptations induced by exercise training on hepatic mitochondria. In the liver, mitochondrial ATP production serves other functions during exercise. The liver plays a vital role in maintaining circulating blood glucose levels through the activation of gluconeogenesis during times of fasting or exercise. Hepatic mitochondria fuel these energy costly gluconeogenic processes with ATP formation in part through the oxidation of fatty acids that are lipolyzed from hepatic and adipose stores (6).
Emerging evidence suggests that exercise training can induce adaptations in markers of hepatic mitochondrial content and function. Eight weeks of treadmill running has been shown to increase hepatic cytochrome c oxidase activity (29) and increase the activity of mitochondrial complexes I, IV, and V in the liver compared with sedentary rats (37). In addition, ten weeks of resistance training in ovariectomized rats significantly increased hepatic β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity and CPT-1 mRNA expression (12). Furthermore, we have previously shown that voluntary wheel running increased several indices of hepatic mitochondrial content and function, including complete palmitate oxidation, β-HAD and citrate synthase activities, and COX IV-Subunit I and cytochrome c protein content in the Otsuka Long-Evans Tokushima Fatty (OLETF) rat model (31, 33). In addition, we have also found similar results in lean Fischer 344 × Brown Norway F1 hybrid rats who underwent 6 weeks of voluntary wheel running, where hepatic PGC-1α mRNA expression and palmitate oxidation were increased in running compared with sedentary rats (22). In addition, rats that are bred for high running capacity, and therefore have a phenotype characteristic of a exercise trained animal, also display higher palmitate oxidation and mitochondrial content compared to rats bred for low running capacity in a sedentary state (38). In total, these studies suggests that daily exercise leads to enhanced hepatic mitochondrial function; however, more thorough studies need to be conducted to elucidate the different aspects of mitochondrial function that are influenced by exercise training.
The purpose of this investigation was to examine and compare the impact of voluntary wheel running and daily treadmill exercise on hepatic mitochondrial content and function, as assessed by mitochondrial respiration, palmitate and pyruvate oxidation, and mitochondrial enzyme activities, in healthy Sprague Dawley rats. Endurance and interval sprint training treadmill exercise, two common forms of exercise training, were used because these two forms of exercise tend to rely on different substrate use (fatty acids vs. glucose, respectively). Furthermore, we sought to maximize the hepatic gluconeogenic effect in one of two voluntary wheel running groups by removing the food just before the dark cycle, therefore causing the rats to run nightly in the fasted state. We hypothesized that the combination of exercise and fasting would maximize the need for hepatic gluconeogenesis to maintain euglycemia, resulting in additional mitochondrial adaptations in the liver. We also hypothesized that while all forms of training would have beneficial effects on hepatic mitochondria, voluntary wheel running would have the greatest impact due to a higher volume of daily exercise.
METHODS
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia and adhered to the animal care standards of the American College of Sports Medicine. Forty-six male Sprague Dawley rats (12 weeks of age) were randomly assigned (n=8-10 per group) into sedentary (SED), voluntary wheel running (VWR), voluntary wheel running with overnight fasting (VWR-OF), treadmill endurance training (TM-END), or treadmill interval sprint training (TM-IST) groups for a 4 week intervention. All rats were individually housed during the intervention in temperature controlled animal quarters (21°C) with a 0600-1800 light and 1800-0600 dark cycle. All groups were provided standard rodent chow (Formulab 5008; Purina Mills, Brentwood, MO) for ad libitum feeding in new cages at the beginning of each week. Rats assigned to the voluntary wheel running groups (VWR, VWR-OF) were housed in cages equipped with voluntary running wheels. Running wheel revolutions were monitored and counted continuously through the 4 week intervention using VitalView software (VitalView, Version 4.2, 2007; Mini Mitter Company, Inc., Bend, OR). The food of the VWR-OF rats was pulled at 1700 and returned the next morning at 0800. The TM-END and TM-IST animals were acclimated to the treadmill for five days at a speed 15 m/min for 5-10 min. The first week and a half of the intervention was a ramp-up phase for both treadmill groups. The TM-END group started at 20 m/min for 10 minutes at a 12% gradient and gradually increased to 30m/min for 60 minutes (12% gradient), 5 days/week for the remainder of the 4 week intervention. In a similar fashion, the TM-IST groups started with 6 sprints at 35 m/min, 1 minute in duration (12 % gradient), and 4.5 minutes rest in between sprints, then gradually increased to six 2.5 minutes sprints at 50m/min (12% gradient) with 4.5 minutes rest in between sprints. Both treadmill groups completed the exercise training in the morning hours while still in the fed state. Body mass and food consumption were measured weekly throughout the investigation. To calculate feeding efficiency, a measurement that allows for the assessment of energy expenditure without direct or indirect calorimetry, the amount of body weight gained was divided by the amount of food consumed (g gain/g intake). At 16 weeks of age, rats were anesthetized with pentobarbital sodium (100 mg/kg) and then exsanguinated by removal of the heart 24 hours after locking of wheels (VWR, VWR-OF) or the last bout of treadmill exercise (TM-END, TM-IST). Animals from each group (SED, VWR, VWR-OF, TM-END, and TM-IST) were killed following an 18 hour fast. Retroperitoneal, epididymal, and omental adipose tissue fat pads were excised from exsanguinated rats and weighed.
Tissue homogenization and mitochondrial isolation procedures
Livers were quickly excised from anesthetized rats and either flash-frozen in liquid nitrogen, or placed in ice-cold isolation buffer (in mM: 220 Mannitol, 70 sucrose, 10 tris-base, 1 EDTA; pH 7.4). Hepatic mitochondria were prepared as previously described (23, 34). Briefly, 1 gram of liver was minced in ice cold mitochondrial isolation buffer (220 mM Mannitol, 70mM Sucrose, 10 mM Tris-base and 1 mM EDTA, pH 7.4), transferred to a 30ml glass tube, on ice, and homogenized with a teflon pestle. The homogenate was then transferred to a 15-ml conical tube and centrifuged (1,500 g, 10 min, 4°C). The pellet was discarded and the supernatant was centrifuged (8,000 g, 10 min, 4°C). The pellet was then resuspended in mitochondrial isolation buffer, homogenized in a glass tube using a glass pestle, and centrifuged (6,000 g, 10 min, 4°C). The pellet was again resuspended in mitochondrial isolation buffer containing fatty acid-free 0.1% BSA, homogenized with glass on glass homogenization, and centrifuged (4,000 g, 10 min, 4°C). Finally, the supernatant was discarded and the mitochondrial pellet was then split and placed in either 1000μl of SET buffer (250 mM Sucrose, 1 mM EDTA, 10 mM Trizma Hydrochloride, and 2 mM ATP, pH 7.4) for palmitate and pyruvate oxidation experiments, or resuspended in MiPO3 buffer (0.5 mM EGTA, 3 mM MgCl2·6H20, 60 mM K-lactobionate, 20 mM Taurine, 10 mM KH2P04, 20mM HEPES, 110 mM Sucrose, 1g/l BSA, 20 mM Histidine, 20 μM vitamin E succinate, 3 mM glutathione, 1 μM leupeptine, 2 mM glutamate, 2 mM malate, 2 mM Mg-ATP) for mitochondrial respiration. Protein concentration was determined by BCA assay.
Palmitate and pyruvate oxidation
Oxidation of [1-14C] palmitate (American Radiochemicals; St. Louis, MO), [1-14C] pyruvate (PerkinElmer; Boston, MA), and [2-14C] pyruvate (PerkinElmer; Boston, MA) were measured in fresh isolated hepatic mitochondria preparations. The collection and measurement of 14C palmitate oxidation allowed for the estimation of complete fatty acid oxidation and was conducted as previously described (31) with the exception of a slight modification with the addition of 2mM ADP. As previously described (23), pyruvate ([1-14C] and [2-14C]) were oxidized to 14CO2 by isolated hepatic mitochondria in the appropriate reaction buffer. [1-14C] pyruvate oxidation was used as an index of pyruvate dehydrogenase activity and [2-14C] pyruvate oxidation as an index of tricarboxylic acid (TCA) cycle flux (25).
Mitochondrial respiration
Mitochondrial respiration was assessed using high-resolution respirometry (Oroboros Oxygraph-2k; Oroboros Instruments; Innsbruck, Austria). Isolated mitochondria (100-150 μg protein) were initially placed in respiration chambers in respiration media (MiR05; sucrose, 100 mM; K-lactobionate, 60 mM; EGTA, 0.5 mM; MgCl2, 3 mM; taurine, 20 mM; KH2PO4, 10 mM; HEPES, 20 mM; adjusted to pH 7.1 with KOH at 37C; and 1 g/L fatty acid free BSA) for assessment of basal respiration (Basal). Oxygen flux was measured by addition of glutamate (5mM) and malate (2mM) to the chambers in the absence of ADP (GM-State 2) for assessment of State 2 respiration. Oxidative phosphorylation (OXPHOS) with electron flux through complex I was then quantified by titration of ADP (25-125 μM) (GM+ADP: State 3-Complex I) for assessment of State 3 respiration. Maximal ADP respiration with electron flux through both complex I and complex II was assessed by the addition of succinate (10 mM) (Succinate: State 3-Complex I+II). Finally, maximal capacity of the electron transport system was assessed by uncoupling with the addition of FCCP (Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, 0.25 μM) (Uncoupled).
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity
Hepatic citrate synthase and β-HAD activity were measured as previously described by Srere (36) and Bass et al. (4), respectively, as well as previously described by our lab (31).
PEPCK, G6Pase, and PGC-1α mRNA expression
Phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase) mRNA expression was quantified as previously described (32). Briefly, to examine PEPCK and G6Pase, the primer dilution mix [nuclease-free water, and both forward and reverse primers (Sigma), Fast Sybr Green Master Mix kit (ABI)], and cDNA sample (50 ng) were loaded to a 96-well microplate and placed into the Roche Light Cycle 480 system (Roche, Rotkreuz, Switzerland) for polymerization. PGC-1α gene expression was assessed by loading TaqMan Master Mix (ABI), PGC-1α primers and probe (Life technologies), and cDNA samples (250 ng) into a 96-well microplate and placed into the ABI 7500 Fast Sequence Detection System (Applied Biosystems, Carlsbad, CA) for polymerization. Once polymerization was completed, results were quantified using the DdCT technique relative to the 18S housekeeping gene.
Western blotting
Western blot analyses were performed to determine protein content of the following: oxidative phosphorylation (OXPHOS) electron transport chain complexes I through V (MitoProfile Total OXPHOS Rodent WB Antibody Cocktail; Abcam, Cambridge, MA.), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α; EMD Millipore Corp., Billerica, MA), NAD-dependent deacetylase sirtuin-1 (SIRT1; Santa Cruz Biotechnology, Santa Cruz, CA), NAD-dependent deacetylase sirtuin-3 (SIRT3; Cell Signaling, Beverly, MA), cAMP-responsive Element-binding Protein (CREB and phospho-CREB(Ser133); Cell Signaling), AMP-activated protein kinase(AMPK and phospho-AMPK (Thr 172); Cell Signaling), and cytochrome c (Cell Signaling). Phosphorylation status (using phosphospecific antibodies) was calculated from the density of the phosphoprotein band divided by density of the total protein using the appropriate antibody (30, 31). Membranes stained with 0.1% amido-black (Sigma) were quantified to control for differences in protein loading or transfer of band densities as previously described (31).
Statistics
Each outcome measure was examined in eight to ten animals per group. Differences among groups were examined by a one-way analysis of variance (IBM SPSS, version 20.0; SPSS, Chicago, Illinois) and significant main effects (P< 0.05) were followed with Fisher LSD post hoc comparisons. Values are reported as means ± standard errors (SE), and a P value of ≤0.05 denotes a statistically significant difference.
RESULTS
Animal characteristics
VWR and VWR-OF groups ran ~4 km/day throughout the 4 week intervention and while running distance did not differ between groups in weeks 1-3, the VWR-OF group maintained daily running distance in week 4; whereas, the VWR running distance decreased from weeks 3 to weeks 4 (p<0.05; Fig.1A). The TM-END and TM-IST groups ran 1.8 and 0.75 kilometers per day, respectively. Heart weight to body weight ratio was significantly higher in VWR, VWR-OF, and TM-END groups compared with SED (p < 0.05; Fig.1B).
Figure 1.
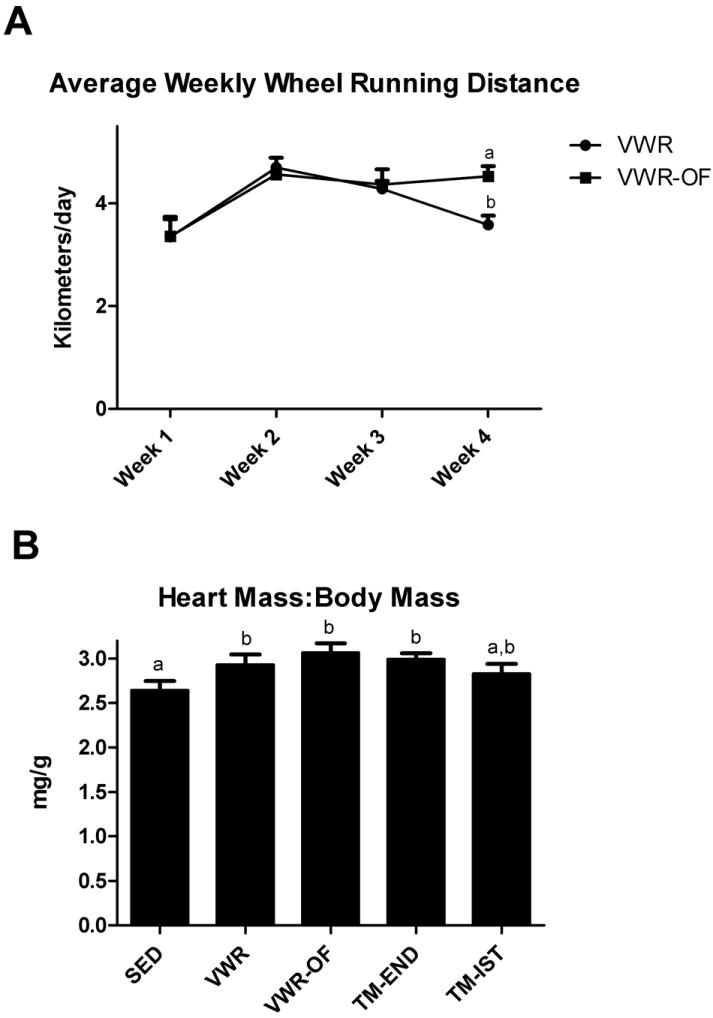
Weekly running distance (A) and heart mass to body mass ratio (B). SED, sedentary; VWR, voluntary wheel running; VWR-OF, voluntary wheel running overnight fasted; TM-END, treadmill endurance; TM-IST, treadmill interval sprint training. Values are means ± SE (n= 8-10). Values with different letters are significantly different (P<0.05).
The SED and VWR groups both weighed significantly more than the VWR-OF, TM-END, and TM-IST groups (Table 1). The SED group also developed significantly larger total fat pad (retroperitoneal, epididymal, and omental) mass than any exercise group (VWR, VWR-OF, TM-END, TM-IST; p≤0.05), with VWR also being greater than VWR-OF, TM-END, and TM-IST groups (p≤0.05; Table 1). VWR rats consumed significantly more food per week than all other groups, while the SED group consumed significantly more food than VWR-OF, TM-END, and TM-IST; Table 1. In addition, food consumption relative to body weight was significantly greater in the VWR rats compared to the SED, VWR-OF, TM-END, and TM-IST groups. Furthermore, both the SED and VWR groups had significantly higher feeding efficiency than either treadmill group (TM-END, TM-IST; Table 1).
Table 1.
Animal characteristics and hepatic mitochondrial enzyme activities.
| Variable | SED | VWR | VWR-OF | TM-END | TM-IST |
|---|---|---|---|---|---|
| Body Weight (g) | 512.3 ± 19.8 a | 491.2 ± 18.9 a | 431.8 ± 14.5 b | 430.4 ± 8.9 b | 423.1 ± 10.0 b |
| Fat Pad Mass (g) | 23.4 ± 2.1 a | 15.3 ± 2.3 b | 8.81 ± 1.6 c | 10.3 ± 0.7 b,c | 10.5 ± 1.0 b,c |
| Food Consumption (g)/week | 189.4 ± 4.4 a | 214.8 ± 4.8 b | 168.1 ± 6.7 c | 152.3 ± 6.3 d | 147.3 ± 3.3 d |
| Food Consumption/Body weight (g per wk/g body wt) | 0.39 ± 0.01 a | 0.46 ± 0.01 b | 0.38 ± 0.02 a,c | 0.35 ± 0.02 c | 0.34 ± 0.01 c |
| Feeding Efficiency | 0.14 ± 0.02 a | 0.12 ± 0.01 a | 0.08 ± 0.03 a,b | 0.03 ± 0.03 b | 0.04 ± 0.01 b |
| β-HAD, Relative to SED (%) (Whole Homogenate) | 100.0 ± 6.3 | 86.3 ± 5.2 | 89.8 ± 7.8 | 96.7 ± 7.6 | 97.1 ± 6.9 |
| β-HAD, Relative to SED (%) (Isolated Mitochondria) | 100.0 ± 4.7 | 99.9 ± 5.6 | 101.6 ± 7.3 | 101.6 ± 7.3 | 95.4 ± 4.8 |
| Citrate synthase, nmol·min-1·μg-1 (Whole Homogenate) | 47.3 ± 0.8 a | 50.7 ± 1.1 b | 49.8 ± 1.6 a,b | 46.6 ± 0.6 a | 46.7 ± 1.1 a |
| Citrate synthase, nmol·min-1·μg-1 (Isolated Mitochondria) | 77.0 ± 2.5 | 81.3 ± 2.1 | 84.2 ± 3.5 | 81.9 ± 8.0 | 79.7 ± 2.7 |
Values are means ± SE; n= 8-10 rats per group. SED, sedentary; VWR, voluntary wheel running; VWR-OF, voluntary wheel running overnight fasted; TM-END, treadmill endurance; TM-IST, treadmill interval sprint training. Fat pad mass was determined through the summation of omental, epididymal, and retroperitoneal fat pads. Values with different letters indicate significant differences (P≤0.05).
Markers of hepatic mitochondrial function
Oxidative phosphorylation (OXPHOS) with electron flux through complex I (GM+ADP: State 3-Complex I) and maximal uncoupled mitochondrial respiration (FCCP: Uncoupled) were significantly (p<0.05) increased in all 4 exercise groups compared with SED animals (Figure 2A). In addition, maximal ADP respiration with electron flux through both complex I and complex II (Succinate: State 3-Complex I+II) was significantly increased in the VWR and VWR-OF groups but not in the treadmill trained animals. [1-14C] pyruvate oxidation, an index of pyruvate dehydrogenase activity, was significantly greater in the VWR-OF, TM-END, and TM-IST groups compared to SED (p≤0.05; Fig.2B). Furthermore, [2-14C] pyruvate oxidation, an index of TCA cycle flux, tended to be higher in the exercise groups compared to the SED rats as reflected by 30-40% increases in the VWR, TM-END, and TM-IST groups, but this did not reach statistical significance (p=0.075; Fig 2C). Moreover, changes in [1-14C] palmitate oxidation did not reach statistical significance despite a 40% increase in the VWR compared to the SED group (Fig 2D). Whereas, incomplete palmitate oxidation was significantly reduced in VWR-OF, TM-END, and TM-IST animals compared to the SED group (p<0.05; Fig.2E). Additionally, citrate synthase activity in whole liver homogenate was significantly higher in the VWR group compared with SED (p<0.05, Table 1), while β-HAD activity did not differ among groups (Table 1).
Figure 2.
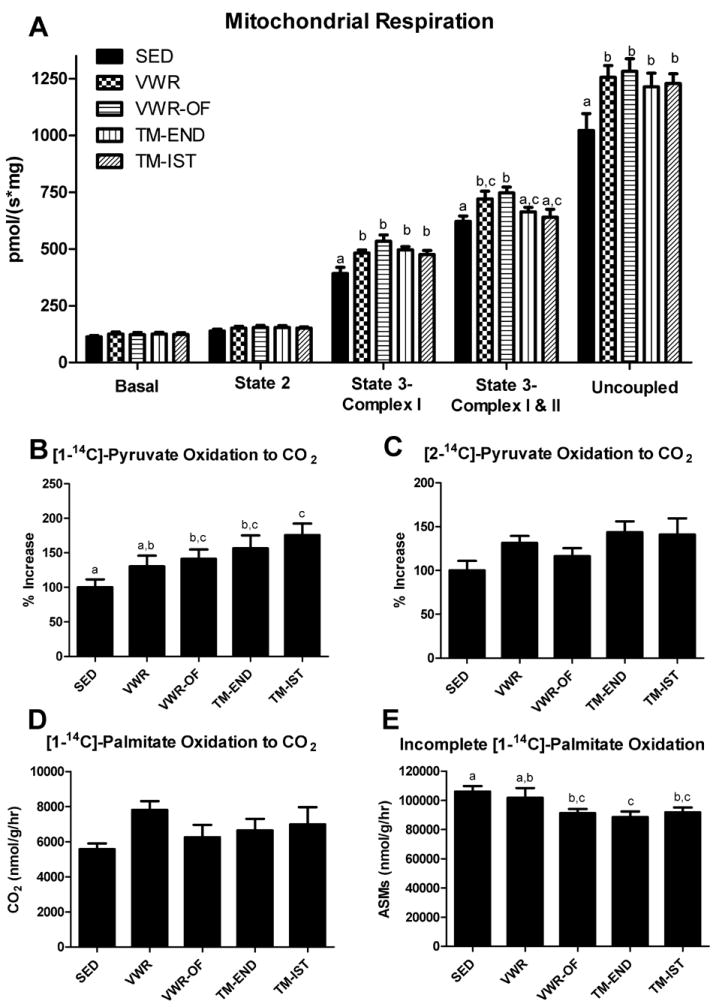
Effects of various exercise modalities on hepatic mitochondrial respiration (A), [1-14C]-pyruvate oxidation to CO2 (B), [2-14C]-pyruvate oxidation to CO2 (C), [1-14C]-palmitate oxidation to CO2 (D), and incomplete [1-14C]-palmitate oxidation (E), in isolated mitochondria. Values are means ± SE (n= 8-10). Values with different letters are significantly different (P<0.05).
Markers of hepatic mitochondrial content and biogenesis
Hepatic cytochrome c protein content, a marker of mitochondrial content, was significantly increased in the VWF-OF group only (p<0.05 vs. SED; Fig. 3A). PCG-1α protein content was assessed due to its role in mitochondrial biogenesis. Neither four weeks of voluntary wheel running nor treadmill training was sufficient to alter hepatic PGC-1α protein content (Fig. 3B). In addition, no differences were detected among groups for hepatic SIRT-1 protein content (Fig. 3C), a protein responsible for deacetylating and increasing PGC-1α activation. We then measured hepatic SIRT3, a mitochondrial deacetylase that activates numerous mitochondrial proteins, to assess the potential activation of multiple mitochondrial proteins in response to exercise. SIRT3 protein content measured in isolated mitochondria was ~2-fold higher in the VWR, VWR-OF, and TM-END groups compared to the SED group (p<0.05, Fig. 3D). Despite the increase in mitochondrial SIRT3, we found no differences in the acetylation status of the mitochondrial proteins with a western blot for acetylated-lysine (data not shown). There are reservations regarding sensitivity of this technique and it is possible that the acetylation status of specific mitochondrial proteins was changed but not detected using this method. Examination of the protein content of individual complexes in the electron transport chain (OXPHOS), as they are markers of mitochondrial content and potential targets of SIRT-3, revealed no significant effect of the various exercise training interventions (Figure 3E). Finally, we also witnessed no differences in hepatic AMPK activation (phosphorylation status) among any of the exercise groups (data not shown).
Figure 3.
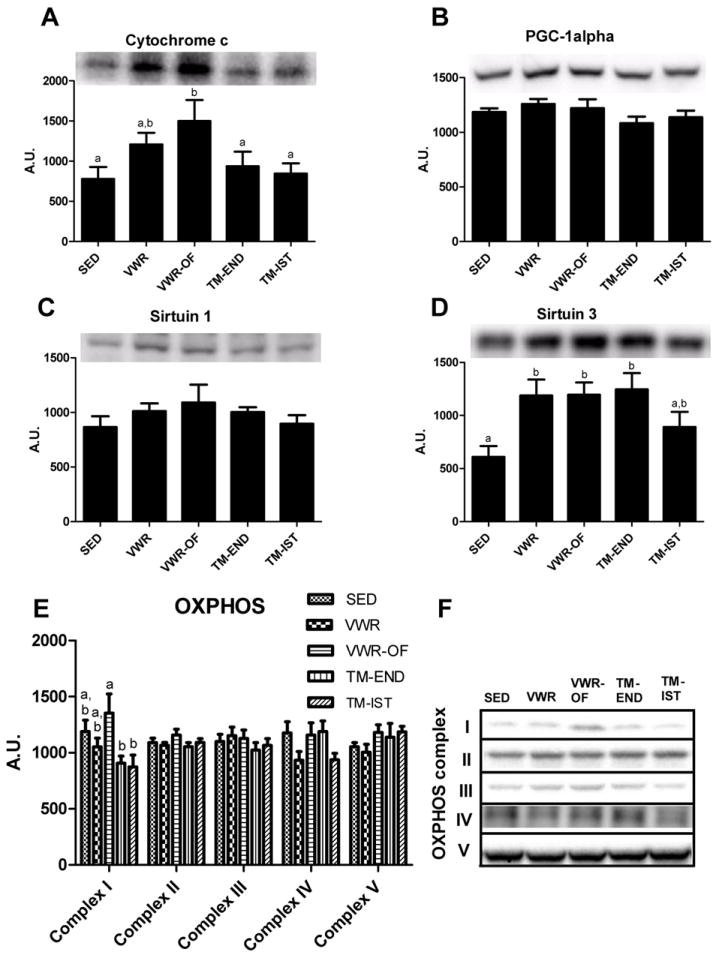
Effects of various exercise modalities on hepatic cytochrome c protein content (A), content PGC-1α protein content (B), SIRT1 protein content (C), SIRT3 protein content measured in isolated mitochondria (D) and oxidative phosphorylation (OXPHOS) complex I-V protein content (E). Representative Western blots shown in (F). Values are means ± SE (n= 8-10). Values with different letters are significantly different (P<0.05). AU, arbitrary units; PGC-1α, peroxisome proliferator-activated receptor coactivator-1α.
Gluconeogenesis and the transcription factor CREB
To determine if the VWR-OF treatment was effective in stimulating a pathway unique to gluconeogenesis, we measured the phosphorylation and activation of CREB, a transcription factor that upregulates gluconeogenic genes. Voluntary wheel running with overnight fasting and treadmill endurance running caused greater than 2-fold increases in the ratio of phosphorylated CREB to CREB protein content compared with SED group (p≤0.05; Fig.4A). PGC-1α, PEPCK, and G6Pase mRNA expression were measured to examine whether the increases in phosphorylated CREB protein content resulted in upregulation of genes important to mitochondrial biogenesis and gluconeogenesis. PGC-1α gene expression followed a similar trend as phosphorylated CREB protein content, with increases seen in VWR-OF and TM-END animals (p<0.05; Fig 4B). In addition, PEPCK mRNA expression was significantly elevated only in the VWR-OF rats compared to the SED (Fig. 4C). G6Pase mRNA expression did not differ among groups (Fig. 4D).
Figure 4.
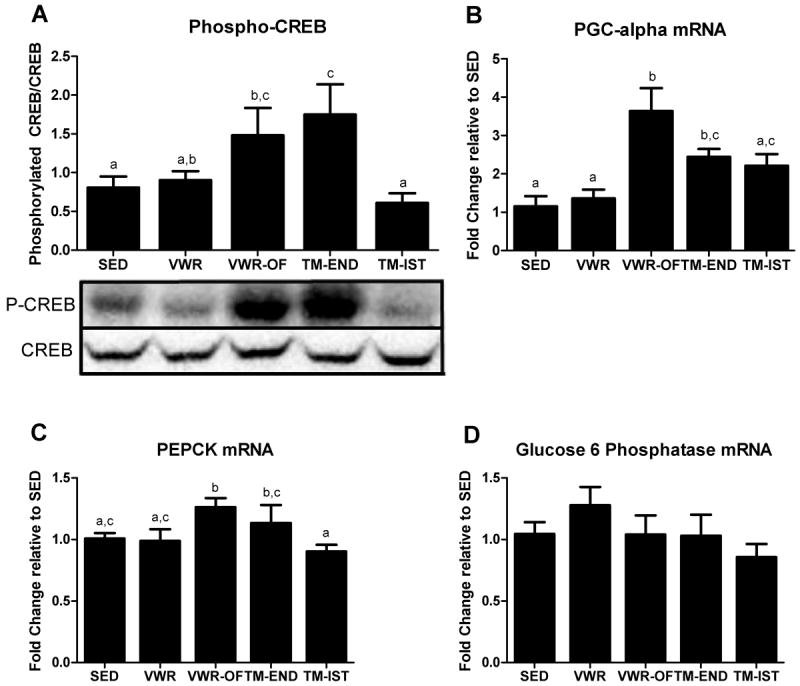
Effects of various exercise modalities on hepatic cAMP-responsive element-binding protein (CREB) protein content (A), PGC-1α mRNA expression (B), PEPCK mRNA expression (C), and glucose 6 phosphatase mRNA expression (D). Values are means ± SE (n= 8-10). Values with different letters are significantly different (P<0.05).
DISCUSSION
It is well known that exercise training increases mitochondrial content (11), as well as improves mitochondrial function (10, 18, 21) in skeletal muscle. Surprisingly, little is known about the effects exercise training has on hepatic mitochondria. To our knowledge, this is the first study to examine the effects of multiple modalities of exercise training (voluntary wheel running and treadmill training) on hepatic mitochondrial content and function in healthy rats. A novel finding of this study is that different exercise modalities resulted in greater hepatic mitochondrial respiration and that each modality elicits effects on different indices of mitochondrial function. It also was found that exercise-induced increases in indices of hepatic mitochondrial function were not dependent on increases in hepatic mitochondrial content.
One of the most accurate methods used to assess mitochondrial function is the measurement of mitochondrial respiration (27). Here we report novel findings that regardless of the training stimuli, the exercise intensity, or the fasting state of the animal, all 4 exercise groups (VWR, VWR-OF, TM-END, and TM-IST) significantly increased ADP-stimulated oxidative phosphorylation and maximal uncoupled hepatic mitochondrial respiration. These findings contradict those by Ascensao et al. (3), who found that state 3 and state 4 respiration were lower after 5 weeks of treadmill training compared to the sedentary condition. Discrepancies between findings may be due to differences in respiration protocols. Furthermore, we found that mitochondrial respiration increased despite a lack of change in protein content in the electron transport chain complexes for our treadmill training groups compared to the SED group. These data suggest that complex activities increased independent of content. Findings from Sun et al. (37) demonstrate that 8 weeks of treadmill training increased the activity of complexes I, IV, and V in the liver compared with sedentary rats. Unfortunately, OXPHOS protein content was not measured in their study to determine if there is a correlation between complex content and function.
In the current study, palmitate oxidation tended to be elevated only in the VWR group (+40%) compared to the sedentary rats; whereas, there was no apparent changes in the other exercising groups. Given our previously observations with 6, 16, and 36 weeks of voluntary wheel running showing increases in hepatic palmitate oxidation (22, 31, 33), it is possible that 4 weeks of exercise training is insufficient to elicit increases in this index of hepatic mitochondrial function. However, we found that voluntary wheel running in combination with overnight fasting and treadmill training (END, IST) resulted in significant decreases in incomplete palmitate oxidation. This is a unique observation given that it previously has been demonstrated that incomplete fatty acid oxidation products are linked to metabolic dysfunction and insulin resistance (19, 20).
Both carbohydrate and fatty acid sources are ultimately transformed to acetyl-CoA and feed into the TCA cycle. Endurance exercise training has been shown to increase TCA cycle flux in skeletal muscle (5). Here we found that VWR, TM-END, and TM-IST groups tended (p=0.075) to increase [2-14C] pyruvate oxidation, an index of TCA cycle flux. In addition, the VWR-OF, TM-END, and TM-IST groups exhibited significant increases in [1-14C]pyruvate oxidation, an index of PDH activity, compared to the SED rats. The increase in PDH activity in these groups suggests greater reliance on carbohydrate as an energy source compared to the SED and VWR groups. The factors driving these differences in substrate oxidation are unknown, but may be related to differences in substrate utilization patterns due to the various exercise modalities and disparities in food intake. The ratio of phosphorylated CREB to total CREB protein content is another sign of different metabolic regulation between groups. Phosphorylated CREB was not elevated in the VWR rats (group which had the greatest food intake) but was elevated in the VWR-OF and TM-END groups compared to the SED rats. CREB is a transcription factor that, once phosphorylated, is activated and upregulates gluconeogenic genes during times of metabolic stress (2, 16). Therefore, the increased phosphorylated CREB protein content suggests that VWR-OF and TM-END interventions resulted in greater reliance on gluconeogenic processes. This is further supported by the finding that PEPCK mRNA expression was significantly elevated in the VWR-OF groups. Conversely, G6Pase mRNA expression was not elevated in any group. This observation that some exercise stimuli (VWR-OF and TM-END) increase the ratio of phosphorylated CREB to CREB protein content and PEPCK mRNA expression (VWR-OF only), but not G6Pase may suggest that gluconeogenic genes do not all respond in the same manner. Evidence of these differences in gluconeogenic enzyme responses to different stimuli has been previously reported (14).
We found that voluntary wheel running led to increases in cytochrome c protein content as well as citrate synthase activity (VWR group only). These findings are in agreement with previous rodent exercise training studies (9, 14). Interestingly, Haase et al. (14) found that 5 weeks of treadmill training increased cytochrome c in wild-type mice. This effect was abolished in PGC-1α knockout mice, indicating the potential necessity of PGC-1α for increases in cytochrome c. However, in the current study, hepatic PGC-1α protein content or protein content of the deacetylase SIRT-1 was not different among groups, yet protein content of cytochrome c was elevated in the VWR groups. One possibility for this discrepancy is that there was already enough SIRT-1 protein content in liver and that the activity of this pre-existing deactylase increased and activated PGC-1α without a need for increased PGC-1α protein levels. Future work is needed to examine the transcriptional regulation of changes in hepatic mitochondrial function induced by exercise.
Post-translational modifications could be another factor playing a role in exercise-induced hepatic mitochondrial changes. In response to metabolic stressors, Sirtuin 3 deacetylates and activates certain complexes in the electron transport chain, as well as key proteins and enzymes involved in ATP production (1, 7, 13, 17, 24). Furthermore, it has been shown that in the absence of SIRT 3, hepatic ATP levels drop more than 50%, suggesting that SIRT 3 is essential for maintaining ATP levels (1). There is also some evidence that SIRT3 is capable of activating AMPK (26, 28, 35), which can initiate energy producing processes such as fatty acid oxidation (15). Here, increases in Sirtuin 3 protein content in the VWR, VWR-OF and TM-END groups, suggesting that these exercise interventions may be enhancing hepatic mitochondrial function through SIRT3-dependent deacetylation of mitochondrial proteins. However, western blot analysis for acetylated-lysine content in the mitochondrial proteins failed to detect significant differences among the groups. Future investigation is warranted to examine the role of SIRT3 in exercise-induced hepatic mitochondrial adaptations.
Hepatic gluconeogenesis is an energy costly process and requires increases in mitochondrial respiration to generate ATP. The VWR-OF group was added to the current investigation to determine whether fasting in combination with exercise training would invoke the need for increased hepatic gluconeogenesis and further hepatic mitochondrial adaptations over VWR alone. The VWR-OF and VWR both resulted in similar increases in hepatic mitochondrial State 3-Complex I & II and maximal uncoupled respiration. However, VWR-OF animals exhibited significant increases in [1-14C] pyruvate oxidation and significant reductions in incomplete palmitate oxidation compared with SED animal, which were not apparent in the VWR rats. Moreover, VWR-OF was the only intervention to significantly increase hepatic cytochrome c protein content. Furthermore, the VWR-OF group had greater PEPCK and PGC-1α mRNA expression than the VWR group, likely due to the greater increases seen in CREB phosphorylation. These data suggests that 4 weeks of wheel running in a fasting condition evokes greater stimulation of gluconeogenic processes, likely leading to enhancement of hepatic mitochondrial content and function.
In conclusion, results from the present investigation indicate that various forms of exercise training increase indices of hepatic mitochondria metabolism and that these changes are not entirely dependent upon changes in mitochondrial content. Regardless of the training stimuli, the exercise intensity, or the fasting state of the animal, exercise significantly improved hepatic mitochondrial respiration. Furthermore, exercise in the setting of a fasting condition appeared to upregulate genes related to gluconeogenesis and mitochondrial biogenesis, suggesting potentially greater hepatic mitochondrial adaptation with more prolonged exercise training.
Acknowledgments
This work was supported by NIH grants T32 AR 048523-07 (JAF and EMM), DK-088940 (JPT), and VHA-CDA2 1299-02 (RSR). The authors would like to thank Tzu-Wen Liu, Timothy Tan, Raisa Buenaventura, and Kelly Stromsdorfer for excellent technical assistance to this work. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447–52. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascensao A, Goncalves IO, Lumini-Oliveira J, Marques-Aleixo I, Dos Passos E, Rocha-Rodrigues S, Machado NG, Moreira AC, Oliveira PJ, Torrella JR, Magalhaes J. Endurance training and chronic intermittent hypoxia modulate in vitro salicylate-induced hepatic mitochondrial dysfunction. Mitochondrion. 2012;12(6):607–16. doi: 10.1016/j.mito.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 5.Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci U S A. 2008;105(43):16701–6. doi: 10.1073/pnas.0808889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5(4):313–20. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49(2):304–11. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constable SH, Favier RJ, McLane JA, Fell RD, Chen M, Holloszy JO. Energy metabolism in contracting rat skeletal muscle: adaptation to exercise training. Am J Physiol. 1987;253(2 Pt 1):C316–22. doi: 10.1152/ajpcell.1987.253.2.C316. [DOI] [PubMed] [Google Scholar]
- 9.da Silva LA, Pinho CA, Rocha LG, Tuon T, Silveira PC, Pinho RA. Effect of different models of physical exercise on oxidative stress markers in mouse liver. Appl Physiol Nutr Metab. 2009;34(1):60–5. doi: 10.1139/H08-132. [DOI] [PubMed] [Google Scholar]
- 10.Daussin FN, Rasseneur L, Bouitbir J, Charles AL, Dufour SP, Geny B, Burelle Y, Richard R. Different timing of changes in mitochondrial functions following endurance training. Med Sci Sports Exerc. 2012;44(2):217–24. doi: 10.1249/MSS.0b013e31822b0bd4. [DOI] [PubMed] [Google Scholar]
- 11.Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209(2):539–54. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- 12.Domingos MM, Rodrigues MF, Stotzer US, Bertucci DR, Souza MV, Marine DA, Gatto Cdo V, de Araujo HS, de Andrade Perez SE. Resistance training restores the gene expression of molecules related to fat oxidation and lipogenesis in the liver of ovariectomized rats. Eur J Appl Physiol. 2012;112(4):1437–44. doi: 10.1007/s00421-011-2098-6. [DOI] [PubMed] [Google Scholar]
- 13.Green MF, Hirschey MD. SIRT3 weighs heavily in the metabolic balance: a new role for SIRT3 in metabolic syndrome. J Gerontol A Biol Sci Med Sci. 2013;68(2):105–7. doi: 10.1093/gerona/gls132. [DOI] [PubMed] [Google Scholar]
- 14.Haase TN, Ringholm S, Leick L, Bienso RS, Kiilerich K, Johansen S, Nielsen MM, Wojtaszewski JF, Hidalgo J, Pedersen PA, Pilegaard H. Role of PGC-1alpha in exercise and fasting-induced adaptations in mouse liver. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1501–9. doi: 10.1152/ajpregu.00775.2010. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246(2):259–73. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 16.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 17.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloszy JO. Adaptation of skeletal muscle to endurance exercise. Med Sci Sports. 1975;7(3):155–64. [PubMed] [Google Scholar]
- 19.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280(39):33588–98. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 20.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol. 1980;48(1):23–8. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- 22.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol (1985) 2009;106(1):161–8. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1alpha overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303(8):G979–92. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284(34):22840–52. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1(9):771–83. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry CG, Kane DA, Lanza IR, Neufer PD. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62(4):1041–53. doi: 10.2337/db12-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285(5):3133–44. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiles JL, Huertas JR, Manas M, Ochoa JJ, Battino M, Mataix J. Dietary fat type and regular exercise affect mitochondrial composition and function depending on specific tissue in the rat. J Bioenerg Biomembr. 2001;33(2):127–34. doi: 10.1023/a:1010700515071. [DOI] [PubMed] [Google Scholar]
- 30.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008;586(Pt 17):4241–9. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–26. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 32.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab. 2010;298(6):E1179–87. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G874–83. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz-Ramirez A, Chavez-Salgado M, Peneda-Flores JA, Zapata E, Masso F, El-Hafidi M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am J Physiol Endocrinol Metab. 2011;301(6):E1198–207. doi: 10.1152/ajpendo.00631.2010. [DOI] [PubMed] [Google Scholar]
- 35.Shi T, Fan GQ, Xiao SD. SIRT3 reduces lipid accumulation via AMPK activation in human hepatic cells. J Dig Dis. 2010;11(1):55–62. doi: 10.1111/j.1751-2980.2009.00416.x. [DOI] [PubMed] [Google Scholar]
- 36.Srere PA. Citrate synthase. Methods Enzymology. 1969;13:3–5. [Google Scholar]
- 37.Sun L, Shen W, Liu Z, Guan S, Liu J, Ding S. Endurance exercise causes mitochondrial and oxidative stress in rat liver: effects of a combination of mitochondrial targeting nutrients. Life Sci. 2010;86(1-2):39–44. doi: 10.1016/j.lfs.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–16. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]


