Abstract
Background
Cell migration and adhesion are essential in intestinal epithelial wound healing and recovery from injury. Focal adhesion kinase (FAK) plays an important role in cell-extra cellular matrix (ECM) signal transduction. We have previously shown that heparin-binding epidermal growth factor-like growth factor (HB-EGF) promotes intestinal epithelial cell (IEC) migration and adhesion in vitro. The current study was designed to determine whether FAK is involved in HB-EGF-induced IEC migration and adhesion.
Materials and Methods
A scrape wound healing model of rat intestinal epithelial cells (RIE-1 cells) was used to examine the effect of HB-EGF on FAK-dependent cell migration in vitro. Immunofluorescence and Western blot analyses were performed to evaluate the effect of HB-EGF on the expression of phosphorylated FAK (p-FAK). Cell adhesion assays were performed to determine the role of FAK in HB-EGF-induced cell adhesion on fibronectin (FN).
Results
HB-EGF significantly increased healing after scrape wounding, an effect that was reversed in the presence of a FAK inhibitor (FAK I-14) (both with p<0.05). HB-EGF increased p-FAK expression, and induced p-FAK redistribution and actin reorganization in migrating RIE-1 cells. Cell adhesion and spreading on FN were significantly increased by HB-EGF (p<0.05). FAK I-14 significantly inhibited both intrinsic and HB-EGF-induced cell adhesion and spreading on FN (both with p<0.05).
Conclusions
FAK phosphorylation and FAK-mediated signal transduction play essential roles in HB-EGF-mediated IEC migration and adhesion.
Keywords: HB-EGF, cell migration, cell adhesion, focal adhesion kinase, wound healing
INTRODUCTION
The gastrointestinal epithelium functions as an important barrier that separates luminal contents from the underlying tissue compartment, and is vital in maintaining mucosal homeostasis.1–3 Mucosal wounds associated with inflammatory disorders compromise this critical epithelial barrier, undermine gut barrier function and allow bacterial translocation and absorption of toxins, antigens, proteases and other macromolecules, leading to local infection followed by distant organ pathology. In response to injury, rapid re-establishment of epithelial barrier function by intestinal epithelial cells (IEC) depends on migration of uninjured IEC to cover denuded sections of basement membrane. 1, 4, 5 This process is known as intestinal restitution.6, 7
IEC migration is a mitosis independent process that starts as early as a few minutes after injury and is completed within 6–12 hours.8 To initiate migration, cells polarize, extend protrusions (lamellopodia) in a particular direction, and form adhesions in the direction of migration. Focal adhesions (FA) are composed of various structural proteins and represent sites where a number of intra- and extracellular signaling events regulating cell migration take place.9, 10 A key player involved in the control of the interaction between cells and the extracellular matrix (ECM) is focal adhesion kinase (FAK), a tyrosine kinase that is localized to cellular focal contact sites, and is the pivotal molecule that controls FA formation.11–13 Upon stimulation, FAK is phosphorylated at Tyr397,a step that is necessary for the full activation of the kinase domain.14 Activated FAK partners with cell membrane integrins with the assistance of other proteins such as paxillin and vinculin, resulting in FA formation, cell adhesion and cell migration.10, 15
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a member of the epidermal growth factor (EGF) family that was initially identified in the conditioned medium of cultured human macrophages,16 and then found to be a member of the EGF family.17 During the past 20 years, we have investigated the roles of HB-EGF in protecting the intestines in various models of intestinal injury including NEC,18–22 ischemia/reperfusion injury,23, 24 and hemorrhagic shock and resuscitation.25–27 Our previously studies have demonstrated that HB-EGF promotes intestinal restitution in murine NEC and intestinal ischemia/reperfusion models in vivo, and in an IEC scrape wound healing model in vitro.28,24, 29 We have also demonstrated that HB-EGF promotes enterocyte migration, in part, by affecting integrin-extracellular matrix (ECM) interactions and intercellular adhesions.28 Numerous studies in the last 20 years have established FAK as a central mediator of integrin signaling, as well as an important component of signaling by other cell surface receptors in the regulation of cell migration in many cell types.10, 14, 30, 31 The current study was designed to examine the effect of HB-EGF on FAK in IEC, and to explore the role of FAK in HB-EGF-induced IEC migration and adhesion.
MATERIALS and METHODS
Materials
Tyr-397 Phosphorylated FAK (p-FAK) antibody and the FAK inhibitor 14 (FAK I-14)were from Santa Cruz Biotechnology, Inc.(Santa Cruz, CA, USA). RhodaminePhalloidin was from Cytoskeleton Inc. (Denver, CO, USA). HRP-conjugated goat anti-rabbit antibody, Cy3 and Alexa-488 secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA, USA). Human plasma fibronectin (FN) was from Millipore (Billerica, MA, USA). Mounting medium for fluorescence with DAPI was from Vector Laboratories Inc. (Burlingame, CA, USA). Radio Immuno Precipitation Assay (RIPA) buffer, protease inhibitor cocktail and the BCA protein assay kit were from Thermo Scientific (Rockford, IL, USA). Phosphatase inhibitor cocktail was Sigma-Aldrich Corp (St Louis, MO, USA). The ECL Plus system was from Amersham Biosciences (Piscataway, NJ, USA). All experiments used human recombinant HB-EGF corresponding to amino acids 74–148 of the mature HB-EGF protein that was produced using a Pichiapastoris expression system (Trillium Therapeutics, Toronto, Canada). The EGF receptor (EGFR) inhibitor AG1478 [4-(3-chloroanilino)-6, 7-dimethoxyquinazoline] was from Calbiochem (San Diego, CA, USA). The doses of HB-EGF and AG 1478 chosen were based on our previous studies.24, 28, 29, 32
Cell Culture
The RIE-1 cell line was kindly provided by Dr. John Barnard (Columbus, OH, USA). Cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100U/ml penicillin and 100μg/ml streptomycin at 37°C in 5% CO2. All experiments were performed on cells between passages 4–10.
Scrape Wound Healing Assay
The scrape wound healing assay was performed as we have previously described.24, 28, 29 Briefly, cells were grown in 24-well plates until confluent and then serum-starved overnight. The bottom of each plate was marked by drawing two crossed lines across the plate diameter, and then a 100 μL sterile pipette tip was used to create a scrape wound perpendicular to the marked lines. After scraping, cells were immediately treated with either: 1) regular control culture medium, 2) HB-EGF (10 ng/ml), 3) the epithelial growth factor receptor (EGFR) inhibitor AG1478 (500 nmol/L), or 4) AG1478 followed 30 min later by HB-EGF. To assess the effect of FAK activation on HB-EGF-induced cell migration, scraped cells were treated with FAK I-14 (2μM), with some cells receiving HB-EGF (10 ng/ml) 60 min later. Non-treated cells served as a negative control. Cell migration was quantified at various time points (6h, 12h, 18h, 24h, 28h). Photographs were taken immediately after wounding (0 h) or at the indicated time points after wounding (IT). The width of the wound was measured across each of the two marked lines in each well (2 fields/well) using Axiovision 3.1 software (Carl Zeiss Inc., Thornwood, NY). Each experiment was performed 3 times in duplicate. The percent of wound healing was calculated as: Wound healing % = (W0h) – (WIT) where W0h and WIT represent the average wound width/well (measured across the pre-marked lines) at 0h and at the indicated time points respectively. The cells were then fixed for immunofluorescent staining or harvested for protein analysis.
Western Blot Analysis
Cells were lysed in RIPA buffer supplemented with phosphatase inhibitor cocktail and protease inhibitor cocktail. Protein concentration was measured using a BCA protein assay kit. Protein extracts were subjected to SDS-PAGE and proteins transferred to nitrocellulose membranes using transfer buffer (25 mM Tris, pH 8.8, 192 mM glycine). Nitrocellulose membranes were incubated in PBS with 0.1% Tween-20 (PBST) containing 5% skim milk for 30min at 37°C to block non-specific binding. Membranes were incubated with p-FAK (Tyr-397) antibodies at 4°C overnight followed by HRP conjugated secondary antibody at room temperature (RT) for 1 h. Blots were developed using the ECL Plus system with quantification performed using Image J software. Values were normalized to β-actin and expressed as mean ± SD.
Fluorescent Immunohistochemistry and F-actin Filament Staining
RIE-1 cells were seeded in 8-well culture slide chambers until confluent, maintained in serum-free medium overnight, and then subjected to scrape wounding. After wounding, cells were treated with HB-EGF (10 ng/ml), AG1478 (500 nM), or with AG1478 (500 nM) followed 30 min later by HB-EGF (10 ng/ml) for 18 h, and were then fixed with 4% paraformaldehyde in PBS for 20 min. Cells were permeabilized by incubating in 0.1% Triton X-100 for 10 min and blocked with 1% bovine serum albumin (BSA) in PBST for 30min. Cells were incubated with p-FAK (Tyr-397) antibody at 4°C overnight, rinsed with PBST, and in cubated with secondary antibody labeled with Alexa-488 at RT for 1 h. For labeling of actin filaments, cells were incubated with RhodaminePhalloidin (100nM) in 1% BSA at RT for 30 min. Mounting medium containing DAPI was used to visualize nuclei. Fluorescent staining was imaged with a Zeiss LSM 710 confocal imaging system (Carl Zeiss Inc., Thornwood, NY) across the pre-marked lines as mentioned previously.28
Cell Adhesion and Spreading Assay
96 well flat bottom microwell plates were pre-coated with fibronectin (FN) (10µg/ml) or BSA (10 µg/ml) at 4°C overnight, and blocked with 1% BSA at RT for 30 min. Serum-starved cells were detached for 5 min with 0.25% trypsin to prepare single cell suspensions in DMEM with 2% FBS. Cells were seeded into the wells (5×104 cells per well) and incubated with the following reagents at 37°C in 5% CO 2:1) no addition (control), 2) HB-EGF (10 ng/ml), 3) AG1478 (500 nM), 4) AG1478 (500 nM) followed 30 min later by HB-EGF (10 ng/ml), or 4) FAK I-14 (2μM), 5) FAK I-14 (2μM)followed 60 min later by HB-EGF (10 ng/ml). After 15, 30, or 60 min, wells were gently washed 3 times with PBS to remove non-adherent cells. Adherent cells were fixed with 95% ethanol for 10 min, stained with 0.1% crystal violet, and photographed using a Zeiss AxioSkop 2 Plus microscope (Carl Zeiss Inc., Thornwood, New York). The dye absorbed by adherent nuclei was extracted with 0.2% Triton X-100, and absorbance was measured using a plate reader with a 570 nm filter set. Values were normalized to untreated control cells and expressed as mean ± SD. Adherent cells were also fixed with 4% paraformaldehyde for immunofluorescent staining using p-FAK (Tyr- 397) antibodies and RhodaminePhalloidin. To quantify cell spreading, cells were stained with RhodaminePhalloidin, three separate fields were photographed, and cells that showed a clear extension of lamellipodia were counted. Each experiment was performed in triplicate wells and was repeated three times.
RESULTS
HB-EGF promotes RIE-1 cell migration via activation of EGFR
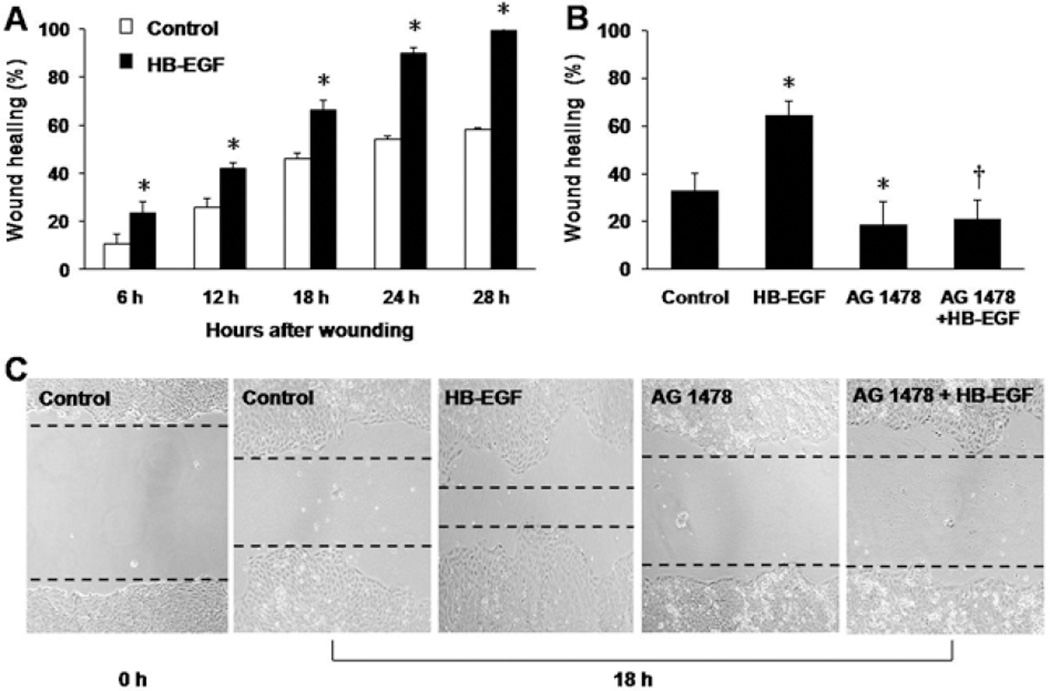
RIE-1 cells subjected to scrape wounding were used to examine the role of HB-EGF in cell migration. Addition of HB-EGF significantly increased RIE-1 cell migration after scraping at all time points: 23.52% ± 4.75%vs. 10.53% ± 4.21%, p=0.036 at 6 h; 61.92% ± 2.57% vs. 25.78% ± 3.71%, p=0.023 at 12 h; 79.01% ± 4.04% vs. 46.15% ± 2.48%, p=0.031 at 18 h; 89.92% ± 2.38% vs. 54.30% ± 1.14%, p=0.011 at 24 h; 99.37% ± 1.09% vs. 58.25% ± 0.84%, p=0.002 at 28 h) (Figure 1A). Eighteen hours after scraping, the addition of AG 1478 significantly inhibited both intrinsic (18.67% ± 9.74% vs. 32.98% ± 15.42%, p=0.031) and HB-EGF-induced cell migration (20.88% ± 8.22% vs. 64.24% ± 16.61%, p=0.021) (Figure 1B,C).
Figure 1.
RIE-1 cell migration after scrape wounding in the presence of EGFR inhibition. (A) Time course of RIE-1 cell wound healing in the presence or absence of HB-EGF in vitro. *p<0.05, vs. control. (B) Effect of HB-EGF on wound healing of RIE-1 cells 18 h after scraping. *p<0.05, vs. control; † p<0.01, vs. HB-EGF. (C) Representative images of RIE-1 cells 18 h after scrape wounding. From left to right: 0 h after scrape wounding of untreated RIE-1 cells; 18 h after wounding of untreated cells, HB-EGF-treated cells, AG1478-treated cells and cells treated with AG1478 plus HB-EGF. The black dotted lines present the wound edges at 18 h. Scale bar = 200 μm.
HB-EGF promotes cell migration of RIE-1 cells after scrape wounding via activation of FAK
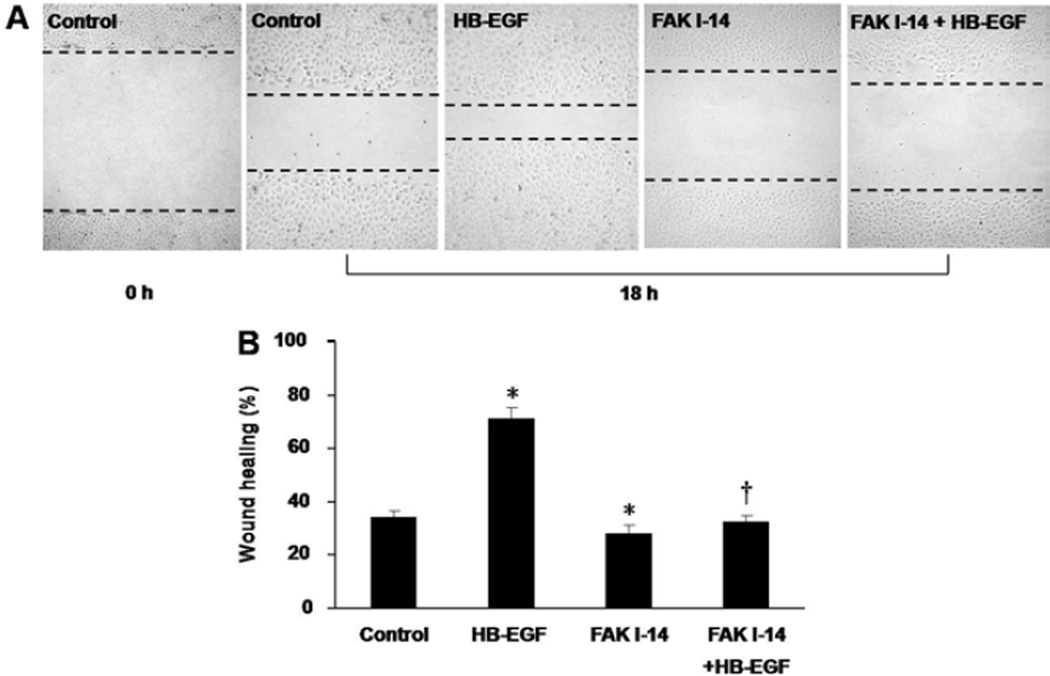
The scrape wounding assay was also used to investigate the role of p-FAK in HB-EGF-induced cell migration. Eighteen hours after scraping, wound healing was significantly increased in the presence of HB-EGF (70.91% ± 3.47% vs. 34.38% ± 2.43%, p=0.007), and significantly decreased in the presence of FAK- I 14 (28.25% ± 6.28% vs. 34.38% ± 2.43%, p=0.047) (Figure 2). Compared to cells treated with HB-EGF alone, treatment of cells with FAK 1–14 prevented HB-EGF-induced cell migration (30.39% ± 7.71% vs. 70.91% ± 3.47%, p=0.011).
Figure 2.
RIE-1 cell migration after scrape wounding in the presence of p-FAK inhibition. (A) Representative images of RIE-1 cells 18 h after scrape wounding. From left to right: 0 h after scrape wounding of untreated RIE-1 cells; 18 h after wounding of untreated cells, HB-EGF-treated cells, FAK1-14-treated cells and cells treated with FAK1-14 plus HB-EGF. The black dotted lines present the wound edges at 18 h. Scale bar = 200μm. (B) Effect of p-FAK inhibition on RIE-1 cell migration 18 h after scrape wounding. *p<0.05, vs. control; † p<0.05 vs. HB-EGF.
HB-EGF induces F-actin filament reorganization and increases the expression and polarization of p-FAK in migrating RIE-1 cells in an ErbB-1 dependent fashion
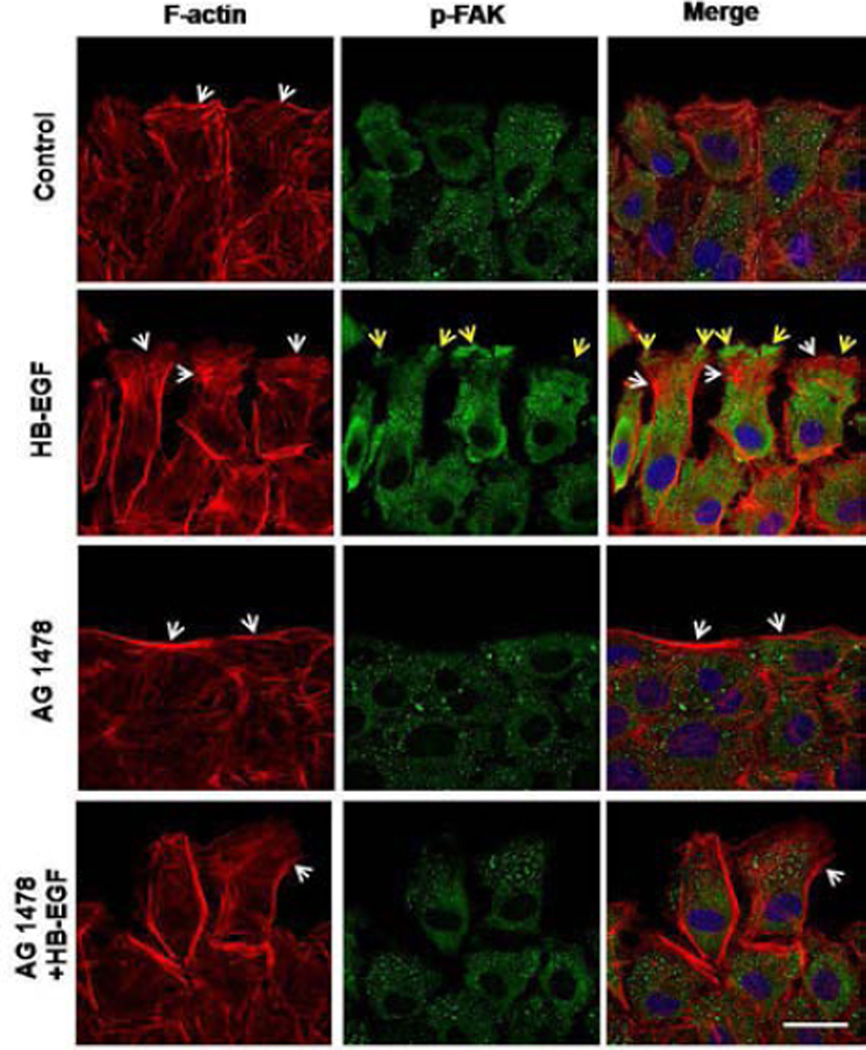
To investigate the role of HB-EGF in regulating actin remodeling and p-FAK expression during cell migration, we examined the effect of HB-EGF on the distribution and localization of p-FAK and actin cytoskeleton rearrangements in migrating RIE-1 cells after scrape wounding. Eighteen hours after scrape wounding, at the foremost edge of migrating RIE-1 cells, control RIE-1 cells had intrinsic organization of F-actin and formed a few lamellipodia, with p-FAK distributed equally throughout the cytoplasm (Figure 3). Addition of HB-EGF increased the number of lamellipodia and resulted in significantly increased accumulation of p-FAK at the foremost edge of the migrating cells. Cells exposed to AG 1478 had significantly reduced numbers of lamellipodia and reduced staining of p-FAK compared with control cells. Treatment of cells with AG 1478 inhibited both intrinsic and HB-EGF-induced F-actin reorganization and p-FAK redistribution.
Figure 3.
Distribution of F-actin filaments and p-FAK after scrape wounding. Shown are representative immunofluorescent images of F-actin filaments (red) and p-FAK (green) in migrating RIE-1 cells 18 h after scrape wounding. At the wound edges, the white arrows indicate lamellipodia, and the yellow arrows indicate polarization of p-FAK to the foremost edge of the migrating RIE-1 cells. Scale bar = 20 μm.
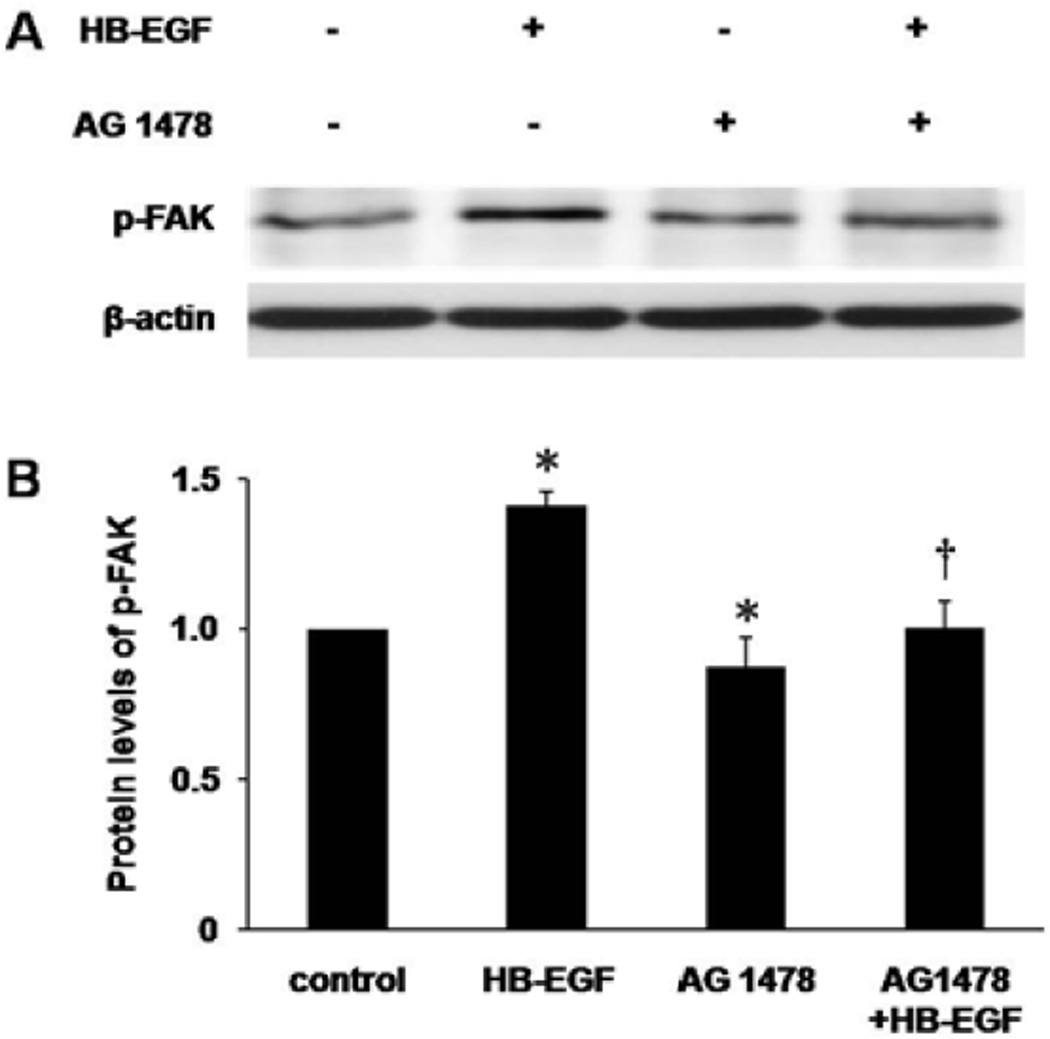
We next examined changes in p-FAK protein levels in RIE-1 cells after scrape wounding as determined by Western blotting. Eighteen hours after scrape wounding, addition of HB-EGF significantly increased relative protein levels of p-FAK (1.42 ± 0.05 vs. 1.00 ± 0.01, p=0.019), whereas blocking of EGFR activity with AG1478 inhibited the expression of p-FAK (0.83 ± 0.08 vs. 1.00 ± 0.01, p=0.046) and also abolished HB-EGF-induced phosphorylation of FAK (0.93 ± 0.07 vs. 1.42 ± 0.05, p=0.025) (Figure 4).
Figure 4.
p-FAK protein levels in RIE-1 cells as determined by Western blotting. (A) Western blotting of p-FAK 18 h after scrape wounding. (B) Densitometric quantification of protein levels of p-FAK. The protein level of p-FAK in control group was defined as 1.0. *p<0.05 vs. control; † p<0.05 vs. HB-EGF.
HB-EGF promotes cell adhesion and spreading of RIE-1 cells on fibronectin (FN) via activation of FAK
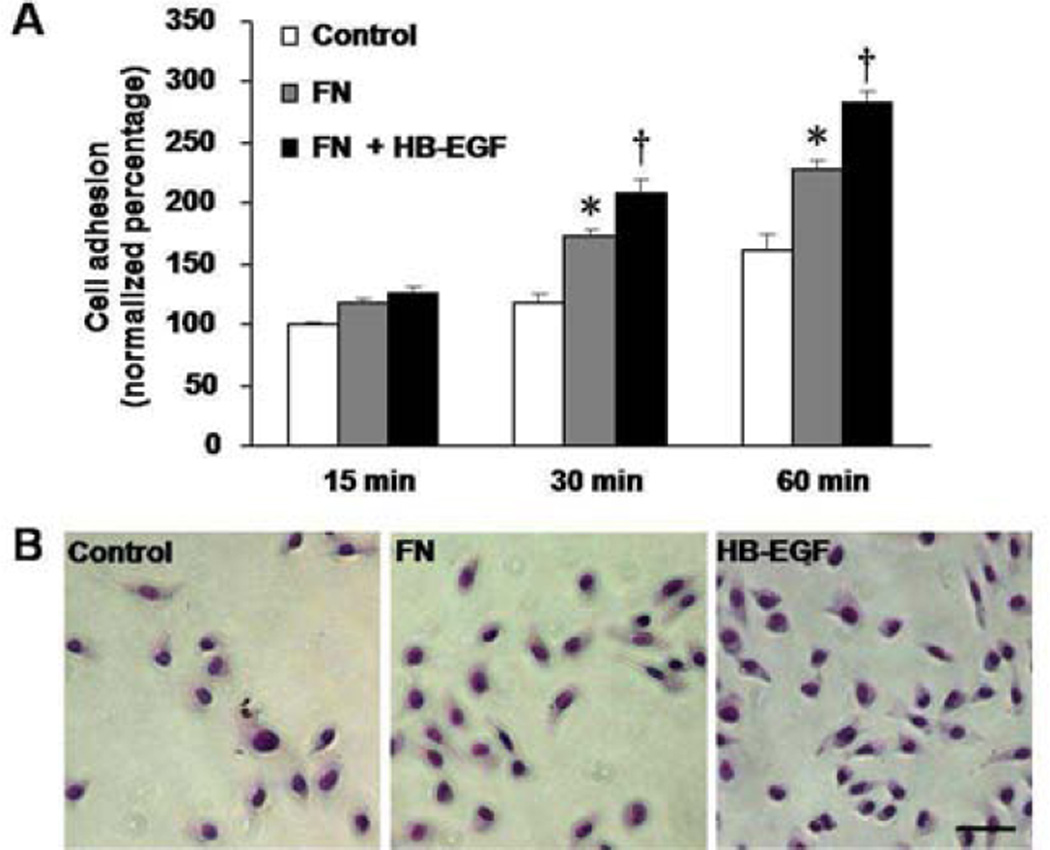
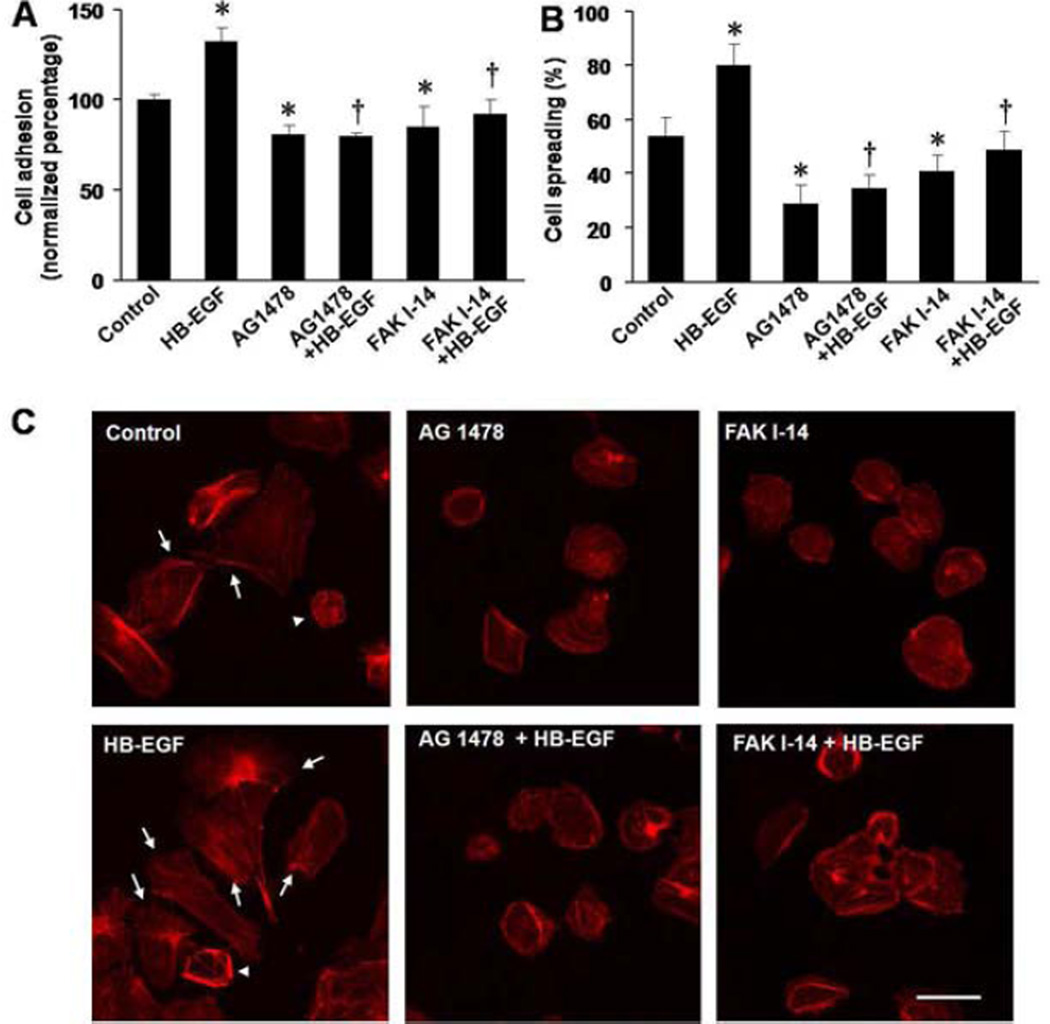
We next determined whether inhibition of p-FAK affected HB-EGF-mediated cell adhesion and spreading on extracellular substrates. First we investigated the role of FN on RIE-1 cell adhesion. Compared with control cells plated in the absence of FN, RIE-1 cell adhesion was significantly increased at 30 min (172.84% ± 5.25% vs. 117.16% ± 3.70%, p=0.015) and 60 min (229.02% ± 10.43% vs. 160.39% ± 6.79%, p=0.028) after incubation on FN coated plates, and was further enhanced when cells were treated with HB-EGF at 30 min (209.31% ± 6.12% vs. 172.84% ± 5.25%, p=0.031) and 60 min (283.53% ± 10.29% vs. 229.02% ± 10.43%, p=0.030) (Figure 5A,B). After 60 min of incubation, HB-EGF treatment significantly increased not only cell adhesion on FN (132.37% ± 7.83% vs. 100.00% ± 3.54%, p=0.033) (Figure 6A) but also significantly increased cell spreading on FN (79.92% ± 8.11% vs. 53.50% ± 7.59%, p=0.041) (Figure 6B). Inhibition of EGFR activation with AG 1478, or inhibition of p-FAK with FAK I-14, significantly decreased intrinsic cell adhesion (AG1478: 70.42% ± 5.72% vs. 100.00% ± 3.54%, p=0.035; FAK 1–14: 80.53% ± 10.68% vs. 100.00% ± 3.54%, p=0.038), as well as cell spreading on FN (AG1478: 28.74% ± 7.20% vs. 53.50% ± 7.59%, p=0.039; FAK I-14: 40.58% ± 3.52% vs. 53.50% ± 7.59%, p=0.041) compared to control cells, and also decreased HB-EGF-induced cell adhesion (AG1478: 79.51% ± 2.47% vs. 132.37% ± 7.83%, p=0.020; FAK I-14: 85.71% ± 5.15% vs. 132.37% ± 7.83%, p=0.030) and spreading on FN (AG1478: 34.43% ± 5.41% vs. 79.92% ± 8.11%, p=0.023; FAK I-14: 51.35% ± 7.11% vs. 79.92% ± 8.11%, p=0.038 (Figure 6A-C).
Figure 5.
RIE-1 cell adhesion on fibronectin (FN). (A) RIE-1 cells adhesion on FN is shown at different time points. *p<0.05vs. control cells plated in the absence of FN; † p<0.05 vs. cells grown on FN in the absence of HB-EGF. (B) Representative images ofRIE-1 cell adhesion after 60 min of incubation. Scale bar = 30 μm.
Figure 6.
RIE-1 cell adhesion and spreading on FN in the presence of EGFR and p-FAK inhibition. (A) Effect of EGFR and p-FAK inhibition on RIE-1 cell adherence to FN-coated plates after 60min of incubation.*p<0.05 vs. control cells; † p<0.05vs. HB-EGF-treated cells. (B) Effect of EGFR and p-FAK inhibition on RIE-1 cell spreading on FN after 60 min after incubation.*p<0.05vs. control cells; † p<0.05vs. HB-EGF-treated cells. (C) Representative images of the RIE-1 cells grown on FN. Cell spreading was defined by formation and extension of lamellipodia, where F-actin (red staining) formed thick bundles (arrows). Cells without lamellipodia did not spread (arrowheads). Scale bar = 25 μm.
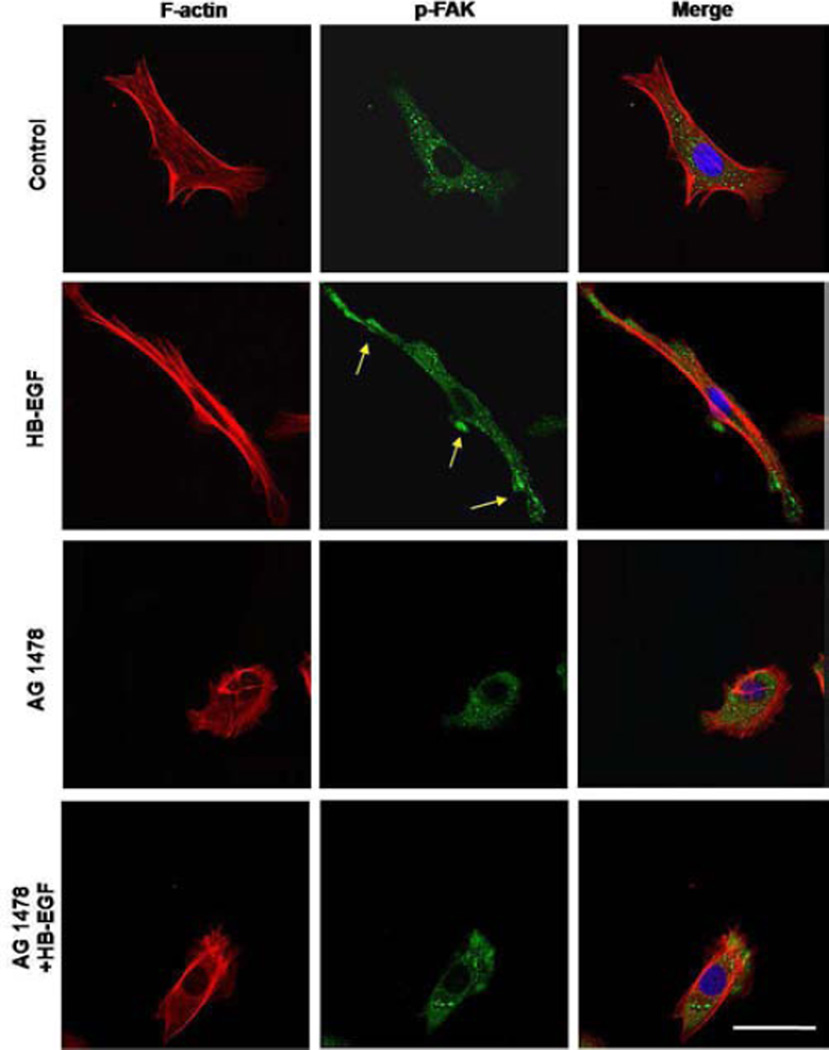
HB-EGF induces p-FAK redistribution in RIE-1 cells grown on FN
We next examined p-FAK localization and actin skeleton organization in RIE-1 cells adhering to FN. In control RIE-1 cells, p-FAK was distributed equally throughout the cytoplasm, with actin fibers forming lamellipodia (Figure 7). Addition of HB-EGF resulted polarized p-FAK at the cell periphery, and promoted cell spreading on FN. Addition of AG 1478 to cells treated with or without HB-EGF significantly inhibited cell spreading on FN and prevented the redistribution of p-FAK to the cell periphery.
Figure 7.
Distribution of F-actin filaments and p-FAK in RIE-1 cells grown on FN. Shown are representative immunofluorescent images of F-actin filaments (red) and p-FAK (green) in migrating RIE-1 cells grown on FN. Yellow arrows indicate polarized p-FAK at the cell periphery. Scale bar = 30 μm.
DISCUSSION
Cell migration and adhesion are essential components of many biological processes that are critically involved in cell growth, embryonic development, immunity, angiogenesis, and wound healing.10, 33, 34 These processes are mediated by key cytoskeletal structures and are controlled by signal transductions that are organized in lamellipodial extensions. These signals are received by cells through contact with the extracellular matrix, in combination with exposure to soluble molecules, lipids, or growth factors.30, 35, 36 Cell recognition of the ECM is initiated by the membrane receptor integrin, accompanied by a series of complex molecular events regulating intracellular signaling processes.37–39 One of the key elements in intracellular signaling is FAK.39, 40 FAK is a nonreceptor protein-tyrosine kinase that is one component of the focal adhesion complex that plays a major role in signaling pathways, especially integrin-mediated signal transduction.14, 31, 41 FAK also participates in signal transduction by G-protein-coupled receptors, such as angiotensin II, receptor tyrosine kinases, and the platelet-derived growth factor (PDGF) receptor.42, 43 Many studies have demonstrated that FAK may function as a convergence point for signaling pathways triggered by integrins and other humoral factors that are important in the regulation of cell function.10, 44, 45 Our previous studies have confirmed that HB-EGF promotes cell migration by affecting integrin-extracellular matrix interactions, suggesting that FAK may play a crucial role in HB-EGF-induced intestinal restitution. However, evidence confirming a relationship between HB-EGF-induced intestinal restitution and FAK was previously lacking.
The current study used a scrape wounding model to provide direct evidence that FAK activation is an essential factor in HB-EGF-induced cell migration. We found that HB-EGF promotes enterocyte migration in an experimental model of wound healing. During enterocyte migration, HB-EGF induced F-actin reorganization associated with increased protein levels and cell peripheral polarization of phosphorylated FAK. In addition, inhibition of FAK activity significantly reversed HB-EGF-mediated IEC migration, consistent with the findings of others that FAK is a critical regulator of cell migration in other cell types.14, 34, 43, 46 We have previously shown that the ability of HB-EGF to affect enterocyte migration is EGFR (ErbB-1)-dependent.28 Our current results confirmed that EGFR blockade not only inhibited HB-EGF-induced cell migration, but also reversed HB-EGF-induced F-actin reorganization and FAK activation. Taken together, these findings demonstrate that HB-EGF promotes cell migration through an EGFR-dependent pathway via activation of FAK.
The interaction of cells with various ECM molecules plays a critical role in both tissue construction and regulation of cellular functions, including cell adhesion, migration, proliferation, and differentiation.10, 31, 34 FN is one of the signals in the ECM that is essential for embryonic development, and is associated with wound healing and angiogenesis.47 The importance of FAK on signal transduction has been emphasized in many earlier studies.10, 15, 31, 45 In addition, we have previously confirmed that HB-EGF promotes RIE-1 cell adhesion and spreading on FN.28 However, evidence confirming a relationship between FAK and HB-EGF-induced cell-ECM interactions is still lacking. We showed in this study that HB-EGF promoted IEC adhesion and spreading on FN, and induced phosphorylated FAK redistribution to the cell periphery, which were dramatically reversed by EGFR blockade or FAK inhibition, indicating that HB-EGF promotes IEC adhesion and spreading on FN though EGFR and via activation of FAK. Multiple downstream signaling pathways including paxillin and members of the Rho family have been identified that mediate FAK regulation of migration and adhesion in various cells,10 and these downstream molecules may be investigated in our future studies of HB-EGF-mediated intestinal restitution.
In summary, we have demonstrated the critical role of FAK activation in HB-EGF-induced cell migration and adhesion. These findings, in combination with our previous studies demonstrating that HB-EGF promotes intestinal restitution in vivo,48, 49 support a potential future therapeutic role for HB-EGF in the promotion of cell migration and adhesion in processes such as wound healing and recovery from injury.
ACKNOWLEDGEMENTS
The authors thank Dr. John Barnard for supplying RIE-1 cells, Jixin Yang, Yu Zhou, Li Chen, and Chun-Liang Chen for helpful suggestions and discussions, and Amanda Darbyshire for general assistance. This work was supported by NIH R01 DK74611 and R01 GM61193 (GEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Y Su: Designed methods and experiments, carried out the laboratory experiments, analyzed the data, interpreted the results and wrote the paper.
G Besner: Defined the research theme, designed experiments, and critically proofread and revised the paper.
REFERENCES
- 1.Rankin CR, Hilgarth RS, Leoni G, et al. Annexin A2 regulates beta1 integrin internalization and intestinal epithelial cell migration. J Biol Chem. 2013;288(21):15229–15239. doi: 10.1074/jbc.M112.440909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simoncini T, Scorticati C, Mannella P, et al. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20(8):1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- 3.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22(2):83–87. doi: 10.1053/j.sempedsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore R, Carlson S, Madara JL. Villus contraction aids repair of intestinal epithelium after injury. Am J Physiol. 1989;257(2 Pt 1):G274–G283. doi: 10.1152/ajpgi.1989.257.2.G274. [DOI] [PubMed] [Google Scholar]
- 5.Levinson H, Moyer KE, Saggers GC, Ehrlich HP. Calmodulin-myosin light chain kinase inhibition changes fibroblast-populated collagen lattice contraction, cell migration, focal adhesion formation, and wound contraction. Wound Repair Regen. 2004;12(5):505–511. doi: 10.1111/j.1067-1927.2004.012502.x. [DOI] [PubMed] [Google Scholar]
- 6.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992;263(3 Pt 1):G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- 7.Moore R, Madri J, Carlson S, Madara JL. Collagens facilitate epithelial migration in restitution of native guinea pig intestinal epithelium. Gastroenterology. 1992;102(1):119–130. doi: 10.1016/0016-5085(92)91791-2. [DOI] [PubMed] [Google Scholar]
- 8.Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol. 1997;273(2 Pt 1):G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- 9.Avraham HK, Lee TH, Koh Y, et al. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J Biol Chem. 2003;278(38):36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63(8):610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen-Hillel E, Mintz R, Meshel T, Garty BZ, Ben-Baruch A. Cell migration to the chemokine CXCL8: paxillin is activated and regulates adhesion and cell motility. Cell Mol Life Sci. 2009;66(5):884–899. doi: 10.1007/s00018-009-8447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5(7):505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 13.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540(1):1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Wang L, Huo Y, Peng X, Xia C, Tang C. Activated focal adhesion kinase involved in adhesion and migration of vascular smooth muscle cells stimulated by fibronectin. Chin Med J (Engl) 2002;115(4):494–497. [PubMed] [Google Scholar]
- 15.Lin CL, Zhang ZX, Tan Z. [Mechanisms of focal adhesion kinase in the proliferation of human pulmonary artery smooth cells under hypoxia] Zhonghua Yi Xue Za Zhi. 2011;91(32):2274–2277. [PubMed] [Google Scholar]
- 16.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1(11):811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 18.Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg. 2005;14(3):167–174. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41(1):144–149. doi: 10.1016/j.jpedsurg.2005.10.018. discussion 44-9. [DOI] [PubMed] [Google Scholar]
- 20.Radulescu A, Zhang HY, Yu X, et al. Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J Pediatr Surg. 2010;45(10):1933–1939. doi: 10.1016/j.jpedsurg.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CL, Yu X, James IO, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012;92(3):331–344. doi: 10.1038/labinvest.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Watkins D, Chen CL, Bhushan B, Zhou Y, Besner GE. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. 2012;215(4):534–545. doi: 10.1016/j.jamcollsurg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia G, Lara-Marquez M, Luquette MH, Glenn S, Haque A, Besner GE. Heparin-binding EGF-like growth factor decreases inducible nitric oxide synthase and nitric oxide production after intestinal ischemia/reperfusion injury. Antioxid Redox Signal. 2001;3(5):919–930. doi: 10.1089/15230860152665073. [DOI] [PubMed] [Google Scholar]
- 24.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129(2):609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 25.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142(2):234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HY, Radulescu A, Chen Y, Besner GE. HB-EGF improves intestinal microcirculation after hemorrhagic shock. J Surg Res. 2011;171(1):218–225. doi: 10.1016/j.jss.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocourt DV, Mehta VB, Besner GE. Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J Surg Res. 2007;139(2):269–273. doi: 10.1016/j.jss.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y, Yang J, Besner GE. HB-EGF promotes intestinal restitution by affecting integrin-extracellular matrix interactions and intercellular adhesions. Growth Factors. 2013;31(1):39–55. doi: 10.3109/08977194.2012.755966. [DOI] [PubMed] [Google Scholar]
- 29.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25(4):253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 30.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 31.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(Pt 8):1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 32.Mehta VB, Besner GE. Heparin-binding epidermal growth factor-like growth factor inhibits cytokine-induced NF-kappa B activation and nitric oxide production via activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2005;175(3):1911–1918. doi: 10.4049/jimmunol.175.3.1911. [DOI] [PubMed] [Google Scholar]
- 33.Singer SJ, Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Jung ID, Chang WK, et al. p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase. Exp Cell Res. 2005;307(2):315–328. doi: 10.1016/j.yexcr.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Rhoads JM, Chen W, Gookin J, et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut. 2004;53(4):514–522. doi: 10.1136/gut.2003.027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 37.Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16(1):52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.McNamara LE, McMurray RJ, Biggs MJ, Kantawong F, Oreffo RO, Dalby MJ. Nanotopographical control of stem cell differentiation. J Tissue Eng. 2010;2010 doi: 10.4061/2010/120623. 120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren XR, Ming GL, Xie Y, et al. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7(11):1204–1212. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- 40.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14(1):92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71(3-4):435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 43.Manso AM, Kang SM, Plotnikov SV, et al. Cardiac fibroblasts require focal adhesion kinase for normal proliferation and migration. Am J Physiol Heart Circ Physiol. 2009;296(3):H627–H638. doi: 10.1152/ajpheart.00444.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu P, Yu DM, Qi JC, et al. [High D-glucose alters PI3K and Akt signaling and leads to endothelial cell migration, proliferation and angiogenesis dysfunction] Zhonghua Yi Xue Za Zhi. 2006;86(48):3425–3430. [PubMed] [Google Scholar]
- 45.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 46.Xiao R, Zhao Y, Wang LJ, Li WP. Effects of Roundabout 5 on adhesion, invasion and potential motility of human tongue carcinoma Tb cells. Chin Med J (Engl) 2011;124(15):2367–2371. [PubMed] [Google Scholar]
- 47.Huveneers S, Truong H, Fassler R, Sonnenberg A, Danen EH. Binding of soluble fibronectin to integrin alpha5 beta1 - link to focal adhesion redistribution and contractile shape. J Cell Sci. 2008;121(Pt 15):2452–2462. doi: 10.1242/jcs.033001. [DOI] [PubMed] [Google Scholar]
- 48.Radulescu A, Zhang HY, Chen CL, et al. Heparin-binding EGF-like growth factor promotes intestinal anastomotic healing. J Surg Res. 2011;171(2):540–550. doi: 10.1016/j.jss.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Radulescu A, Chen CL, Zhang HY, James IO, Besner GE. Heparin-binding epidermal growth factor-like growth factor improves intestinal barrier function and reduces mortality in a murine model of peritonitis. Surgery. 2013;153(1):52–62. doi: 10.1016/j.surg.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]