Abstract
Background
In addition to tremor, patients with essential tremor (ET) may exhibit non-motor features, including a range of cognitive deficits. Several prospective, population-based epidemiological studies have reported an association between ET and incident dementia, especially Alzheimer's disease (AD). Moreover, in a brain repository-based study, a larger than expected proportion of ET patients also developed pathological changes characteristic of progressive supranuclear palsy, further suggesting a link between ET and tau pathology.
Methods
We selected a group of ET patients that were free of dementia clinically and without AD on postmortem examination. Our hypothesis was that neuronal tauopathic burden would be higher in the brains of these ET patients compared to controls. We compared Braak stage for neuronal tangles and Consortium to Establish a Registry for Alzheimer's Disease (CERAD) scores for neuritic plaques in the two groups.
Results
The two groups were similar in age (82.6±6.0 vs. 80.4±8.1, p=0.22). The 40 ET patients had a higher Braak neurofibrillary stage than 32 controls (means: 2.2 ± 1.2 vs. 1.2 ± 1.1; medians: 2.0 vs. 1.0, p <0.001). Meanwhile, CERAD scores for neuritic plaques were similar in patients and controls (means: 0.6 ± 0.9 vs. 0.5 ± 0.6; medians: 0.0 vs. 0.0, p = 0.83).
Conclusion
While ET itself is not a tauopathy (i.e., a neurodegenerative disorder among whose main features are accumulation of hyperphosphorylated tau protein), ET may predispose individuals to accumulate more widespread cellular tau aggregates, and thus tau could play a central role in the cognitive impairment that can accompany ET.
Keywords: Essential tremor, Dementia, Non-motor, Alzheimer's Disease, Braak, Tau, Neurofibrillary tangle
Introduction
In addition to motor features, essential tremor (ET) patients may exhibit a range of cognitive deficits that are in excess of those seen in age-matched control subjects [1]. Several prospective, population-based epidemiological studies have reported an association between ET and incident dementia, especially Alzheimer's disease (AD) [2].Moreover, a brain repository-based study reported the development of pathological changes characteristic of progressive supranuclear palsy (PSP) in a larger than expected proportion of ET patients, further suggesting the presence of links between ET and tau burden [3].
To further explore links between ET and Alzheimer's type pathological changes, and especially tau burden, we compared Braak neurofibrillary tangle stage [4] and Consortium to Establish a Registry for Alzheimer's Disease (CERAD)[5] scores for neuritic plaques in non-demented ET patients and controls. Our hypothesis was that neuronal tangle burden rather than neuritic plaque burden would be increased in non-demented ET patients compared to controls.
Methods
Subjects
This study was conducted at the Essential Tremor Centralized Brain Repository (ETCBR), Columbia University Medical Center. The ETCBR is a centralized repository for the prospective collection of brains from ET patients in the United States. Participants signed informed consent approved by the CUMC Internal Review Board.
ET diagnoses were carefully assigned using three sequential methods, as described [6]. Every six months, patients completed a follow-up telephone questionnaire, which included a series of screening questions for Parkinson's disease (PD) and dystonia. A follow-up videotaped neurological examination was performed if any screening question was positive for PD or dystonia, or if hand-drawn spirals showed signs of micrographia.
The ET patients underwent a brief cognitive assessment upon enrollment and every six months, which consisted of short Telephone Interviews (TICS) for Cognitive Status (range = 0 - 9) performed at both times [7] and the Mini-Mental Status Examination (MMSE) performed upon enrollment (range = 0 – 30) [8]. Furthermore, the patients were asked to self-report a diagnosis of dementia. Clinical dementia was defined as any self-report of dementia or a Mini-Mental Status score <25 [9]. Between 2003 and mid-2013, 112 ET patients died and their brains were prospectively collected.
Sixty-seven available normal elderly control brains from the New York Brain Bank, mainly derived from the Alzheimer's Disease Research Center at CUMC and the Washington Heights Inwood Columbia Aging Project, did not have clinical diagnoses of dementia, AD, ET, or PD, and were without neuropathological diagnoses of neurodegenerative disease. Controls underwent detailed cognitive assessments by neuropsychological testers, including an extensive neuropsychological battery and either the MMSE and/or the Short Blessed Test [10] every 12-18 months.
Tissue processing
The New York Brain Bank operates under approval of the Institutional Review Board of CUMC. All brains were well-characterized including standardized, complete neuropathological assessment and determination of any pathological findings in paraffin embedded tissue sections (see nybb.hs.columbia.edu). This consisted of a comprehensive neuropathological histologic evaluation with Luxol Fast Blue-Hematoxylin Eosin (LH&E), modified Bielschowsky, and immunohistochemical staining for hyperphosphorylated tau (AT8) (1:200, Thermo Scientific, Rockford IL) (including hippocampus, globus pallidus, putamen, caudate nucleus, amygdala, thalamus, subthalamic nucleus, mesencephalon with red nucleus, pons, medulla oblongata, cerebellum with dentate nucleus, and cerebral cortex), beta-amyloid (1:400, Biocare Medical, Concord, CA) (including cerebral cortex, hippocampal formation, caudate nucleus, putamen, and thalamus) and alpha-synuclein (1:40, Leica, Buffalo Grove, IL) (including cerebral cortex, hippocampal formation, globus pallidus, putamen, amygdala, midbrain with substantia nigra, pons with the locus ceruleus, medulla with the dorsal vagal nucleus, and olfactory bulbs). A CERAD score for neuritic plaques [5] and Braak stage for neurofibrillary tangles [4] was assigned to each brain. AD (low, intermediate, high-likelihood) was defined using NIA-Reagan criteria [11]. Postmortem interval (PMI) was the number of hours between death and placement of brain in a cold room or upon ice.
Final Sample
From the 112 ET brains, we excluded 28 with additional clinical diagnoses other than/in addition to ET (2 with dystonia, 1 with AD) or the presence of definite pathological findings that do not occur in brains from patients with pure ET (n = 25 with either PD, PSP, or corticobasal degeneration). Additionally, we excluded 3 with a pathological diagnosis of intermediate or high-likelihood AD, and 13 with a missing cognitive assessment. We were able to age-match a final sample of 40 ET patients with 32 controls who had complete clinical and pathological data.
Statistical Analyses
Analyses were performed using SPSS (version 21.0). Clinical characteristics of ET patients and controls were compared using Student's t-tests and chi-square tests. Since Braak stage and CERAD scores were not normally distributed, a non-parametric test (Mann-Whitney test) was used when comparing stages/scores in patients vs. controls. We also reported Braak stage and CERAD score as categorical variables, comparing the proportions with each stage/score in patients vs. controls using a chi-square test or Fischer's exact test. Spearman's correlation coefficients were used to test for a correlation between Braak stage and CERAD scores. Braak stage had 7 levels (0 to 6) but CERAD score had only 4 levels (0, A [1], B [2], C [3]). We also performed several secondary analyses. First, we excluded 4 additional ET patients with diagnoses of low-likelihood AD on postmortem examination, to further ensure that the study sample was AD-free. Second, to reduce the effects of potential selection bias caused by the different cognitive assessments between patients and controls, we repeated our main analysis including only the top quartile of patients and controls based on their most recent TICS and the Short Blessed test scores. To assess the independent association of Braak stage and CERAD score with diagnosis (ET patient vs. control), a logistic regression model was performed in which the ET patient-control status was the outcome variable, and Braak stage and CERAD score were the independent variables, adjusting for age and education.
Results
None of the selected 40 ET patients or 32 controls had clinical dementia or pathological changes that met the criteria of AD. Patients and controls were similar in age, gender and brain weight; PMIs in controls were longer than in patients (Table 1). None had Lewy bodies.
Table 1.
Clinical and Pathological Characteristics in ET Patients and Controls
| ET (n = 40) | Controls (n = 32) | p value | |
|---|---|---|---|
|
Clinical
| |||
| Age in years | 82.6 ± 6.0 | 80.4 ± 8.1 | 0.22a |
| Education (years) | 14.3 ± 2.7 | 15.7 ± 3.5 | 0.13a |
| Female gender | 23 (57.5) | 15 (46.9) | 0.37b |
| Age of tremor onset (years) | 39.3 ± 20.9 | Not applicable | Not applicable |
| Tremor duration (years) | 41.9 ± 21.4 | Not applicable | Not applicable |
|
Brain Pathology
| |||
| PMI (hours) | 3.5 ± 3.7 | 8.7 ± 9.9 | 0.008a |
| Brain Weight (grams) | 1223 ± 162 | 1215 ± 128 | 0.82a |
| Braak Stage | |||
| Mean | 2.2 ± 1.2 | 1.2 ± 1.1 | |
| Median | 2.0 | 1.0 | <0.001c |
| 0 | 3 (7.5) | 10 (31.3) | 0.018d |
| 1 | 8 (20.0) | 11 (34.4) | |
| 2 | 15 (37.5) | 8 (25.0) | |
| 3 | 9 (22.5) | 2 (6.3) | |
| 4 | 4 (10.0) | 1 (3.1) | |
| 5 | 1 (2.5) | 0 (0) | |
| 6 | 0 (0) | 0 (0) | |
| CERAD score | |||
| Mean | 0.6 ± 0.9 | 0.5 ± 0.6 | |
| Median | 0.0 | 0.0 | 0.83c |
| 0 | 25 (62.5) | 18 (56.3) | 0.40d |
| 1 | 10 (25.0) | 12 (37.5) | |
| 2 | 2 (5.0) | 2 (6.3) | |
| 3 | 3 (7.5) | 0 (0) | |
Values are mean ± standard deviation or numbers (percentage).
Abbreviations: CERAD, Consortium to Establish a Registry for AD; PMI, postmortem interval
Student's t test, Subjects were matched on age
Chi-square test
Mann-Whitney test
Fisher's exact test
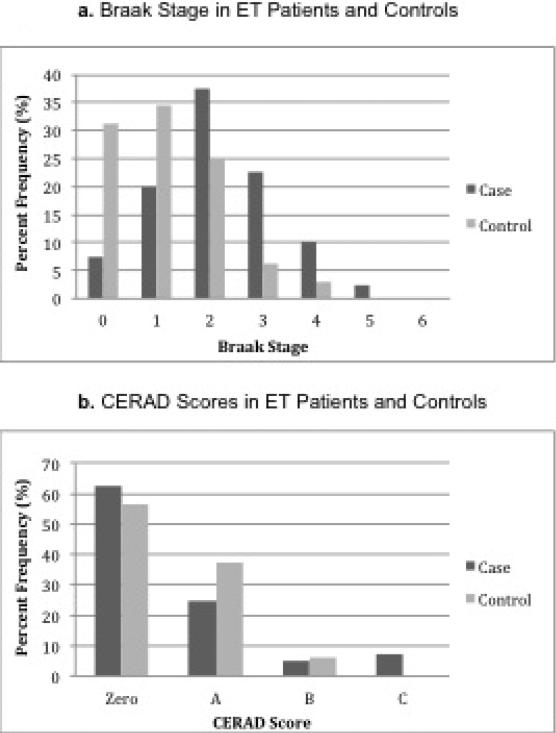
ET brains had a higher Braak stage than control brains (means: 2.2 ± 1.2 vs. 1.2 ± 1.1; medians: 2.0 vs. 1.0, p < 0.001) (Table 1). We also treated Braak stage for neuronal tangles as a categorical variable, and compared the proportion at each stage in patients vs. controls; the patient-control difference remained robust (p = 0.018, Table 1, Figure 1a). CERAD scores in ET patients and controls were similar (means: 0.6 ± 0.9 vs. 0.5 ± 0.6; medians: 0.0 vs. 0.0, p = 0.83) (Table 1). The overall proportion at each CERAD stage (zero, A, B, C) was also similar in patients vs. controls (p = 0.40, Table 1, Figure 1b).
Figure.
Braak staqe and CERAD scores by diagnosis.
Although we had already excluded patients and controls with postmortem diagnoses of intermediate or high-likelihood AD, we also performed an analysis in which we excluded 4 additional ET patients with postmortem diagnoses of low-likelihood AD. The patient-control difference in Braak stage persisted (means: 2.0 ± 1.1 vs. 1.2 ± 1.1; medians: 2.0 vs. 1.0, p = 0.002) and CERAD scores again remained similar (means: 0.5 ± 0.6 vs. 0.4 ± 0.8; medians: 0.0 vs. 0.0, p = 0.36). We also repeated the analysis, including only the top quartile of patients and controls based on their most recent cognitive screen (Short Blessed Test or MMS), and results were similar (mean Braak stages: 2.1 ± 1.1 vs. 1.2 ± 0.8; medians: 2.0 vs. 1.0, p = 0.009, and mean CERAD scores: 0.5 ± 0.7 vs. 0.6 ± 0.9; median scores: 0.0 vs. 0.0, p = 0.99).
Overall, Braak stage and CERAD scores were correlated with one another (Spearman’s r = 0.46, p<0.001).
We assessed the role of potential confounders. In our controls, Braak stage was not associated with Short Blessed Test scores (Spearman's r = -0.22, p = 0.39), Folstein MMSE (Spearman's r = 0.39, p = 0.30), years of education (Spearman's r = -0.23, p = 0.34), gender (Mann-Whitney p = 0.50), PMI (Spearman's r = -0.05, p = 0.80), or brain weight (Spearman's r = -0.13, p = 0.48). Braak stage and age were correlated (Spearman's r = 0.54, p = 0.009). There was no correlation between CERAD score and Short Blessed Test scores (Spearman's r = -0.13, p = 0.63), years of education (Spearman's r = 0.09, p = 0.72), gender (Mann-Whitney p = 0.32), PMI (Spearman's r = -0.02, p = 0.90), or brain weight (Spearman's r = -0.12, p = 0.52). There was a correlation between CERAD score and age (Spearman's r = 0.47, p < 0.001). A logistic regression model was performed in which ET patient-control status was the outcome variable, and Braak stage and CERAD score were the independent variables, adjusting for age and years of education. Braak stage was significantly associated with ET patient-control status (p = 0.023) whereas CERAD score was not (p = 0.58).
Discussion
The central finding of this study is that neuronal tangles are more prevalent in brains from non-demented ET patients than in brains from non-demented controls, whereas the extent of the neuritic plaque burden was similar. In a prior study, we reported similar results (i.e., higher Braak but not higher CERAD scores in ET cases than in controls); however, the sample size was considerably smaller and more important, we made no systematic attempt to exclude demented patients as we did here [12]. The current study considered only ET patients without clinical dementia or pathological diagnoses of AD. While it is possible that some of these carefully selected ET patients had mild cognitive impairments and, had they lived longer, they may have developed AD, at the time of death, they were free of AD.
The biological ramifications of these findings must be considered. The extent, if any, to which this increased neuronal tangle burden we observed in ET is responsible for the observed associations between ET, AD and PSP in clinical-epidemiological studies is not known, but an intriguing possibility is raised by our study.
The study had limitations. First, we assessed cognitive status in ET patients using screening questionnaires. Hence, it is possible that some patients could have had cognitive dysfunction or early dementia, since our screening tool may not be adequately sensitive. Regardless, we excluded any patients who had intermediate or high-likelihood AD on postmortem examination, and in a secondary analysis even excluded those with low-likelihood AD. Hence, even if some mildly clinically demented ET patients had been included, they did not have a pathological diagnosis of AD. Second, patients and controls did not have the same cognitive evaluation; it is possible that controls were more thoroughly scrutinized for and excluded if they had mild cognitive problems than were the ET patients. Our secondary analysis that only included those patients and controls with the highest quartile of scores in their most recent cognitive screen was aimed to minimize the potential effect of this possible source of selection bias; in that analysis, the patient-control difference persisted. The study also had several strengths. First, this study presented a large series of ET brains that received clinical-cognitive assessments during life and a detailed postmortem assessment, ensuring that our sample comprised patients without AD. Second, we carefully matched these patients by age to a group of controls that was also free of dementia. Finally, brains were prospectively collected and Alzheimer's- related pathologies were scored by the same senior neuropathologist using standardized methods.
In summary, while ET itself is not a tauopathy (i.e., a neurodegenerative disorder among whose main features are the accumulation of hyperphosphorylated tau), ET may predispose individuals to accumulate more widespread cellular tau aggregates, and thus tau burden could play a central role in the cognitive impairment that can accompany ET.
Acknowledgements
This research was supported by R01 NS42859, P50AG008702, and UL1RR024156 (National Institutes of Health, Bethesda, MD), and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benito-León J, Louis ED, Sánchez-Ferro Á , Bermejo-Pareja F. Rate of cognitive decline during the premotor phase of essential tremor: a prospective study. Neurology. 2013;81(1):60–6. doi: 10.1212/WNL.0b013e318297ef2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73(8):621–5. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis ED, Babij R, Ma K, Cortes E, Vonsattel JPG. Essential tremor followed by progressive supranuclear palsy: Postmortem reports of eleven patients. J Neuropathol Exp Neurol. 2013;72:8–17. doi: 10.1097/NEN.0b013e31827ae56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibril- lary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to establish a registry for Alzheimer's Disease (CerAD). Part II. Standardization of the neuropathologic assessment of Alzhei-mer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 6.Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control Brains. Brain. 2013;136(10):3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–7. [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. ‘Mini- mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Roalf DR, Moberg PJ, Xie SX, Wolk DA. Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9(5):529–37. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 11.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 12.Louis ED, Faust PL, et al. Neuropathological Changes in Essential Tremor: 33 Cases Compared With 21 Controls. Brain. 2007;130(12):3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]