Abstract
Objectives
To compare the extent to which different combinations of objectively measured sedentary behavior (SB) and physical activity contribute to cardiometabolic health.
Design and Methods
A population representative sample of 5,268 individuals, aged 20-85 years, was included from the combined 2003-2006 NHANES datasets. Activity categories were created on the combined basis of objectively measured SB and moderate-to-vigorous physical activity (MVPA) tertiles. Cardiometabolic abnormalities included elevated blood pressure, levels of triglycerides, fasting plasma glucose, C-reactive protein, homeostasis model assessment (HOMA) of insulin resistance value, and low HDL-cholesterol level. BMI, and DXA-derived percent body fat (% BF) and android adiposity were also compared across groups. Predictors for a metabolically abnormal phenotype (≥3 cardiometabolic abnormalities, or insulin resistance) were determined.
Results
Adults with the least SB and greatest MVPA exhibited the healthiest cardiometabolic profiles, whereas adults with the greatest SB and lowest MVPA were older and had elevated risk. Time spent in SB was not a predictor of the metabolically abnormal phenotype when MVPA was accounted for. Adults with the highest MVPA across SB tertiles did not differ markedly in prevalence of obesity, adiposity, and/or serum cardiometabolic risk factors; however, less MVPA was associated with substantial elevations of obesity and cardiometabolic risk. Android adiposity (per kilogram) was independently associated with the metabolically abnormal phenotype in both men (OR: 2.36 [95% CI, 1.76-3.17], p<0.001) and women (OR: 2.00 [95% CI, 1.63-2.45], p<0.001). Among women, greater SB, and less lifestyle moderate activity and MVPA were each independently associated with the metabolically abnormal phenotype, whereas only less MVPA was associated with it in men.
Conclusions
MVPA is a strong predictor of cardiometabolic health among adults, independent of time spent in SB.
Keywords: exercise, accelerometry, adiposity, sitting time, insulin resistance
Introduction
The American Medical Association has recently recognized obesity as a disease (7). Although excess adiposity indeed contributes to a pathophysiologic milieu, the adipogenic process leading to obesity is merely a natural consequence of chronic, dysregulated energy partitioning. Despite the well-established trajectory of cardiometabolic health decline that coincides with increases in adiposity, there is significant ongoing debate pertaining to the optimal clinical method for screening obesity and the inherent risk of morbidity and mortality. Since body mass index (BMI) lacks sensitivity to accurately detect non-obese individuals with excess body fat (18), there is an obvious public health exigency to improve screening for cardiometabolic abnormalities (3, 24) across body phenotypes, and perhaps more importantly, to better understand the modifiable explanatory drivers that mediate the link with cardiometabolic disease.
Over-nutrition and lack of daily physical activity have received the bulk of attention as underlying factors to potentiate risk of obesity and clustering of metabolic abnormalities. However, and especially during the past few years, the topic of excessive chronic sedentary behavior (SB) has received a great deal of attention as a strong independent driver for diseases and early mortality (4, 6, 10, 14, 17, 23). The bulk of this work has demonstrated a robust link between subjectively measured physical inactivity (e.g., television viewing time), and health risk, even after adjustment for BMI and self-reported physical activity. This suggests one of two potentially significant public health messages regarding activity participation: (1) habitual physical activity and exercise cannot protect against the negative consequences stemming from excessive SB, or (2) individuals who engage in large volumes of SB are at exaggerated risk because they are also less likely to engage in physical activity. Regardless of the interpretation, these messages are extremely important and provide the foundation for lifestyle modification and public health interventions. However, what remains to be determined is how different combinations of objectively measured SB and intensity-specific physical activity patterns contribute to protection against or potentiation of cardiometabolic disease. Therefore, the purpose of this study was to examine cardiometabolic profiles for differing volumes of SB, within each category of moderate-to-vigorous physical activity (MVPA), as well as to determine the independent associations of sedentary time, activity accumulation, and various measures of adiposity with the metabolically abnormal phenotype.
Methods
Study Design and Sample
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status of adults and children in the United States. The NHANES 2003/2004 and 2005/2006 surveys were specifically chosen based on their wealth of relevant information pertaining to body composition and android adiposity, objective physical activity counts, and markers of cardiometabolic health. Of the 9,515 screened participants in the NHANES 2003-2006 who were 20 years and older, 5,268 had valid data from dual-energy x-ray absorptiometry (DXA), at least 4-days of objectively measured activity, and the necessary blood samples obtained after an overnight fast and/or had non-fasting samples obtained for high-sensitivity C-reactive Protein (hsCRP) and high-density lipoprotein- (HDL-)cholesterol. Subjects were excluded on the basis of BMI<18.5 kg/m2. Similarly, for all race/ethnicities that were missing or coded as “Other Race - Including Multi-Racial,” we chose to exclude these subjects (this represented <5% of the available sample). This study was approved for exemption from full institutional review board (IRB) review.
Demographic and Anthropometrics Factors
Socio-demographic characteristics were all assessed by self-report during the in-home interview. Age was used as both a continuous factor, as well as a categorical factor: (1) ≥ 20 years and < 40 years, (2) ≥ 40 years and < 60 years, and (3) ≥ 60 years. Race/ethnicity was categorized as: (1) non-Hispanic white, (2) non-Hispanic black, and (3) Mexican American or other Hispanic. Annual household income was categorized as: (1) ≤ $24,999, (2) $25,000-$54,999, and (3) ≥ $55,000. Education was categorized as: (1) less than high school graduate, (2) high school graduate/general educational development (GED) or equivalent, and/or some college or Associate's degree (e.g., A.A. A.S.), and (3) college graduate or above.
Weight was measured using a digital Toledo scale (Mettler-Toledo International, Inc., Columbus, OH), and participants wore only underwear, gown, and foam slippers. Height was measured using a fixed stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Standard categories were applied to determine if each participant was normal weight (18.5-24.9), overweight (25-29.9), or obese (≥ 30). Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest. Standard cut points for abdominal obesity in men (> 102 cm) and women (> 88 cm) were used, as outlined by the ATP III report (16).
Body composition and android adiposity
The NHANES DXA scans were administered using a Hologic QDR-4500A fan-beam densitometer with Hologic software (Hologic Corp., Bedford, MA). Total lean mass, excluding bone mass, total fat mass and total percent body fat (%BF) were reported. Obesity was defined with sex-specific cutoffs, at a level (≥ 25% BF for men and ≥ 35% BF for women) associated with increased cardiometabolic risk, and frequently used in the literature (5, 18, 20, 21). In addition, the Hologic APEX software computed adipose tissue within the android area of a total body DXA scan. Located within the abdomen, android area is roughly the area around the waist between the mid-point of the lumbar spine and the top of the pelvis. Android adipose tissue thus represents the combined total of subcutaneous and visceral adipose tissue in the android anatomic region, and is presented in total grams.
Cardiometabolic Parameters
Resting systolic and diastolic blood pressures were measured three to four times with a mercury sphygmomanometer by trained staff. Non-fasting serum measures of HDL-cholesterol and high sensitivity C-reactive protein (hsCRP) concentrations were measured. Additionally, fasting measures were obtained from participants examined in the morning session, for triglycerides, plasma glucose, and insulin. The homeostasis model assessment (HOMA) was calculated according to the formula: [Insulin0 (μU/ml) × Glucose0 (mmol/l)]/22.5 (15).
The Metabolically Abnormal Phenotype
Cardiometabolic abnormalities included elevated blood pressure, elevated levels of triglycerides, fasting plasma glucose, C-reactive protein, elevated HOMA value, and low high-density lipoprotein cholesterol level. Subjects were classified with/without the metabolically abnormal phenotype (≥3 cardiometabolic abnormalities or insulin resistance), on the basis of presence of any three or more abnormal findings from the following: (1) elevated triglycerides (≥150 mg/dL [1.7 mmol/L]); (2) reduced HDL-cholesterol (<40 mg/dL [1.0 mmol/L] in men; <50 mg/dL [1.3 mmol/L] in women); (3) hypertension (≥ 130 mm Hg systolic and/or ≥85 mm Hg diastolic); (4) elevated fasting glucose (≥ 100 mg/dL); (5) elevated C-reactive protein (≥ 0.30 mg/L); and/or (6) HOMA score of ≥5.9, as recently determined and validated against hyperinsulinemic-euglycemic clamp by Tam and colleagues (22). Any subject with missing cardiometabolic abnormalities was excluded from these analyses.
Objective Activity Assessment
Habitual physical activity and SB were assessed in NHANES with an accelerometer (Actigraph 7164; Actigraph, LLC, Pensacola, FL), which provided an objective estimate of the intensity of bodily movement. The accelerometer was worn on the right hip during waking hours by participants for 7 days. In order to represent an individual's typical behavior in the assessment of activity, at least 4 days of monitoring with at least 10 hours per day were necessary for inclusion. Lack of, or minimal movement (i.e. <100 counts per minute (cpm)) recorded by the accelerometer was used to derive the non-sleeping time spent in SB, as previously documented (11, 13). Accelerometer counts were also used to classify all worn time as time spent in light-intensity activity (100–759 cpm), lifestyle moderate activity (760-2019 cpm) (2, 12), moderate-intensity physical activity (2020-5998 cpm), vigorous-intensity physical activity (exercise, ≥5999 cpm) and MVPA (i.e., the combined time in moderate- and vigorous-intensity physical activity). Total daily minutes of SB and each activity category were summed from all time spent and averaged across all valid days. However, since subjects wore the monitors for differing amounts of time, proportion of wear-time values were calculated for each subject to account for total number of minutes spent in SB and each activity category, relative to total time spent wearing the accelerometer. Activity categories were defined on the combined basis of SB and MVPA tertiles (i.e., 9 total categories).
Statistical Analysis
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). To obtain population-representative findings, analyses were conducted using both fasting and non-fasting sample weights for the combined 2003/2004 and 2005/2006 NHANES cycles, which account for the complex survey design (including oversampling), survey nonresponse, and post-stratification. Descriptive characteristics were examined by combining MVPA and SB tertiles, and are provided as means, standard errors, and percentages. Differences in these characteristics across activity categories were tested using linear regression (proc surveyreg) and logistic regression (proc surveylogistic) for continuous and categorical variables respectively, after creating appropriate categories and dummy coding for each. To assess the odds of the metabolically abnormal phenotype, separately weighted, unadjusted and adjusted logistic regression models were performed by gender. The effects of sedentary behavior and each activity category were assessed with unadjusted and adjusted models.
Correlations between measures of adiposity (%BF and android adiposity) and indicators of adiposity (BMI) were all high (r = 0.70-0.88; p<0.001). Therefore, various collinearity diagnostics were performed. Since collinearity diagnostics are not available in the SAS logit or surveylogistic statements, and moreover, considering that the issue of collinearity is an issue of the explanatory variables (and not the dependent variable), it was possible to estimate an equivalent model using multiple linear regression. In doing so, the “tol” “vif” and “collinoint” options were used to formally examine the extent of collinearity between predictors in the full adjusted models. Despite the fact that there were high correlation coefficients between measures of BMI, % body fat, and android adiposity, there was only a single variance inflation factor (VIF) >5 (android adiposity VIF: 7.1 for males; 6.3 for females). Moreover, despite the fact that two tolerance values (BMI and android adiposity) were <0.40, which is also indicative of multicollinearity, there were no eigenvalues greater than 30 (all <10). However, due to the potential collinearity issues between BMI and android adiposity, separate adjusted regression models were completed for each, interchangeably.
Results
SB was categorized as low (≤415 minutes for women; and ≤416 for men), moderate (>415 minutes and ≤ 548 minutes for women; and >416 and ≤534 minutes for men), and high (>548 minutes for women; and >534 minutes for men). Similarly, MVPA was categorized as low (≤5 minutes for women; and ≤10 for men), moderate (>5 minutes and ≤ 17 minutes for women; and >10 and ≤32 minutes for men), and high (>17 minutes for women; and >32 minutes for men).
Descriptive data are presented as weighted means, standard errors, and percentages by tertiles of SB and MVPA in Table 1. Adults with the least SB and greatest MVPA were younger and exhibited the healthiest cardiometabolic profiles, whereas adults with the greatest SB and lowest MVPA were older and had significantly elevated risk. Adults with the highest MVPA (∼43-48 min) across SB tertiles did not differ in prevalence of obesity, adiposity, and/or serum cardiometabolic risk factors; however, less MVPA was reflective of significant elevations of obesity and cardiometabolic risk, regardless of SB.
Table 1. Cardiometabolic and activity characteristics of the study population by combined MVPA and SB tertiles.
| High MVPA | Moderate MVPA | Low MVPA | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Low SB n=767 | Moderate SB n=581 | High SB n=552 | Low SB n=663 | Moderate SB n=636 | High SB n=584 | Low SB n=367 | Moderate SB n=543 | High SB n=575 | |
|
|
|||||||||
| Female, % | 45.8 | 55.5* | 52.7§ | 54.4‡ | 47.8‡ | 47.6§ | 53.9¶ | 48.9 | 52.1a |
| Age, years | 39.75 (0.51) | 42.56 (0.75)* | 42.85 (0.65)§ | 44.40 (0.75)‡ | 46.07 (0.57)‡ | 48.77 (0.61)†§‡ | 56.27 (1.33)a¶ | 62.23 (0.93)*a¶ | 63.60 (0.74)§¶ |
| Body Mass Index (BMI), kg/m2 | 26.90 (0.27) | 26.89 (0.26) | 26.81 (0.25) | 28.64 (0.33)‡ | 28.83 (0.29)‡ | 29.24 (0.44)‡ | 29.94 (0.42)a¶ | 29.59 (0.35)¶ | 29.50 (0.30)¶ |
| Obesity (BMI >30), % | 26.9 | 25.9 | 20.2 | 33.9‡ | 37.1‡ | 34.9‡ | 39.8¶ | 40.0¶ | 37.0¶ |
| Body Fat, % | 31.06 (0.43) | 32.47 (0.54) | 32.60 (0.36)§ | 34.51 (0.48)‡ | 34.82 (0.35)‡ | 35.02 (0.47)‡ | 37.55 (0.47)a¶ | 36.75 (0.46)a¶ | 37.86 (0.43)a¶ |
| Obesity (%BF-sex specific), % | 66.5 | 65.9 | 65.6 | 78.7‡ | 79.6‡ | 80.1‡ | 86.4a¶ | 87.9a¶ | 87.7a¶ |
| WC, cm | 92.72 (0.53) | 92.55 (0.75) | 92.64 (0.67) | 97.47 (0.95)‡ | 98.85 (0.86)‡ | 100.11 (0.76)§‡ | 103.17 (0.93)a¶ | 103.08 (0.87)a¶ | 103.04 (0.69)a¶ |
| Abdominal Obesity (sex-specific WC), % | 41.8 | 43.2 | 39.8 | 55.6‡ | 54.2‡ | 58.1‡ | 67.1a¶ | 68.9a¶ | 69.0a¶ |
| Adroid Adiposity, grams | 2017.77 (50.9) | 2045.42 (57.8) | 2038.35 (59.4) | 2492.56 (82.2)‡ | 2514.81 (62.9)‡ | 2581.37 (62.7)‡ | 2771.22 (88.9)a¶ | 2734.20 (93.4)¶ | 2770.41 (58.9)a¶ |
| Objectively Measure d Activity Categories | |||||||||
| Sedentary (<100 cpm), min (% wear time) | 329.36 (42.0) | 474.44 (52.4)* | 657.58 (60.5)†§ | 339.93 (48.1)‡ | 475.87 (57.8)* | 661.47 (64.4)†§ | 351.73 (55.6)a¶ | 480.60 (65.2)*¶ | 683.51 (71.2)†§¶ |
| Light (100 - 759 cpm), min (% wear time) | 272.67 (34.2) | 285.71 (30.7)* | 290.60 (26.1)§ | 266.25 (36.7) | 258.34 (30.4)‡ | 276.16 (26.4)†§‡ | 234.21 (35.9)a¶ | 221.40 (28.9)a¶ | 239.21 (24.5)†a¶ |
| Lifestyle Moderate (760 - 2019 cpm), min (% we ar time) | 142.17 (17.6) | 115.95 (12.3)* | 104.54 (9.3)†§ | 95.04 (13.1)‡ | 85.01 (10.0)*‡ | 79.88 (7.7)†§‡ | 51.74 (7.9)a¶ | 42.37 (5.5)*a¶ | 38.96 (4.0)§¶ |
| Moderate/Vigorous PA (>2020 cpm), min (% wear time) | 48.36 (6.2) | 42.26 (4.6)* | 43.68 (4.1)§ | 14.70 (2.1)‡ | 15.53 (1.9)‡ | 15.38 (1.6)‡ | 3.63 (0.6)a¶ | 3.23 (0.4)a¶ | 2.99 (0.3)§¶ |
| Glucose, mg/dL | 99.30 (1.87) | 96.80 (1.07) | 95.46 (0.89) | 100.21 (1.52) | 103.69 (2.22)‡ | 102.75 (1.92)‡ | 108.84 (2.28)a¶ | 111.97 (2.49)a¶ | 112.29 (1.99)a¶ |
| Insulin, μU/mL | 8.22 (0.37) | 8.34 (0.38) | 9.96 (0.57)†§ | 11.51 (0.76)‡ | 11.86 (0.89)‡ | 10.80 (0.53) | 13.51 (0.84)¶ | 14.75 (0.92)a¶ | 13.15 (0.86)a¶ |
| HOMA | 2.05 (0.11) | 2.07 (0.11) | 2.44 (0.16) | 2.87 (0.20)‡ | 3.25 (0.32)‡ | 2.82 (0.17) | 3.85 (0.31)¶ | 4.09 (0.27)a¶ | 4.04 (0.28)a¶ |
| Triglycerides, mg/dL | 119.11 (5.37) | 120.03 (4.42) | 122.03 (4.92) | 143.66 (3.89)‡ | 142.67 (7.54)‡ | 144.73 (6.82)‡ | 155.56 (6.47)¶ | 150.74 (7.29)¶ | 154.12 (4.93)¶ |
| Total Cholesterol, mg/dL | 199.92 (2.34) | 199.59 (2.37) | 197.28 (2.15) | 207.12 (3.67) | 205.36 (2.92) | 202.24 (3.58) | 210.40 (2.07)¶ | 204.89 (2.31) | 201.00 (2.57) |
| HDL-Cholesterol, mg/dL | 56.24 (0.81) | 57.17 (0.79) | 57.17 (0.87) | 54.20 (0.69) | 53.32 (0.58)‡ | 52.96 (0.59)‡ | 53.39 (0.94)¶ | 53.31 (0.64)¶ | 53.25 (0.72)¶ |
| hsCRP, mg/dL | 0.29 (0.02) | 0.30 (0.04) | 0.29 (0.02) | 0.47 (0.06)‡ | 0.42 (0.02) | 0.42 (0.03)‡ | 0.60 (0.07)¶ | 0.53 (0.06)¶ | 0.56 (0.04)a¶ |
| Systolic Blood Pressure, mm Hg | 119.13 (0.69) | 119.38 (0.87) | 118.51 (0.89) | 122.69 (0.66)‡ | 122.81 (0.85)‡ | 124.29 (1.09)‡ | 131.17 (1.17)a¶ | 134.67 (1.12)*a¶ | 133.90 (1.56)a¶ |
| Diastolic Blood Pressure, mm Hg | 71.36 (0.51) | 71.82 (0.51) | 71.62 (0.55) | 72.81 (0.47) | 73.47 (0.59)‡ | 73.82 (0.44)‡ | 73.18 (0.77) | 72.05 (0.51) | 72.24 (0.70) |
Abreviations: BMI-body mass index; %BF-percentage of fat; WC-waist circumference; HOMA-Homeostasis Model of Assessment; HDL-high density lipoprotein; hsCRP-high sensitivity C-reactive protein; cpmcounts per minute; PA-physical activity; MVPA-moderate/vigorous physical activity.
Significant difference within MVPA categories: Low SB vs Moderate SB.
Significant difference within MVPA categories: Moderate SB vs High SB.
Significant difference within MVPA categories: Low SB vs High SB.
Significant difference between equivalent SB categories: High MVPA vs Moderate MVPA.
Significant difference between equivalent SB categories: High MVPA vs Low MVPA.
Significant difference between equivalent SB categories: Moderate MVPA vs Low MVPA.
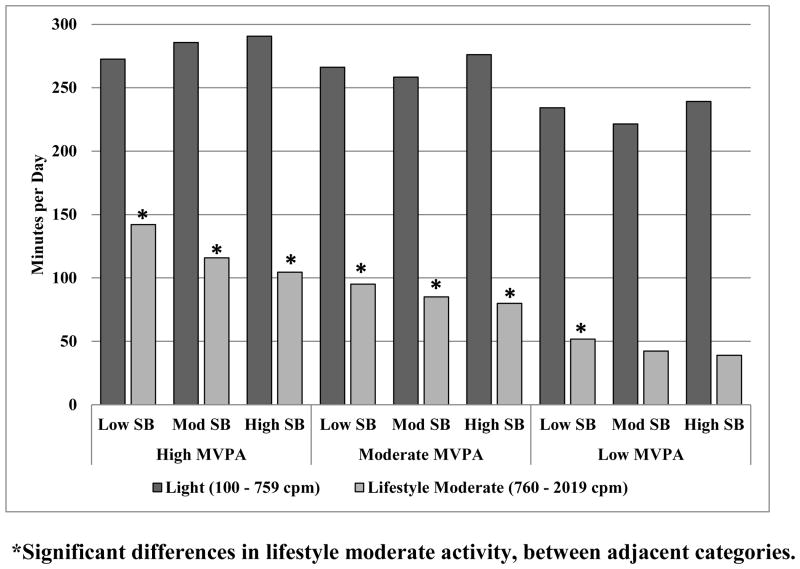
A significant trend of decreased time spent in lifestyle moderate activity across tertiles of MVPA was observed, such that individuals with the highest MVPA also spent significantly more time (p<0.001) in lifestyle moderate activity (men: 136.17 min. (2.84); and women: 108.99 min. (2.65)), than both adults in the middle (men: 94.05 min. (1.66); and women 79.05 min (0.95)) and lowest tertiles of MVPA (men: 48.59 min. (1.10); and women 38.74 min. (1.10)) (Figure 1).
Figure 1.
Differences in lifestyle moderate activity and light activity, across MVPA and SB tertiles.
The Metabolically Abnormal Phenotype
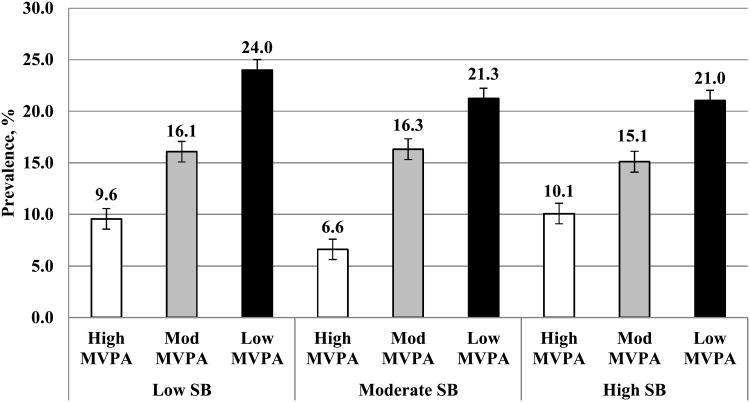
Prevalence of the metabolically abnormal phenotype was 17.3% in men and 16.5% in women. Age-adjusted prevalence was significantly greater for individuals with the lowest MVPA (21-24%; p<0.001), as compared to moderate MVPA (15.1-16.3%) and high MVPA (6.6-10.1%) (Figure 2). Among adults with the highest MVPA (∼43-48 min), there were no differences in age-adjusted prevalence of the metabolically abnormal phenotype across SB tertiles (p>0.05).
Figure 2.
Age-adjusted prevalence differences in the metabolically abnormal phenotype across MVPA and SB tertiles.
Table 2 provides the unadjusted odds ratios (OR) for individual predictors of the metabolically abnormal phenotype, as well as separate models adjusted for potential covariates. In the unadjusted model, greater age, lower education, lower annual income, higher BMIs, higher %BF, greater android adiposity, greater sedentary behavior, less lifestyle moderate activity, and less MVPA were each individually associated with higher odds of being metabolically abnormal. BMI for obesity (≥30 kg/m2) carried the strongest OR of 7.45 (95% CI, 5.57-9.97) and 9.74 (95% CI, 7.61-12.48) for men and women, respectively. After adjustment for all model predictors, greater age (both men and women), less education (both men and women), higher BMI (both men and women, in the respective models), and android adiposity per kg (both men and women, in the respective models) were significantly associated with higher odds of the metabolically abnormal status. Further, after adjusting for all activity categories, the associations of both sedentary behavior and lifestyle moderate activity with the metabolically abnormal phenotype remained significant only among women. Conversely, lower MVPA was a significant predictor in both men and women.
Table 2. Unadjusted and multivariable-adjusted odds ratios for the metabolically abnormal phenotype.
| Unadjusted | Multivariable Adjusteda | Multivariable Adjustedb | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model Predictors | Men | Women | Men | Women | Men | Women |
| Age group, year | ||||||
| 20-39 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 40-59 | 1.93 (1.52-2.44) | 2.25 (1.80-2.82) | 1.41 (1.07-1.92) | 1.52 (1.04-2.22) | 1.56 (1.04-2.10) | 1.81 (1.11-2.95) |
| ≥60 | 2.42 (1.92-3.05) | 3.55 (2.72-4.62) | 1.16 (0.78-1.73) | 2.10 (1.34-3.27) | 1.51 (1.00-2.38) | 2.84 (1.76-4.56) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | 0.82 (0.66-1.02) | 1.08 (0.86-1.34) | 0.80 (0.59-1.10) | 0.98 (0.72-1.32) | 0.97 (0.71-1.33) | 1.17 (0.85-1.63) |
| Hispanic or Mexican American | 0.96 (0.77-1.19) | 1.08 (0.77-1.54) | 1.15 (0.86-1.53) | 1.13 (0.69-1.87) | 1.42 (1.03-1.97) | 1.47 (0.90-2.41) |
| Highest level of education | ||||||
| < HS Graduate | 1.53 (1.18-1.99) | 2.61 (1.72-3.95) | 1.86 (1.10-3.13) | 1.79 (1.03-3.11) | 2.06 (1.10-3.85) | 2.31 (1.27-4.21) |
| HS Graduate and/or Some College | 1.55 (1.19-2.01) | 1.80 (1.29-2.51) | 1.76 (1.16-2.68) | 1.44 (0.93-2.23) | 1.85 (1.09-3.15) | 1.59 (1.00-2.54) |
| ≥ College Graduate | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Annual Household Income | ||||||
| < $24,999 | 1.34 (1.14-1.61) | 1.81 (1.40-2.35) | 1.23 (0.81-1.87) | 0.87 (0.59-1.28) | 1.22 (0.77-1.91) | 0.79 (0.54-1.17) |
| $25,000 - $54,999 | 1.19 (0.95-1.49) | 1.54 (1.20-1.98) | 1.18 (0.91-1.52) | 1.02 (0.71-1.45) | 1.06 (0.76-1.46) | 1.01 (0.68-1.48) |
| ≥ $55,999 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Body Mass Index (BMI) categories | ||||||
| Normal (18.5-24.9) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | - | - |
| Overweight (25.0-29.9) | 3.19 (2.44-4.18) | 4.24 (3.15-5.70) | 2.35 (1.39-3.96) | 3.95 (2.48-6.25) | - | - |
| Obese (≥30.0) | 7.45 (5.57-9.97) | 9.74 (7.61-12.48) | 4.25 (2.24-8.10) | 8.08 (5.16-12.65) | - | - |
| Total Perce nt Body Fatc | ||||||
| Percent Body Fat, per 10% | 3.66 (3.07-4.37) | 3.64 (3.12-4.26) | 1.79 (1.00-2.43) | 1.31 (0.93-1.85) | 0.60 (0.33-1.08) | 0.83 (0.53-1.28) |
| Total Android Adiposity | ||||||
| Android Adiposity, per 1 kg | 1.94 (1.74-2.17) | 2.00 (1.86-2.13) | - | - | 2.36 (1.76-3.17) | 2.00 (1.63-2.45) |
| Sedentary Behavior (<100 counts per min) | ||||||
| 1st Tertile (least SB) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2nd Tertile | 0.99 (0.72-1.35) | 1.47 (1.07-2.01) | 0.88 (0.63-1.22) | 1.33 (0.90-1.96) | 0.83 (0.59-1.16) | 1.72 (1.15-2.57) |
| 3 rd Tertile | 1.05 (0.82-1.34) | 1.23 (0.88-1.72) | 1.06 (0.78-1.44) | 0.98 (0.63-1.53) | 1.18 (0.85-1.64) | 1.31 (0.82-2.10) |
| Light Activity (100 - 759 cpm) | ||||||
| 1st Tertile | 1.55 (1.21-1.99) | 1.72 (1.34-2.22) | 1.35 (0.97-1.88) | 1.15 (0.72-1.82) | 1.41 (0.91-2.21) | 1.31 (0.79-2.15) |
| 2nd Tertile | 1.22 (0.92-1.61) | 1.33 (0.84-1.52) | 1.27 (0.87-1.84) | 0.98 (0.69-1.39) | 1.25 (0.81-1.93) | 0.97 (0.65-1.46) |
| 3 rd Tertile (most activity) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Lifestyle Moderate (760 - 2019 cpm) | ||||||
| 1st Tertile | 2.23 (1.61-3.09) | 3.30 (2.55-4.28) | 1.24 (0.75-2.04) | 1.99 (1.13-3.51) | 1.03 (0.61-1.74) | 2.06 (1.08-3.72) |
| 2nd Tertile | 1.49 (1.04-2.16) | 1.58 (1.19-2.11) | 1.07 (0.71-1.61) | 1.30 (0.88-1.93) | 0.95 (0.62-1.43) | 1.34 (0.87-2.04) |
| 3 rd Tertile (most activity) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Moderate/Vigorous PA (≥2020 cpm) | ||||||
| 1st Tertile | 3.29 (2.36-4.57) | 3.86 (2.86-5.21) | 1.56 (1.01-2.55) | 1.40 (1.00-2.08) | 1.74 (1.07-2.83) | 1.10 (0.77-1.59) |
| 2nd Tertile | 1.81 (1.35-2.44) | 2.36 (1.69-3.29) | 1.33 (0.91-1.94) | 1.45 (1.10-2.01) | 1.58 (1.07-2.33) | 1.35 (1.01-1.80) |
| 3 rd Tertile (most activity) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
Abbreviations: HS-High School; BMI-Body mass index; BF-Body fat; SB-Sedentary behavior; PA-Physical activity
Metabolically abnormal is defined as ≥ 3 cardiometabolic abnormalities, including elevated blood pressure; elevated levels of triglycerides, fasting plasma glucose, and C-reactive protein; elevated homeostasis model assessment (HOMA) of insulin resistance value; and low high-density lipoprotein cholesterol level.
Each factor in the table is adjusted for every other factor, except android adiposity.
Each factor in the table is adjusted for every other factor, except BMI.
Total percent body fat was included in the unadjusted and adjusted multivariable models, and is presented by 10% unit increments.
Discussion
This study presents a comprehensive examination of cardiometabolic health profiles across combinations of objectively measured sedentary time and MVPA. The primary finding of this study was that adults with the highest MVPA (∼43-48 min/day) across low-, med- and high-SB tertiles did not differ markedly in prevalence of obesity, adiposity, and/or serum cardiometabolic risk factors. Conversely, less MVPA was reflective of substantial elevations of obesity and cardiometabolic risk, regardless of time spent in SB. As expected, adults with the least SB and greatest amount of MVPA were younger and exhibited the healthiest cardiometabolic phenotypes, whereas adults with the greatest SB and least time in MVPA were older and had significantly elevated prevalence of obesity by BMI, WC, and %BF, as well as greater risk across virtually every clinical cardiometabolic parameter. Interestingly, a significant trend of decreased time spent in lifestyle moderate activity across lower tertiles of MVPA was found, such that individuals with the highest MVPA also spent significantly more time in lifestyle moderate activity than adults in the middle and lowest tertiles of MVPA. This is aligned with a recent study which demonstrated that MVPA and lifestyle activity accumulate in similar patterns and are highly correlated (1). It is quite possible that this combined tendency toward elevated MVPA and lifestyle moderate activity may have provided substantial additive health benefits, regardless of the amount of time spent in SB or light activity.
These results are somewhat contradictory to another recent NHANES study which revealed that, among older adults (≥ 65 years), the link between SB and various cardiometabolic health outcomes was not modified by the level of MVPA participation (8). Those findings were supportive of recent work (23) suggesting independent associations between SB, physical activity, and cardiometabolic outcomes, and that sufficient MVPA may not ameliorate the negative contribution of SB on cardiometabolic risk. When adjusting for age in the current study, prevalence of cardiometabolic abnormalities decreased slightly across SB and MVPA tertiles; however, a significant trend remained wherein adults with higher MVPA had much lower prevalence of the metabolically abnormal phenotype, irrespective of time spent in SB. It is plausible that the contribution of SB on health risk is exaggerated in high risk populations (e.g. older adults and mobility disabilities sub-populations), especially if physical activity is very low across all intensities. Additional research is warranted to identify population and patient-specific activity strategies that are sustainable.
Moreover, our results seem to suggest a sex-specific pattern of risk for cardiometabolic health. After adjusting for all potential predictors and with regard to activity compartmentalization, less time spent in SB as well as greater lifestyle moderate activity and MVPA were all associated with lower odds of being metabolically abnormal in women, whereas only greater MVPA was significant in men. In these analyses, light activity was not significantly associated with the metabolically abnormal criteria in men or women. Thus, replacing sedentary time with light activity may not confer the positive health benefits that have been widely purported in recent years. Rather, it appears there is ample evidence to support the value of greater lifestyle moderate activity and MVPA as potent healthy behavior strategies. These findings support the recommendations outlined by the 2008 Physical Activity Guidelines for Americans (PAGA) (5) and the American College of Sports Medicine (ACSM) (9), which advocated the importance achieving at least 150 minutes of MVPA per week.
The strongest factors associated with the metabolically abnormal phenotype in both men and women were BMI and android adiposity, even after adjustment for all sociodemographic factors, %BF, SB, and all activity categories. BMI and android adiposity were highly correlated, and thus were used separately in the logistic regression models. Interestingly, once android adiposity was accounted for, the association between %BF and the metabolically abnormal status was completely eliminated. This finding suggests that incorporating body composition as a predictor for cardiometabolic disease may be useful only as a proxy representing increased abdominal adiposity. Thus, in addition to recommending regular physical activity, this study supports the clinical value of screening both BMI and abdominal adiposity with waist circumference monitoring. However, as with all cross-sectional investigations, a limitation of this study is the inability to disentangle the cause-effect relationship between predictors and outcomes. Indeed whether excessive SB “causes” obesity, or obesity itself is a cause of increased SB (i.e., reverse causality), is an interesting and complex topic (19). Findings do, however, support the relative value of physical activity accumulation, independent of SB and obesity, to attenuate risk.
Conclusions
More MVPA and less adiposity are strong factors associated with cardiometabolic health among adults, and this is independent of age, %BF, and time spent in SB. Striking differences between men and women were observed for the associations between android adiposity and cardiometabolic abnormalities. Specifically, although android adiposity plays an important contributing role in the negative cardiometabolic milieu for both men and women, it seems to be stronger independent predictor among women. Moreover, adults who accumulated the greatest MVPA also tended to do more lifestyle moderate activity, and there is clearly an additive cardiometabolic benefit that could not likely be equaled by simply replacing SB with light activity. Behavioral interventions to increase moderate and intense physical activity, reduce BMI and abdominal adiposity, and decrease sedentary behavior are certainly warranted.
Acknowledgments
The authors would like to acknowledge the University of Texas Medical Branch Claude D. Pepper Center (P30-AG024832), and the Michigan Institute for Clinical & Health Research (2UL1TR000433-06). The results of the present study do not constitute endorsement by ACSM.
Funding/Support: This study was funded by the U.S. National Institutes of Health: R24 HD065702-03 (M. Peterson & S. Al Snih). I-Min Lee was supported in part by grant CA154647 from the U.S. National Institutes of Health.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Role of the Sponsors: The funders had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
References
- 1.Camhi S, Sisson S, Johnson W, Katzmarzyk P, Tudor-Locke C. Accelerometer determined lifestyle physical activities. J Phys Act Health. 2011;8(3):382–9. doi: 10.1123/jpah.8.3.382. [DOI] [PubMed] [Google Scholar]
- 2.Camhi SM, Sisson SB, Johnson WD, Katzmarzyk PT, Tudor-Locke C. Accelerometer determined moderate intensity lifestyle activity and cardiometabolic health. Preventive medicine. 2011;52(5):358–60. doi: 10.1016/j.ypmed.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Carnethon MR, De Chavez PJD, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA: the journal of the American Medical Association. 2012;308(6):581–90. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charansonney OL, Despres JP. Disease prevention--should we target obesity or sedentary lifestyle? Nat Rev Cardiol. 2010;7(8):468–72. doi: 10.1038/nrcardio.2010.68. [DOI] [PubMed] [Google Scholar]
- 5.Committee PAGA. 2008 Physical Activity Guidelines for Americans. Washington DC: USDHHS; 2008. [Google Scholar]
- 6.Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. 2013;97(3):487–96. doi: 10.3945/ajcn.112.046854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frellick M. AMA declares obesity a disease Medscape Medical News 2013. 2013 Jul; Available from: http://www.medscape.com/viewarticle/806566.
- 8.Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary Behavior, Physical, Activity and Markers of Health in Older Adults. Med Sci Sports Exerc. doi: 10.1249/MSS.0b013e318288a1e5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sport Exer. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 11.Maher CA, Mire E, Harrington DM, Staiano AE, Katzmarzyk PT. The independent and combined associations of physical activity and sedentary behavior with obesity in adults: NHANES 2003-06. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–22. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 13.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. American journal of epidemiology. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. American Journal of Clinical Nutrition. 2012;95(2):437–45. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 17.Newton RL, Jr, Han H, Zderic T, Hamilton M. The energy expenditure of sedentary behavior: a whole room calorimeter study. PLoS One. 2013;8(5):e63171. doi: 10.1371/journal.pone.0063171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 34(5):791–9. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 19.Pulsford RM, Stamatakis E, Britton AR, Brunner EJ, Hillsdon MM. Sitting Behavior and Obesity Evidence from the Whitehall II Study. American Journal of Preventive Medicine. 2013;44(2):132–8. doi: 10.1016/j.amepre.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. European heart journal. 2007;28(17):2087–93. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 21.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PloS one. 2010;5(5):e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam CS, Ravussin E. Response to Comment on: Tam et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35:1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]; Diabetes Care. 2013;36(1):e11. [Google Scholar]
- 23.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 24.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Archives of internal medicine. 2008;168(15):1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]