Abstract
Introduction
Skeletal muscle ischemia reperfusion injury (I-R) is a complex injury process that includes damage to the sarcolemmal membrane, contributing to necrosis and apoptosis. MG53, a muscle-specific TRIM family protein, has been shown to be essential for regulating membrane repair and has been shown to be protective against cardiac I-R and various forms of skeletal muscle injury. The purpose of this study was to determine if recombinant human MG53 (rhMG53) administration offered protection against I-R.
Methods
rhMG53 was administered to rats immediately before tourniquet-induced ischemia and again immediately before reperfusion. Two days later muscle damage was assessed histologically.
Results
rhMG53 offered no protective effect, as evidenced primarily by similar Evans blue dye inclusion in the muscles of rats administered rhMG53 or saline.
Discussion
Administration of rhMG53 does not offer protection against I-R in rat skeletal muscle. Additional studies are required to determine if the lack of a response is species-specific.
Keywords: muscle injury, ischemia reperfusion, MG53, TRIM72, tourniquet
Introduction
Skeletal muscle disease1 and trauma2 can involve tissue ischemia and reperfusion injury (I-R), potentially leading to permanent loss of limb function, amputation, or even death. I-R is a complex injury that results in vascular, neural, and muscular damage.3 In myofibers, free radicals generated during I-R promote membrane damage,4 inciting a cascade of events that can lead to necrosis and apoptosis.1 As such, increasing the antioxidant capacity of skeletal muscle prior to I-R injury is effective at mitigating tissue damage.5 However, with trauma it may not be feasible to combat initial free radical production, raising the need to identify effective therapies directed at enhancing membrane repair.6
Recently, Cai et al.7 identified mitsugumin 53 (MG53 or TRIM72), a muscle-specific TRIM family protein which is essential in regulating membrane repair in coordination with dysferlin.7,8 This is further illustrated by the progressive muscle weakness and defective muscle repair after exercise or injury in MG53 knock-out mice.9 Interestingly, delivery of recombinant human MG53 (rhMG53) to mechanically injured C2C12 myotubes in vitro enhanced cell membrane repair.10 Moreover, rhMG53 delivery to mdx and wild-type mice improved the capacity to repair membranes damaged by eccentric contractions or cardiotoxin.11,10 Based on these observations, we hypothesized that delivery of rhMG53 would ameliorate skeletal muscle damage secondary to I-R injury.
Methods
Adult male Sprague-Dawley rats (403 ± 15 g) were divided into 2 groups (n=7 per group): 1) MG53-treated (MG53); and 2) saline-treated controls (Saline). Both groups underwent 3 hr of pneumatic tourniquet (TK) induced I-R.6 Lyophilized rhMG53 (TRIM-edicine, Inc.) was dissolved in sterile saline (2 mg/ml) and administered via tail vein injection (6 mg/kg body wt) 5 min prior to TK inflation and 5 min prior to removal. Two days after injury, lower leg tissues were harvested. Frozen tibialis anterior (TA) muscle sections stained with H&E were scored13,14 by a certified veterinary pathologist blind to the treatment, and the prevalence of damaged fibers was quantitated from 10 10x images from each muscle. Approximately 800 fibers were counted per muscle.12 Muscle fiber cell membrane integrity was determined histologically in TA muscles by microscopic visualization of Evans blue dye (EBD; 1% w/v; i.p. 24 hr before injury) inclusion within damaged cells.7 The area fraction of EBD was calculated in 8 10x images from each muscle. Gastrocnemius muscles were weighed before and after drying at ~50°C for 7 days, and the wet to dry weight ratio served as an index of edema. To ensure that the batch of rhMG53 protein had not degraded, a 30μg protein sample was separated using SDS-PAGE, transferred to a nitrocellulose membrane, and stained with Ponceau. Additionally, rhMG53-pretreated (0.2 mg/mL; 10) C2C12 myotubes were exposed to 300μM H2O2 for 18 hours and then re-incubated in rhMG53 and assayed for viability 24 hours later using an XTT Cell Proliferation Assay Kit (ATCC). Statistical differences between groups were determined using independent t-tests for continuous data and Mann–Whitney U tests for interval data (α<0.05).
Results
The magnitude of sarcolemmal damage, as evidenced by intracellular EBD inclusion, was similar between Saline and rhMG53 treated rats; 50.9±4.8 and 55.8±3.1% of the total fibers had EBD staining (P=0.404), respectively (Fig. 1A–C). In a separate analysis, 43.2±6.9 and 52.1±7.3% of the fibers counted were injured in H&E sections from Saline and MG53 groups (P=0.425), respectively (Fig. 1D–F). Pathologist scoring of H&E stained sections (Suppl. Fig. 1A–D) also revealed no difference between groups in the extent of edema, degeneration, necrosis, or inflammation. Similar levels of edema occurred in both groups as indicated by an identical increase of 46% in the wet weight of the injured TA relative to its contralateral uninjured muscle (P = 0.976). rhMG53 was not degraded, as a large band of protein was observed at approximately 53 kDa (Suppl. Fig. 1E). In vitro C2C12 myotube viability following exposure to H2O2 was improved with application of rhMG53 (Suppl. Fig. 1F).
Figure 1.
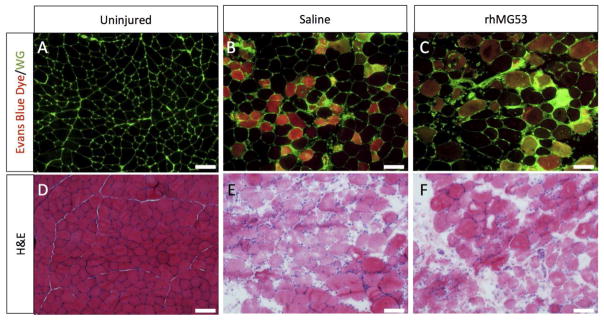
rhMG53 administration does not attenuate muscle fiber damage following I-R injury in rats. A-C) Cross-sections of the TA muscles were stained with wheat germ agglutinin conjugated with Alexa fluor 488 (green). Fibers with EBD inclusion fluoresce red. D–F) TA cross-sections were stained with H&E. The scale bars for all images equal 100 μm.
Discussion
These findings do not support the hypothesis that administration of rhMG53 to skeletal muscle undergoing I-R attenuates tissue injury. To the contrary, all indices measured indicated that I-R induced equally severe muscle injury with or without administration of rhMG53, although it did improve C2C12 myotube viability upon H2O2 exposure in vitro (Suppl. Fig. 1). These results are in contrast to those previously reported, which indicated a therapeutic effect of rhMG53 in cardiac I-R13,14,10 and other forms of skeletal muscle injury in mice. 7,9,15,10
The explanation for the current findings is not obvious. The 3-hour ischemia time prior to reperfusion produces severe skeletal muscle injury,16,6,3 which resulted in loss of sarcolemmal integrity and signs of fiber injury in over 50% of the TA muscle by 2 days post-injury (Fig. 1). The possibility exists that the injury severity was beyond therapeutic benefit, or possibly rat muscle contains higher intrinsic protective function than mouse muscle. Clearly, acute administration of rhMG53 (8 mg/Kg; i.m.) attenuated the extent of muscle injury out to 7 days after cardiotoxin injection in mice, which resulted in sarcolemmal damage in approximately 80% of muscle fibers.10 The dose of 6 mg/Kg used in this study was within the range of 4–8 mg/Kg, which has been reported to have positive results with intravenous administration in vivo.10,15 Further, it is unlikely that the timing of the administration of rhMG53 was responsible for the disparate findings, as the dosing regimen used in this study was designed to provide the best chance of showing a benefit. That is, peak plasma levels of rhMG53 occur 4 hr after intravenous injection and fall to 20% at 6 hr post-injection;10 at 3 hr, the time of the second dose of MG53, plasma levels would be at approximately 92% of peak. This strategy maximized exposure to rhMG53 during the early phase of reperfusion - the time associated with the majority of I-R associated damage.1
It is possible that additional pretreatments in the hours or days preceding the injury may be required to induce beneficial effects, but this would be irrelevant for most clinical applications, particularly for trauma. Additionally, intramuscular injection of rhMG53 could be explored in future studies. Finally, studies that have shown protection against muscle injury with rhMG53 have involved mice10,15, suggesting the possibility of species differences.
Supplementary Material
Acknowledgments
This work was funded by the U.S. Army Medical Research and Medical Command (grant: F_025_2010_USAISR) awarded to TJW, and National Institutes of Health grant awarded to JM. We would like to thank Ms. Melissa Sanchez for her technical support and LTC J Scot Estep, DVM, for pathology support. All animal protocols were approved by the US Army Institute of Surgical Research Animal Care and Use Committee. This study adhered to National Institutes of Health guidelines for the care and use of laboratory animals (DHHS Publication, NIH, 86 to 23). All components of this study including the decision where to publish were at the sole discretion of the authors. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense (AR 360-5) or the United States Government. Some of the authors are employees of the U.S. government, and this work was prepared as part of their official duties.
Abbreviations
- EBD
Evans blue dye
- H&E
Hematoxylin and eosin
- i.m
Intramuscular
- i.v.
Intravenous
- I-R
Ischemia-reperfusion injury
- MG53
Mitsugumin 53
- rhMG53
Recombinant human Mitsugumin 53
- TA
Tibialis anterior muscle
- TRIM
Tripartite motif
References
- 1.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10(6):620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in iraq and afghanistan. Ann Surg. 2011;253(6):1184–1189. doi: 10.1097/SLA.0b013e31820752e3. [DOI] [PubMed] [Google Scholar]
- 3.Criswell TL, Corona BT, Ward CL, Miller M, Patel M, Wang Z, Christ GJ, Soker S. Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol. 2012;180(2):787–797. doi: 10.1016/j.ajpath.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Heppenstall RB, Scott R, Sapega A, Park YS, Chance B. A comparative study of the tolerance of skeletal muscle to ischemia. Tourniquet application compared with acute compartment syndrome. J Bone Joint Surg Am. 1986;68(6):820–828. [PubMed] [Google Scholar]
- 5.Bolcal C, Yildirim V, Doganci S, Sargin M, Aydin A, Eken A, Ozal E, Kuralay E, Demirkilic U, Tatar H. Protective effects of antioxidant medications on limb ischemia reperfusion injury. J Surg Res. 2007;139(2):274–279. doi: 10.1016/j.jss.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 6.Walters TJ, Mase VJ, Jr, Roe JL, Dubick MA, Christy RJ. Poloxamer-188 reduces muscular edema after tourniquet-induced ischemia-reperfusion injury in rats. J Trauma. 2011;70(5):1192–1197. doi: 10.1097/TA.0b013e318217879a. [DOI] [PubMed] [Google Scholar]
- 7.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11(1):56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lek A, Evesson FJ, Lemckert FA, Redpath GM, Lueders AK, Turnbull L, Whitchurch CB, North KN, Cooper ST. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(12):5085–5094. doi: 10.1523/JNEUROSCI.3560-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284(23):15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4(139):139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisleder N, Lin P, Zhao X, Orange M, Zhu H, Ma J. Visualization of MG53-mediated cell membrane repair using in vivo and in vitro systems. J Vis Exp. 2011;(52) doi: 10.3791/2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack MC, Kwon E, Eberlin KR, Randolph M, Friend DS, Thomas AC, Watkins MT, Austen WG., Jr Development of reproducible histologic injury severity scores: skeletal muscle reperfusion injury. Surgery. 2008;143(1):126–133. doi: 10.1016/j.surg.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107(1):76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 14.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121(23):2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, Cao CM, Xiao RP. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res. 2011;91(1):108–115. doi: 10.1093/cvr/cvr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JG, Lee J, Roe J, Tromberg BJ, Brenner M, Walters TJ. Hemodynamic changes in rat leg muscles during tourniquet-induced ischemia-reperfusion injury observed by near-infrared spectroscopy. Physiol Meas. 2009;30(7):529–540. doi: 10.1088/0967-3334/30/7/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


