Graphical abstract
Highlights
-
•
Until 2013, class II proteins had only been found in flaviviruses and alphaviruses.
-
•
A class II fusion protein was recently discovered in the unrelated phlebovirus genus.
-
•
Within the same family as alphaviruses, rubella virus has a divergent class II fold.
-
•
Pestiviruses, although they are Flaviviridae, have fusion proteins from a novel class.
-
•
Viral class II proteins may originate from cellular class II fusion protein ancestors.
Abstract
Enveloped viruses must fuse their lipid membrane to a cellular membrane to deliver the viral genome into the cytoplasm for replication. Viral envelope proteins catalyze this critical membrane fusion event. They fall into at least three distinct structural classes. Class II fusion proteins have a conserved three-domain architecture and are found in many important viral pathogens. Until 2013, class II proteins had only been found in flaviviruses and alphaviruses. However, in 2013 a class II fusion protein was discovered in the unrelated phlebovirus genus, and two unexpectedly divergent envelope proteins were identified in families that also contain prototypical class II proteins. The structural relationships of newly identified class II proteins, reviewed herein, shift the paradigm for how these proteins evolved.
Current Opinion in Virology 2014, 5:34–41
This review comes from a themed issue on Virus structure and function
Edited by Wah Chiu, Thibaut Crépin and Rob WH Ruigrok
For a complete overview see the Issue and the Editorial
Available online 11th February 2014
1879-6257/$ – see front matter, © 2014 Elsevier B.V. All rights reserved.
Introduction
Viral envelope proteins are the principal effectors of virus assembly and cell entry. Enveloped viruses must fuse their lipid membrane with a host-cell membrane in order to deliver their genome into the cytoplasm for replication. This membrane fusion event is catalyzed by viral envelope proteins. Viruses also rely on their envelope proteins to recognize host cells by binding cellular receptors. Envelope proteins shield viruses from the immune system and bear most of the neutralizing antibody epitopes against any given virus. The envelope proteins of many viruses form a rigid outer structural shell, which usually takes the form of a quasi-spherical icosahedral assembly.
Viral membrane fusion proteins fall into at least three distinct structural classes. The influenza virus hemagglutinin (HA) is the prototype of “class I” fusion proteins [1], which encompass those of other orthomyxoviruses and paramyxoviruses, retroviruses, filoviruses, and coronaviruses [2]. The unifying structural feature of class I fusion proteins is a core consisting of three bundled α-helices [3, 4]. Class II fusion proteins are a structurally unrelated class found in flaviviruses, alphaviruses, and most recently in rubella virus (sole member of the rubivirus genus) and Rift Valley fever virus (from the phlebovirus genus) [4, 5••, 6••]. Class II proteins share a three-domain architecture consisting almost entirely of β-strands, with tightly folded “fusion loops” in the central domain serving as the anchor in the cellular membrane targeted for fusion (Figure 1 ). Class III fusion proteins, found in herpesviruses, rhabdoviruses and baculoviruses, possess structural features from both class I proteins (a core three-helix bundle) and from class II proteins (a central β-stranded fusion domain) [7].
Figure 1.
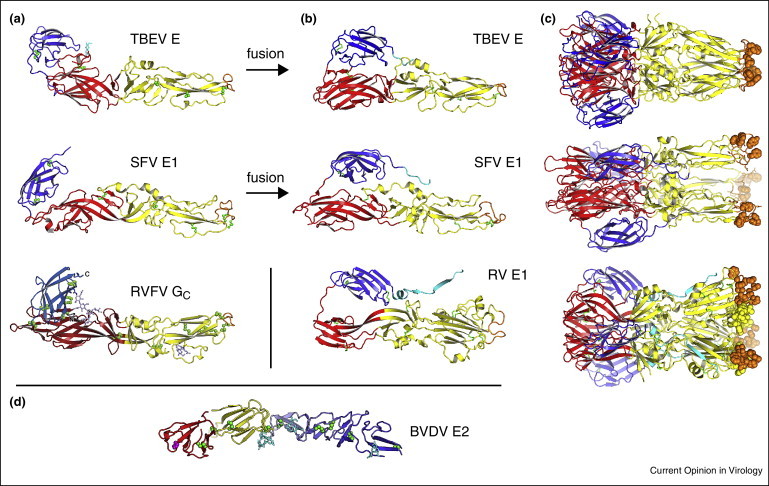
Representative class II membrane fusion glycoproteins in their prefusion and postfusion conformations. (a) The class II fold consists of three domains. A β-sandwich domain (red) organizes the structure; an elongated domain (yellow) bears a hydrophobic “fusion loop” (orange) at its tip, which serves as an anchor in the target cellular membrane; an Ig-like domain (blue) contains the structural determinants of cellular tropism and virulence, as well as most neutralizing antibody epitopes. The following viral fusion proteins are shown in their prefusion conformation: E from the flavivirus tick-borne encephalitis virus (TBEV) [11]; E1 from the alphavirus Semliki Forest virus (SFV) [19]; Gc from Rift Valley fever virus (RVFV), a phlebovirus from the Bunyaviridae family [5••]. (b) Class II proteins in their postfusion conformation. Shown here are TBEV E [42], SFV E1 [43] and E1 from rubella virus (RV) [6••]. Class II proteins are trimeric in the postfusion conformation, (c). (d) Envelope protein E2 from bovine viral diarrhea virus (BVDV) has a novel fold despite being in the Flaviviridae family (genus pestivirus) [9••, 10••].
Until recently, class II proteins had only been found in flaviviruses and alphaviruses (in the Flaviviridae and Togaviridae families, respectively), which share many key characteristics. Indeed viruses from these two genera all have positive-stranded RNA genomes of 11–12 kilobases with similar gene organizations, icosahedral outer protein shells with a diameter of approximately 50 nm, and lifecycles that alternate between vertebrates and arthropod vectors [8]. The most plausible evolutionary model had thus been one in which flaviviruses and alphaviruses evolved from a common ancestor virus. However, a class II fusion protein was recently discovered in the unrelated Bunyaviridae family [5••]. Conversely, divergent fusion protein architectures have emerged within the Flaviviridae and Togaviridae families in which the prototypical class II proteins were first identified [6••, 9••, 10••]. Together, these recent discoveries shift the evolutionary paradigm from a divergent model (common ancestor virus), to a model in which class II fusion proteins evolved independently by borrowing from a common (or related) ancestral class II cellular membrane fusion protein.
Unifying structural features of class II envelope proteins
The class II fusion protein fold was first discovered in glycoprotein E from tick-borne encephalitis virus, a member of the Flaviviridae family [11]. The E proteins from other flaviviruses were subsequently found to have very similar structures [12, 13, 14, 15, 16, 17, 18], and the E1 proteins from three alphaviruses (Semliki Forest, Sindbis and Chikungunya viruses) have the same fold despite a lack of sequence similarity to flavivirus E proteins (Figure 1) [19, 20, 21]. The envelope proteins from flaviviruses and alphaviruses assemble into icosahedral outer shells, but the mode of assembly differs in the two families, with alphaviruses forming canonical (T = 4) quasi-equivalent assemblies [19, 22•, 23•] and flaviviruses forming unusual non-equivalent icosahedral assemblies [24, 25, 26•]. Class II proteins are anchored in the viral membrane via a C-terminal transmembrane anchor, which is linked by a flexible “stem” region to the ectodomain (Figure 2 ). The ectodomain consists of three domains: a β-barrel (domain I); an elongated, mostly β-stranded domain bearing a tightly folded “fusion loop” that inserts into the target cellular membrane (domain II); and an IgC-like module that bears the epitopes responsible for cellular tropism and efficient antibody neutralization (domain III) [11, 27, 28, 29]. Remarkably, despite evidence that domain III is directly involved in cellular attachment of flaviviruses [30, 31], no receptors that bind to class II proteins in flaviviruses or alphaviruses have yet been identified. However, protein–glycan interactions involving class II glycoproteins have been shown to contribute to attachment (but not endocytosis [32, 33]) of certain flaviviruses in a subset of host-cell types. These interactions involve the C-type lectins DC-SIGN and L-SIGN [15, 34, 35, 36], mannose receptor [37], and cell-surface heparan sulfate [38]. In alphaviruses, it is the non-fusogenic E2 spike protein that mediates receptor binding but interestingly E2 also recognizes DC-SIGN, L-SIGN and heparan sulfate [39, 40].
Figure 2.
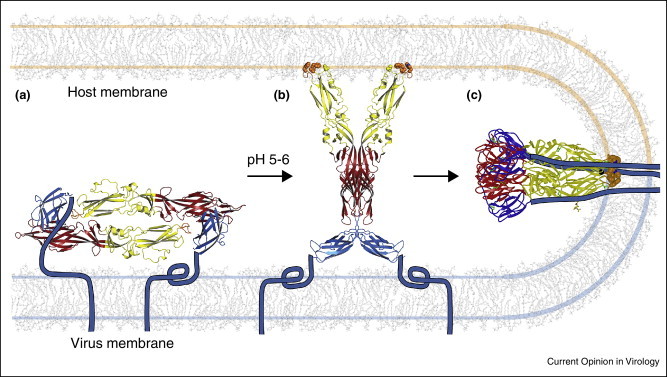
Membrane fusion by class II envelope proteins. (a) The protein forms dimers in the outer protein shell of the virion. The “stem-anchor” (cyan) tethers the protein to the viral membrane. Gc from Rift Valley fever virus (RVFV) is shown as an example [5••]. (b) The protein responds to the reduced pH of an endosomal compartment with a hinge motion that exposes the hydrophobic fusion loop (orange). The fusion loop inserts into the cell membrane. A crystal structure of RVFV Gc proposed to correspond to this “prehairpin” intermediate is shown [5••]. (c) The protein then folds back on itself, directing the fusion loop toward the transmembrane anchor. The refolding energy bends the apposed membranes. Creation of additional trimer contacts between the stem-anchor and the ectodomain leads to fusion of the viral and cellular membranes. The postfusion conformation of dengue type 2 virus is shown [41].
Crystal structures of various class II envelope proteins before and after the conformational change that catalyzes membrane fusion provide a molecular outline of the fusion mechanism (Figure 1) [11, 12, 13, 14, 15, 19, 20, 21, 41, 42, 43, 44, 45]. Complementing these prefusion and postfusion structures, structures thought to represent fusion intermediates provide invaluable insights on the steps required for fusion [5••, 20, 45, 46]. In the mechanism that is emerging (Figure 2), the fusion protein responds to the reduced pH of an endosomal compartment with a motion that breaks most or all of the intersubunit contacts in the outer protein shell, exposing a hydrophobic “fusion loop”, which spontaneously inserts into the outer bilayer leaflet of the host-cell membrane [5••, 20, 41, 47, 48]. The fusion protein then folds back on itself, directing its transmembrane anchor toward the fusion loop. This fold-back forces the host-cell membrane (held by the fusion loop) and the viral membrane (held by the transmembrane anchor) against each other, resulting in fusion of the two membranes. The ectodomains of class II fusion proteins are either monomers or dimers in the prefusion conformation, but always form trimers in the postfusion conformation (Figure 1). The mechanism and structural basis of membrane fusion are conserved in all class II proteins examined to date. Indeed the overall topology of dual membrane anchors being driven toward each other by a fold-back of the fusogen appears to be conserved in all viral fusion proteins (reviewed in Refs. [3, 4]).
Until 2013, class II proteins had only been found in flaviviruses and alphaviruses, but recent studies suggest that the class II fold is more widely prevalent than previously anticipated. Indeed, Dessau and Modis showed that glycoprotein C (Gc) from Rift Valley fever virus (RVFV) is a class II fusion protein [5••]. RVFV belongs to the phlebovirus genus in the Bunyaviridae family, which is unrelated to flaviviruses or alphaviruses. Moreover, rubella virus E1 was shown to have a class II fold, albeit with a more divergent structure than expected for a virus in the same Togaviridae family as alphaviruses [6••]. However, despite the presence of some novel structural features in both RVFV Gc and rubella E1, the two proteins still possess each of the core structural features (described earlier in this section) that unify class II fusion proteins (Figure 1, Figure 2). These parallels even extend to receptor binding in the case of phleboviruses, since RVFV and Uukuniemi virus were recently shown to utilize DC-SIGN as a receptor [49]. In the case of rubella, myelin oligodendrocyte glycoprotein (MOG) was recently identified as a putative receptor for E1 [50], making MOG the first receptor reported to bind to a class II protein via protein–protein interactions.
Unexpected similarities in class II proteins from flaviviruses and phleboviruses
The identification in 2013 of a class II fusion protein in RVFV [5••], although it had been predicted by amino acid analysis [51], was nevertheless unexpected because phleboviruses such as RVFV do not have any of the key characteristics shared by flaviviruses and alphaviruses. Phleboviruses have segmented negative-sense and ambi-sense RNA genomes, undergo membrane fusion much later in late endosomes [52], and their envelope proteins form much larger (103 nm diameter) T = 12 icosahedral lattices [53, 54] with a novel mode of assembly [5••]. The structure of RVFV Gc is strikingly similar to flavivirus E structures (especially dengue E), more similar in fact than flavivirus and alphavirus envelope proteins are to each other. The most notable similarity is that Gc forms dimers that have the same head-to-tail configuration as flavivirus E dimers, with the fusion loop buried at the dimer interface (Figure 3 ). This is particularly surprising given that the E dimer is the building block of the flavivirus non-equivalent “herringbone” assembly, which is very distinct from the T = 12 phlebovirus assembly, although interestingly a non-equivalent configuration has been proposed for the latter [5••]. Another noteworthy similarity between Gc and E is the fusion loop, which has the same tightly folded glycine-rich structure in the two proteins (Figure 3). Together, the structural similarities of Gc and E are strongly suggestive of some sort of evolutionary link between the Bunyaviridae and Flaviviridae families.
Figure 3.
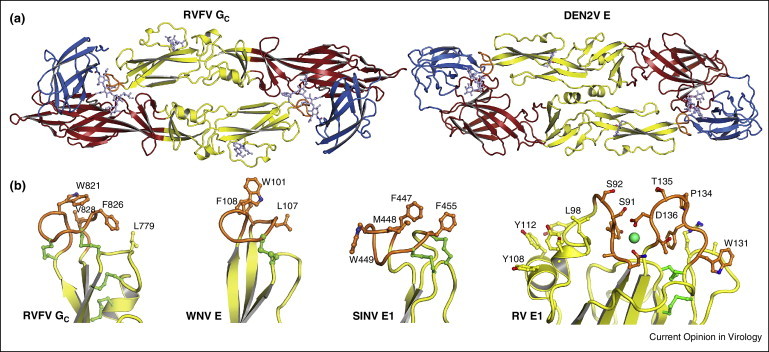
Conserved structural features in class II fusion proteins. (a) Gc from Rift Valley fever virus (RVFV) crystallizes in a dimeric head-to-tail configuration [5••]. The Gc dimers are strikingly similar to the flavivirus E dimers (dengue type 2 E shown here [12]). E dimers are the building block of the icosahedral outer protein shell in flaviviruses [26]. (b) The fusion loop serves as the anchor in the target cellular membrane during the fusion reaction (see Figure 2). The structure of the fusion loop is highly conserved in class II fusion proteins. Shown here are the fusion loops of, from left to right, RVFV Gc [5••], Sindbis virus (SINV) E1 [20], West Nile virus (WNV) E [13], and rubella virus (RV) E1 [6••].
Divergence of the class II fold within the Togaviridae family
In another recent advance, the E1 protein of rubella virus was found to have the most divergent class II fold identified so far. This was unexpected given that rubella virus belongs to the same Togaviridae family as alphaviruses. The most notable differences of the rubella E1 structure, which was crystallized in the trimeric postfusion conformation, are in domain II (Figure 1). Domain II is larger due to three insertions. Instead of a single 10–15-amino acid fusion loop, rubella E1 has two fusion loops that project a total of 15 aromatic side chains (mainly tyrosines) for interaction the cellular membrane (Figure 3) [6••]. A metal ion (Na+ or Ca2+) is coordinated between the two fusion loops and bound Ca2+ allows rubella E1 to bind lipid membranes a neutral pH [6••]. There are no metal sites in the other class II fusion proteins, or in the fusion motifs of any other viral fusion protein reported to date. Another distinctive feature of the rubella E1 structure is that domain III is swapped in the E1 trimer, occupying the position of domain III from the neighboring subunit in the flavivirus and alphavirus postfusion E trimers. Additionally, the rubella E1 structure includes the stem (Figure 1), which connects domain III to the transmembrane anchor and is either absent or mostly disordered in the structures of other class II proteins. Lastly, rubella virus particles exhibit a large degree of pleomorphy [55•], making rubella E1 the only class II fusion protein known not to form an icosahedral assembly.
A new envelope protein fold in the Flaviviridae family
The Flaviviridae family contains four genera: flavivirus, pestivirus, pegivirus (GB viruses) and hepaciviruses (hepatitis C viruses) [8]. Until 2013, envelope protein structures were available only from the flavivirus genus. Envelope proteins from pestiviruses and hepaciviruses had been predicted to have class II folds based on the disulfide bonding pattern [56] and on amino acid sequence analyses of the E1 and E2 envelope proteins [57]. It was therefore surprising when two groups discovered in 2013 that the larger envelope protein, E2, from the pestivirus BVDV (bovine viral diarrhea virus) is not a class II fusion protein. Instead BVDV E2 has a novel fold, suggesting that pestiviruses have a non-class II fusion machinery. Since E1, with its 174-amino acid ectodomain, is too small to be a class II fusogen, the E2 structure appears to define a new structural class of fusion proteins (Figure 4 ) [9••, 10••]. The structure of BVDV E2 provides an even more striking example than rubella E1 of how structurally divergent viral envelope proteins can be within a single virus family.
Figure 4.
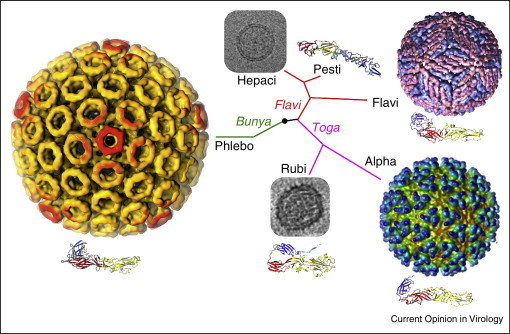
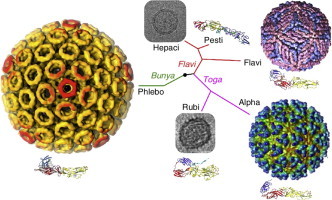
Structural relationships of viruses that contain class II fusion proteins. The class II fold is highly conserved in flaviviruses, alphaviruses and phleboviruses, even though these viruses differ in their genomic organization, coding strategies and outer protein shell assemblies. These three genera have in common that they have lifecycles that alternate between vertebrate and arthropod hosts. Rubella virus (RV) E1 has the most divergent class II fold even though rubella belongs to the same family as alphaviruses (Togaviridae). Glycoprotein E2 from the pestivirus bovine viral diarrhea virus has a novel fold even though pestiviruses belong to the same family as flaviviruses (Flaviviridae) [9••, 10••]. Rubella virus and pestiviruses, and their close relatives the hepaciviruses, have in common that they infect strictly vertebrate hosts, and also that they do not form rigid icosahedral outer protein shells. Thus, structural conservation in viral fusion proteins does not correlate with overall phylogenetic relatedness. The virus particles shown here are, clockwise from top right, dengue virus, Semliki Forest virus, RV, Rift Valley fever virus and hepatitis C virus (HCV). The electron micrographs of RV [55•] and HCV [61] are not drawn to scale with the particles in color. The phylogenetic tree is based on qualitative structural and genetic relationships between envelope proteins and is not based on a quantitative phylogenetic analysis.
Evolutionary implications of the structural relationships between class II proteins
The discovery of a class II fusion protein in a phlebovirus [5••], in a virus family otherwise unrelated to flaviviruses and alphaviruses, reveals that the class II fold is more prevalent and more widely distributed across virus families than was previously anticipated. The striking structural similarity between the flavivirus E proteins and RVFV G — which extends to the mode of dimerization even though E and Gc dimers form different types of icosahedral lattices — is strongly suggestive of a common evolutionary origin for certain envelope proteins within the Bunyaviridae and Flaviviridae families. But what is the nature of this link? The two virus families clearly differ in their genomic organization, coding strategies and outer protein shell assemblies (Figure 4). In the light of these differences it is tempting to speculate that, rather than diverging from a common ancestor virus, class II fusion proteins may instead have evolved independently from a common (or related) and as yet unidentified ancestral cellular class II membrane fusion protein. The concept of independent transmission of class II fusion proteins from hosts to viruses is supported by the discovery that certain viruses within the same family with similar genomic organizations can have distinct fusion machineries. Indeed, pestiviruses have a non-class II fusion machinery distinct from that of flaviviruses even though the two genera are adjacent to each other in phylogenetic tree of the Flaviviridae family [9••]. Thus, although pestiviruses and flaviviruses may have evolved from a common ancestor virus, they evidently borrowed their fusion machineries from different sources. These could presumably be different host fusion proteins, but alternatively different virus species could conceivably have borrowed fusion proteins from each other during co-infections with multiple viruses. The conservation of an α-helical coiled coil architecture in class I viral proteins and in the SNARE family of intracellular vesicle fusion proteins provides a compelling precedent for the evolutionary transfer of a structural membrane fusion fold between host and virus during evolution. Although similarities between class I fusion proteins and SNAREs have long been recognized [58], the link was further strengthened by a recent study demonstrating that a paramyxovirus class I fusion protein resembles SNAREs in that it has α-helical transmembrane anchors in both membranes before fusion, with subsequent zippering of the coiled coils during fusion resulting in a bundle of helical hairpins that extends across the fused membrane [59•, 60].
Alphaviruses and flaviviruses seem to have undergone a more conservative evolution, despite belonging to different families. The discovery of a divergent class II fold in rubella virus within the same family as alphaviruses (Togaviridae) was therefore unexpected [6••]. Notably, the more canonical class II folds have all been found in viruses alternating between arthropod and vertebrate hosts, whereas rubella virus infects only humans. The structural conservation of class II proteins in viruses with vertebrate-arthropod lifecycles may reflect more stringent evolutionary restraints exerted on these viruses. Rubella virus, along with pestiviruses and hepaciviruses, each have a single vertebrate host with which they seem to have co-evolved more rapidly.
Together, the structural relationships that have emerged between envelope proteins across different virus families are consistent with an evolutionary model in which class II fusion proteins originate from an as yet unidentified set of ancestral class II membrane fusion proteins in the host. Moreover, fusion proteins appear to have been transferred as independent modules, implying that the class II membrane fusion fold may have been hijacked by different viruses at different times throughout evolution.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Disease Award, and NIH grant R01 GM102869 to Y.M.
References
- 1.Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 2.Lamb R.A., Jardetzky T.S. Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol. 2007;17:427–436. doi: 10.1016/j.sbi.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schibli D.J., Weissenhorn W. Class I and class II viral fusion protein structures reveal similar principles in membrane fusion. Mol Membr Biol. 2004;21:361–371. doi: 10.1080/09687860400017784. [DOI] [PubMed] [Google Scholar]
- 4.Kielian M., Rey F.A. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Dessau M., Modis Y. Crystal structure of glycoprotein C from Rift Valley fever virus. Proc Natl Acad Sci U S A. 2013;110:1696–1701. doi: 10.1073/pnas.1217780110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that the Gc envelope protein from Rift Valley fever virus (from the Bunyaviridae family) has a class II fold with striking resemblances to that of E from dengue and other flaviviruses, including a propensity to form head-to-tail dimers with a hydrophobic membrane anchor, or fusion loop buried at the dimer interface. RVFV Gc was the first class II protein identified in a virus family otherwise unrelated to flaviviruses and alphaviruses, suggesting that class II proteins may have been transferred as independent modules during evolution from a host or another virus.
- 6••.DuBois R.M., Vaney M.C., Tortorici M.A., Kurdi R.A., Barba-Spaeth G., Krey T., Rey F.A. Functional and evolutionary insight from the crystal structure of rubella virus protein E1. Nature. 2013;493:552–556. doi: 10.1038/nature11741. [DOI] [PubMed] [Google Scholar]; This study showed that the E1 envelope protein from rubella virus has the most structurally divergent class II fold identified so far. This was unexpected because rubella virus is in the same Togaviridae family as alphaviruses, which have canonical class II proteins. Rubella E1 is the first class II fusion protein to be identified in a virus that that does not alternate between vertebrate and arthropod hosts — rubella virus only infects humans. This suggests that the envelope proteins of viruses with both insect and vertebrate hosts may be subject to more stringent evolutionary restraints.
- 7.Backovic M., Jardetzky T.S. Class III viral membrane fusion proteins. Adv Exp Med Biol. 2011;714:91–101. doi: 10.1007/978-94-007-0782-5_3. [DOI] [PubMed] [Google Scholar]
- 8.Lindenbach B.D., Murray C.L., Thiel H.J., Rice C.M. Flaviviridae. In: Knipe D.M., Howley P.M., editors. vol. 1 (Sixth Edition) Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 712–746. (Fields Virology). [Google Scholar]
- 9••.Li Y., Wang J., Kanai R., Modis Y. Crystal structure of glycoprotein E2 from bovine viral diarrhea virus. Proc Natl Acad Sci U S A. 2013;110:6805–6810. doi: 10.1073/pnas.1300524110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study and the study by El Omari et al. [10••] were the first to show that the pestivirus BVDV (bovine viral diarrhea virus) has a novel type of fusion machinery. This was unexpected because pestiviruses belong to the same family as flaviviruses, which have canonical class II fusion proteins. This supports the evolutionary model in which envelope proteins can be transferred from the host (or another virus) as independent modules.
- 10••.El Omari K., Iourin O., Harlos K., Grimes J.M., Stuart D.I. Structure of a pestivirus envelope glycoprotein E2 clarifies its role in cell entry. Cell Rep. 2013;3:30–35. doi: 10.1016/j.celrep.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; See annotation for Li et al. [9••].
- 11.Rey F.A., Heinz F.X., Mandl C., Kunz C., Harrison S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 12.Modis Y., Ogata S., Clements D., Harrison S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A. 2003;100:6986–6990. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai R., Kar K., Anthony K., Gould L.H., Ledizet M., Fikrig E., Marasco W.A., Koski R.A., Modis Y. Crystal structure of west nile virus envelope glycoprotein reveals viral surface epitopes. J Virol. 2006;80:11000–11008. doi: 10.1128/JVI.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luca V.C., AbiMansour J., Nelson C.A., Fremont D.H. Crystal structure of the Japanese encephalitis virus envelope protein. J Virol. 2012;86:2337–2346. doi: 10.1128/JVI.06072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modis Y., Ogata S., Clements D., Harrison S.C. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79:1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nybakken G.E., Nelson C.A., Chen B.R., Diamond M.S., Fremont D.H. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80:11467–11474. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Zhang W., Ogata S., Clements D., Strauss J.H., Baker T.S., Kuhn R.J., Rossmann M.G. Conformational changes of the flavivirus E glycoprotein. Structure (Camb) 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cockburn J.J., Navarro Sanchez M.E., Goncalvez A.P., Zaitseva E., Stura E.A., Kikuti C.M., Duquerroy S., Dussart P., Chernomordik L.V., Lai C.J. Structural insights into the neutralization mechanism of a higher primate antibody against dengue virus. EMBO J. 2012;31:767–779. doi: 10.1038/emboj.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lescar J., Roussel A., Wien M.W., Navaza J., Fuller S.D., Wengler G., Rey F.A. The Fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell. 2001;105:137–148. doi: 10.1016/s0092-8674(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 20.Li L., Jose J., Xiang Y., Kuhn R.J., Rossmann M.G. Structural changes of envelope proteins during alphavirus fusion. Nature. 2010;468:705–708. doi: 10.1038/nature09546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voss J.E., Vaney M.C., Duquerroy S., Vonrhein C., Girard-Blanc C., Crublet E., Thompson A., Bricogne G., Rey F.A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature. 2010;468:709–712. doi: 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]
- 22•.Zhang R., Hryc C.F., Cong Y., Liu X., Jakana J., Gorchakov R., Baker M.L., Weaver S.C., Chiu W. 4.4 A cryo-EM structure of an enveloped alphavirus Venezuelan equine encephalitis virus. EMBO J. 2011;30:3854–3863. doi: 10.1038/emboj.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study and the study by Kostyuchenko et al. [23•] reveal the structure of an alphavirus particle in unprecendented detail. The electron microscopy structure clearly resolves the transmembrane helices and cytoplasmic tails of the class II fusion protein E1, and of the receptor binding envelope protein E2. These features were missing in previously determined crystal structures.
- 23•.Kostyuchenko V.A., Jakana J., Liu X., Haddow A.D., Aung M., Weaver S.C., Chiu W., Lok S.M. The structure of barmah forest virus as revealed by cryo-electron microscopy at a 6-angstrom resolution has detailed transmembrane protein architecture and interactions. J Virol. 2011;85:9327–9333. doi: 10.1128/JVI.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; See annotation for Zhang et al. [22•].
- 24.Mukhopadhyay S., Kim B.S., Chipman P.R., Rossmann M.G., Kuhn R.J. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Zhang X., Ge P., Yu X., Brannan J.M., Bi G., Zhang Q., Schein S., Zhou Z.H. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat Struct Mol Biol. 2013;20:105–110. doi: 10.1038/nsmb.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals the structure of a flavivirus particle in unprecendented detail, at near-atomic resolution. The electron microscopy structure reveals features that were missing in previously determined crystal structures, including the unusual truncated helical hairpin transmembrane anchor of the class II fusogen, E, and a segment of envelope protein M that contains three pH-sensing histidines and prevents E from prematurely exposing its fusion loop.
- 27.Crill W.D., Roehrig J.T. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol. 2001;75:7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliphant T., Engle M., Nybakken G.E., Doane C., Johnson S., Huang L., Gorlatov S., Mehlhop E., Marri A., Chung K.M. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nybakken G.E., Oliphant T., Johnson S., Burke S., Diamond M.S., Fremont D.H. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung J.J., Hsieh M.T., Young M.J., Kao C.L., King C.C., Chang W. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J Virol. 2004;78:378–388. doi: 10.1128/JVI.78.1.378-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crill W.D., Wichman H.A., Bull J.J. Evolutionary reversals during viral adaptation to alternating hosts. Genetics. 2000;154:27–37. doi: 10.1093/genetics/154.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan W.H., Navarro-Sanchez E., Dumortier H., Decossas M., Vachon H., dos Santos F.B., Fridman H.W., Rey F.A., Harris E., Despres P. Dermal-type macrophages expressing CD209/DC-SIGN show inherent resistance to dengue virus growth. PLoS Negl Trop Dis. 2008;2:e311. doi: 10.1371/journal.pntd.0000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozach P.Y., Burleigh L., Staropoli I., Navarro-Sanchez E., Harriague J., Virelizier J.L., Rey F.A., Despres P., Arenzana-Seisdedos F., Amara A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem. 2005;280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 34.Davis C.W., Nguyen H.Y., Hanna S.L., Sanchez M.D., Doms R.W., Pierson T.C. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro-Sanchez E., Altmeyer R., Amara A., Schwartz O., Fieschi F., Virelizier J.L., Arenzana-Seisdedos F., Despres P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W., Eller M.A., Pattanapanyasat K., Sarasombath S., Birx D.L. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller J.L., de Wet B.J., Martinez-Pomares L., Radcliffe C.M., Dwek R.A., Rudd P.M., Gordon S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Maguire T., Hileman R.E., Fromm J.R., Esko J.D., Linhardt R.J., Marks R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 39.Klimstra W.B., Nangle E.M., Smith M.S., Yurochko A.D., Ryman K.D. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klimstra W.B., Ryman K.D., Johnston R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modis Y., Ogata S., Clements D., Harrison S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 42.Bressanelli S., Stiasny K., Allison S.L., Stura E.A., Duquerroy S., Lescar J., Heinz F.X., Rey F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbons D.L., Vaney M.C., Roussel A., Vigouroux A., Reilly B., Lepault J., Kielian M., Rey F.A. Conformational change and protein–protein interactions of the fusion protein of Semliki Forest virus. Nature. 2004;427:320–325. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]
- 44.Luca V.C., Nelson C.A., Fremont D.H. Structure of the St. Louis encephalitis virus postfusion envelope trimer. J Virol. 2013;87:818–828. doi: 10.1128/JVI.01950-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein D.E., Choi J.L., Harrison S.C. Structure of a dengue virus envelope protein late-stage fusion intermediate. J Virol. 2013;87:2287–2290. doi: 10.1128/JVI.02957-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-San Martin C., Sosa H., Kielian M. A stable prefusion intermediate of the alphavirus fusion protein reveals critical features of class II membrane fusion. Cell Host Microbe. 2008;4:600–608. doi: 10.1016/j.chom.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiasny K., Kossl C., Lepault J., Rey F.A., Heinz F.X. Characterization of a structural intermediate of flavivirus membrane fusion. PLoS Pathog. 2007;3:e20. doi: 10.1371/journal.ppat.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nour A.M., Li Y., Wolenski J., Modis Y. Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS Pathog. 2013;9:e5851003. doi: 10.1371/journal.ppat.1003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lozach P.Y., Kuhbacher A., Meier R., Mancini R., Bitto D., Bouloy M., Helenius A. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Cong H., Jiang Y., Tien P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J Virol. 2011;85:11038–11047. doi: 10.1128/JVI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garry C.E., Garry R.F. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of Bunyaviruses are class II viral fusion protein (beta-penetrenes) Theor Biol Med Model. 2004;1:10. doi: 10.1186/1742-4682-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozach P.Y., Mancini R., Bitto D., Meier R., Oestereich L., Overby A.K., Pettersson R.F., Helenius A. Entry of bunyaviruses into mammalian cells. Cell Host Microbe. 2010;7:488–499. doi: 10.1016/j.chom.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overby A.K., Pettersson R.F., Grunewald K., Huiskonen J.T. Insights into bunyavirus architecture from electron cryotomography of Uukuniemi virus. Proc Natl Acad Sci U S A. 2008;105:2375–2379. doi: 10.1073/pnas.0708738105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huiskonen J.T., Overby A.K., Weber F., Grunewald K. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: evidence for GN-GC glycoprotein heterodimers. J Virol. 2009;83:3762–3769. doi: 10.1128/JVI.02483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Battisti A.J., Yoder J.D., Plevka P., Winkler D.C., Prasad V.M., Kuhn R.J., Frey T.K., Steven A.C., Rossmann M.G. Cryo-electron tomography of rubella virus. J Virol. 2012;86:11078–11085. doi: 10.1128/JVI.01390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use electron cryomicroscopy to examine the structure of rubella virions. The rubella virus envelope proteins, E1 and E2, are shown to be organized into extended rows on the viral surface. Although many virions were approximately spherical, they exhibited a large degree of pleomorphy, lacked icosahedral organization and had a different mode of assembly to alphaviruses despite being in the same Togaviridae family.
- 56.Krey T., d’Alayer J., Kikuti C.M., Saulnier A., Damier-Piolle L., Petitpas I., Johansson D.X., Tawar R.G., Baron B., Robert B. The disulfide bonds in glycoprotein E2 of hepatitis C virus reveal the tertiary organization of the molecule. PLoS Pathog. 2010;6:e1000762. doi: 10.1371/journal.ppat.1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garry R.F., Dash S. Proteomics computational analyses suggest that hepatitis C virus E1 and pestivirus E2 envelope glycoproteins are truncated class II fusion proteins. Virology. 2003;307:255–265. doi: 10.1016/s0042-6822(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 58.Skehel J.J., Wiley D.C. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- 59•.Donald J.E., Zhang Y., Fiorin G., Carnevale V., Slochower D.R., Gai F., Klein M.L., DeGrado W.F. Transmembrane orientation and possible role of the fusogenic peptide from parainfluenza virus 5 (PIV5) in promoting fusion. Proc Natl Acad Sci U S A. 2011;108:3958–3963. doi: 10.1073/pnas.1019668108. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies new parallels between class I envelope proteins and cellular SNARE fusion proteins. The authors show that the N-terminal fusion peptide of a paramyxovirus class I fusion protein forms a transmembrane α-helix after membrane insertion. This helix extends the helical coiled coil core of the fusion protein and contributes to the zippering of the coiled coils during fusion. In the trimeric postfusion conformation, the N-terminal and C-terminal helices form a bundle that extends across the fused membrane. SNAREs also have N-terminal and C-terminal transmembrane helices that contribute to the fusogenic zippering of helices. The additional parallels between class I proteins and SNAREs strengthen the case for an evolutionary link between these two protein families, providing a possible precedent for host-to-virus transfer of fusion proteins during evolution.
- 60.Stein A., Weber G., Wahl M.C., Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–528. doi: 10.1038/nature08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gastaminza P., Dryden K.A., Boyd B., Wood M.R., Law M., Yeager M., Chisari F.V. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84:10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


