Abstract
Mono-2-ethyhexyl phthalate (MEHP) is a metabolite of a plasticizer found in many consumer products. MEHP inhibits mouse ovarian follicle growth by reducing 17β-estradiol (E2) production. Yet, whether MEHP causes follicle death (atresia) is unclear. We hypothesized that MEHP causes atresia by altering apoptosis gene expression, and that E2 co-treatment blocks these effects. Follicles were exposed to MEHP (0.36–36 µM) ± E2 for 48–96h to determine the effect of MEHP ± E2 on atresia and gene expression. MEHP increased atresia, but this effect was blocked by co-treatment with E2. MEHP increased the expression of the pro-apoptotic gene Aifm1, but decreased that of the pro-apoptotic gene Bok and the anti-apoptotic gene Bcl2l10. E2 interfered with MEHP-induced changes in Aifm1 and Bcl2l10. Our findings suggest that decreased E2 levels are required for MEHP-induced follicle atresia and that Aifm1, Bok, and Bcl2l10 are involved in this process.
Keywords: Ovary, phthalate, follicle, estradiol, apoptosis
1. Introduction
Phthalates are a group of chemicals, some of which are commonly regarded as endocrine disruptors for their ability to interfere with hormone-regulated processes (reviewed in [1–3]). Due to their ubiquitous presence in many consumer products, humans are exposed to phthalates via ingestion, inhalation, intravenous, and dermal contact. Continuous daily exposure in humans has been demonstrated by epidemiological studies showing that more than 75% of human spot urine samples tested have detectable levels of phthalates [4].
Di-2-ethylhexyl phthalate (DEHP) and its immediate metabolite, mono-2-ethylhexyl phthalate (MEHP) are two of the most studied phthalates. DEHP is commonly found in construction materials and in PVC products including clothing, food packaging, children toys, and medical devices (reviewed in [3]). Most humans are exposed to DEHP via the diet (oral route) and via medical devices. Upon entering the body, DEHP is rapidly converted into MEHP by the action of esterases [5]. Thus, MEHP is commonly regarded as the toxic metabolite of DEHP. DEHP exposure in the United States has been estimated to range between 0.7–3.6 µg/kg of body weight per day (µg/kg/d) when calculated from urinary levels of MEHP and secondary metabolites [6, 7], or between 8.2–18.9 µg/kg/d when based on DEHP concentration in food [7]. Higher exposure to DEHP has been reported in patients undergoing medical treatments such as parenteral nutrition in neonates (20 mg/d), blood transfusions in adults (> 4 mg/kg/d), and kidney dialysis (0.8 mg/kg/treatment; reviewed in [3]). Occupational exposure to DEHP has been estimated to range between 0.5–170 µg/kg/d [8]. In fact, epidemiological studies have shown associations between increased phthalate exposure and increased miscarriage rates, and increased urinary phthalate levels and increased pregnancy complications [9, 10].
Further, various animal studies have demonstrated that DEHP is a female reproductive toxicant. One main outcome of DEHP exposure in rats has been decreased serum 17β-estradiol (E2) levels [11–16]. Studies have also reported that DEHP exposure in rats results in prolonged estrous cycles and decreased ovulation rates [12], altered circulating follicle-stimulating hormone levels [12, 13, 16], altered circulating luteinizing hormone levels [14, 15], altered testosterone and progesterone [15, 16] levels, and increased numbers of atretic ovarian follicles [16].
Ovarian follicles exist in various developmental stages of which, the antral follicle is the most mature type. Antral follicles are the main source of ovarian E2 and the only follicle type capable of ovulation upon proper stimulation. Therefore, damage to the antral follicle population can lead to E2 deficiency, anovulation, and ultimately, to infertility. E2 deficiency may also increase a woman’s risk for disorders such as osteoporosis, cardiovascular disease, and depression [17–19]. Although many follicles are present within the ovary, only a few will grow to a mature stage and become ovulated. The majority of follicles die by a programmed cell death process known as follicular atresia [20, 21]. Follicular atresia occurs by apoptosis and, like in other tissues, it is regulated by a strict balance between pro-apoptotic proteins, including BCL2-associated X protein (BAX), BCL2-related ovarian killer protein (BOK), and BH3 interacting domain death agonist (BID) and anti-apoptotic proteins, including B cell leukemia/lymphoma 2 (BCL2) and Bcl2-like 10 (BCL2L10; for review see [22]).
Previous work from our group demonstrated that both DEHP (2.6–256 µM; 96 h) and MEHP (0.36–36 µM; 96 h) inhibit the growth of antral follicles in vitro [23–25], down-regulate cell cycle gene and aromatase mRNA, and decrease production of E2 by antral follicles [23]. Further, previous studies showed that supplementing MEHP-treated follicles with E2 reverses inhibition of antral follicle growth and restores cell cycle gene expression [23]. While these previous studies suggest that MEHP inhibits follicle growth by reducing E2 production by antral follicles, it is unclear whether MEHP causes antral follicle death and if so, whether MEHP-induced follicle death could be prevented by E2 supplementation. Therefore, the present study was designed to test the hypothesis that MEHP causes antral follicle atresia and that this can be prevented by co-treating follicles with E2. To test our hypothesis, we exposed individual mouse antral follicles to increasing concentrations of MEHP and determined the effect of MEHP on antral follicle atresia. We also determined the effect of co-treatment with E2 on the ability of MEHP to induce antral follicle atresia. To further understand the mechanism by which MEHP causes antral follicle atresia, we evaluated the effect of MEHP on the expression of apoptosis-related genes.
2. Materials and Methods
2.1 Reagents
MEHP was obtained from AccuStandard (New Haven, CT), dimethylsulfoxide (DMSO), ITS (insulin, transferrin, selenium), and penicillin/streptomycin, and 17β-estradiol (E2) were obtained from Sigma-Aldrich (St. Louis, MO). Alpha-minimal essential media (α-MEM) was obtained from Life Technologies (Grand Island, NY). Human recombinant follicle-stimulating hormone (rFSH) was obtained from Dr. A.F. Parlow from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA) and charcoal-stripped fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA).
2.2 Animals
Cycling female CD-1 mice (age 35–39 days) were obtained from Charles River Laboratories (Charles River, CA). Animals were housed four mice per cage at the University of Illinois College of Veterinary Medicine Central Animal Facility. Animals were subjected to 12L:12D cycles, water and food were provided ad libitum, and temperature was maintained at 22 ± 1°C. Animals were allowed to acclimate for at least 48 h before use and were euthanized at 35–39 days of age by carbon dioxide inhalation followed by cervical dislocation. The ovaries were removed and antral follicles mechanically isolated. All experiments and methods involving animals conformed to the Guide for the Care and Use of Experimental Animals [26] and were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.3 MEHP treatments
All MEHP treatments were prepared in supplemented α-MEM (5% FBS, 1% ITS, 1% penicillin/streptomycin, and 5 IU/mL rFSH). Stock solutions of various concentrations of MEHP were prepared using DMSO as a solvent (0.017 M, 0.17 M, and 1.7 M) to ensure that an equal volume of solvent could be added to each well. A stock of E2 was used to prepare final working concentrations of E2 in culture of 1 nM and 10 nM as described previously [23]. An additional set of stocks of MEHP (final concentration 36 µM) and E2 (final concentrations 1 and 10 nM) containing 0.375% DMSO was prepared for the E2 co-treatment groups to ensure that the final concentration of solvent in the culture was also 0.075%. MEHP and E2 doses were selected based on previous work showing MEHP-induced changes in antral follicle growth, cell cycle gene and aromatase mRNA expression, and E2 production [23]. We have previously observed that cultured mouse antral follicles will produce levels of E2 that range from 267.83 to 3098.7 pg/mL, which are equivalent to 0.98 and 11.4 nM.
2.4 Antral Follicle Culture
Antral follicles were mechanically isolated from mouse ovaries based on relative size (200–350 µm) and placed in culture as described previously [23, 27]. Each follicle culture experiment consisted of 8–12 follicles per treatment. Treatment groups included a control for culture conditions that consisted of supplemented media only (non-treated control), a vehicle control consisting of DMSO (0.075%), and MEHP at final concentrations of 0.36, 3.6, and 36 µM. For the E2 co-treatment experiments, follicles were treated with MEHP at 36 µM, E2 at 1 and 10 nM, and MEHP and E2 (1 and 10 nM) together. The effect of MEHP and E2 co-treatment on antral follicle atresia was evaluated in 96 h cultures. Gene expression experiments were conducted on 48 h cultures, a time preceding the onset of follicular atresia in MEHP-treated follicles.
2.5 Histological Evaluation of Antral Follicle Atresia
At the end of the 96 h culture period, media were removed, and each individual follicle was processed for analysis of atresia as previously described [27, 28]. Each follicle section was examined for level of atresia as evidenced by the presence of apoptotic bodies and reported as an average of all ratings observed throughout the follicle. Follicles were rated on a scale of 1–4 based on the presence of apoptotic bodies within the follicle: 1 for healthy, 2 for 1–10%, 3 for 10–30%, and 4 for more than 30% apoptotic bodies present in the follicle. All atresia ratings were assigned by two individuals without knowledge of treatment group.
2.6 Real-time PCR
At the end of the 96 h culture period, follicles were immediately snap frozen and stored at −80°C for subsequent real-time PCR (qPCR) analysis. Total RNA was extracted from pooled follicles (8–12 follicles per treatment) using RNeasy Micro kits (Qiagen, Valencia, CA) and incubated with DNAse (Qiagen, 15 min) to eliminate genomic DNA. The RNA concentration of each sample was determined using a Nanodrop ND1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). RNA samples (50 ng) were reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Each cDNA sample was diluted 1:4 with nuclease-free water prior to analysis. All qPCR experiments were carried out using a CFX96 Real-time System C1000 Thermal Cycler (Bio-Rad) and SYBR green as the real-time probe. Reactions were prepared and subjected to the qPCR program described in Craig et al. [27, 29]. Primers were designed using PrimerBLAST [30] and validated by observing a single peak following melt curve analysis and a single product of the right size by agarose gel electrophoresis. Table 1 shows the sequences for the selected primers. Expression data were analyzed with REST2009 software (Qiagen, Hilden, Germany). The housekeeping gene β-actin did not change under the tested conditions and thus, was used as a reference gene for normalizing the gene expression data. Data are reported as mean relative mRNA expression ratios from three separate follicle culture experiments.
Table 1.
Real-time PCR Primer Information
| Accession No. | Gene name | Abbreviation | Forward | Reverse |
|---|---|---|---|---|
| NM_007393.3 | actin, beta | Actb | GGGCACAGTGTGGGTGAC | CTGGCACCACACCTTCTAC |
| NM_012019.2 | apoptosis-inducing factor, mitochondrion-associated 1, nuclear gene encoding mitochondrial protein | Aifm1 | AGGACGGTGAGCAACATGAA | GTTCTATCCACCCATCCCGC |
| NM_009741.3 | B cell leukemia/lymphoma 2 | Bcl2 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
| NM_007527.3 | BCL2-associated X protein | Bax | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG |
| NM_013479.2 | Bcl2-like 10 | Bcl2l10 | CGCTACACACACTGACTGGA | CTTTAGGATCCCCTGCCCTG |
| NM_016778.2 | BCL2-related ovarian killer protein | Bok | CTGCCCCTGGAGGACGCTTG | CCGTCACCACAGGCTCCGAC |
| NM_007544.3 | BH3 interacting domain death agonist | Bid | AGCAAATGTTCCCTCCGCTTCTGT | GTAGGCTGTGGCGGCTCGTG |
| NM_001025296.1 | DNA fragmentation factor, alpha subunit, transcript variant 1 | Dffa | GCCAGATCCTTACCACACTGA | TTATGTCCCAGCTCAGAGCGA |
| NM_080637.3 | NME/NM23 family member 5 | Nme5 | CGGACAGCTTAAGGGCGATA | CACGGCTGGAAACATGAACC |
| NM_013693.2 | tumor necrosis factor | Tnf | GATCGGTCCCCAAAGGGATG | TTTGCTACGACGTGGGCTAC |
2.7 Statistical Analysis
For all comparisons, statistical significance was assigned at p≤0.05. The effects of treatments on antral follicle atresia (atresia rating data) were compared by ANOVA followed by Tukey’s post hoc tests using SPSS statistical software (SPSS Inc., Chicago, IL). Gene expression data were compared using the data analysis feature of REST2009 software (http://rest.gene-quantification.info).
3. Results
3.1 Effect of MEHP on antral follicle atresia
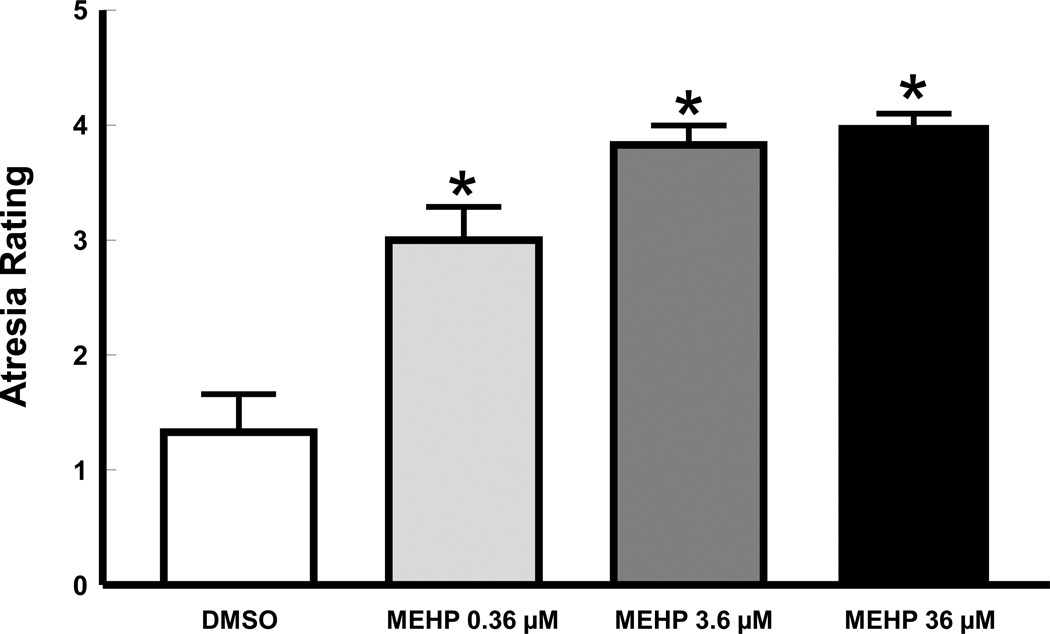
We hypothesized that MEHP exposure induces follicular death (atresia). To determine the effect of MEHP treatment on the health of antral follicles, we treated antral follicles with vehicle control (DMSO) or increasing concentrations of MEHP (0.36, 3.6, and 36 µM) for a total of 96 h and then subjected the follicles to histological evaluation. All follicles treated with MEHP had significantly more apoptotic bodies (indicators of antral follicle atresia) than follicles treated with vehicle (p≤0.05; Figure 1).
Figure 1. Effect of MEHP on mouse antral follicle atresia rating.
Mouse antral follicles were isolated and treated for 96 h and their atresia rating determined as described in sections 2.4 and 2.5. Data were derived from 3–4 follicles per group obtained from at least three separate culture experiments. Data were represented by mean ± SEM and were analyzed using ANOVA followed by Tukey’s post hoc test with significance set at p≤0.05. Asterisks indicate significant differences when compared to vehicle (DMSO).
3.2 Effect of E2 co-treatment on MEHP-induced antral follicle atresia
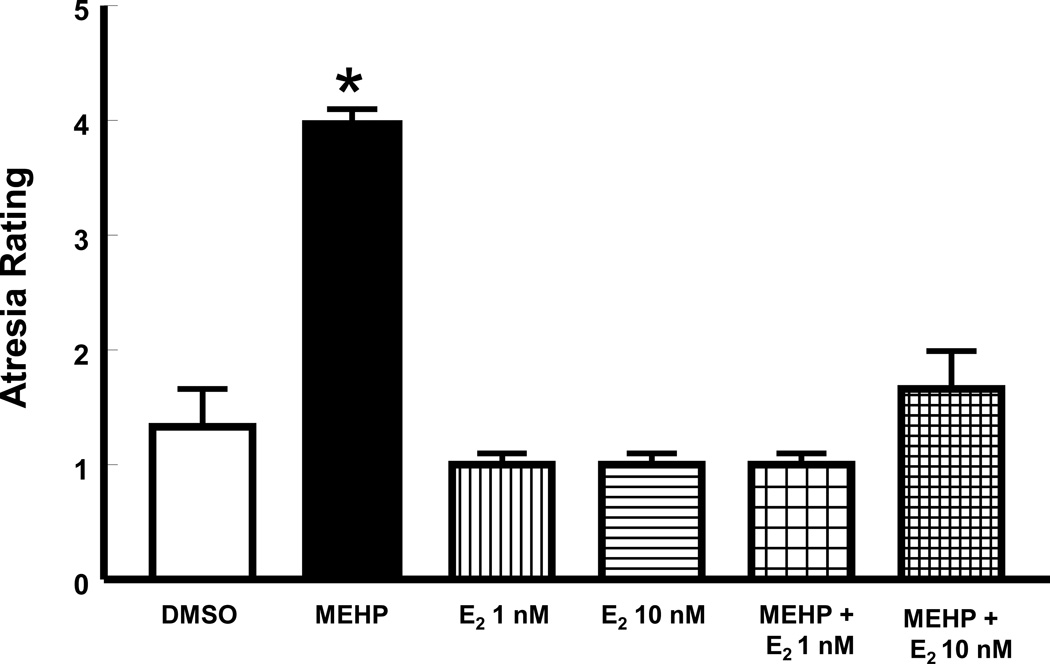
Given that previous studies indicate that E2 co-treatment protects antral follicles from MEHP-induced growth inhibition of antral follicles [23], we hypothesized that E2 co-treatment would also protect follicles against MEHP-induced atresia. We tested our hypothesis by treating antral follicles with vehicle, MEHP at 36 µM, E2 (1 and 10 nM), and MEHP together with E2 for 96 h. Consistent with the data shown in Figure 1, MEHP (36 µM) significantly increased the number of apoptotic bodies, resulting in higher atresia ratings for MEHP-treated follicles compared to vehicle controls (p≤0.05). E2 alone had no effect on atresia rating, but when given together with MEHP, it prevented MEHP from inducing antral follicle atresia (p≤0.05; Figure 2).
Figure 2. Effect of co-treatment with E2 on MEHP-induced antral follicle atresia.
Mouse antral follicles were isolated, treated with vehicle, MEHP, E2, or MEHP and E2 for 96 h, and their atresia rating determined (see sections 2.4 and 2.5 for details). Data were derived from 3–4 follicles per group obtained from at least three separate culture experiments. Data were represented by mean ± SEM and were analyzed using ANOVA followed by Tukey’s post hoc test with significance set at p≤0.05. Asterisk indicates significant difference when compared to DMSO.
3.3 Effect of MEHP and E2 co-treatment on the expression of genes that regulate apoptosis
To gain insight into how MEHP induces atresia in antral follicles and how E2 may block this effect, we conducted experiments in which we treated follicles with MEHP ± E2 and then compared the expression of various mRNAs previously identified as being involved in the process of chemical-induced ovarian follicle apoptosis in mice. The selected genes included Bax, Bok, Bid, and Bcl2 [27, 31–33]. In a preliminary study, we compared mRNA expression in vehicle-treated antral follicles and MEHP-treated follicles using an apoptosis-specific qPCR array (data not shown). Various novel genes not previously described as being altered in chemical-induced ovarian follicle apoptosis were identified and selected for further analysis here. These genes included mitochondrial apoptosis-inducing factor 1 (Aifm1), DNA fragmentation factor, alpha subunit (Dffa), Bcl2-like 10 (Bcl2l10), tumor necrosis factor (Tnf), and NME/NM23 family member 5 (Nme5).
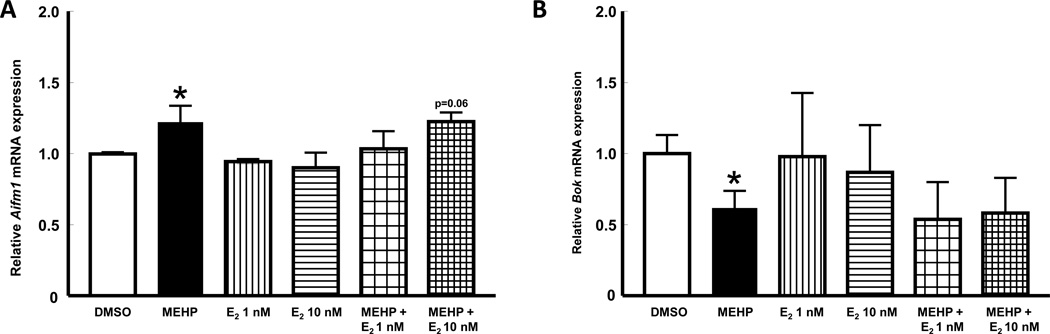
The expression of the pro-apoptotic factor Aifm1 was significantly increased by MEHP treatment when compared to control (p≤0.05; Figure 3A). Treatment with E2 alone did not have an impact on Aifm1 expression. However, Aifm1 expression was not different from that in control when antral follicles were co-treated with MEHP + E2 at 1 nM. Aifm1 expression was similar between MEHP and MEHP + E2 at 10 nM (p>0.05), but levels in MEHP + E2 at 10 nM were not significantly different from those in vehicle controls (p=0.06). The expression of the pro-apoptotic factor Bok was significantly decreased by MEHP treatment when compared to vehicle control (p≤0.05; Figure 3B). Bok mRNA levels were not affected by treatment with E2 alone or co-treatment with MEHP (p>0.05). Finally, the expression of mRNAs encoding the pro-apoptotic factors Bax, Bid, Tnf, and Dffa was not affected by MEHP, E2 alone, or the combination of both when compared to vehicle controls (p>0.05; data not shown).
Figure 3. Effect of co-treatment with MEHP and E2 on the expression of mRNAs that encode proteins that favor apoptosis.
Mouse antral follicles were isolated, treated with vehicle, MEHP, E2, or MEHP and E2 for 48 h, and processed for qPCR gene expression analysis (see sections 2.4 and 2.6 for details). Data represent mean relative expression ± SEM obtained from three separate culture experiments, each with 12 follicles per treatment. Statistical significance was set at p≤0.05 and difference between treatments and vehicle are indicated by the asterisks. Trends between a treatment and vehicle are indicated by the actual p-value (e.g., p=0.06). (A) expression of Aifm1 (mitochondrial apoptosis-inducing factor 1) and (B) expression of Bok (BCL2-related ovarian killer protein).
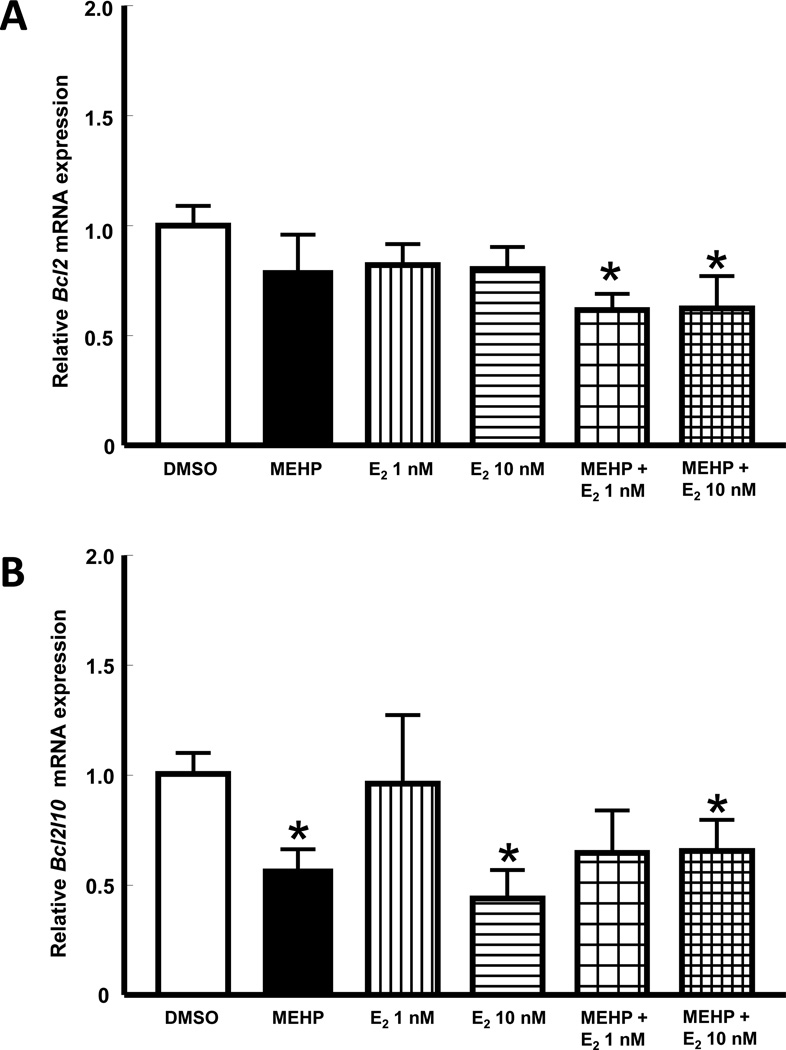
Compared to vehicle control, the expression of the anti-apoptotic factor Bcl2 was not affected by treatment with MEHP or E2 alone (p>0.05), but was significantly down regulated when antral follicles were co-treated with MEHP plus E2 (p≤0.05; Figure 4A). The expression of the anti-apoptotic factor Bcl2l10 was significantly decreased by MEHP treatment when compared to that in vehicle control follicles (p≤0.05). E2 treatment had dose-dependent effects on Bcl2l10 expression. Specifically, Bcl2l10 expression was not affected by E2 at 1 nM, but was significantly reduced by E2 at 10 nM. Interestingly, follicles co-treated with MEHP and E2 at 1 nM exhibited Bcl2l10 expression that was similar to control follicles, while follicles treated with MEHP and E2 at 10 nM, like MEHP-treated follicles, had significantly decreased Bcl2l10 expression when compared to vehicle controls (p≤0.05; Figure 4B). Finally, the expression of the anti-apoptotic factor Nme5 mRNA was not affected by MEHP, E2 alone, or MEHP plus E2 (p>0.05; data not shown).
Figure 4. Effect of co-treatment with MEHP and E2 on the expression of mRNAs that encode proteins that prevent apoptosis.
Mouse antral follicles were isolated, treated with vehicle, MEHP, E2, or MEHP and E2 for 48 h, and processed for qPCR gene expression analysis (see sections 2.4 and 2.6 for details). Data represent mean relative expression ± SEM obtained from three separate culture experiments, each with 12 follicles per treatment. Statistical significance was set at p≤0.05 and difference between treatments and vehicle are indicated by the asterisks. (A) expression of Bcl2 (B cell leukemia/lymphoma 2), (B) expression of Bcl2l10 (Bcl2-like 10).
4. Discussion
We have shown that MEHP treatment for 96 h increases atresia in mouse antral follicles and that this effect is prevented by co-treatment with E2. Our findings also show that MEHP treatment alters the expression of some apoptosis-related genes following in vitro exposure for 48 h. Surprisingly, not all MEHP-induced changes in gene expression were prevented by E2 co-treatment and some genes responded to E2 alone and/or in combination with MEHP despite not being affected by treatment with MEHP alone.
To our knowledge, our study is the first to evaluate mouse antral follicle atresia histologically following exposure to MEHP in vitro. Our observations in mouse antral follicles are consistent with previous reports of increased apoptosis in MEHP-treated preantral follicles from rats [34], granulosa cells from DEHP-treated mice [35], and equine cumulus cells treated with DEHP [36]. Thus, our findings and those of others indicate that MEHP interferes with ovarian function by promoting ovarian follicle death via apoptosis. Furthermore, our results suggest that this effect of MEHP can be prevented by co-treatment with E2.
One major goal of this work was to uncover which factors are involved in the mechanism by which MEHP induces atresia in antral follicles. Thus, we compared the expression of various genes involved in the regulation of apoptosis between MEHP-treated and control-treated follicles at 48 h. When compared to vehicle controls, MEHP-treated follicles expressed significantly higher levels of Aifm1 at 48 h. The Aifm1 gene encodes the protein AIF, which is a caspase-independent programmed cell death regulator (for review, see [37]). AIF has been shown to play an important role in multiple neuronal death pathways [38] and it has been shown to be released from the mitochondria and translocated into the nucleus to execute DNA fragmentation [39–41]. The present study reports increased expression of Aifm1 in response to MEHP treatment in ovarian follicles. Previously, microarray analysis revealed that bisphenol A (BPA), another endocrine disruptor that causes antral follicle atresia, alters Aifm1 expression in the ovarian adenocarcinoma cell line BG-1 [42]. Furthermore, Aifm1 mRNA expression has been shown to increase in SCC-4 human tongue squamous carcinoma cells in response to the natural alkaloid berberine [43]. It is possible that the MEHP-induced increase in Aifm1 expression could lead to increased AIF protein expression and increased DNA fragmentation in MEHP-treated follicles. Thus, future studies characterizing the involvement of AIF in MEHP-induced antral follicle atresia should be conducted because understanding the role of AIF in ovarian cell death will provide valuable information about non-traditional apoptotic pathways (e.g. caspase-independent) in reproductive tissues.
MEHP treatment for 48 h resulted in decreased expression of Bok mRNA, a pro-apoptotic factor, previously shown to be up-regulated in response to methoxychlor which induces antral follicle atresia in mice [31]. No other published studies have evaluated the effect of MEHP on Bok expression in ovarian cells; however, one study reported that exposure to BPA decreased the expression of Bok mRNA in the human ovarian carcinoma cell line OVCAR-3 [44]. Although results from Ptak et al. [44] suggest that BPA-induced down-regulation of Bok in OVCAR-3 cells is part of a pro-survival mechanism, our results suggest that this may not be the case in antral follicles. This idea is based on the observation that even though Bok mRNA is down-regulated at 48 h, MEHP-treated follicles still undergo atresia as evidenced by increased presence of apoptotic bodies at 96 h. Future studies will be needed to further understand the role of Bok down-regulation in MEHP-induced antral follicle toxicity.
In the present study, MEHP-treated follicles expressed significantly less Bcl2l10 mRNA than vehicle controls. The role of BCL2L10 protein in apoptosis has been described as controversial [45] based on evidence suggesting both pro- and anti-apoptotic activities in various cell lines. Specifically, BCL2L10 has been reported to be both pro- [46, 47] and anti-apoptotic [48] in human embryonic kidney 293T (HEK293T). BCL2L10 was also shown to block apoptosis in MCF-7 cells overexpressing the pro-apoptotic proteins BAK and BIK and to protect in interleukin-3 (IL-3)-dependent prolymphocytic and bone-marrow derived cell lines [49]. However, BLC2L10 is mostly regarded as an anti-apoptotic factor. Thus, our observations support the idea that MEHP promotes atresia of antral follicles by decreasing the expression of anti-apoptotic genes. Interestingly, BCL2L10 has also been shown to be involved in the regulation of meiosis and the intracellular structure of mouse oocytes [45]. Therefore, future studies will be critical to understand the consequences of MEHP treatment on oocyte health and to differentiate these effects from those on the somatic cells of the follicle.
MEHP treatment did not cause significant alterations in the expression of Bax, Bid, and Bcl2. These observations were unexpected since these genes were selected based on evidence showing that their expression is altered by other chemicals that cause atresia in mouse antral follicles [27, 31–33]. Previous studies have also reported that MEHP increases Bax and decreases Bcl2 mRNA expression in histiocytic lymphoma U937 cells (300 µM for 20 h; [50] and mouse antral follicles (36 µM for 96 h) [25]. Thus, it is important to note that these differences in the effect of MEHP on the expression of Bax and Bcl2 mRNAs versus our study may be due to differences in the tissues studied (U937 cells vs. ovarian antral follicles), the length of exposure (48 vs. 96 h), and the concentrations studied (300 µM vs. 0.36–36 µM). To date, no studies have reported on the effect of MEHP treatment on Bid mRNA expression, but at least one study has reported that MEHP leads to increased cleavage of BID protein in bone marrow B cells [51]. Perhaps, post-translational, rather than transcriptional, changes to BID are more relevant to MEHP-induced antral follicle toxicity and, thus, should be explored in future studies.
Proper E2 levels promote follicle growth and inhibit follicle atresia (reviewed in [52]). E2 is thought to exert its role in promoting folliculogenesis by favoring granulosa cell proliferation and inhibiting apoptosis signals [52]. Various studies have reported that E2 favors cell proliferation and survival by activating the transcription of factors required for progression through the cell cycle, while repressing others that cause cell cycle arrest and apoptosis [53–57]. Various studies have demonstrated that MEHP decreases E2 production by down-regulating the expression of aromatase in rat granulosa cells [58, 59], human granulosa-lutein cells [60] and mouse antral follicles [23]. It is thought that MEHP down-regulates aromatase expression by disrupting FSH receptor signaling [61] and/or modulating peroxisome-proliferator activated receptors (PPARs; [62]) in rat Sertoli and granulosa cells, respectively. Previous work from our group has shown that co-treating with E2 partially restores follicular growth and cell cycle gene expression in MEHP-treated antral follicles [23]. We have expanded on this work to demonstrate that co-treatment with E2 also prevents MEHP-induced antral follicle atresia. Thus, our data showing that E2 replacement prevents MEHP-induced growth inhibition and increased atresia suggests that these adverse outcomes are caused by the ability of MEHP to decrease E2 levels.
We also investigated the effect of E2 co-treatment on MEHP-related changes in expression of apoptosis-related factors. We observed that the effects of E2 co-treatment on MEHP-induced increased expression of Aifm1 were dependent on the concentration of the E2 supplement. Although no previous studies have evaluated the effect of MEHP, E2, or both together on Aifm1 expression in ovarian follicles, previous studies have reported decreased Aifm1 expression in response to E2 treatment in the ovarian adenocarcinoma cell line BG-1 [42] and in MCF7 breast cancer cells [63]. These observations are interesting because we did not observe significant changes in Aimf1 expression in follicles treated with E2 alone. Still, it is possible that MEHP causes up-regulation of Aimf1 as a result of decreasing E2 production by antral follicles, but the detailed mechanism of how E2 regulates Aifm1 expression and the importance of this regulation in determining ovarian follicle survival remains to be determined.
Treatment with E2 at 1 and 10 nM did not affect the expression of Bok in mouse antral follicles. This result is interesting because E2 has been shown to decrease the expression of Bok mRNA in the mouse uterus [64] and MCF7 breast cancer cells [63]. Here, co-treating follicles with MEHP plus E2 resulted in intermediate levels of Bok mRNA that were visually similar to those in MEHP-treated follicles, but not statistically different from those in vehicle controls. Therefore, our data suggest that MEHP may act like E2 in regulating Bok mRNA expression in mouse antral follicles, but further studies will be needed to explore this possibility.
Finally, we observed that MEHP decreases the expression of Bcl2l10, but does not affect the expression of Bcl2 mRNA. To our surprise, E2 alone at 10 nM and the combination of MEHP and E2 at 10 nM had the same effect on Bcl2l10 expression. Further, Bcl2 expression was significantly down-regulated only in follicles treated with both MEHP and E2. It is difficult to explain these changes based on previous work because this is the first study evaluating the effect of MEHP and E2 on Bcl2l10 expression. However, an interaction between E2 treatment and Bcl2l10 expression has been reported in MCF7 cells [63], but contrary to our observations, Bcl2l10 expression was increased by E2 in that study. Similarly, Bcl2 mRNA expression has been shown to be increased by E2 treatment in ovarian surface epithelium cells [65] and in T-helper type 2 cells [66]. Our results suggest that the co-treatment of MEHP and E2 modifies the individual effects of these chemicals on Bcl2l10 mRNA expression. Teasing out the mechanism by which this occurs is undoubtedly a promising topic for future studies.
To our knowledge, this study constitutes the first report of increased antral follicle atresia following MEHP exposure that also documents the effects of E2 co-treatment on MEHP-induced antral follicle atresia. This work expands our understanding of the mechanism by which MEHP causes antral follicle death and further supports the idea that decreased E2 levels are required for MEHP-induced antral follicle toxicity. However, much remains to be elucidated about the detailed mechanisms of action of MEHP and the involvement of key pro- and anti-apoptotic factors in this process.
HIGHLIGHTS.
MEHP (0.36–36 µM) induces atresia in mouse ovarian antral follicles.
E2 (1–10 nM) blocks MEHP-induced follicle atresia.
E2 may block MEHP-induced atresia via changes in Aifm1, Bok, and Bcl2l10 expression.
Acknowledgements
The authors thank Timothy DelValle and Dr. Liying Gao for technical help. This work was supported by National Institute on Environmental Health grants K99ES021467 (ZRC), R01ES019178 (JAF), and T32ES007326 (RKG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zelieann R. Craig, Email: zr.craig@arizona.edu.
Jeffrey Singh, Email: jsingh0922@gmail.com.
Rupesh K. Gupta, Email: drrupesh@yahoo.com.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- 1.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. A mixture of five phthalates esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 2.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, et al. Reproductive and Developmental Toxicity of Phthalates. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2009;12:225–249. doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- 4.Third National Report on Human Exposure to Environmental Chemicals. 2005 NCEH Pub. No. 05-0570.
- 5.Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, et al. NTP Center for the evaluation of risks to human reproduction; Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reprod Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 6.Wittassek M, Wiesmüller GA, Koch HM, Eckert R, Dobler L, Müller J, et al. Internal phthalate exposure over the last two decades -- A retrospective human biomonitoring study. Int J Hyg Environ Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Clark K, Cousins IT, Mackay D. Anonymous The Handbook of Environmental Chemistry. New York: Springer-Verlag; 2003. Assessment of critical exposure pathways; pp. 227–262. [Google Scholar]
- 8.Hines E, Calafat A, Silva M, Mendola P, Fenton S. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspect. 2009;117:86–92. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldyreva MV, Klimova TS, Iziumova AS, Timofeevskaia LA. The effect of phthalate plasticizers on the generative function. Gig Tr Prof Zabol. 1975;19:25–29. [PubMed] [Google Scholar]
- 10.Tabacova S, Little R, Balabaeva L. Maternal exposure to phthalates and complications of pregnancy. Epidemiology. 1999;10:S127. [Google Scholar]
- 11.Laskey J, Berman E. Steroidogenic assessment using ovary culture in cycling rats: effects of bis (2-diethylhexyl) phthalate on ovarian steroid production. Reprod Toxicol. 1993;7:25–33. doi: 10.1016/0890-6238(93)90006-s. [DOI] [PubMed] [Google Scholar]
- 12.Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128:216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- 13.Hirosawa N, Yano K, Suzuki Y, Sakamoto Y. Endocrine disrupting effect of di-(2-ethylhexyl)phthalate on female rats and proteome analyses of their pituitaries. Proteomics. 2006;6:958–971. doi: 10.1002/pmic.200401344. [DOI] [PubMed] [Google Scholar]
- 14.Ma M, Kondo T, Ban S, Umemura T, Kurahashi N, Takeda M, et al. Exposure of prepubertal female rats to inhaled di(2-ethylhexyl)phthalate affects the onset of puberty and postpubertal reproductive functions. Toxicol Sci. 2006;93:164–171. doi: 10.1093/toxsci/kfl036. [DOI] [PubMed] [Google Scholar]
- 15.Svechnikova I, Svechnikov K, Söder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol. 2007;194:603–609. doi: 10.1677/JOE-07-0238. [DOI] [PubMed] [Google Scholar]
- 16.Ma M, Zhang Y, Pei X, Duan Z. Effects of di-(2-ethylhexyl) phthalate exposure on reproductive development and PPARs in prepubertal female rats. Wei Sheng Yan Jiu. 2011;40:688–692. [PubMed] [Google Scholar]
- 17.Bagur AC, Mautzlen C. Risk for developing osteoporosis in untreated premature menopause. Calcif Tiss Int. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- 18.Dennerstein L, Lehert P, Burger H, Dudley E. Mood and the menopausal transition. J Nerv Ment Dis. 1999;187:685–691. doi: 10.1097/00005053-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson J, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 20.Hirshfield A. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 21.Hirshfield A. Size-frequency analysis of atresia in cycling rats. Biol Reprod. 1988;38:1181–1188. doi: 10.1095/biolreprod38.5.1181. [DOI] [PubMed] [Google Scholar]
- 22.Hengartner M. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol. 2010;242:224–230. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Craig Z, Basavarajappa M, Gupta R, Flaws J. Di (2-ethylhexyl) phthalate inhibits growth of mouse antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol. 2012;258:288–295. doi: 10.1016/j.taap.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Craig Z, Basavarajappa M, Hafner K, Flaws J. Mono (2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012;87:152. doi: 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Laboratory AR. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- 27.Craig Z, Hannon P, Wang W, Ziv-Gal A, Flaws J. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod. 2013;88:23. doi: 10.1095/biolreprod.112.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- 29.Craig Z, Leslie T, Hatfield K, Gupta R, Flaws J. Mono-hydroxy methoxychlor alters levels of key sex steroids and steroidogenic enzymes in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2010;249:107–113. doi: 10.1016/j.taap.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134–145. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basavarajappa MS, Karman BN, Wang W, Gupta RK, Flaws JA. Methoxychlor induces atresia by altering Bcl2 factors and inducing caspase activity in mouse ovarian antral follicles in vitro. Reprod Toxicol. 2012;34:545–551. doi: 10.1016/j.reprotox.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peretz J, Craig Z, Flaws J. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012;87:63. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulose T, Hannon P, Peretz J, Craig Z, Flaws J. Estrogen receptor alpha overexpressing mouse antral follicles are sensitive to atresia induced by methoxychlor and its metabolites. Reprod Toxicol. 2012;33:353–360. doi: 10.1016/j.reprotox.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inada H, Chihara K, Yamashita A, Miyawaki I, Fukuda C, Tateishi Y, et al. Evaluation of ovarian toxicity of mono-(2-ethylhexyl) phthalate (MEHP) using cultured rat ovarian follicles. J Toxicol Sci. 2012;37:483–490. doi: 10.2131/jts.37.483. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Liu T, Zhou L, He J, Ye L. Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol. 2012;34:869–875. doi: 10.1016/j.etap.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Ambruosi B, Uranio M, Sardanelli A, Pocar P, Martino N, Paternoster M, et al. In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model. PLoS One. 2011;6:e27452. doi: 10.1371/journal.pone.0027452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cregan S, Dawson V, Slack R. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 38.Cheung E, Melanson-Drapeau L, Cregan S, Vanderluit J, Ferguson K, McIntosh W, et al. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci. 2005;25:1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzo H, Susin P, Penninger J, Kromer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- 40.Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 41.Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 42.Hwang K, Park S, Yi B, Choi K. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab Anim Res. 2011;27:99–107. doi: 10.5625/lar.2011.27.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho Y, Lu C, Yang J, Chiang J, Li T, Ip S, et al. Bernerine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009;29:4063–4070. [PubMed] [Google Scholar]
- 44.Ptak A, Wróbel A, Gregoraszczuk E. Effect of bisphenol-A on expression of selected genes involved in cell cycle and apoptosis in the OVCAR-3 cell line. Toxicol Lett. 2011;202:30–35. doi: 10.1016/j.toxlet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Yoon S, Kim E, Kim Y, Lee H, Kim K, Bae J. Role of Bcl2-like 10 (Bcl2l10) in regulating mouse oocyte maturation. Biol Reprod. 2009;81:497–506. doi: 10.1095/biolreprod.108.073759. [DOI] [PubMed] [Google Scholar]
- 46.Inohara N, Gourley T, Carrio R, Muñiz M, Merino J, Garcia I, et al. Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J Biol Chem. 1998;273:32479–32486. doi: 10.1074/jbc.273.49.32479. [DOI] [PubMed] [Google Scholar]
- 47.Lee R, Chen J, Matthews C, McDougall J, Neiman P. Characterization of NR13-related human cell death regulator, Boo/Diva, in normal and cancer tissues. Biochem Biophys Acta. 2001;1520:187–194. doi: 10.1016/s0167-4781(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 48.Ke N, Godzik A, Reed J. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J Biol Chem. 2001;276:12481–12484. doi: 10.1074/jbc.C000871200. [DOI] [PubMed] [Google Scholar]
- 49.Song Q, Kuang Y, Dixit V, Vincenz C. Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO J. 1999;18:167–178. doi: 10.1093/emboj/18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokoyama Y, Okubo T, Kano I, Sato S, Kano K. Induction of apoptosis by mono(2-ethylhexyl)phthalate (MEHP) in U937 cells. Toxicol Lett. 2003;144:371–381. doi: 10.1016/s0378-4274(03)00256-x. [DOI] [PubMed] [Google Scholar]
- 51.Bissonnette S, Teague J, Sherr D, Schlezinger J. An endogenous prostaglandin enhances environmental phthalate-induced apoptosis in bone marrow B cells: activation of distinct but overlapping pathways. J Immunol. 2008;181:1728–1736. doi: 10.4049/jimmunol.181.3.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenfeld C, Wagner J, Roberts R, Lubahn D. Intraovarian actions of oestrogen. Reproduction. 2001;122:215–226. doi: 10.1530/rep.0.1220215. [DOI] [PubMed] [Google Scholar]
- 53.Prall O, Sarcevic B, Musgrove E, Watts C, Sutherland R. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 54.Foster J, Henley D, Bukovsky A, Seth P, Wimalasena J. Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol Cell Biol. 2001;21:794–810. doi: 10.1128/MCB.21.3.794-810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabbah M, Courilleau D, Mester J, Redeulih G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci U S A. 1999;96:11217–11222. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi V, Borgo R, et al. Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Mol Cell Biol. 2004;24:7260–7274. doi: 10.1128/MCB.24.16.7260-7274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foster J, Fernando R, Ishida N, Nakayama K, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–41366. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- 58.Lovekamp T, Davis B. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172:217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- 59.Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994;128:224–228. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- 60.Reinsberg J, Wegener-Toper P, van der Ven K, van der Ven H, Klingmueller D. Effect of mono-(2-ethylhexyl) phthalate on steroid production of human granulosa cells. Toxicol Appl Pharmacol. 2009;239:116–123. doi: 10.1016/j.taap.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Grasso P, Heindel J, Powell C, Reichert LJ. Effects of mono(2-ethylhexyl) phthalate, a testicular toxicant, on follicle-stimulating hormone binding to membranes from cultured rat Sertoli cells. Biol Reprod. 1993;48:454–459. doi: 10.1095/biolreprod48.3.454. [DOI] [PubMed] [Google Scholar]
- 62.Lovekamp-Swan T, Jetten A, Davis B. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl)phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201:133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 63.Sengupta S, Obiorah I, Maximov P, Curpan R, Jordan V. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169:167–178. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moggs J, Tinwell H, Spurway T, Chang H, Pate I, Lim F, et al. Phenotypic anchoring of gene expression changes during estrogen-induced uterine growth. Environ Health Perspect. 2004;112:1589–1606. doi: 10.1289/txg.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi K, Kang S, Tai C, Auersperg N, Leung P. Estradiol up-regulates antiapoptotic Bcl-2 messenger ribonucleic acid and protein in tumorigenic ovarian surface epithelium cells. Endocrinology. 2001;142:2351–2360. doi: 10.1210/endo.142.6.8144. [DOI] [PubMed] [Google Scholar]
- 66.Huber S, Kupperman J, Newell M. Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl 2 expression. Lupus. 1999;8:384–387. doi: 10.1177/096120339900800511. [DOI] [PubMed] [Google Scholar]