Abstract
The current study examined the effects of pheromonal exposure on adult neurogenesis and revealed the role of the olfactory pathways on adult neurogenesis and behavior in the socially monogamous prairie vole (Microtus ochrogaster). Subjects were injected with a cell proliferation marker, 5-bromo-2’-deoxyuridine (BrdU), and then exposed to their own soiled bedding or bedding soiled by a same- or opposite-sex conspecific. Exposure to opposite-sex bedding increased BrdU-labeling in the amygdala (AMY), but not the dentate gyrus (DG), of female, but not male, voles—indicating a sex-, stimulus-, and brain region-specific effect. The removal of the main olfactory bulbs or lesioning the vomeronasal organ (VNOX) in females reduced BrdU-labeling in the AMY and DG as well as inhibited the male bedding-induced BrdU-labeling in the AMY—revealing the importance of an intact olfactory pathway on amygdaloid neurogenesis. VNOX increased anxiety-like behavior and altered social preference, but it did not affect social recognition memory in female voles. VNOX also reduced the percentage of BrdU-labeled cells that co-expressed the neuronal marker TuJ1 in the AMY, but not the DG. Together, our data indicate the importance of the olfactory pathway in mediating brain plasticity in the limbic system as well as its role in behavior.
Keywords: Amygdala, hippocampus, BrdU, anxiety, vomeronasal organ, social preference, pheromones
Introduction
The detection of olfactory stimuli is essential for resource acquisition (e.g., food and shelter) and avoidance of predation (Wyatt, 2003; Jacobs, 2012). Olfaction is also involved in mate choice, partner bonding, and parent-offspring attachment (Wyatt, 2003; Jacobs, 2012). While there are various definitions, pheromones are commonly defined as chemosensory stimuli conveying information among conspecifics (Dulac & Torello, 2003; Brennan & Keverne, 2004). The main olfactory epithelium and vomeronasal organ (VNO) detect such stimuli and project via the main olfactory (MOB) and accessory olfactory (AOB) bulbs, respectively, to limbic brain structures, which mediate various social behaviors (Kollack-Walker & Newman, 1995; Lonstein et al., 1998; Baum & Kelliher, 2009). Pheromonal exposure affects physiology and behavior (Taylor et al., 1992; Kelliher & Wersinger, 2009). It can also alter adult neurogenesis—the generation and functional integration of new neurons in adulthood.
The subventricular zone (SVZ) and the dentate gyrus (DG) give rise to adult-generated cells that differentiate into neurons and integrate into the MOB and DG, respectively (Ming & Song, 2005). Male pheromone exposure increases SVZ cell proliferation in female mice and prairie voles (Microtus ochrogaster) (Smith et al., 2001; Mak et al., 2007). Male pheromones also increase cell survival in the MOB and AOB of female mice (Mak et al., 2007; Oboti et al., 2009). Such pheromone exposure also increases hippocampal adult neurogenesis—a phenomenon that is dependent on an intact MOB pathway (Mak et al., 2007). While adult-generated cells also occur in non-traditional neurogenic brain regions along the olfactory pathway, namely the amygdala (AMY), medial preoptic area, and hypothalamus (Huang et al., 1998; Fowler et al., 2002; Baum & Kelliher, 2009), little is known about the effects of pheromonal exposure on adult neurogenesis within these limbic brain regions.
The current study assessed the effects of pheromones on adult neurogenesis in the AOB, MOB, and limbic brain regions that belong to the olfactory pathways in the prairie vole. The prairie vole is particularly sensitive to changes in the social environment and thus has been used to study the effects of the social environment on adult neurogenesis. For example, social isolation in females and fatherhood reduce AMY and DG cell survival (Lieberwirth et al., 2012; Lieberwirth et al., 2013). Furthermore, male pheromone exposure leads to an increase in SVZ cell proliferation in female prairie voles (Smith et al., 2001). In the current study, voles were exposed to their own bedding or to bedding soiled by a conspecific to assess the pheromonal effect on the number of adult-generated cells in the MOB, AOB, and limbic brain regions of the olfactory pathways. Furthermore, MOB and VNO lesions were used to assess whether an intact olfactory pathway is necessary for pheromone-induced changes in the number of adult-generated cells. Lastly, we assessed whether the disruption of the VNO pathway affects various behaviors (including anxiety-like and social behaviors). Investigating the effects of a disrupted olfactory pathway on adult neurogenesis and on behavior will guide further research to establish a potential functional link between adult neurogenesis and behavior.
Materials and Methods
Subjects
Subjects (90–120 days of age) were sexually naïve male and female prairie voles (M. ochrogaster) that were the F3 generation of a laboratory breeding colony started from field-captured animals. Subjects were pair-housed with a same-sex conspecific in plastic cages (18 × 29 × 13 (H) cm) that contained cedar chip bedding. Water and food were provided ad libitum. Females and males were housed in separate colony rooms on a 14:10 hr light:dark cycle with lights on at 0730. The room temperature was kept at 21±1 °C. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Florida State University and were conducted in accordance with the guidelines set forth by the National Institute of Health.
BrdU injections, bedding exposure, and brain perfusion
All subject pairs were transferred to clean cages prior to the start of the experiment and housed individually. Twenty-four hours later, subjects were injected with a cell proliferation marker, 5-bromo-2’-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO) once a day, at 24-hr intervals, for 3 consecutive days. BrdU injections were given intraperitoneally (ip; 150 mg/kg body weight). Throughout the 3-day BrdU injection schedule, subjects were exposed daily in their home cages to bedding soiled by the same stimulus animal. The soiled bedding (approximately 100 g) was added to the subjects’ cage each morning and remained there until the end of the experiment.
Twenty-four hours after the last BrdU injection, subjects were deeply anesthetized and then perfused through the ascending aorta with 0.9 % saline followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were removed and post-fixed in 4 % paraformaldehyde for 2 hr at 4 °C. Thereafter, brains were transferred into 30 % sucrose in PB at 4 °C. Using a freezing microtome, the brains were cut coronally (40-µm section thickness) and sections were stored in 0.1 M PB with 1 % sodium azide until processed for immunohistochemistry.
Experimental design
Experiment 1: This experiment was designed to examine the effects of bedding exposure on BrdU-labeling in the brains of male and female prairie voles. Subjects were randomly assigned to one of three treatment groups that were exposed to (1) their own bedding (Own; male: n = 6, female: n = 5), (2) bedding from a same-sex individual (Same; male: n = 6, female: n = 7), or (3) bedding from an opposite-sex individual (Opp; male: n = 7, female: n = 8). For the latter two groups, the bedding used was obtained from a same- or opposite-sex individual that was not a sibling of the subject. After 3 days of BrdU injections and bedding exposure, subjects were perfused, and their brains were processed for BrdU immunohistochemistry.
Experiment 2: The second experiment examined the role of the main and accessory olfactory systems on bedding-induced BrdU-labeling in female prairie voles. Female subjects were randomly assigned to one of three treatment groups: (1) intact (Control), (2) olfactory bulbectomy (OBX), and (3) vomeronasal organ lesion (VNOX). Following surgery, subjects were allowed to recover for three weeks before being further divided into two subgroups that were exposed to either their own bedding (Own; Control: n = 8, OBX: n = 7, VNOX: n = 6) or to male bedding (Male; Control: n = 6, OBX: n = 10, VNOX: n = 8), respectively, for 3 days. As described in Experiment 1, all subjects received daily BrdU injections throughout the bedding exposure. Thereafter, subjects were perfused and the brains were processed for BrdU immunohistochemistry.
Experiment 3: As the AMY, especially the medial (MeA) and cortical (CoA) subregions, has been implicated in olfactory discrimination/memory and social behaviors (Kirkpatrick et al., 1994a; Ferguson et al., 2001; Maras & Petrulis, 2006), we examined the effects of VNOX on social and anxiety-like behaviors. In addition, we evaluated its effects on BrdU-labeling and neuronal differentiation. We did not include an OBX group in this experiment, as OBX has been recognized as a reliable model for depression and in turn would lead to distinct behavioral changes (Morales-Medina et al., 2013). Female subjects were randomly assigned to one of two groups that received sham surgery (Sham; n = 13) or VNOX (n = 9). After a three-week recovery period, subjects were injected with BrdU for 3 consecutive days. Thereafter, they underwent behavioral testing for social recognition (day 1), social preference (day 2), as well as locomotion and anxiety-like behaviors (day 3). Locomotion and anxiety-like behaviors were examined in the open field (OF) and elevated plus maze (EPM) tests on day 3 with a 6-hr interval. One hour following behavioral testing, subjects were perfused. One set of brain sections was processed for BrdU single labeling and another set was processed for BrdU/TuJ1/NG2 triple fluorescent labeling (see below).
Surgical procedures
OBX was performed using a previously established procedure (Kirkpatrick et al., 1994b). Briefly, subjects were anesthetized with sodium pentobarbital (100 mg/kg body weight), and a small window was made in the skull above the MOBs. While looking through a dissection microscope, the MOB was bilaterally vacuum-aspirated. This surgical procedure also removed the AOBs that reside bilaterally on the dorsal-posterior region of the MOBs. For the sham surgery, the olfactory bulbs were exposed but were left undisturbed. After the surgery, the skin incision above the window in the skull was sutured, and the subjects were allowed to recover for three weeks, before being assigned to experiments. Successful OBX was verified when the brains were extracted after perfusion.
VNOX was performed using an established procedure (Curtis et al., 2001). Subjects were anesthetized and their VNO was approached via the roof of the mouth. After exposure of the incisive bone, blunt dissection was used to expose the VNO capsule. A small burr was used to enlarge the incisive foramen, through which the entire VNO capsule was removed. The incision at the roof of the mouth was carefully sutured. For the sham lesion, the incisive bone was exposed and then the incision at the roof of the mouth was sutured. Subjects were allowed to recover for three weeks. Successful VNOX was verified histologically by staining MOB sections using 0.2 % thionin staining and confirm the absence of glomeruli staining in the AOB (Curtis et al., 2001).
Behavioral testing
The social recognition (SOC) test was performed as described in our previous study (Lieberwirth et al., 2012). Briefly, subjects were placed individually into a testing cage (25 × 45 × 20 (H) cm) and allowed to habituate for 10 min. Then, an unrelated juvenile female (30–40 days of age) was introduced into the cage for a 5-min exposure (trial 1, T1). The juvenile was removed for 30 min and then re-introduced for another 5-min exposure (trial 2, T2). This process was repeated 3 times (T1, T2, and T3). During the fourth exposure period (trial 4, new), an unrelated juvenile female was introduced for 5 min. All behavioral interactions were video-recorded. The duration and frequency of the subject’s olfactory investigation of the juvenile (including naso-nasal and naso-genital sniffing) were quantified.
The social preference (SOP) test was performed using a testing apparatus that consisted of 3 plexiglass chambers (18 × 29 × 13 (H) cm). Two clear hollow tubes connected the central chamber (empty) to two stimulus chambers, one of which contained an unfamiliar adult female and the other chamber contained an unfamiliar adult male. Both stimulus animals were loosely tethered to their chambers. At the beginning of the test, each subject was placed into the central chamber and then allowed to move freely throughout the apparatus. The 1-hr test was video-recorded. The subjects’ durations spent in each chamber and in side-by-side contact with the stimulus animals as well as the frequencies of the subjects’ cage entries were scored.
The open field (OF) test was performed using an established method (Pan et al., 2009). The apparatus measured 56 × 56 × 20 (H) cm and had a visual line grid that divided the apparatus into 16 squares, each measuring 14 × 14 cm. The subject was placed in the center of the apparatus, and the behavior was video-recorded for 10 min. The frequencies of center and corner entries, the durations spent in the center and corner squares, and the total numbers of line crosses were quantified.
The elevated plus maze (EPM) test was also performed using an established method (Pan et al., 2009). The testing apparatus was elevated 45 cm off the ground and consisted of two open (3.5 × 6.5 cm) and two closed (35 × 6.5 × 15 (H) cm) arms that crossed in the middle. Each subject was placed onto the center of the EPM facing a closed arm. The 5-minute test was video-recorded. The latency entering the open arm as well as the durations and frequencies in the open and closed arms were recorded. Subsequently, the percent of open to total arm entries and duration were calculated.
Immunohistochemistry
BrdU immunohistochemistry was performed on 40-µm floating sections at 120-µm intervals, using an established protocol (Lieberwirth et al., 2012). Briefly, sections were treated with 2 N HCl in 0.1 M PB at 37 °C for 30 min. After rinsing in 0.1 M PB, sections were incubated in 0.3 % H2O2 in 0.1 M PB for 10 min followed by blocking in 10 % normal goat serum (NGS) in 0.3 % Triton in 0.1 M PB (TPB) for 1 hr. Subsequently, the sections were incubated in rat anti-BrdU primary antibody (1:8000, Accurate, Westbury, NY) in 0.3 % TPB with 2 % NGS at 4 °C overnight. Then, sections were rinsed and incubated in biotinylated goat anti-rat IgG (1:300, Vector, Burlingame, CA) in 0.3 % TPB with 2 % NGS for 2 hr. Thereafter, sections were incubated in ABC Vector Elite in 0.1 M PB for 90 min and BrdU immunostaining was revealed using 3’-diaminobenzidine (DAB, Vector).
BrdU, TuJ1, and NG2 triple fluorescent immunohistochemistry was performed on floating sections at 120-µm intervals from the DG and AMY regions of the subjects in Experiment 3 using an established method (Fowler et al., 2005). The mouse anti-TuJ1 monoclonal IgG recognizes a neuron-specific class III β-tubulin, which is an early marker of neuronal differentiation (Lee et al., 1990; Alexander et al., 1991; Pencea et al., 2001). The rabbit anti-NG2 polyclonal IgG recognizes NG2, an integral membrane proteoglycan expressed in glial progenitor cells (Nishiyama et al., 1995). Briefly, sections were blocked in 10 % normal donkey serum (NDS) in 0.1 % TPB and then incubated in a cocktail of rat anti-BrdU (1:800), mouse anti-TuJ1 (1:500, Covance), and rabbit anti-NG2 (1:400, Millipore, Temecula, CA) in 0.1 % TPB with 2 % NDS at 4 °C for 48 hr. After rinsing in 0.1 % TPB, sections were incubated in a secondary antibody cocktail with 1:200 dilution of Cy3-conjugated donkey anti-rat IgG (Jackson Immunoresearch, West Grove, PA), Alexa Fluor 647-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch), and Alexa Fluor 488-conjugated donkey anti-mouse IgG (Jackson Immunoresearch) in 0.1 % TPB with 2 % NDS for 2 hr at room temperature. Thereafter, sections were rinsed, mounted on slides in slowfade (Life Technology, Grand Island, NY), and coverslipped.
Data quantification and analysis
BrdU-immunoreactive (BrdU-ir) cells were examined in the granular cell layer of the MOB and AOB; the DG, including the granular (GCL), polymorphic (Pol), and molecular (MCL) cell layers; and the AMY, including the cortical (CoA), medial (MeA), and central (CeA) subnuclei. Cell counts were conducted on multiple brain sections in each brain area (MOB and AOB: 6 sections; DG: 4 sections; and AMY: 4 sections). Cell numbers and region volumes were quantified bilaterally by using a Zeiss Axioskop II microscope with the optical fractionator workflow (for stereological sampling parameters see Table 1) of the Stereo Investigator software (MBF Bioscience, Chicago, IL), as described previously (Lieberwirth et al., 2012). Differences in the density of BrdU-ir cells across treatment groups in each brain region were analyzed using a two-way ANOVA (sex × bedding for Experiment 1 & treatment × bedding for Experiment 2) followed by Student-Neuman-Keul’s (SNK) post-hoc tests. In Experiment 2, differences between the OBX sham and VNOX sham animals were first compared by an independent sample t-test. As no group differences were found, these animals were combined into a single control group for the two-way ANOVA test. In Experiment 3, individual cells stained for BrdU, BrdU/TuJ1, or BrdU/NG2, were quantified in the DG, MeA, and CoA. Cells were visualized under 40 × magnification with a Zeiss LSM510 confocal microscope. Percentages of BrdU-ir cells co-labeled for TuJ1 or NG2 were calculated, and group differences were analyzed by independent sample t-tests. Group differences across the behavioral measurements in the OF and EPM tests were analyzed by independent sample t-tests. Data in the SOP test were analyzed by a two-way ANOVA (treatment × stimulus) followed by SNK post-hoc test. Data obtained in the SOC test were analyzed using a repeated measure ANOVA, followed by SNK post-hoc tests. The criterion for significance was set at p < 0.05.
Table 1.
Stereological quantification parameters
| Brain Area | # of Sections |
Counting Frame (in µm) |
Grid (in µm) |
Paxinos & Watson Plate # |
|---|---|---|---|---|
| Olfactory bulb | 6 | 30 × 30 | 43 × 43 | 1 |
| Amygdala | 4 | 50 × 50 | 71 × 71 | 27 – 33 |
| Hippocampus | 4 | 30 × 30 | 43 × 43 | 29 – 35 |
Note: The average mounted section thickness was about 22 µm and the optical dissector height was set to 15 µm. The average Gunderson coefficient of error was less than 0.2 across all brain regions.
Results
Exposure to conspecific’s bedding facilitates BrdU-labeling in a stimulus-, sex-, and brain region-specific manner
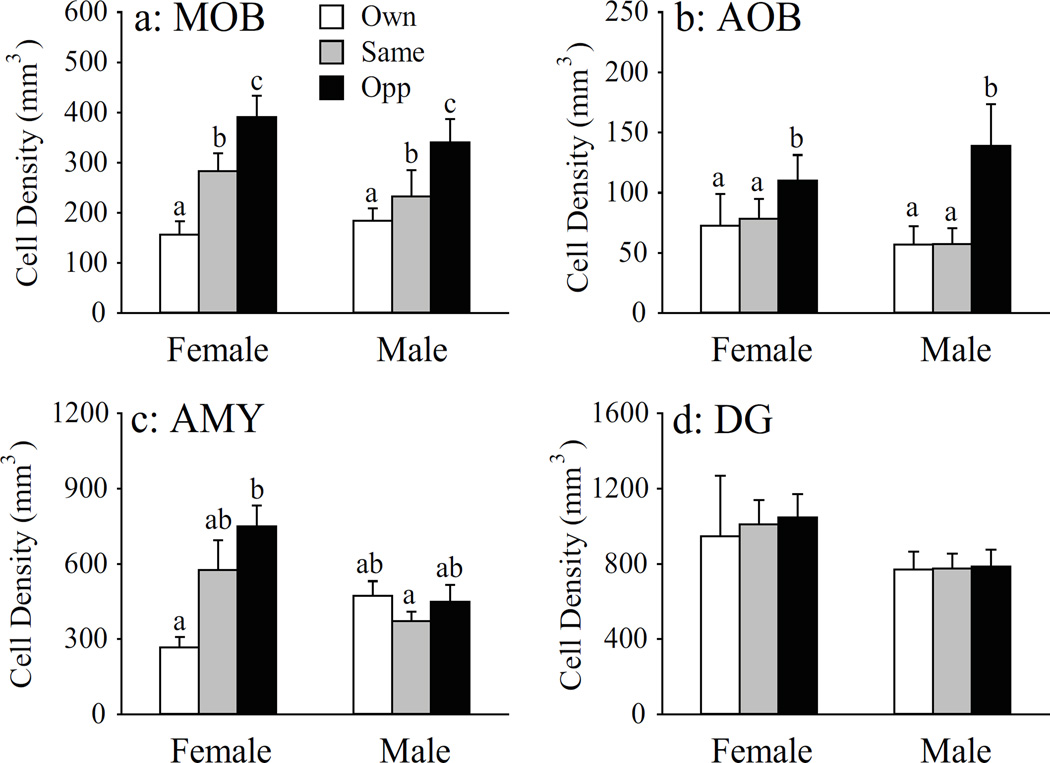
Exposure to bedding soiled by a conspecific increased the density of BrdU-ir cells in a brain region-specific manner, as treatment effects were found in the MOB, AOB, and AMY, but not DG. In the MOB, bedding exposure showed a graded effect on BrdU-ir labeling (F(2, 33) = 11.23, p < 0.01). Compared to the control group exposed to its own bedding, exposure to same-sex bedding increased the density of BrdU-ir cells in the MOB, and this bedding-induced increase was even larger in response to opposite-sex bedding (Fig. 1a). Exposure to opposite-sex bedding increased the density of BrdU-ir cells in the AOB compared to the treatment groups that were exposed to their own or same-sex bedding (F(2, 32) = 4.49, p < 0.05), while the latter two groups did not differ from each other (Fig. 1b). No sex differences and treatment by sex interactions were found in the density of BrdU-ir cells in either the MOB or AOB. In the AMY, a significant treatment effect (F(2, 32) = 3.93, p < 0.05) and treatment by sex interaction (F(2, 32) = 5.12, p < 0.01) were found for BrdU-labeling. In female voles, exposure to male bedding, but not female bedding, significantly increased the density of BrdU-ir cells compared to the control animals exposed to their own bedding (Fig. 1c & Fig. 2a-c). Such bedding effects were not found in male voles (Fig. 2e-g). In the subnuclei of the AMY, exposure to male bedding increased the density of BrdU-ir cells in the MeA and CoA, but not CeA, of females, compared to the control females exposed to their own bedding (Table 2). Finally, there were no effects of treatment, sex, and treatment by sex interaction on the density of BrdU-ir cells in the DG (Fig. 1d & Fig. 2h-m).
Figure 1.
Bedding exposure affects BrdU-labeling in a stimulus-, sex-, and brain region-specific manner. Opposite-sex bedding significantly increased the density of BrdU-labeled cells in the main olfactory bulb (MOB) in female and male prairie voles (a). Same-sex bedding induced an increase intermediate to control and opposite-sex bedding levels. Only opposite-sex bedding significantly increased the density of BrdU-labeled cells in the accessory olfactory bulbs (AOB) of female and male prairie voles (b). Opposite-sex bedding also significantly increased BrdU-labeling in the female, but not male, amygdala (AMY) (c). Exposure to conspecific bedding did not alter the level of BrdU-labeling in the dentate gyrus (DG) of the hippocampus (d). Error bars represent SEM. Alphabetic letters indicate results from the SNK post-hoc tests. Bars with different letters differ significantly from each other.
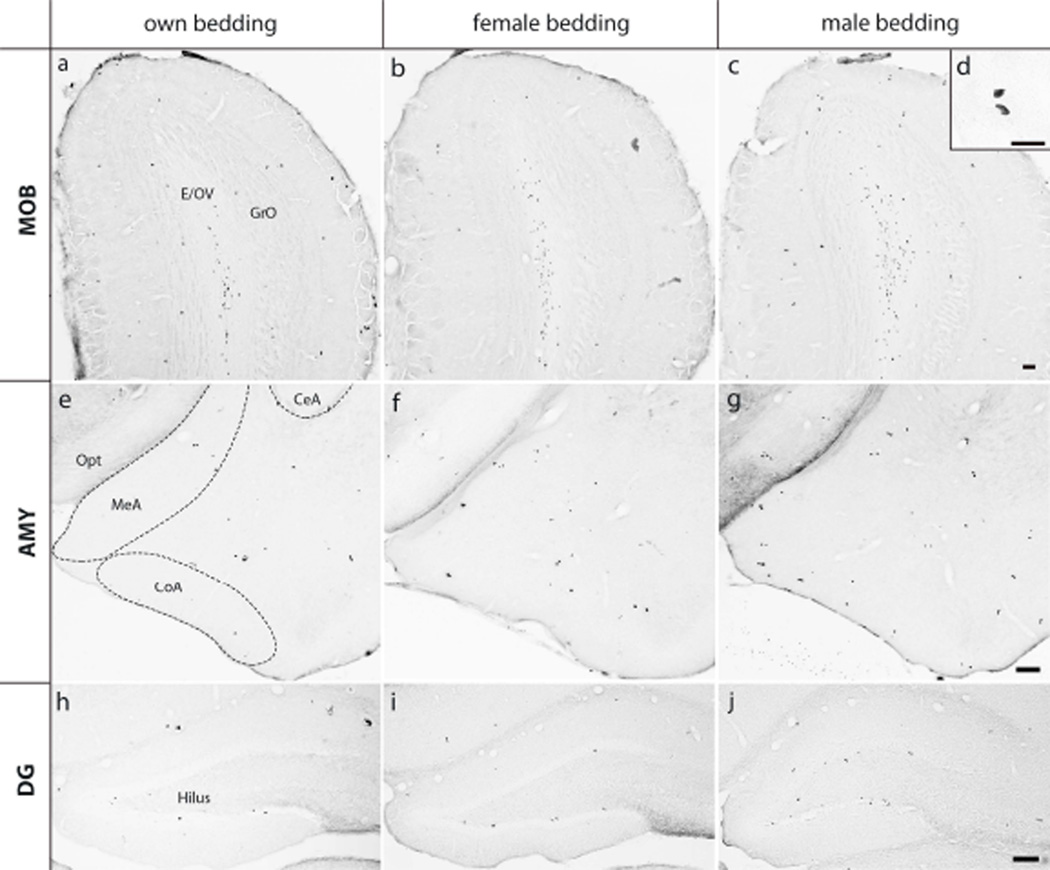
Figure 2.
Photo micrographics illustrating BrdU-labeled cells in the main olfactory bulb (MOB; a-d), amygdala (AMG; e-g), and dentate gyrus of the hippocampus (DG; h-j) in female prairie voles that were exposed to own bedding (a, e & h), female bedding (b, f & i), or male bedding (c, g & j). Scale bar = 100 µm. The insert panel (d) shows high magnification of BrdU-labeled cells in the MOB (scale bar = 25 µm). CeA: central nucleus, CoA: anterior corticol nucleus, and MeA: medial nucleus of the amygdala; E: ependyma & subependymal layer and GrO: granular cell layer of the main olfactory bulb; OV: olfactory ventricle; opt: optic tract.
Table 2.
Density of BrdU-ir cells (#/mm3) in the subnuclei of the amygdala in female and male prairie voles.
| Female |
Male |
ANOVA (p) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Bedding | Own | Same | Opposite | Own | Same | Opposite | Sex | Group | S × G |
| MeA | 340.1±59.7a* | 582.2±151.0ab | 910.6±158.1b | 427.3±115.9a | 395.2±65.3a | 365.6±52.9a | 0.05 | Ns | 0.05 |
| CoA | 366.6±43.8a | 1016.2±219.2ab | 1418.3±195.3b | 932.7±118.2ab | 631.4±72.2a | 828.4±161.9ab | ns | 0.05 | 0.01 |
| CeA | 189.6±55.2** | 355.2±71.7 | 393.4±52.0 | 321.2±45.0 | 232.4±41.0 | 307.1±46.3 | ns | Ns | ns |
Alphabetic letters indicate group differences following a SNK posthoc test.
Mean ± SEM.
The olfactory systems mediate male bedding facilitated BrdU-labeling in the AMY
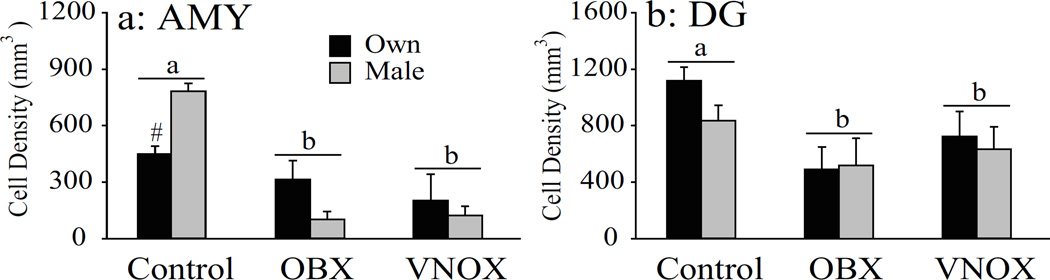
Following OBX or VNOX, female voles showed a significant decrease in the density of BrdU-ir cells in the AMY compared to the intact group (F(2, 33) = 22.50, p < 0.01; Fig. 3a). In addition, exposure to male bedding increased the density of BrdU-ir cells in intact animals, but not in animals that received OBX or VNOX (F(2, 33) = 7.32, p < 0.01, Fig. 3a). The BrdU-ir data in the MeA, CoA, and CeA subnuclei of the AMY were summarized in Table 3. In the DG, similarly to the AMY, both OBX and VNOX groups had a lower density of BrdU-labeled cells than the intact group (F(2, 39) = 7.52, p < 0.01, Fig. 3b). Exposure to male bedding was not effective in altering BrdU-labeling in the DG.
Figure 3.
The effect of bedding exposure on BrdU-labeling depends on an intact olfactory pathway. Both olfactory bulb lesions (OBX) and vomeronasal organ removal (VNOX) resulted in a reduction of BrdU-labeling in the amygdala (AMY) in female prairie voles (a). In addition, exposure to male-bedding induced an increase in BrdU-labeling in the AMY only in intact, but not OBX and VNOX, females. OBX and VNOX also reduced BrdU-labeling in the dentate gyrus (DG), but bedding exposure did not alter BrdU-labeling in the DG in any groups (b). Error bars represent SEM. Alphabetic letters indicate results from the SNK post-hoc tests. Bars with different letters differ significantly from each other.
Table 3.
Density of BrdU-ir cells (#/mm3) in the subnuclei of the amygdala in female prairie voles.
| Control |
OBX |
VNOX |
ANOVA (p) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Bedding | Own | Male | Own | Male | Own | Male | Lesion | Group | S × G |
| MeA | 364.9±42.3a* | 765.0±114.4b | 235.1±98.6a | 267.2±112.5a | 279.2±92.5a | 123.8±41.2a | 0.01 | ns | 0.05 |
| CoA | 708.4±129.9b** | 1330.7±52.4c | 402.1±185.3ab | 159.7±64.0a | 501.1±157.5ab | 156.7±65.0a | 0.01 | ns | 0.01 |
| CeA | 319.5±27.1b | 471.3±68.2c | 64.4±53.4a | 61.5±21.0a | 207.1±64.1ab | 104.2±44.0a | 0.01 | ns | 0.05 |
Alphabetic letters indicate group differences following a SNK posthoc test.
Mean ± SEM.
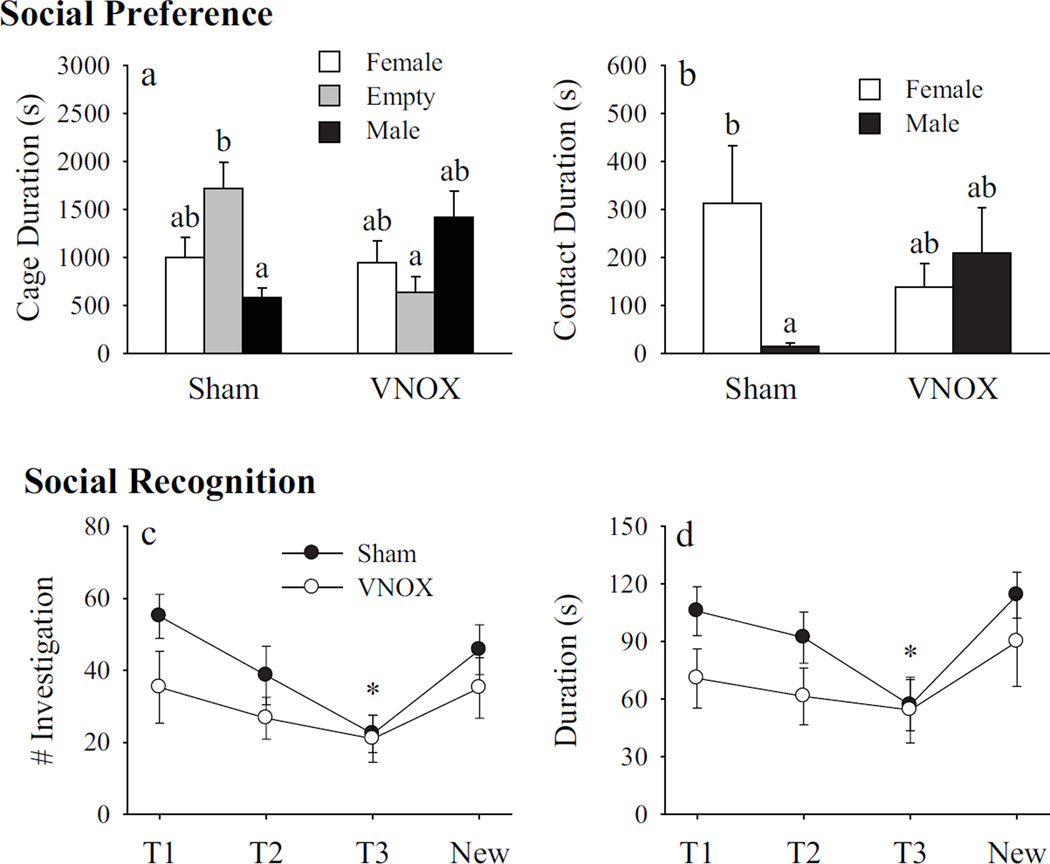
VNOX induces anxiety-like behavior and alters social affiliation without affecting social recognition
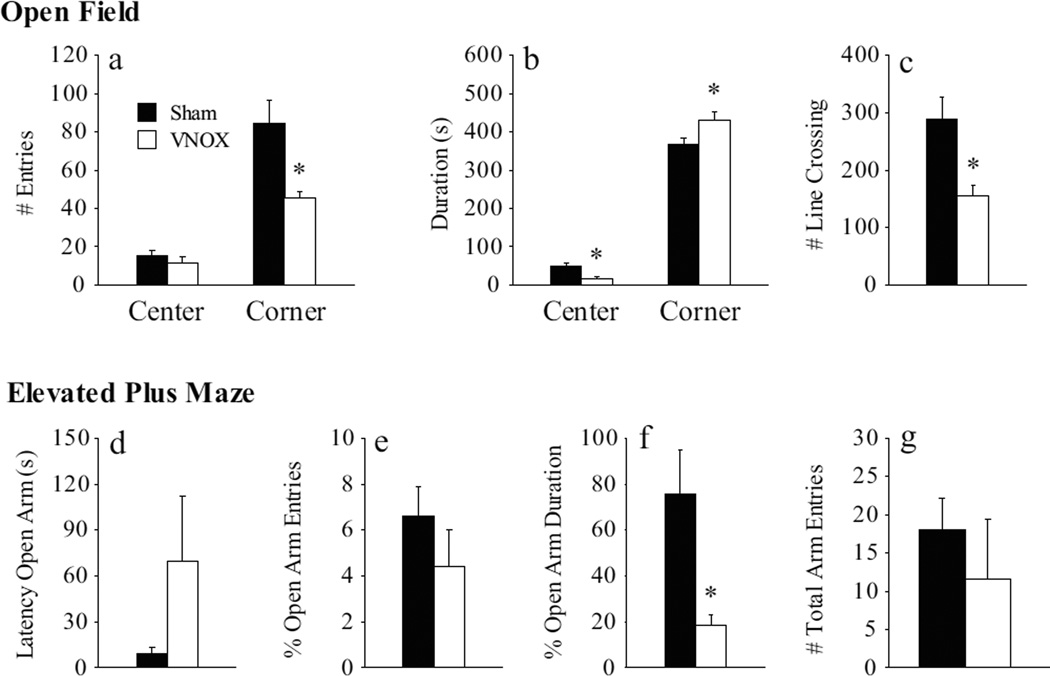
Anxiety-like behavior was significantly increased by VNOX in female prairie voles. In the OF test, VNOX females showed a lower frequency (t(18) = 2.36, p < 0.05; Fig. 4a), but a longer duration (t(18) = 2.25, p < 0.05), of corner squares entries compared to the sham-lesioned controls (Fig. 4b). In addition, the VNOX females spent less time in the center of the OF arena (t(18) = 2.55, p < 0.05) than the control females. VNOX females also showed a decrease in locomotor activity indicated by a lower number of total line crossings compared to controls (t(18) = 2.46, p < 0.05; Fig. 4c). In the EPM test, VNOX females spent a lower percentage of time in the open arms (t(17) = 2.24, p < 0.05, Fig. 4f), compared to the controls. Although VNOX females tended to show a longer latency to enter the open arm (t(18) = 1.81, p = 0.09, Fig. 4d) and to have fewer open arm entries (t(18) =1.86, p = 0.08, Fig. 4g) compared to control females, these differences did not reach statistical significance.
Figure 4.
Vomeronasal organ removal (VNOX) induced a subtle increase in anxiety-like behaviors. In the open field test, VNOX females had less corner entries (a), spent less time in the center but more time in the corners (b), and had reduced line crosses in the arena (c) compared to SHAM animals. In the elevated plus maze, VNOX females showed a decrease in open arm duration (f) than SHAM females. The two groups did not differ in other behavioral measurements. *: p<0.05. Error bars represent SEM.
In the SOP test, a significant interaction between treatment and stimulus animals was found in the cage duration (F(2, 61) = 8.77, p < 0.01). The SNK post-hoc test indicated that sham-lesioned subjects spent more time in the empty cage than in the male cage. In addition, sham-lesioned subjects spent more time in the empty cage than did VNOX subjects (Fig. 5a). No group differences were found in the VNOX subjects (Fig. 5a). A significant interaction between treatment and stimulus animals was also found in side-by-side contact time (F(1, 40) = 3.83, p < 0.05). The SNK post-hoc test indicated that sham-lesioned subjects spent more time in side-by-side contact with the female than male conspecific, whereas such a preference was not found in VNOX subjects (Fig. 5b).
Figure 5.
Vomeronasal organ removal (VNOX) altered social preference in a three-chambered social preference test without affecting social recognition. In the social preference test, SHAM females spent more time in the empty cage than the cage holding a male stimulus animal, such preference was not observed in VNOX females (a). Furthermore, SHAM females showed a longer duration of side-by-side contact with the female as compared to the male stimulus; whereas VNOX females spend a similar amount of time in side-by-side contact with the female and male stimulus (b). In the social recognition test, each female was exposed to the same juvenile three times (T1, T2, and T3) and then to a new juvenile (New). The inter-exposure interval was 30 min. VNOX and SHAM females did not differ in the frequency (c) and duration (d) of olfactory investigation of the juvenile conspecific. In both treatment groups, trial 3 (T3) was significantly lower in frequency and duration than the other trials. *: p<0.05. Error bars represent SEM.
In the SOC test, there were main effects of exposure (T1, T2, T3, and new) on both the frequency (F(3, 12) = 7.28, p < 0.01) and duration (F(3, 12) = 5.04, p < 0.05) of olfactory investigation (Fig. 5c & d). The frequency and duration scores of T3 were significantly lower than those observed during the other exposure points. No treatment (sham vs VNOX) effect and treatment by test interactions were found for the frequency and duration of olfactory investigation.
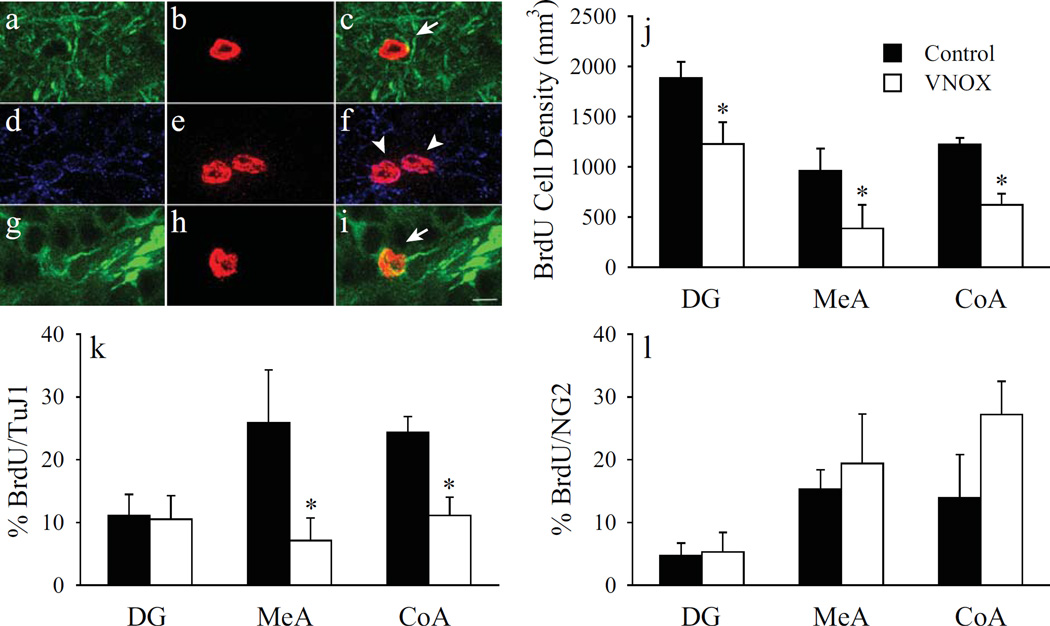
VNOX impairs cell proliferation and neuronal differentiation in the AMY
BrdU single labeling in Experiment 3 replicated the results in Experiment 2; VNOX significantly reduced the density of BrdU-ir cells in the DG (t(16) = 2.41, p < 0.05), MeA (t(15) = 2.36, p < 0.05), and CoA (t(15) = 2.20, p < 0.05), compared to sham-lesioned controls (Fig. 6j). For the brain sections processed for BrdU/TuJ1/NG2 triple fluorescent labeling, numbers of BrdU-ir cells were quantified in the DG (VNOX: 237, Sham-lesioned: 193), MeA (VNOX: 138, Sham-lesioned: 101), and CoA (VNOX: 166, Sham-lesioned: 132). VNOX significantly reduced the number of neuronally differentiated cells in a brain region-specific manner. The percentage of BrdU-ir cells co-stained with TuJ1 was significantly decreased in both the MeA (t(8) = 2.35, p < 0.05; Fig. 6a-c) and CoA (t(9) = 2.57, p < 0.05), but not DG (Fig. 6g-i), of VNOX females compared to sham-lesioned controls (Fig. 6k). No group differences were found in the number of BrdU/NG2 double-labeled cells in any of the examined brain areas (Fig. 6d-f & 6l).
Figure 6.
Stacked confocal microscopy images illustrating fluorescent-labeled cells in the amygdala (AMY, a-f) and dentate gyrus of the hippocampus (DG, g-i). Cells were labeled for BrdU (red), the neuronal marker TuJ1 (green), and the glial marker NG2 (purple). Arrows indicate BrdU/TuJ1 double-labeled cells (c, i). Arrow heads indicate BrdU/NG2 double-labeled cells (f). Scale bar = 10 µm. Vomeronasal organ removal (VNOX) decreased the number of BrdU-labeled cells in the DG, as well as the medial (MeA) and anterior cortical (CoA) nuclei of the AMY (j). VNOX also significantly reduced the percentage of BrdU-labeled cells that co-labeled for TuJ1 in the MeA and CoA, but not DG (k). VNOX had no effect cells double-labeled for BrdU and NG2 (l).
Discussion
The exposure to chemosensory stimuli affects adult neurogenesis. For example, exposure to male pheromones increases the cell proliferation in the SVZ of female mice and prairie voles (Smith et al., 2001; Mak et al., 2007). Exposure to male pheromones also increases the cell survival in the MOB and AOB as well as the neuronal differentiation in the MOB of female mice (Mak et al., 2007; Oboti et al., 2009). Consistent with and in extension of these findings, data from the present study showed that the exposure to pheromones affects the number of adult-generated cells in the brain of the socially monogamous prairie vole in a stimulus- and brain region-specific manner. Specifically, the MOB of female and male voles responded to pheromones from same- and opposite-sex conspecifics, the AOB only responded to an opposite-sex stimulus, whereas the DG showed no response. Interestingly, only chemosensory stimuli from an opposite-sex conspecific enhanced the BrdU-labeling in the AMY, particularly in the MeA and CoA, of female, but not male, prairie voles, indicating a sex-specific effect.
Both the MOB and AOB have been shown to respond to pheromones (Veyrac et al., 2011); however, the anatomical differences in the main and accessory olfactory pathways may mediate functional differences, which in turn, may underlie the observed stimulus-specific response (Meredith, 1998; Brennan & Kendrick, 2006). The AOB receives inputs from the VNO and projects to the AMY, in particular to the MeA and CoA (Brennan & Kendrick, 2006). The AOB may be especially sensitive to reproductive stimuli (i.e., pheromones of opposite-sex, rather than same-sex, conspecifics), as the AOB likely modulates reproductive behavior through its projections to the MPOA and hypothalamus via the MeA (Meredith, 1998; Choi et al., 2005; Brennan & Kendrick, 2006; Samuelsen & Meredith, 2009). Additional evidence of the involvement of the AOB in mediating reproductive behavior comes from studies showing that mating experience increased the number of adult-generated cells in the AOB of female and male mice (Corona et al., 2011; Portillo et al., 2012).
Although the pheromonal effects on adult neurogenesis have been studied in several rodent species (for review see Lieberwirth & Wang, 2012), very few studies have examined brain areas along the olfactory pathways downstream from the MOB and AOB. One interesting finding in the present study is the stimulus- and sex-specific response in the AMY of prairie voles to the exposure of conspecific bedding. The AMY showed an increase in BrdU-labeling in response to the chemosensory stimuli from an opposite-sex conspecific only in female, but not male, voles. In addition, this effect was subregion-specific; it was only observed in the MeA and CoA—brain regions that receive direct inputs from the AOB—but not the CeA. It should be noted that anatomical sex differences have been found in the AMY. For example, in rats and mice, males and females differ in the amygdalar volume (Hines et al., 1992; Ahmed et al., 2008; Morris et al., 2008) as well as the number of amygdaloid cells that express certain neurochemical phenotypes such as vasopressin and oxytocin (De Vries, 1984; Caffe et al., 1989; De Vries & Miller, 1998). It is possible that such sex differences in the AMY may determine its sensitivity and responsiveness to chemosensory stimuli between males and females. Such differences in the neuroanatomy of the AMY have been attributed to sex-specific reproductive behaviors (Segovia & Guillamon, 1993; Guillamon & Segovia, 1997; Keller et al., 2009). It should be noted that female prairie voles lack an estrous cycle and are induced into behavioral estrus in response to interactions with a male or male-associated sensory cues (Cohen-Parsons & Carter, 1987). As this male-induced behavioral estrus is associated with various physiological changes (Dluzen & Carter, 1979; Hnatczuk & Morrell, 1995), we cannot exclude the possibility that the observed increase in BrdU-labeling in response to male bedding exposure is in part due to estrus induction (Smith et al., 2001). A caveat in the present study should be mentioned. The opposite-sex, but not same-sex, stimulus animals were housed in a different room from the subjects. Therefore, it is possible that novelty associated with male bedding exposure may also play a role in influencing the BrdU-labeling in the AMY of female voles, although this did not seem to be the case in male voles.
The effects of chemosensory stimuli on adult neurogenesis depend on an intact olfactory pathway (Mak et al., 2007). Congruent with the finding that OBX reduces hippocampal adult neurogenesis (Jaako-Movits et al., 2006; Keilhoff et al., 2006; Morales-Medina et al., 2013), our data showed that OBX decreased BrdU-labeling in the DG as well as in the AMY in female prairie voles. The olfactory bulbs have direct projections to the AMY (Brennan & Kendrick, 2006), which, in turn, projects to the DG (Pikkarainen et al., 1999; Pitkanen et al., 2000). Our data support the notion that an intact olfactory pathway plays an important role in regulating basal levels of cell proliferation in adult animals (Mak et al., 2007; Lieberwirth & Wang, 2012; current study). In addition, control, but not OBX, females showed increased BrdU-labeling in the AMY in response to male bedding exposure, suggesting that an intact olfactory pathway is also essential for the chemosensory stimulus to affect cell proliferation (Mak et al., 2007; Lieberwirth & Wang, 2012; current study). Interestingly, this effect is brain region-specific as the bedding exposure altered cell proliferation in the AMY—an area downstream primarily from the olfactory bulbs, but not in the DG—an area downstream further from the AMY. The underlying mechanisms need to be further examined.
Our data also show that VNOX females had almost identical patterns of BrdU-labeling in the DG and AMY as OBX females. Because OBX by vacuum-aspiration also led to the damage of the AOB—an area that receives direct inputs from the VNO (Brennan & Kendrick, 2006), our data suggest that the accessory olfactory pathway likely plays an important role in mediating the pheromone-induced changes in adult-generated cells, although we cannot exclude the possibility that the main olfactory pathway may also be involved. It should also be mentioned that VNOX females showed a decrease in the percentage of BrdU-labeled cells that were co-labeled with TuJ1 in the AMY, but not DG, indicating an effect on adult neurogenesis selectively in downstream brain areas along the accessory olfactory pathway. Future studies should focus on assessing whether these immature neurons display long-term survival and become functionally integrated into the existing circuitry. Interestingly, previous research has suggested that immature DG neurons display distinct characteristics from mature neurons that may potentially allow these immature neurons to influence the existing circuitry independent of reaching maturity or long-term survival (Liu et al., 1996; Wang et al., 2000; Cameron & McKay, 2001; Zhao et al., 2008). Thus, it may be of interest to assess whether these immature AMY neurons may similarly play a functional role. It will also be interesting to reveal the phenotypes of BrdU-ir cells in the VNOX and control animals without behavioral testing, as the behavioral testing itself may have affected the findings.
It is worth to mention that although studies on adult neurogenesis have focused primarily on the DG and SVZ (see reviews by Fowler et al., 2008; Lieberwirth & Wang, 2012), newly proliferated cells have been reported in the adult AMY in several mammalian species including voles (Fowler et al., 2003; Lieberwirth et al., 2012), rats (Keilhoff et al., 2006), mice (Okuda et al., 2009), hamsters (Antzoulatos et al., 2008), and monkeys (Bernier et al., 2002; Marlatt et al., 2011). In prairie voles, 30-min after a BrdU injection, BrdU-ir cells were found in the AMY, and some of these cells were co-labeled for immature neuronal and glial markers (Fowler et al., 2003). These data clearly indicate the presence of neuronal and glial progenitors that are locally dividing within the AMY, although one cannot exclude the possibility that these progenitor cells are migrating into the AMY from other brain regions (e.g., SVZ) (Marlatt et al., 2011).
In addition to changes in BrdU-labeling, VNOX also affected behaviors in female prairie voles. VNOX caused a subtle increase in anxiety-like behaviors as assessed in both the OF and EPM tests. These data are consistent with previous findings that the ablation of the VNO increased anxiety-like behaviors in the OF and light-dark box tests in mice (Liu et al., 2010). In addition to the increase in anxiety-like behaviors, VNOX resulted in a reduction in the measure of locomotion in the OF, while no such decrease was observed in the EPM. Therefore, we cannot fully rule out that a decrease in exploration in VNOX females may have at least in part contributed to these findings. The VNO and its associated accessory olfactory pathway are critical for a variety of social behaviors. For example, a genetically compromised VNO system in male mice caused alterations in aggressive behavior and sexual behavior (Leypold et al., 2002). The ablation of the VNO resulted in a reduction of urine marking in male mice (Liu et al., 2010) and prevented mating-induced partner preference formation in female prairie voles (Curtis et al., 2001).
In the current study, VNOX females displayed alterations in social preferences. Control females preferred the empty chamber over the chamber holding the male conspecific, following VNOX surgery such a preference disappeared. Furthermore, control females showed a preference to spend more time in side-by-side contact with the female compared to the male stimulus animal. VNOX animals did not show such a preference. Interestingly, VNOX did not affect social recognition memory, which is supported by previous studies that have shown that VNOX does not impair mate recognition or social discrimination (Petrulis et al., 1999; Woodley et al., 2004; Keller et al., 2006; Liu et al., 2010). Therefore, it seems unlikely that a deficit in social discrimination underlies the altered social preference following VNOX. One possible explanation for the lack of preference is that VNOX might not interfere with the detection of chemical differences between pheromones of conspecifics, while interfering with the extraction of the biological information from the pheromone (Liu et al., 2010).
Recent research has suggested that adult-generated cells may play an essential role in mediating behavioral functions (Imayoshi et al., 2009). For example, adult-generated MOB neurons are involved in olfactory discrimination, odor recognition, as well as recognition of conspecifics (Rochefort et al., 2002; Enwere et al., 2004; Mak & Weiss, 2010; Alonso et al., 2012). Similarly, adult-generated neurons in the DG have been implicated in hippocampal functions including spatial learning, object recognition, and associate memory formation (Shors et al., 2001; Clelland et al., 2009; Jessberger et al., 2009). It is reasonable to speculate that new neurons in the adult AMY, in particular in the MeA and CoA, may mediate its functions in social behavior (Kirkpatrick et al., 1994a; Ferguson et al., 2001; Maras & Petrulis, 2008). It should also be pointed out, however, that our data are only correlational: olfactory disruption due to VNOX (and OBX) may affect behavior via non-neurogenesis-dependent pathways. Therefore, future studies are needed to investigate the causal relationship, if any, between adult neurogenesis within the AMY and behavior (Mercadante et al., 2008).
Acknowledgements
We are grateful to Kelly Lei, Adam S. Smith, Brennan Paedae, and Manal Tabbaa for their critical reading of the manuscript and for the technical assistance provided by Sarah Huth. This research was supported by NIH grants MHR01-89852 and MHR01-58616 to ZXW.
Footnotes
Conflict of Interest
The authors have no financial disclosures and have no potential conflicts of interest.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci U S A. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Lepousez G, Wagner S, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci. 2012;15 doi: 10.1038/nn.3108. 897-U127. [DOI] [PubMed] [Google Scholar]
- Antzoulatos E, Magorien JE, Wood RI. Cell proliferation and survival in the mating circuit of adult male hamsters: Effects of testosterone and sexual behavior. Horm Behav. 2008;54:735–740. doi: 10.1016/j.yhbeh.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: Attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–R89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Caffe AR, Van Ryen PC, Van der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiol Behav. 1987;39:309–314. doi: 10.1016/0031-9384(87)90227-7. [DOI] [PubMed] [Google Scholar]
- Corona R, Larriva-Sahd J, Paredes RG. Paced-mating increases the number of adult new born cells in the internal cellular (granular) layer of the accessory olfactory bulb. PLoS One. 2011;6:e19380. doi: 10.1371/journal.pone.0019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt matinginduced pair bonding in female prairie voles (Microtus ochrogaster) Brain Res. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in the brain : The relation between structure and function. Amsterdam ; New York : New York, NY, U.S.A.: Elsevier ; Sole distributors for the U.S.A. and Canada, Elsevier Science Pub. Co; 1984. [Google Scholar]
- De Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog Brain Res. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Carter CS. Ovarian hormones regulating sexual and social behaviors in female prairie voles, Microtus ochrogaster. Physiol Behav. 1979;23:597–600. doi: 10.1016/0031-9384(79)90063-5. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: From genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamon A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44:377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Hnatczuk OC, Morrell JI. Interaction of male sensory cues and estradiol in the induction of estrus in the prairie vole. Physiol Behav. 1995;58:785–790. doi: 10.1016/0031-9384(95)00132-3. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51:379–386. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Jaako-Movits K, Zharkovsky T, Pedersen M, Zharkovsky A. Decreased hippocampal neurogenesis following olfactory bulbectomy is reversed by repeated citalopram administration. Cell Mol Neurobiol. 2006;26:1559–1570. doi: 10.1007/s10571-006-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF. From chemotaxis to the cognitive map: The function of olfaction. Proc Natl Acad Sci U S A. 2012;109:10693–10700. doi: 10.1073/pnas.1201880109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Bernstein HG, Wolf G. Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology. 2006;31:1165–1176. doi: 10.1038/sj.npp.1300924. [DOI] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Wersinger SR. Olfactory regulation of the sexual behavior and reproductive physiology of the laboratory mouse: Effects and neural mechanisms. Ilar J. 2009;50:28–42. doi: 10.1093/ilar.50.1.28. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): Behavioral and anatomical specificity. Behav Neurosci. 1994a;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Williams JR, Slotnick BM, Carter CS. Olfactory bulbectomy decreases social behavior in male prairie voles (M. ochrogaster) Physiol Behav. 1994b;55:885–889. doi: 10.1016/0031-9384(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Lee MK, Rebhun LI, Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci U S A. 1990;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Y, Jia X, Liu Y, Wang Z. Fatherhood reduces the survival of adult-generated cells and affects various types of behavior in the prairie vole (Microtus ochrogaster) Eur J Neurosci. 2013;38:3345–3355. doi: 10.1111/ejn.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The social environment and neurogenesis in the adult mammalian brain. Front Hum Neurosci. 2012;6:118. doi: 10.3389/fnhum.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Lio PA, Pasternak JF, Trommer BL. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J Neurophysiol. 1996;76:1074–1088. doi: 10.1152/jn.1996.76.2.1074. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Zhang JX, Zhang JH, Bao WD, Liu DZ. Vomeronasal organ ablation elicits chemosensory dysfunction and abnormal behavior in mice. J Ethol. 2010;28:263–271. [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: Possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The posteromedial cortical amygdala regulates copulatory behavior, but not sexual odor preference, in the male Syrian hamster (Mesocricetus auratus) Neuroscience. 2008;156:425–435. doi: 10.1016/j.neuroscience.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Philippens I, Manders E, Czeh B, Joels M, Krugers H, Lucassen PJ. Distinct structural plasticity in the hippocampus and amygdala of the middle-aged common marmoset (Callithrix jacchus) Exp Neurol. 2011;230:291–301. doi: 10.1016/j.expneurol.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Mercadante MT, Cysneiros RM, Schwartzman JS, Arida RM, Cavalheiro EA, Scorza FA. Neurogenesis in the amygdala: a new etiologic hypothesis of autism? Medical hypotheses. 2008;70:352–357. doi: 10.1016/j.mehy.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Juarez I, Venancio-Garcia E, Cabrera SN, Menard C, Yu W, Flores G, Mechawar N, Quirion R. Impaired structural hippocampal plasticity is associated with emotional and memory deficits in the olfactory bulbectomized rat. Neuroscience. 2013;236:233–243. doi: 10.1016/j.neuroscience.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Stallcup WB. Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell. 1995;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboti L, Savalli G, Giachino C, De Marchis S, Panzica GC, Fasolo A, Peretto P. Integration and sensory experience-dependent survival of newlygenerated neurons in the accessory olfactory bulb of female mice. Eur J Neurosci. 2009;29:679–692. doi: 10.1111/j.1460-9568.2009.06614.x. [DOI] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J Neurosci Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brainderived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiol Behav. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Portillo W, Unda N, Camacho FJ, Sanchez M, Corona R, Arzate DM, Diaz NF, Paredes RG. Sexual activity increases the number of newborn cells in the accessory olfactory bulb of male rats. Front Neuroanat. 2012;6 doi: 10.3389/fnana.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neuroscience. 2009;164:1468–1476. doi: 10.1016/j.neuroscience.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia S, Guillamon A. Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Res Brain Res Rev. 1993;18:51–74. doi: 10.1016/0165-0173(93)90007-m. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Taylor SA, Salo AL, Dewsbury DA. Estrus induction in four species of voles (Microtus) J Comp Psychol. 1992;106:366–373. doi: 10.1037/0735-7036.106.4.366. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Wang G, Baum MJ, Bakker J. The main and accessory olfactory systems of female mice are activated differentially by dominant versus subordinate male urinary odors. Brain Res. 2011;1402:20–29. doi: 10.1016/j.brainres.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chem Senses. 2004;29:659–669. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour communication by smell and taste. Cambridge, UK ; New York: Cambridge University Press; 2003. [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]