Abstract
Introduction
Many clinicopathological studies do not specify the presence of other pathologies located within the brain, so disease heterogeneity may be under appreciated.
Objective
The purpose of this study was to determine the frequencies of concomitant pathologies among parkinsonian disorders.
Methods
Data from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), an ongoing longitudinal clinical-neuropathological study, was used to analyze concomitant pathologies, including Alzheimer’s disease (AD), argyrophilic grains (Arg), cerebral amyloid angiopathy (CAA), cerebral white matter rarefaction (CWMR) and overlap of each parkinsonian disorder in clinico-pathologically defined Parkinson’s disease (PD; N=140), dementia with Lewy bodies (DLB; N=90), progressive supranuclear palsy (PSP; N=64), multiple system atrophy (MSA; N=6), corticobasal degeneration (CBD; N=7); and normal elderly (controls; N=166).
Results
Of the neuropathologically-confirmed PD cases, 38% had a concomitant diagnosis of AD, 9% PSP, 25% Arg, and 44% CWMR, and 24% CAA. For DLB, 89% had AD, 1% PSP, 21% Arg, and 51% CWMR, and 50% CAA. For PSP cases, 36% had AD, 20% PD, 1% DLB, 44% Arg, 52% CWMR and 25% CAA. Similar heterogeneity was seen for MSA and CBD cases. Many cases had more than one of the above additional diagnoses.
Conclusions
These data demonstrate a great deal of concomitant pathologies among different types of parkinsonian disorders; this may help explain the heterogeneity of clinical findings.
Keywords: Argyrophilic grains, White Matter Rarefaction, Alzheimer’s disease, cerebral amyloid angiopathy, neoplasms, vascular dementia, Parkinson’s disease
1. Introduction
Parkinsonian disorders are clinically characterized by tremor, rigidity, postural instability and bradykinesia. There are also other clinical features within these disorders, but these signs and symptoms, especially early in their disease courses have great overlap. Pathologically, each parkinsonian disorder is principally defined by its proteinopathy, cellular morphology and topographical location within the brain. Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) are both considered tauopathies, where aggregates of abnormally phosphorylated tau are found within glia and neurons in specific brain regions. However in PSP, the defining morphology is tufted astrocytes while in CBD it is astrocytic plaques. Both conditions have neurofibrillary tangles and pre-tangles, as well and other glial tau aggregates in the form of thorned astrocytes and oligodendroglial coiled bodies [1]. Multiple system atrophy (MSA), Parkinson’s disease (PD), and dementia with Lewy bodies (DLB) are all considered synucleinopathies, and are defined pathologically by aggregates of the pre-synaptic protein α-synuclein. In MSA, the aggregates are present in oligodendrocytes as well as neurons, while in PD, and DLB they are predominantly within neurons, both as the classical perikaryal cytoplasmic inclusions and in nerve fibers within the neuropil [2, 3]. There is evidence in PD that α-synuclein aggregates first in the brainstem while in DLB the limbic region is likely to be affected first [4]. In both conditions, end stage cases have a wide brain distribution that includes the entire cerebral cortex [4]. Despite these tendencies, there are large overlaps in lesion distribution and, by convention, they are separated somewhat arbitrarily by the relative onset of parkinsonism and dementia [2, 3, 5].
Outside of the defining histopathology for these disorders, other pathologies are known to commonly co-exist. Given many parkinsonian disorders typically manifest later in life, it is not surprising multiple pathologies may co-exist, including cerebral white matter rarefaction (CWMR), argyrophilic grains (Arg), cerebral amyloid angiopathy (CAA), and the plaques and tangles of Alzheimer’s disease (AD). CWMR, known in the radiological literature as leuko-araiosis, white matter hyperintensities or “small vessel ischemic injury”, is commonly found in older individuals and its presence has been correlated with gait abnormalities [6]. In Parkinson’s disease, 30–50% of cases are known to have CMWR; and CMWR in PD can be associated with more severe gait problems, bradykinesia and postural instability [7, 8]. Argyrophilic grains are present at a frequency of 16–22% in normal elderly persons [9], and there are reports of these deposits in some subjects with parkinsonism [9, 10]. Cerebrovascular lesions, including CAA, have been thought to be associated with AD but also present in normal older individuals and has been reported in some types of parkinsonism [11–13]. Alzheimer’s disease histopathology has also been studied extensively as an overlapping pathology with many parkinsonisms, especially DLB [3, 5].
No study to date has pursued a comprehensive examination of the frequencies of concomitant pathologies among all parkinsonian disorders. The purpose of this study was to determine if concomitant pathologies have higher frequencies in certain parkinsonian disorders as compared to older subjects who were free of significant clinical neurological findings.
2. Methods
2.1 Case selection
Cases were selected from Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) and the Banner Sun Health Research Institute (BSHRI), Brain and Body Donation Program (BBDP), a longitudinal clinicopathological study of normal aging, dementia and parkinsonism [14]. The BBDP database was queried for cases that had come to autopsy January 1997 thru December 2012 having a clinicopathological diagnosis of one of the following parkinsonian disorders: PD, DLB, MSA, PSP, and/or CBD. Concomitant pathologies in cases lacking a defined clinico-neuropathological diagnosis (controls) were also assessed. Annualized visits and exams for cognition and movement disorders and criteria for clinical diagnoses are previously described [14]. All experiments were conducted in accordance with the Declaration of Helsinki. The operations of the Brain and Body Donation Program are approved by the Banner Health Institutional Review Board and all participants or next of kin gave informed consent.
2.2 Clinical and neuropathological assessment
All cases underwent autopsy and a standardized neuropathological assessment, resulting in the assignment of pathological diagnoses. The final clinicopathological characterizations were based on both the neuropathology and clinical characteristics as obtained from standardized neurological assessments as well as review of private medical records. Standard criteria for these diagnoses have been reviewed elsewhere [15, 16].
For clinicopathological diagnoses, subjects received a diagnosis of PD if they had two or more cardinal clinical signs (rest tremor, bradykinesia, rigidity) as well as Lewy bodies and pigmented neuron loss in the substantia nigra. A diagnosis of PSP was made if the subject had neurofibrillary tangles and tufted astrocytes present in several of the following areas: frontal cortex, putamen, pallidum, subthalamic nucleus, substantia nigra, dentate nucleus, inferior olivary nucleus, or pontine nuclei [15, 17, 18]. A diagnosis of CBD was made if there was a presence in the cerebral cortex of swollen, achromatic neurons (or “ballooned” neurons) and astrocytic plaques [15, 19]. A diagnosis of MSA was made using the 1999 consensus criteria and glial cytoplasmic inclusions containing α-synuclein deposits were present [15, 20]. Vascular dementia (VaD) was diagnosed according to criteria set forth by Roman and colleagues.[21] Subjects received a diagnosis of AD if they had a clinical history of dementia and were classified as “intermediate” or “high” according to the NIA-Reagan criteria [22]. DLB was diagnosed according to consensus criteria published by the Dementia with Lewy Bodies Consortium [3].
Tissue processing methods have been previously described [14]. Briefly, the cerebrum was cut in the coronal plane at the time of brain removal into 1 cm thick slices and then divided into left and right halves. The slices from the right half were frozen between slabs of dry ice while the slices from the left half were fixed by immersion in neutral-buffered 4% formaldehyde for 48 hours at 4 degrees C. Formaldehyde-fixed paraffin-embedded sections were stained with hematoxylin and eosin to detect any neoplasms and vascular lesions. Large-format, 40–80 µm-thick formaldehyde-fixed sections were subject to a series of 4 stains: hematoxylin and eosin, Gallyas, Thioflavin-S, and Campbell-Switzer methods. Hematoxylin and eosin on large format section was used for CWMR grading and detection of vascular lesions. Neurofibrillary tangles, astrocytic plaques, tufted astrocytes and argyrophilic grains were detected with Gallyas silver stains. Campbell-Switzer silver stains were used to detect amyloid plaques. Thioflavin-S was used as a secondary stain to detected Lewy bodies (LBs), neurofibrillary tangles and amyloid plaques. To detect pathologic α-synuclein, in the form of Lewy bodies (LBs), glial cytoplasmic inclusions and Lewy neurites, formalin-fixed, 5µm paraffin-embedded sections were used for IHC with an antibody against phosphorylated α-synuclein peptide (1:10,000; rabbit polyclonal antihuman phosphoserine 129, gift of Dr. Akiyama) [23]. These processes are done on all brains received by our BBDP [14].
Neuropathological assessment was performed blinded to clinical categorization and diagnosis was assigned by an experienced neuropathologist (TGB). For other pathologies that do not have a clinicopathological definition, methods of diagnoses have been described previously [24]. CWMR was defined as having 26% or more of the centrum semiovale affected in one or more of the following lobes: frontal, parietal, occipital, and temporal; these methods have been published previously [24]. CAA was graded on a 0–3 scale based analogously on CERAD templates [25]. Hippocampal sclerosis (HS) was defined as having complete or near complete neuronal loss in the CA1 and/or dentate gyrus region of the hippocampus in the relative absence of neurofibrillary tangles or ghost tangles [26]. Brain neoplasms were defined as those that were primary or metastatic parenchymal tumors; these did not include meningiomas or schwannomas. Argyrophilic grains (Arg) were defined as typical spindle-shaped structures revealed by the Gallyas silver stain [9]. Incidental LBs (iLBs) were defined as intra-neuronal α-synuclein inclusions being present but not in a distribution and/or possess clinical features warranting a clinicopathological diagnosis of PD, DLB or MSA.
2.3 Statistical analysis
All analyses were conducted with Sigma Plot 12.0 (Systat Software, Inc., San Jose, CA, USA). One-way analysis of variance (ANOVA) was used to test differences between multiple group means, with Dunn’s method for post-hoc paired significance testing. The proportional distribution of categorical variables was analyzed with the chi-squared test. Furthermore, multiple logistic regression analyses were used to understand if frequencies of concomitant pathologies differed within each group after adjusting for age and gender. Statistical significance was defined as a P value < 0.05.
3. Results
Table 1 lists demographic data for all groups analyzed. Parkinson disease (PD) cases had longer disease durations when compared to DLB, PSP, MSA, and CBD. Control and PSP cases were significantly older at death than PD, CBD and MSA (Table 1). Controls had a significantly lower average score on the Motor Part III of the Unified Parkinson’s disease (UPDRS) before death when compared to PD, DLB, PSP and MSA (Table 1). There were no significant differences with respect to time from the last UPDRS until death and no significant differences in gender ratios across all groups.
Table 1.
Demographics of examined groups in the Brain and Body donation program. All neurodegenerative disease groups are not mutually exclusive. All data are listed as mean ± standard deviation.
| PD | DLB | PSP | MSA | CBD | Controls | |
|---|---|---|---|---|---|---|
| Total | 140 | 90 | 64 | 7 | 6 | 166 |
| % Male | 67.1% | 56.7% | 64.1% | 42.9% | 50.0% | 56.0% |
| age of death | 80±6.5 | 81±7.2 | 84±8.5* | 74±7.4 | 75±5.7 | 83±9.3* |
| disease duration | 15±2.5┼ | 7±4.1 | 8±6.2 | 5±1.7 | 7±1.9 | n/a |
| Last UPDRS | 42±20.3 | 35±26.8 | 33±19.5 | 43±28.2 | n/a*** | 8±6.8** |
| months prior to death | 12±11.5 | 19±14.4 | 33±19.5 | 17±12.1 | n/a*** | 16±16.2 |
PD had significant greater disease duration when compared to DLB, PSP, CBD, and MSA cases.
PSP and control cases were significantly older than PD, CBD, and MSA cases at death.
Controls had a significantly lower average UPDRS score before death when compared to PD, DLB, PSP, and MSA.
Only one CBD case had a record of a UPDRS.
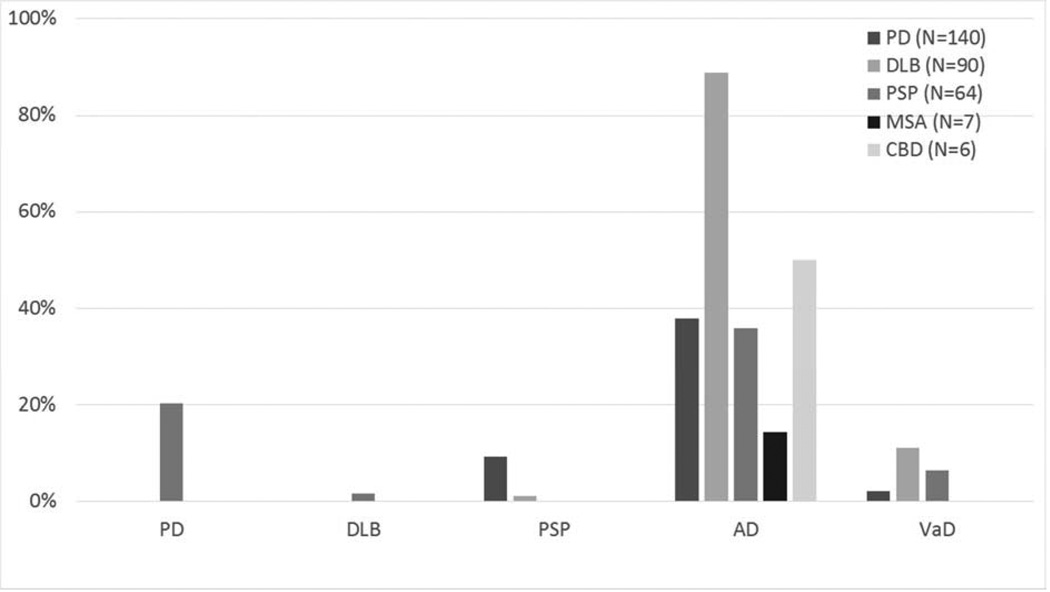
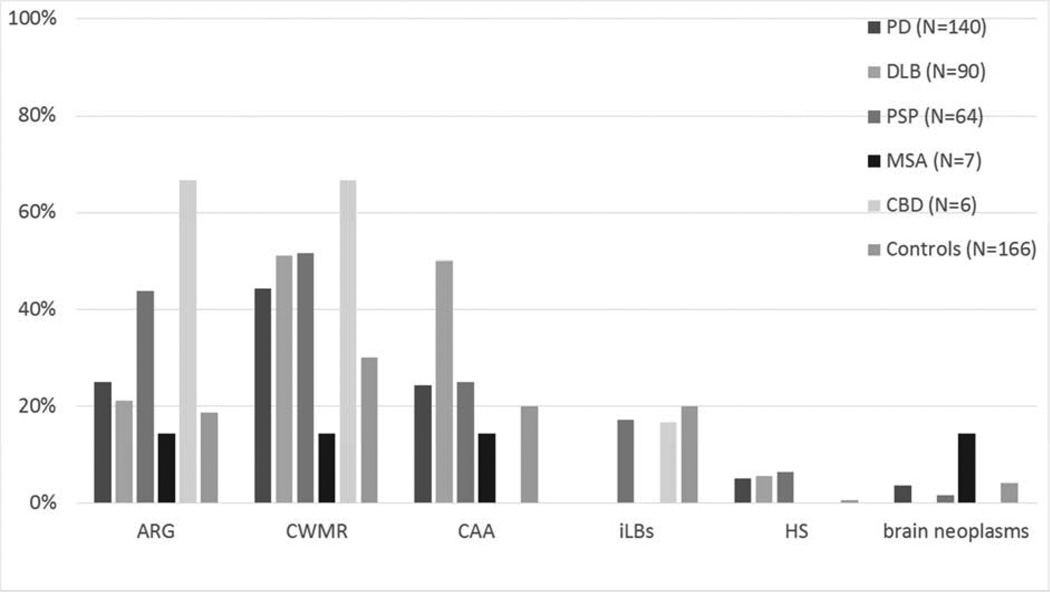
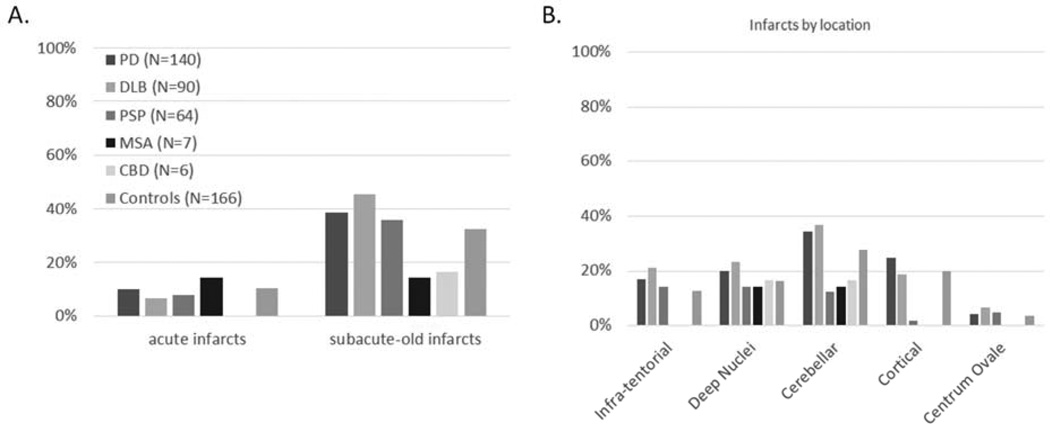
Frequencies of certain concomitant clinicopathological diagnoses among the parkinsonian disorders are located in Figure 1. The only significantly comorbidity was AD, which was more frequent in DLB as compared to PD, PSP, MSA and CBD, even after adjusting for age and gender. Subsequent analysis, dividing all PD by the presence or absence of dementia, showed PD with dementia had a higher frequency of AD when compared to PD without dementia, PSP, MSA, and CBD (PD subgroups not shown). Figure 2 shows the frequencies of other pathologies examined including Arg, CWMR, CAA, iLBs, HS, and brain neoplasms among the parkinsonian disorders and controls. None of these other pathologies had different frequencies among groups. Increased age was the driving factor for nearly all concomitant pathologies; for Arg, CAA, CWMR, and HS (p values < 0.009). Frequencies of infarctions and the location of the infarction among the parkinsonian disorders and controls are located in Figure 3. There were no statistical differences among any groups with respect to the presence or location of infarcts.
Fig. 1.
Frequencies of certain concomitant clinicopathological diagnoses among parkinsonian disorders. Many cases had more than one of the concomitant diagnosis listed on the x-axis. *The DLB group contained significantly higher frequencies of concomitant AD when compared to other groups.
Fig. 2.
Frequencies of certain concomitant pathologies among parkinsonian disorders and controls. There were no significant differences among groups. All groups on the x-axis are not mutually exclusive, and there is considerable overlap with concomitant pathologies.
Fig. 3.
Frequencies of the type of infarcts (A) and their location (B) among parkinsonisms and controls. Acute infarcts were not included in locational analysis. Infarct groups are not mutually exclusive. There were no significant differences among groups.
4. Discussion
The present study confirms other reports demonstrating that all parkinsonian disorders have an array of concomitant pathologies- including overlaps of major clinicopathological diagnoses. This study is the first to compare the frequencies of multiple pathologies across all parkinsonian disorders. Although multiple pathologies were found within each disorder, the presence of most concomitant pathologies was no different among different parkinsonian disorders or different from those found in controls. The main factor driving most concomitant pathologies was age, but even after adjusting for age there were significant differences.
Other studies have examined the overlap of clinicopathological defined major neurodegenerative disorders (for review see [27]). In this series, AD was more likely to be a comorbid condition in subjects with DLB than in subjects with other parkinsonian disorders (PSP, MSA, CBD). When the entire PD group was divided by those with and without dementia, it was the PD dementia group that was more likely to have a concomitant diagnosis of AD. Previous studies have demonstrated PD with dementia and DLB have more concomitant AD pathology and the current data support this [28]. Alzheimer’s disease (AD) was still often present to some degree in PSP, MSA, and CBD, and these intersections have been reported [29–31]. Furthermore, more than one parkinsonian disorder was present in the same subjects; this series had 13 cases with PSP and PD and 1 case with PSP and DLB. There are other pathologically confirmed reports of PSP cases with a concomitant diagnosis of PD or DLB [30, 32, 33]. These overlaps are especially critical to report, since in the neurology practice typically only one clinical diagnostic entity is assigned based on parkinsonian symptoms.
There are multiple reports examining cerebrovascular pathologies such as VaD, CAA, CWMR and infarcts in parkinsonian disorders and results have been inconclusive [7, 8, 11–13, 34]. When comparing PD to normal controls, Jellinger and colleagues found the total frequency of cerebrovascular lesions (lacunes, CAA, WMR, old and recent ischemic infarcts and hemorrhages) in PD was higher than in controls, however similar to our results when examining minor to moderate vascular pathologies (such as single infarctions or moderate to severe CAA) there were no differences [13]. This group also conducted a similar study in DLB, finding the presence of cerebrovascular lesions was lower in DLB than in PD and controls [12]. Although in the current study there are high percentages of cases with infarctions, there were low frequencies of VaD- indicating that many of the vascular pathologies in this series were minor and perhaps non-contributing to disease processes. A clinical radiological study on white matter hyperintensities demonstrated patients with MSA had severe changes compared to controls [34]. The current study did not find significant differences between groups with respect to any cerebrovascular pathology examined, suggesting that any particular parkinsonism is neither protective nor a risk factor for cerebrovascular pathologies. We would like to caution that our series is much smaller than previous studies. Furthermore, results may vary based on sampling procedures. In the current study, large format sections (40–80 µm) of each cerebral lobe, as well as 5 µm paraffin sections of representative brain regions were used. A recent study conducted on estimation of whole-brain microinfarct burden indicated that observing 1 or 2 microinfarcts in a single 6 µm section implied a maximum-likelihood estimate of 552 or 1,104 microinfarcts throughout the brain [35]. This large window of estimation could explain the inconclusiveness of the frequencies of cerebrovascular pathologies within parkinsonisms. Hippocampal sclerosis (HS) is a common pathological feature in many dementia cases with a frequency of 12 to 13% and has been reported in some parkinsonisms [26, 36]. There were relatively few cases of HS in this series that were concurrent with major parkinsonian disorders, which may explain why no significant differences were detected across groups; the highest frequency of HS was 6.3% in PSP, followed by 5.6% in DLB, and 5% in PD and 0.6% in the control group. As for brain neoplasms, the low presence in the current study although not significantly different than controls, may support a hypothesis of PD being inversely related to cancer; in a meta-analysis of 29 studies PD collectively showed a significant reduced cancer risk [37]. There are reports of Arg in some forms of parkinsonism [9, 10]. Based on the nature of Arg being composed of the tau protein, it has been hypothesized that Arg shares a common biochemical and genetic pathway with other tauopathies such as PSP and CBD [38]. However, we found similar frequencies of Arg within synucleinopathies (MSA, DLB and PD) and tauopathies (PSP and CBD).
A note of caution on interpreting these data is our series selection. Cases from AZSAND and the BBDP are mainly elderly volunteers, typically over the age of 70 at enrollment, living in Maricopa County and metropolitan Phoenix, Arizona. Due emphasis of the program being the study of PD as well as dementia there is a recruitment bias towards enrolling subjects with these disorders, so other parkinsonian disorders, such as PSP, MSA and CBD may be misrepresented. Furthermore, although most of our diseased and control subjects are recruited through community outreach efforts, a significant fraction are recruited from neurologists’ offices and thus extrapolation of these results to the general population should be done with caution. We would have also liked to explore the notion of the significance of each concomitant pathology’s relation to clinical data such as UPDRS scores for each parkinsonian disorder; however, these data were limited by the small number of non-PD parkinsonian cases.
5. Conclusions
The present study highlights that an array of pathologies, outside the realm of those that define each disease, are found within all parkinsonian disorders. There was even overlap within the parkinsonian disorders themselves, which poses the question of whether physicians should consider assigning a second diagnosis when one is suspected. Co-existing pathologies may impact clinical measures and perceived effect sizes in clinical trials. With reports of the diagnostic accuracy of these diseases being less than 100% in the clinical setting [39, 40], concomitant pathologies may be the key to understanding discrepancies between clinical and pathological diagnoses. Given there is a clinical heterogeneity that exists within these disorders and there is a known pathological heterogeneity, larger studies are needed.
Acknowledgements
The authors thank the patients and their families for their participation in the study. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), and the Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol. 2011;7:263–272. doi: 10.1038/nrneurol.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 3.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 4.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 6.Murray ME, Senjem ML, Petersen RC, Hollman JH, Preboske GM, Weigand SD, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccini P, Pavese N, Canapicchi R, Paoli C, Del Dotto P, Puglioli M, et al. White matter hyperintensities in Parkinson's disease. Clinical correlations. Arch Neurol. 1995;52:191–194. doi: 10.1001/archneur.1995.00540260097023. [DOI] [PubMed] [Google Scholar]
- 9.Sabbagh MN, Sandhu SS, Farlow MR, Vedders L, Shill HA, Caviness JN, et al. Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer Dis Assoc Disord. 2009;23:229–233. doi: 10.1097/WAD.0b013e318199d833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuboi Y, Josephs KA, Boeve BF, Litvan I, Caselli RJ, Caviness JN, et al. Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord. 2005;20:982–988. doi: 10.1002/mds.20478. [DOI] [PubMed] [Google Scholar]
- 11.Jellinger KA, Attems J. Prevalence and impact of cerebrovascular pathology in Alzheimer's disease and parkinsonism. Acta Neurol Scand. 2006;114:38–46. doi: 10.1111/j.1600-0404.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 12.Jellinger KA. Prevalence of vascular lesions in dementia with Lewy bodies. A postmortem study. J Neural Transm. 2003;110:771–778. doi: 10.1007/s00702-003-0824-x. [DOI] [PubMed] [Google Scholar]
- 13.Jellinger KA. Prevalence of cerebrovascular lesions in Parkinson's disease. A postmortem study. Acta Neuropathol. 2003;105:415–419. doi: 10.1007/s00401-003-0676-3. [DOI] [PubMed] [Google Scholar]
- 14.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol. 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 16.Ellison D. Neuropathology : a reference text of CNS pathology. 2nd ed. New York: Mosby: Edinburgh; 2004. [Google Scholar]
- 17.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 19.Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology. 1997;48:119–125. doi: 10.1212/wnl.48.1.119. [DOI] [PubMed] [Google Scholar]
- 20.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 21.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 22.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 23.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, et al. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Dugger BN, Clark CM, Serrano G, Mariner M, Bedell BJ, Coleman RE, et al. Neuropathologic heterogeneity does not impair florbetapir-positron emission tomography postmortem correlates. J Neuropathol Exp Neurol. 2014;73:72–80. doi: 10.1097/NEN.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 26.Beach TG, Sue L, Scott S, Layne K, Newell A, Walker D, et al. Hippocampal sclerosis dementia with tauopathy. Brain Pathol. 2003;13:263–278. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong RA, Lantos PL, Cairns NJ. Overlap between neurodegenerative disorders. Neuropathology. 2005;25:111–124. doi: 10.1111/j.1440-1789.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 28.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm. 2002;109:329–339. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- 29.Oshima K, Dickson DW. Cortical Alzheimer type pathology does not influence tau pathology in progressive supranuclear palsy. Int J Clin Exp Pathol. 2009;2:399–406. [PMC free article] [PubMed] [Google Scholar]
- 30.Gearing M, Olson DA, Watts RL, Mirra SS. Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology. 1994;44:1015–1024. doi: 10.1212/wnl.44.6.1015. [DOI] [PubMed] [Google Scholar]
- 31.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS. Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology. 1997;48:959–969. doi: 10.1212/wnl.48.4.959. [DOI] [PubMed] [Google Scholar]
- 32.Mori H, Yoshimura M, Tomonaga M, Yamanouchi H. Progressive supranuclear palsy with Lewy bodies. Acta Neuropathol. 1986;71:344–346. doi: 10.1007/BF00688061. [DOI] [PubMed] [Google Scholar]
- 33.Judkins AR, Forman MS, Uryu K, Hinkle DA, Asbury AK, Lee VM, et al. Co-occurrence of Parkinson's disease with progressive supranuclear palsy. Acta Neuropathol. 2002;103:526–530. doi: 10.1007/s00401-001-0483-7. [DOI] [PubMed] [Google Scholar]
- 34.Umoto M, Miwa H, Ando R, Kajimoto Y, Kondo T. White matter hyperintensities in patients with multiple system atrophy. Parkinsonism Relat Disord. 2012;18:17–20. doi: 10.1016/j.parkreldis.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 2013;80:1365–1369. doi: 10.1212/WNL.0b013e31828c2f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 37.Bajaj A, Driver JA, Schernhammer ES. Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 38.Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol. 2002;61:547–556. doi: 10.1093/jnen/61.6.547. [DOI] [PubMed] [Google Scholar]
- 39.Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–1026. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- 40.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]