Abstract
An interesting paradox has emerged regarding the schizophrenia-spectrum. Put simply, college students with schizotypy (defined as the personality organization reflecting a vulnerability to schizophrenia-spectrum pathology) report experiencing pathology with respect to some key functional domains on a level that is equal to or more severe than older, outpatients with an prolonged psychiatric disorders. Notably, this self-reported pathology is not supported by objective/behavioral performance data, suggesting that the primary deficit is psychological in nature (e.g., metacognition). We evaluated whether this subjective-objective dysjunction extends to quality of life (QOL). Eighty-three college students with schizotypy were compared to 50 outpatients with severe mental illness (SMI) as well as to 82 undergraduate and 34 community control groups in subjective and objective QOL via a modified version of Lehman’s Quality of Life Interview, which covers a range of QOL domains. The schizotypy and SMI group were equally impoverished in all measures of subjective QOL compared to the college and community control groups. In contrast, the schizotypy group was relatively normal in most measures of objective quality of life compared to the SMI group. The subjective-objective dysjunction appears to extend to QOL, and these differences do not appear to reflect a more global negativistic reporting bias.
Keywords: schizophrenia, schizotypy, quality of life, subjective, objective, paradox
1. Introduction
The notion that severity of schizophrenia traits reflects a continuous phenomenon in the general population is well-regarded, and has been a tenant of empirical attempts to understand how the disorder manifests at genotypic (Docherty and Sponheim, 2008), endophenotypic (Lenzenweger et al., 2007) and phenotypic (Cohen and Davis, 2009) levels as well as how schizophrenia liability may contribute to adaptive abilities, such as creativity (Burch et al., 2006) and enhanced academic success (Nettle, 2006). Despite debate as to whether elements of schizophrenia vulnerability are categorical in nature (Meehl, 1962; van Os et al., 2000), there is general agreement that subclinical schizophrenia-like traits are present in a substantial minority of the population, often referred to as “schizotypy” – the personality organization reflecting a vulnerability for schizophrenia (Lenzenweger, 2006). Various methods of identifying individuals with schizotypy exist, though the use of self-report questionnaires is the most common method. It is important to note that many, perhaps most, of these self-report-based schizotypy studies are conducted on college student samples often at relatively selective institutions (at least, in regards to their “research intensive” status and importance among the tens of thousands of colleges and institutions that exist in the world). In this regard, these samples are probably some of the most high-functioning individuals in the schizophrenia-spectrum in terms of cognitive, motivational, financial and social resources.
An interesting paradox regarding these individuals has been revealed in the literature recently (e.g., Auster et al., 2013; Chun et al., In Press; Cohen et al., 2012a). The paradox in question concerns how individuals with self-reported schizotypy, particularly those recruited from relatively demanding universities (and are thus presumably demonstrating academic, social and cognitive functioning that is not demonstrably impaired), resemble outpatients in key self-reported variables. Studies to date suggest this resemblance occurs in three domains. First, college students with schizotypy report experiencing high levels of subjective cognitive complaints (i.e., on the order of two standard deviations) regarding attention, memory, language and other basic neurocognitive abilities relative to college controls (e.g., Chun et al., In Press). This subjective deficit is in striking contrast to their actual performance in these cognitive domains which is generally in the average range. Although there are many accounts in the literature of neurocognitive deficits being associated with college schizotypy (e.g., Gooding et al., 1999), a recent meta-analysis suggested that objective clinical neuropsychological performance is grossly normal across a range of abilities (e.g. memory, attention and language) examined in the literature in college persons with self-reported schizotypy (Chun et al., 2013). Of note, very few group differences in neurocognitive functioning examined in this study demonstrated even a small effect size. In this regard, there appears to be a pronounced dysjunction between subjective and objective domains of cognitive functioning. Second, college schizotypal subjects have reported experiencing in-the-moment anhedonia (i.e., reduced experience of pleasant emotion) during a laboratory emotion-induction task at a level that was more severe than patients with schizophrenia and mood disorders (Cohen et al., 2012a). Conversely, college students with schizotypy have not shown psychophysiological deficits (Gooding et al., 2002) or implicit affective responses (Cohen & Hong, 2011) to emotional stimuli relative to college control groups, and there are accounts of increased psychophysiological activity in some studies (e.g., Karcher & Shean, 2012; but note psychophysiological abnormalities more generally, Ragsdale, Mitchell, Cassisi & Bedwell, 2013; O’Driscoll, Lenzenweger & Holzman, 1998). Thus, there appears to be discrepancy between subjective and objective emotion functioning in college schizotypy. Third, a dysjunction has been reported in olfactory functions (Auster et al., 2013). In this study, college individuals with schizotypy reported lower levels of subjective pleasure when rating a range of olfaction stimuli compared to both patients with schizophrenia and college controls. However, when asked to identify/recognize the olfactory stimuli, college schizotypal individuals performed significantly better than patients with schizophrenia and comparable to nonpsychiatric controls. In sum, across cognitive, emotional and olfactory domains, it appears that college schizotypy is associated with subjective deficits equal to or worse than those seen in outpatients with schizophrenia. However, objective performance deficits do not approximate these subjective deficits. This is paradoxical in the sense that college schizotypal individuals, at least as a group, are presumably more healthy and functional in every conceivable dimension compared to outpatients with SMI.
While it is unclear, at present, what this dysjunction between subjective and objective report in schizotypy means, it raises questions about whether some higher-order cognitive ability (e.g., metacognition, insight, attributions, autobiographical memory) is disturbed in some manner. Understanding this issue could hold important implications for our understanding of the schizophrenia spectrum. For example, deficits in higher-order cognitive abilities or in the systems that regulate them may reflect an important marker for understanding schizophrenia processes and for identifying vulnerable individuals. Clarifying the dysjunction between objective and subjective report in schizotypy may also shed light on how schizophrenia-risk manifests in the general population, and may help explain some of the adaptive features of schizotypy that have been found in the literature, such as enhanced creativity (Miller and Tal, 2007), academic success (Nettle, 2006) and even improved mating abilities (Nettle and Clegg, 2006). Regardless, it is important to further investigate the scope of this dysjunction. The present study sought to determine whether these seemingly paradoxical findings extend to quality of life. We assessed subjective and objective quality of life using a validated instrument in schizotypal college students outpatients with severe mental illness (SMI) and college and community control groups. Both subjective and objective QOL were assessed using self-report, which allows us to examine whether schizotypy is associated with a more general bias towards pathological responding (in which case, they should show SMI-like scores on both scales), or whether their pathological responding, if present, is limited to subjective domains. We predicted that the schizotypy group would be significantly impoverished in subjective but not objective QOL compared to the college and community control groups (at medium-large and negligible-small effect size levels respectively), but show the opposite pattern compared to the SMI group (at negligible-small and medium-large effect size levels respectively).
2. Method
2.1 Subjects
Subjects were recruited from a large public university, a community mental health outpatient clinic, and from the community at large. This study was approved by the responsible Institutional Review Board and consent was obtained for all subjects.
2.1.1 Patient group
The patient groups included 50 adults with Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV; American Psychiatric Association (APA), 1994) diagnosed with schizophrenia (n=28) or unipolar or bipolar (n=22) affective disorders. Note that there was substantial blurring between these diagnostic categories in that a significant portion of individuals diagnosed with schizophrenia also had a history of depression and mania (19% and 22%, respectively), and that a significant portion of patients diagnosed with affective disorders had a history of psychosis (33%). Hence, patients were collapsed into an SMI group. All patients were clinically stable at the time of testing (i.e., Global Assessment of Functioning rating [GAF; APA, 1994] above 30) and were receiving pharmacotherapy under the supervision of a multi-disciplinary team. All patients were prescribed psychotropic medications at the time of testing, and there was considerable variability in type, dosage, and medium (e.g., depot versus oral) across patients. Diagnoses were made based on information obtained from the patients’ medical records and from a structured clinical interview (SCID; First, 1996). Exclusion criteria included: a) documented evidence of mental retardation from the medical records, b) current or historical DSM-IV diagnosis of alcohol or drug abuse suggestive of severe physiological symptoms (e.g., delirium tremens, repeated loss of consciousness), and c) history of significant head trauma (requiring overnight hospitalization). There were no statistically significant differences in any dependent measures between the diagnostic groups (all p’s>.10).
2.1.2 Schizotypy and college control groups
Subjects from the schizotypal and nonpatient control groups were undergraduate freshmen and sophomores (N=10,258) who were approached by email to participate in an online survey. Embedded within this survey were a consent form, basic demographic questions, the Schizotypal Personality Questionnaire – Brief Revised (Cohen et al., 2010; Raine and Benishay, 1995), the Brief Symptom Inventory (Derogatis and Melisaratos, 1983) and infrequency items (Chapman and Chapman, 1983). The final screening sample included 2,300 complete responses. Ten subjects were excluded for invalid profiles, defined as an infrequency score >3. Based on evidence that schizotypy is a construct with a population incidence approaching ten percent (Lenzenweger, 2006), we adopted a conservative strategy where the top five percent of scorers (computed from the ethnicity and gender determined means) on the positive/disorganized (n=51), and/or negative (n=32) subscales were invited to participate in the laboratory phase of the study (see Cohen and Hong, 2011; Cohen et al., 2012b) for elaboration on this methodology). Positive (i.e., ideas or reference, suspiciousness, magical thinking and unusual perceptions), disorganization (i.e., odd speech, eccentric behavior) and negative (i.e., constricted affect, no close friends) subscales were employed. Individuals scoring high on the negative scale were only considered eligible if they a) also showed elevation on the positive or disorganization scales, or b) had a depression scale score from the Brief Symptom Inventory below their gender and ethnicity determined mean. This was done to address concerns that depressive symptoms can give “false positives” on negative schizotypy scales. Control subjects were identified and included based on scores below the ethnicity and gender-determined means for each of the positive, negative, and disorganization SPQ factors (n=485), of which 82 were selected and agreed to participate. Table 1 contains descriptive statistics for the groups.
Table 1.
Descriptive statistics for the demographic and clinical variables across the schizotypy, severe mental illness (SMI), and control (Con) groups.
| Schizotypy (n = 82) | SMI (n = 50) | College Con (n = 82) | Community Con (n = 34) | |
|---|---|---|---|---|
| % Women | 66.70% | 37.00% | 67.60% | 50.00% |
| % Caucasian | 62.70% | 61.60% | 63.50% | 50.00% |
| Psychiatric History | ||||
| % Major Depressive Episode | - | 44.00% | - | 0.00% |
| % Manic Episodes | - | 30.00% | - | 0.00% |
| % Psychosis | - | 68.00% | 0.00% | |
| Age (mean ± SD) | 18.33 ± 3.31 | 42.19 ± 11.24 | 19.49 ± 5.25 | 40.32 ± 12.48 |
| BPRS Factor Scores (mean ± SD) | ||||
| Mania/Excitement | - | 9.97 ± 4.70 | - | - |
| Negative | - | 6.89 ± 3.62 | - | - |
| Positive | - | 10.48 ± 5.00 | - | - |
| Affective | - | 8.28 ± 4.32 | - | - |
| SPQ-BR Scores (mean ± SD) | ||||
| Positive | 28.43 ± 9.65 | - | 6.31 ± 5.19 | - |
| Negative | 12.96 ± 5.90 | - | 2.24 ± 2.18 | - |
| Disorganization | 24.21 ± 5.26 | - | 5.91 ± 4.62 | - |
2.1.3 Community control group
Individuals were recruited from the community for the community control group (n=34). Exclusion criteria were similar to that of the patient group, and also included history of schizophrenia or affective disorder based on information obtained from a SCID interview. Subjects were compensated $40 for their participation.
2.2 Measures
2.2.1 Diagnostic and symptom ratings
The Brief Psychiatric Rating Scale (Lukoff et al., 1986) was used to measure patients’ symptoms. Factor subscale scores reflecting positive (i.e., bizarre behavior, suspiciousness, unusual thought content, disorientation, and hallucinations items), negative (i.e., self-neglect, blunted affect, motor retardation, and emotional withdrawal items), depressive (i.e., depression, guilt, suicidality, and anxiety items), and mania/excitement (i.e., motor hyperactivity, elevated mood, excitement, distractibility, hostility, and grandiosity items) symptoms (Ventura et al., 2000) are reported. Preliminary diagnoses and symptom ratings were made by one of four doctoral level students who were trained to criterion (intra-class correlation coefficient values >.70). Diagnoses and ratings were based on information obtained from medical records, the patients’ treatment teams, and self-report and behavioral observations made during the research interviews. All diagnoses and ratings were videotaped and reviewed during a monthly case conference meeting that was led by a licensed clinical psychologist with considerable diagnostic experience (Alex S. Cohen). Final ratings and diagnoses were recorded when full agreement by the case conference members was made.
2.2.2 Quality of life (QOL)
QOL was measured using a modified self-report version of Lehman’s brief Quality of Life Interview (Lehman, 1995). This version of the QoLI has been used across a number of published studies with a range of clinical and nonclinical/undergraduate samples (Chun et al., In Press; Cohen and Davis, 2009; Heider et al., 2007; Renshaw and Cohen, In Press; Wasserman et al., 2006). Using this measure, QOL is assessed across objective – tapping tangible or behavioral-based phenomenon (e.g., “How often do you talk to a member of your family on the telephone”, “In the past month, did you have enough money for transportation?”) – and subjective – tapping subjective satisfaction (e.g., “Select the item that best describes how you feel about the way things are in general between you and your family”) - domains. Seven areas of quality of life are covered with this instrument. For each area, one item covers subjective quality of life (range for each item from 1 [i.e., “Terrible”] to 7 [i.e., “Delighted”]), and a variable number of items covers objective quality of life – with increasing scores reflecting greater objective QOL. The seven areas include: housing concerns (QOLobjective k [number of items] = 1; scores dichotomized due to limited range), daily activities (QOLobjective k = 5; α = .58; note – we excluded item 8 to improve reliability), family relationships (QOLobjective k = 2; α = .69), social relationships (QOLobjective k = 4; α = .77), financial resources (QOLobjective k = 5; α = .58), legal and safety concerns (QOLobjective k = 3; scores dichotomized due to limited range) and health concerns/resources (QOLobjective k = 1). Composite scores, based on z-scores of each of the individual subscales, were computed separately for the objective (k = 7; α = .66) and subjective (k = 7; α = .78) subscales.
2.3 Analyses
The analyses were conducted in three steps. First, we computed and compared descriptive and clinical variables between the schizotypy, SMI and control groups to determine whether any of these variables needed to be considered in subsequent analyses. Second, we employed two separate group (i.e., schizotypy, SMI, college control, community control) by domain (i.e., housing, daily activities, family interactions, social interactions, financial resources, legal and safety concerns and health concerns/resources) MANOVAs such that subjective and objective QOL domains were analyzed separately as the dependent variables. Finally, we computed total subjective and objective QOL scores by standardizing the individual domain scores and summing them. These scores were then compared across the four groups. The objective housing concerns and legal/safety concern variables showed non-normal distributions and were dichotomized. These variables were analyzed using chi-square analysis. Otherwise, the distributions for all variables were normal, and all analyses employed two-tailed tests.
3. Results
3.1 Descriptive and clinical variables
Sex was statistically different between groups, χ23 =18.71, p < .001, such that women had greater representation in the schizotypy and college control groups than in the outpatient and community control groups. Age was also statistically different between groups, F3,250=158.62, p<.001. Scheffé post-hoc analysis revealed that the college groups were significantly younger than the patient and community control groups (all p’s<.001). The groups did not significantly differ in ethnicity, χ26 =8.40, p=.21. All analyses in this study were recomputed controlling for age and sex without notable change in findings. Within the SMI group, the schizophrenia versus non-schizophrenia subjects did not significantly differ in QOL variables (all p’s>.10).
3.2 Quality of life
Means and standard deviations for the four groups are in Table 2. Omnibus MANOVA revealed that there were significant group differences for both objective, F3,21=13.60, p<.001, and subjective, F3,21=5.09, p<.001, QOL variables. Between-subjects effects were significant for each variable (all p<.05), with the exception of the objective legal measure, χ23 =3.81, p=.28. Post-hoc analyses, using Scheffé tests, revealed the following: a) For the majority of the objective QOL measures (i.e., housing concerns, daily activities, family activities, social activities, financial concerns), the SMI group was significantly lower than the other groups (all p’s<.01), b) Health concerns was the only objective measure where the schizotypy group was abnormal in any regard; they were similar to the SMI group (p=.99) and lower than the college (p<.001) and community (p=.01) control groups, c) for each of the subjective QOL measures, the schizotypy group was significantly lower than the college control group at a trend or better level (all p’s<.06), and they were lower than the community control group for three of the measures (i.e., housing concerns, daily activities and social activities, all p’s<.05), and d) the SMI and schizotypy groups were significantly different for only one of the seven objective QOL measures – schizotypy subjects reported greater satisfaction with their finances than SMI subjects (p=.001), though, as noted above, they were less satisfied with their finances compared to college controls (p=.02). In sum, schizotypy reported better objective quality of life than patients with SMI, but they were largely similar in terms of subjective quality of life.
Table 2.
Mean ± standard deviation for each quality of life subscale (objective and subjective) compared across the schizotypy, severe mental illness and control groups
| Schizotypy | Severe Mental Illness | College Controls | Community Controls | |
|---|---|---|---|---|
| OBJECTIVE | ||||
| Housing Concerns | −1.02 ± 0.15 | −2.06 ± 0.85 | −1.00 ± 0.00 | −1.06 ± 0.24 |
| Daily Activities | 8.99 ± 2.57 | .34 ± 2.34 | 8.77 ± 2.64 | 7.56 ± 4.89 |
| Family Relationships | −4.36 ± 1.56 | −6.00 ± 2.48 | −4.13 ± 1.38 | −4.29 ± 1.95 |
| Social Relationships | −9.37 ± 3.95 | −13.75 ± 4.90 | −7.68 ± 2.19 | −10.00 ± 3.85 |
| Financial Resources | −2.67 ± 1.68 | −4.52 ± 2.31 | −2.18 ± 1.25 | −2.26 ± 1.48 |
| Legal & Safety Concerns | .88 ± .33 | .84 ± .37 | .85 ± .36 | .97 ± .17 |
| Health Concerns | −2.82 ± 0.99 | −2.88 ± 1.32 | −1.88 ± 0.79 | −2.15 ± 0.93 |
| SUBJECTIVE | ||||
| Housing Concerns | 4.53 ± 1.46 | 4.94 ± 1.63 | 5.48 ± 1.15 | 5.47 ± 1.19 |
| Daily Activities | 4.41 ± 1.21 | 4.73 ± 1.46 | 5.40 ± 0.94 | 5.16 ± 0.83 |
| Family Relationships | 4.82 ± 1.46 | 4.50 ± 1.66 | 5.96 ± 1.23 | 5.53 ± 1.26 |
| Social Relationships | 4.58 ± 1.78 | 4.60 ± 1.79 | 5.85 ± 1.13 | 5.59 ± 1.08 |
| Financial Resources | 4.42 ± 1.54 | 3.33 ± 1.82 | 5.20 ± 1.39 | 4.35 ± 1.28 |
| Legal & Safety Concerns | 4.52 ± 1.34 | 4.52 ± 1.92 | 5.13 ± 1.29 | 5.18 ± 1.13 |
| Health Concerns | 4.75 ± 1.24 | 4.65 ± 1.66 | 5.68 ± 1.05 | 5.38 ± 1.13 |
Increasing scores reflect greater quality of life for all objective and subjective measures.
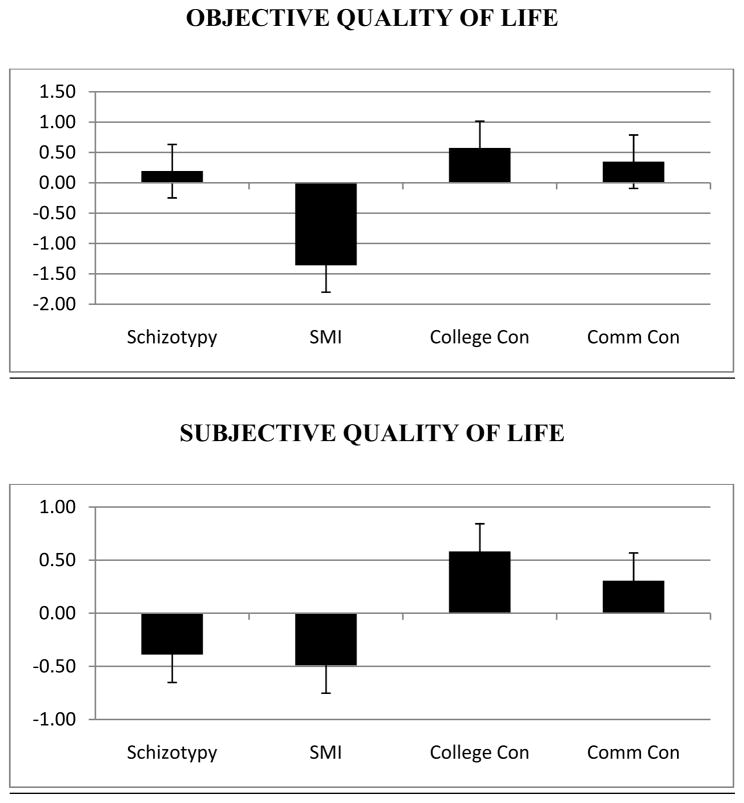
Total subjective and objective QOL scores, in z-score format, were computed and are presented in Figure 1. Both objective and subjective variables were significantly different across the groups, F3,229=88.44, p<.001 and F3,229=21.57, p<.001, respectively. For objective QOL, Scheffé post-hoc tests suggested that the SMI group was significantly lower than the other groups (all p’s<.001). The schizotypy group was also lower in objective QOL than the college control group (p=.01), but was similar to the community control group (p=.78). With respect to subjective QOL, the schizotypy and SMI groups were similar (p=.94), and both groups were significantly lower than the control groups (all p’s<.001) on these measures.
Fig. 1.
Summary objective and subjective quality of life scores (in z-score format) compared across the schizotypy, severe mental illness (SMI) and college (Coll) and community (Comm) control groups.
Importantly, Figure 1 allows for estimation of effect sizes – a potential issue given the variability in sample sizes across groups. In terms of objective quality of life, patients with severe mental illness were poorer than each of the other groups at a large effect size level (Cohen’s d’s>1.98), whereas the differences between the other groups was not (Cohen’s d’s<0.65). Conversely, the difference in subjective quality of life between the schizotypy and patient group was negligible (d=0.10), yet both groups were impoverished compared to the control groups at a large effect size level (all d>0.87).
4. Discussion
The present study tested the hypothesis that individuals with self-reported schizotypy would show subjective quality of life deficits similar in severity to outpatients with SMI, but that objective quality of life would be relatively normal (i.e., similar to college and community control groups). Our results generally supported this hypothesis. The schizotypy group was statistically similar to the SMI group in subjective satisfaction for every QOL domain assessed in this study (despite being low in satisfaction compared to both control groups), yet showed better objective quality of life compared to the SMI group in five of seven domains. These results did not appear to reflect group differences in demographic variables, and examination of the effect sizes suggested that the null results were not a function of limited power (i.e., they were generally in the negligible range). Moreover, it is difficult to argue that the present results reflect a general self-report response bias towards pathology because the schizotypy group was, for the most part, normal in terms of objective QOL, which was also based on self-report.
Why is it that individuals with schizotypy are inherently dissatisfied with their QOL despite being relatively normal in objective QOL compared to their college peers? The answer to this question is, at present, unknown, and is complicated by the lack of a comprehensive model detailing the psychological processes involved in self-reported subjective satisfaction. Personality researchers have proposed that subjective well-being is reflective of trait factors and is relatively stable despite varying life circumstances, health, income and other factors (e.g., Diener and Fujita, 1995). The notion that satisfaction reflects a stable “set point” that is, in large part, heritable (Lykken and Tellegen, 1996) is interesting in that schizotypal features are largely stable (Chapman et al., 1994) and, at least to some degree, heritable (Docherty and Sponheim, 2008; MacDonald et al., 2001). In this regard, subjective dissatisfaction, whether it be in QOL, cognitive concerns (Chun et al., In Press) or in response to laboratory stimuli (Auster et al., 2013; Cohen et al., 2012a), may reflect stable trait features, and perhaps more importantly, a marker of illness vulnerability.
There are also a host of cognitive frameworks that might be useful for understanding the abnormal subjective reports from persons with schizotypy, particularly if their subjective reports are viewed as stemming from beliefs that are generally inconsistent with objective data. From a cognitive therapy perspective, dysfunctional beliefs of individuals with schizotypy are thought to reflect negativistic core beliefs. Beck et al. (2006) note a common core belief of individuals with schizotypal personality disorder as being “I am different, worthless and abnormal”. Biased by this belief, it is plausible that schizotypal individuals report dissatisfaction with their lives despite having similar levels of objective quality of life as their peers. Relatedly, an information-processing perspective may be useful for understanding the subjective deficits in schizotypy. It may be that individuals with schizotypy place abnormally high weight on irrelevant stimuli when deriving their beliefs. For example, the belief that “my social life is unsatisfying” may stem from overemphasizing some information (e.g., “I am not married”) and deemphasizing other information (e.g., “Not many people my age are married”, “I know lots of people”). Although reports of normal neurocognition in schizotypy are quite common in the literature (Chun et al., In Press), abnormalities in information processing, such as latent inhibition (Nelson and Rawlings, 2010) or facial processing biases (Brown and Cohen, 2010), are commonly reported. Similarly, meta-cognition, a construct important in understanding self-disturbances (Mishara, Lysaker & Schwartz, 2014) and subjective recovery/QOL (Kukla, Lysaker & Salyers, 2013) in schizophrenia, has been demonstrated in some studies to be abnormal in schizotypy (e.g., Stirling, Barkus & Lewis, 2007). Clearly, more work is needed to develop a clear mechanistic understanding of subjective quality of life, both in healthy adults and in individuals with schizotypy.
The following limitations warrant mention. First, the QOL measures used in this study, while used in many prior studies, may not have been particularly sensitive and some subscales, notably measuring objective housing and legal/safety concerns, may have suffered from limited range. Despite these psychometric concerns, results still supported our hypotheses. Second, the present study did not take into account medication effects. While all individuals in the SMI group were psychiatrically stable and were being medicated, we were unable to examine how individual medication type and dosage, adherence, and interfering factors (e.g., smoking, illicit substance use) may have affected QOL. Third, the groups were not matched in age or sex. Finally, our use of a self-reported schizotypy sample recruited from one specific college population may not be representative of all college individuals with schizotypy, nor all individuals with schizotypy more generally.
In sum, the present findings provided further evidence of a “paradox” between subjective and objective functions in college schizotypal subjects. We recommend further research on this topic extend into three areas: First, it will be important to document additional domains beyond cognition, emotion, olfaction and quality of life that may, or may not, suffer from this dysjunction. Clarifying this issue will help define the boundaries that characterize schizotypal individuals’ beliefs. Second, it will be important to follow-up on the finding that objectively-defined health was similar between the schizotypy and SMI groups. It is unclear why this is, and more discriminating and sophisticated measures of health will probably be needed to shed light on this issue. Finally, it will be important to identify the mechanism underlying the inaccurate beliefs that individuals with schizotypy show and what psychological systems are implicated in maintaining these beliefs. Considering how these mechanisms may contribute to both dysfunction as well as adaptive elements of schizotypy (e.g., creativity) will be important in future research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Association, A.P. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- Auster T, Brown LA, Cohen AS. Objective and Subjective Olfactory Ratings across the Schizophrenia-Spectrum. 2013. Under Review. [Google Scholar]
- Beck AT, Freeman A, Davis DD. Cognitive Therapy of Personality Disorders. Guilford Press; 2006. [PubMed] [Google Scholar]
- Brown LA, Cohen AS. Facial emotion recognition in schizotypy: the role of accuracy and social cognitive bias. J Int Neuropsychol Soc. 2010;16(3):474–483. doi: 10.1017/S135561771000007X. [DOI] [PubMed] [Google Scholar]
- Burch GS, Pavelis C, Hemsley DR, Corr PJ. Schizotypy and creativity in visual artists. Br J Psychol. 2006;97(Pt 2):177–190. doi: 10.1348/000712605X60030. [DOI] [PubMed] [Google Scholar]
- Chun CA, Minor KS, Cohen AS. Neurocognition in psychometrically-defined schizotypy: We are NOT measuring the “right stuff”. Journal of International Neuropsychological Society. doi: 10.1017/S135561771200152X. In Press. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Unpublished test. Madison, WI: 1983. Infrequency Scale. [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Callaway DA, Najolia GM, Larsen JT, Strauss GP. On “risk” and reward: Investigating state anhedonia in psychometrically defined schizotypy and schizophrenia. J Abnorm Psychol. 2012a;121(2):407–415. doi: 10.1037/a0026155. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Davis TE., 3rd Quality of life across the schizotypy spectrum: findings from a large nonclinical adult sample. Compr Psychiatry. 2009;50(5):408–414. doi: 10.1016/j.comppsych.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Hong SL. Understanding constricted affect in schizotypy through computerized prosodic analysis. Journal of Personality Disorders. 2011;25(4):478–491. doi: 10.1521/pedi.2011.25.4.478. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Matthews RA, Najolia GM, Brown LA. Toward a more psychometrically sound brief measure of schizotypal traits: introducing the SPQ-Brief Revised. Journal of Personality Disorders. 2010;24(4):516–537. doi: 10.1521/pedi.2010.24.4.516. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, Brown LA, Minor KS. Towards a cognitive resource limitations model of diminished expression in schizotypy. J Abnorm Psychol. 2012b;121(1):109–118. doi: 10.1037/a0023599. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychological Medicine. 1983;13(3):595–605. [PubMed] [Google Scholar]
- Diener E, Fujita F. Resources, personal strivings, and subjective well-being: a nomothetic and idiographic approach. J Pers Soc Psychol. 1995;68(5):926–935. doi: 10.1037//0022-3514.68.5.926. [DOI] [PubMed] [Google Scholar]
- Docherty AR, Sponheim SR. Anhedonia as a phenotype for the Val158Met COMT polymorphism in relatives of patients with schizophrenia. Journal of Abnormal Psychology. 2008;117(4):788–798. doi: 10.1037/a0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MBG, Miriam, Spitzer Robert L, Williams Janet B. User’s guide for the Structured Clinical Interview for DSM-IV Axis I disorders - Research Version (SCID-I version 2.0, February 1996 Final Version. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Gooding DC, Davidson RJ, Putnam KM, Tallent KA. Normative emotion-modulated startle response in individuals at risk for schizophrenia-spectrum disorders. Schizophr Res. 2002;57(1):109–120. doi: 10.1016/s0920-9964(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Kwapil TR, Tallent KA. Wisconsin Card Sorting Test deficits in schizotypic individuals. Schizophr Res. 1999;40(3):201–209. doi: 10.1016/s0920-9964(99)00124-3. [DOI] [PubMed] [Google Scholar]
- Heider D, Angermeyer MC, Winkler I, Schomerus G, Bebbington PE, Brugha T, Azorin JM, Toumi M. A prospective study of quality of life in schizophrenia in three European countries. Schizophrenia Research. 2007;93(1):194–202. doi: 10.1016/j.schres.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kukla M, Lysaker PH, Salyers M. Do persons with schizophrenia who have better metacognitive capacity also have a stronger subjective experience of recovery? Psychiatry Research. 2013;209(3):381–385. doi: 10.1016/j.psychres.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Lehman A. Evaluating Quality of Life for Persons with Severe Mental Illness: Assessment Toolkit. Cambridge, Mass: The Evaluation Center at Health Services Research Institute; 1995. [Google Scholar]
- Lenzenweger MF. Schizotaxia, schizotypy, and schizophrenia: Paul E. Meehl’s blueprint for the experimental psychopathology and genetics of schizophrenia. J Abnorm Psychol. 2006;115(2):195–200. doi: 10.1037/0021-843X.115.2.195. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, McLachlan G, Rubin DB. Resolving the latent structure of schizophrenia endophenotypes using expectation-maximization-based finite mixture modeling. J Abnorm Psychol. 2007;116(1):16–29. doi: 10.1037/0021-843X.116.1.16. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale (BPRS) Schizophrenia Bulletin. 1986;12:594–602. [Google Scholar]
- Lykken D, Tellegen A. Happiness is a stochastic phenomenon. Psychological Science. 1996;7(3):186–189. [Google Scholar]
- MacDonald AW, 3rd, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: a community-based twin study. Schizophr Bull. 2001;27(1):47–58. doi: 10.1093/oxfordjournals.schbul.a006859. [DOI] [PubMed] [Google Scholar]
- Meehl P. Schizotaxia, schizotypy, schizophrenia. American Psychology. 1962;17:827–838. [Google Scholar]
- Miller GF, Tal IR. Schizotypy versus openness and intelligence as predictors of creativity. Schizophr Res. 2007;93(1–3):317–324. doi: 10.1016/j.schres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Mishara AL, Lysaker PH, Schwartz Self-disturbances in Schizophrenia: History, Phenomenology, and Relevant Findings From Research on Metacognition. Schizophrenia Bulletin. 2014;40(1):5–12. doi: 10.1093/schbul/sbt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Rawlings D. Relating schizotypy and personality to the phenomenology of creativity. Schizophr Bull. 2010;36(2):388–399. doi: 10.1093/schbul/sbn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D. Schizotypy and mental health amongst poets, visual artists, and mathematicians. Journal of Research in Personality. 2006;40(6):876–890. [Google Scholar]
- Nettle D, Clegg H. Schizotypy, creativity and mating success in humans. Proc Biol Sci. 2006;273(1586):611–615. doi: 10.1098/rspb.2005.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll GA, Lenzenweger MF, Holzman PS. Antisaccades and smooth pursuit eye tracking and schizotypy. Archives of General Psychiatry. 1998;55:837–43. doi: 10.1001/archpsyc.55.9.837. [DOI] [PubMed] [Google Scholar]
- Ragsdale KA, Mitchell J, Cassisi J, Bedwell JS. Comorbidity of schizotypy and psychopathy: Skin conductance to affective pictures. Psychiatry Research. 2013;210:1000–1007. doi: 10.1016/j.psychres.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Raine A, Benishay D. The SPQ-B: A brief screening instrument for schizotypal personality disorder. Journal of Personality Disorders. 1995;9(4):346–355. [Google Scholar]
- Renshaw TL, Cohen AS. Life satisfaction as a distinguishing indicator of college student functioning: Further validation of the two-continua model of mental health. Social Indicators Research; In Press. [Google Scholar]
- Stirling J, Barkus E, Lewis S. Hallucination proneness, schizotypy and meta-cognition. Behavior Research & Therapy. 2007;45(6):1401–1408. doi: 10.1016/j.brat.2006.06.003. [DOI] [PubMed] [Google Scholar]
- van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res. 2000;45(1–2):11–20. doi: 10.1016/s0920-9964(99)00224-8. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: A principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Research. 2000;97(2–3):129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Wasserman DA, Sorensen JL, Delucchi KL, Masson CL, Hall SM. Psychometric evaluation of the Quality of Life Interview, Brief Version, in injection drug users. Psychology of Addictive Behaviors. 2006;20(3):316–321. doi: 10.1037/0893-164X.20.3.316. [DOI] [PubMed] [Google Scholar]