Abstract
Objectives
Generalized Anxiety Disorder is one of the most prevalent anxiety disorders, but its neural basis is relatively understudied. This study aims to characterize the functional connectivity in the Default Mode Network (DMN) in Generalized Anxiety Disorder (GAD) across the lifespan.
Design and settings
Functional and structural MRI data were collected with subjects at rest. We analyzed the resting state functional connectivity patterns in the DMN for twenty-seven GAD participants and thirty-nine non-anxious comparison participants. Using a two-way ANOVA, we explored the interaction between age and GAD status on functional connectivity. In GAD participants we analyzed the correlation of functional connectivity indices with the duration of illness and worry severity.
Results
The age-by-anxiety interaction showed a greater anxiety effect on the functional connectivity between the posterior cingulate seed and the medial prefrontal cortex for the older group relative to the younger participants. Longer duration of illness was positively correlated with greater functional connectivity between the posterior cingulate cortex and the insula. Worry severity was inversely correlated with the functional connectivity between the PCC seed and the medial prefrontal cortex.
Conclusion
The presence of GAD, longer duration of illness and more severe worry exacerbate the effects of age on the functional connectivity in the Default Mode Network. These results support the need for tailored research and interventions in late-life anxiety.
Keywords: Generalized Anxiety Disorder, Functional Connectivity, Resting State, Age effect, Duration of illness, Worry Severity
INTRODUCTION
While anxiety is a universal human experience, over the last decades evidence has accumulated regarding the negative impact of pathological anxiety on mind, body, and quality of life (1). It is not very clear though how the cumulative burden of pathological anxiety affects the brain over the lifespan. In this study we analyze the age-related differences in the neural basis of pathological anxiety and we explore the impact of the duration of illness on the connectivity in the Default Mode Network (DMN).
Different types of anxiety disorders may differ substantially in their neuroanatomical bases (2). Disorders such as simple/social phobia, post-traumatic stress disorder and panic disorder have received a rather extensive neuroanatomical description (3). Generalized anxiety disorder (GAD), defined by excessive and uncontrollable worry, has a less well-defined neural basis. In teenagers and young adults, GAD has been associated with altered anterior cingulate cortex (ACC) activity, including failure to engage the ACC in emotion regulation (4), increased activation in the medial prefrontal (mPFC) and ACC during mood-induction (5), and increased rostral ACC activation associated with better treatment response (6). Limbic changes have also been associated with the neural circuitry of GAD: exaggerated amygdala reactivity has been described following warning cues preceding aversive/neutral pictures (7), and GAD has been associated with disrupted connectivity patterns of the amygdala subregions (8).
The neural network abnormalities described in younger adults might not be entirely translatable into the elderly, given the anatomical and pathophysiological changes observed in the aging brain (9). The few studies that have addressed the neural basis of late-life GAD have described structural changes in the orbital frontal cortex (OFC) (10) and task-related functional changes in the rostral anterior cingulate (ACC) (11).
Of note, while some anxiety disorders, e.g. panic disorder, diminish in severity with aging, GAD persists and new cases develop with aging (12). Thus, GAD is the most prevalent anxiety disorder in the elderly (13). Compared with mid-life GAD, late-life GAD is characterized by a more mediocre treatment response to both pharmacotherapy (14) and cognitive-behavioral therapy (CBT) (15, 16). This difference has been attributed to age-induced neurobiological changes (17), but so far no study has explored head-to-head the neuroanatomical differences in old versus young GAD.
The DMN is an organized functional network of several brain regions: posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), inferior parietal lobule (IPL) and medial temporal regions (MTL) (18). This network shows a high level of functional connectivity at rest and its activity consistently decreases during performance of active tasks such as goal directed cognition and task engagement (18). The DMN is involved in the integration of autobiographical memories and in self-monitoring, in the retrieval and manipulation of past events in an effort to solve problems and develop future plans, and in emotion regulation (19). The connectivity between the PCC and the vmPFC has been associated with self-reflection during resting state (20).
To date, the few studies that have explored the DMN functional connectivity in midlife anxiety (21, 22) or in late-life anxious depression (23) have consistently reported decreased functional connectivity between the PCC and the medial prefrontal cortex. To our knowledge, none of these studies focused on Generalized Anxiety Disorder. Most young adult GAD studies have focused on task-related functional connectivity. The few studies exploring the resting state connectivity reported disruptions in amygdala-based resting state functional connectivity (24) and in the hippocampus-based functional connectivity (25).
While several other functional networks have been recently described (26) such as the salience network and the executive control network, we chose to focus in this study on the DMN given 1) its reliable description in the literature and 2) the lack of data regarding the behavior of DMN in Generalized Anxiety. Thus, in order to address this gap in the literature, we analyzed the PCC functional connectivity maps in the DMN in midlife and late-life GAD. Furthermore, we compared these connectivity maps with the ones obtained in non-anxious participants. We hypothesized that 1) the PCC functional connectivity in the DMN will differ in young vs. old anxious participants; 2) compared with non-anxious participants, anxious participants will have decreased functional connectivity between the PCC and the medial prefrontal cortex.
METHOD
Participants
The data were collected from two studies conducted at the University of Pittsburgh: “Structural and functional neuroanatomy of late-life GAD” (age 60 and over) and “A pilot fMRI study of emotion modulation in midlife anxiety” (age 25 – 49). Subjects were recruited from direct advertisement through fliers, local radio and bus ads, as well as from two research registries affiliated with the University of Pittsburgh: The Advanced Center for Intervention and Services Research in Late-Life Mood Disorders (ACISR) registry and the Clinical and Translational Science Institute (CTSI) registry.
Anxious participants had a principal diagnosis of GAD for at least six months according to the Structured Clinical Interview for DSM-IV (SCID)(27) and a score of 17 or higher on the Hamilton Anxiety Rating Scale (HARS)(28) at the time of scanning. Patients with other anxiety disorders were included if GAD was the principal diagnosis (based on severity and duration, e.g. longer duration of GAD and greater subjective distress). Patients with a past history of alcohol or substance abuse that was in full remission for at least 3 months were also included. Lifetime comorbid unipolar depression was allowed if GAD was the primary diagnosis (based on duration), but subjects with current major depressive disorder at the time of scanning were excluded. Four GAD subjects had panic disorder, one subject had social phobia and five GAD subjects had a history of unipolar depression (in remission).
Other exclusion criteria were lifetime psychosis or bipolar disorder, a diagnosis of dementia, a Mini Mental State Examination score less than 24, suicide risk, ongoing psychotherapy, and current antidepressant or anxiolytic use. All subjects were psychotropic-free at the time of scanning and they underwent a wash out period of two weeks if previously on an antidepressant (six weeks if on fluoxetine).
Participants were allowed to receive non-psychotropic medications. Comparison participants had no history of psychiatric disorders except for history of adjustment disorder. Both studies were approved by the University of Pittsburgh Institutional Review Board.
Additional clinical measures
Besides the HARS and MMSE, participants were further assessed using the self-report Penn State Worry Questionnaire (29) and the GAD Severity Scale (GADSS) (30). The total duration of illness was determined based on participants’ self report.
Data Acquisition
Data acquisition was identical in both studies. Resting state MRI data were acquired during a five minute interval while participants looked at a fixation point on the screen. Participants were instructed to think of nothing in particular during this interval. Resting state data were collected prior to other task-related fMRI data.
Imaging data were collected with a 3Tesla Siemens Trio TIM scanner located in the MR Center at the University of Pittsburgh. T2*-weighted BOLD images were acquired using a gradient-echo echoplanar imaging (EPI) sequence in axial orientation (parallel to AC-PC): TR/TE = 2000/34 ms, Matrix size = 128×128×28, Voxel size = 2×2×3 mm (no gap), flip angle = 900. A total of 150 temporal images were acquired for each subject
The most inferior slice was located below the most inferior aspect of the temporal lobes. High-resolution anatomical images (T1 weighted magnetization-prepared rapid gradient echo MPRAGE) were collected over 4 min 43sec using the following parameters: FOV = 256×254 mm, voxel size 1×1×1 mm; TI = 900 ms, TR/TE = 2/3.43 ms, flip angle = 90.
Image Preprocessing
Functional imaging data were processed and analyzed with Statistical Parametric Mapping 8 (SPM8; Welcome Trust Center for Neuroimaging, London, UK. http://www.fil.ion.ucl.ac.uk/spm/software/spm8) implemented in Matlab (Mathworks, Natick, MA). The temporal functional images for each subject were realigned to the first image using rigid-body transformation. The structural grey matter images were segmented from the MPRAGE image and used to normalize the functional images to the standard Montreal Neurologic Institute (MNI) space. This was done by first registering the grey matter image to the mean realigned functional images using a linear affine transformation; the grey matter image was then normalized to the SPM MNI grey matter template using a non-linear affine transformation. Finally, the same transformation was applied to all the functional images. Normalized functional images were then smoothed using a 10 mm Gaussian smoothing kernel to account for the greater morphologic variability in the elderly sample (31).
Functional Connectivity Analysis
The Robust Weighted Least Square (WLS) toolbox (verison 3.1, SPM8) was used to detect and adjust for artifacts in the fMRI time series data. This method uses the residual variance to weight the images, resulting in an optimal model estimation of noisy data due to motion artifacts (32) (http://www.icn.ucl.ac.uk/motorcontrol/imaging/robustWLS.html).
The posterior cingulate cortex (PCC) was used as the seed region to examine the connectivity within the DMN (18). The left and right posterior cingulate from the Automated Anatomical Labeling atlas (33) (1×1×1 mm) in Colin27 space was down sampled to a voxel resolution of 3.75 × 3.75 × 3.75 mm (left and right PCC combined, 200 voxels). A smaller region-of-interest (ROI) of 39 voxels, centered on the posterior cingulate, was created as seed region performing erosions (2 iterations, 6 connected, 2.5-dimensional) with a 3×3×3 mm voxel structuring element.
For each subject, a reference resting-state time-series was extracted by averaging the time-series for all voxels (resulted from the Robust WLS) within the posterior cingulate seed using the Marsbar plug-in in SPM (34). The resulted time-series were used as regressor in the general linear model of the first-level analysis in SPM8, to generate a connectivity map for each participant. Additionally, to further control for physiological and movement-related variance, we included in the model, as nuisance covariates, the adjusted CSF time-series extracted from a sphere centered in the fourth ventricle (radius = 2 mm, center MNI x/y/z= 0/−43/−26) and the motion correction parameters. Individual PCC connectivity maps were estimated and submitted for level 2 group analysis.
Voxelwise Factorial analysis
Second level analyses were performed using SPM8. The first-level PCC functional connectivity maps were modeled in a 2×2 factorial design where we have explored the effects of the following factors: age (young vs. old), anxiety (GAD vs. non-anxious), and the age x anxiety interaction. The interaction was modeled to test the effect of anxiety on the difference between the two age groups [e.g. younger anxious - younger controls versus older anxious - older controls: YA-YC>OA-OC].
Voxelwise Regression analysis
The first-level functional connectivity maps for the GAD participants were included in four separate regression analyses using as covariates 1) the total duration of illness, 2) the HARS scores, 3) the PSWQ scores, 4) the GADSS scores. We chose to perform separate regression analysis on the HARS scores and the PSWQ scores as the two questionnaires address different aspects of pathological anxiety: HARS elicits mainly global indices of anxiety, including somatic symptoms, while PSWQ was designed to elicit specifically symptoms of pathologic worry. Additionally, we included the GADSS scores to explore the correlation with illness severity.
For both, ANOVA and regression analysis, we corrected for multiple comparisons by using the methods implemented in SPM 8 (false discovery rate FDR and family-wise error FWE). A corrected p≤ 0.05 was deemed significant. An anatomic defined prefrontal mask including the ROIs of interest from the DMN (ventral and dorsal medial prefrontal cortex) was used for small volume correction for the age-by-anxiety interaction analysis.
RESULTS
A total of 53 participants were included in this study. Descriptive statistics are presented in Table 1. As a rule, when referring to “functional connectivity” in this section, we imply the functional connectivity between the PCC seed and the various clusters described below.
Table 1.
Demographic and clinical characteristic of the sample.
| Non-Anxious Comparison Group | Generalized Anxiety Group | Group comparison* (t/Χ2/W, p) | |||
|---|---|---|---|---|---|
| Old (n=21) | Young (n=10) | Old (n=15) | Young (n=9) | ||
| Age (mean, SD) | 70.76±7.33 | 33.6±10.49 | 67.4±6.52 | 31.67±10.06 | t(53)=0.90, p=0.37 |
| Gender (F) | 12 | 6 | 11 | 8 | Χ2(1)=1.86, p=0.17 |
| Race **(W/AA/A) | 18/3/0 | 9/1/0 | 14/1/0 | 6/2/1 |
Fisher’s exact p=0.82 |
| Duration of Illness (median (min, max), years) | n/a | n/a | 8 (1,49) | 11(4,31) | W#=90.5 p=0.80 |
| Mini Mental State Examination [median, (min, max)]## | 30 (28, 30) (n=12) | n/a | 29 (27,30) (n=9) | W#=69, p=0.25 | |
| HARS (mean, SD) | 3.05±1.88 | 2±1.63 | 19.6±2.5 | 22.22±3.49 | t(53)=−26.49 p<0.001 |
| PSWQ (mean, SD) | 31.81±7.73 | 29.5±8.49 | 56.27±11.8 | 65.89±10.04 | t(53)=−10.74, p<0.001 |
| GADSS (mean, SD) | 2.71±2.37 | 2.2±1.99 | 10.33±2.82 | 14.44±3.84 | t(53)=−11.46 p<0.001 |
Non-Anxious Comparison vs. GAD (young and old together), except for duration of illness (old vs. young GAD). HARS=Hamilton Anxiety Rating Scale; PSWQ=Penn State Worry Questionnaire. GADSS= Generalized Anxiety Disorder Severity Scale.
W=White, AA=African American, A=Asian.
Wilcoxon’s rank sum test.
Anxious old vs. control old only
Voxelwise Factorial Analysis
Young vs. older (see Fig 1). Young participants had significantly greater functional connectivity in multiple prefrontal medial prefrontal regions: BA 8 left (t=5.48, df=51, p<0.05 whole-brain FDR corrected, x/y/z = −22/35/43), BA 8 right (t=4.20, df=51, p<0.05 whole-brain FDR corrected, x/y/z = 24/40/43), BA 9 (t=4.2, df=51, p <0.05 whole-brain FDR corrected, x/y/z=21/47/42), BA 10 (t=4.59, df=51, p<0.05 whole-brain FDR corrected, x/y/z = 5/60/24). Older participants had significantly greater functional connectivity in temporo-parietal regions, including the right insula (BA 44 t=4.48, df=51, p<0.05 whole-brain FDR corrected, x/y/z =43/1.05/7; BA 13, t= 5.18, df=51, p<0.05 whole-brain FDR corrected, x/y/z=43/−37/19), left insula (BA 44, t=3.68, df=51, p<0.05 whole-brain FDR corrected, x/y/z= −44,3,8), right middle temporal gyrus (BA 22, t= 4.48, df= 51, p<0.05 whole-brain FDR corrected, x/y/z =39/−58/20), left middle temporal gyrus (BA 39, t=4.51, df =51, p<0.05, whole-brain FDR corrected, x/y/z = −44, −58,8).
Fig. 1.
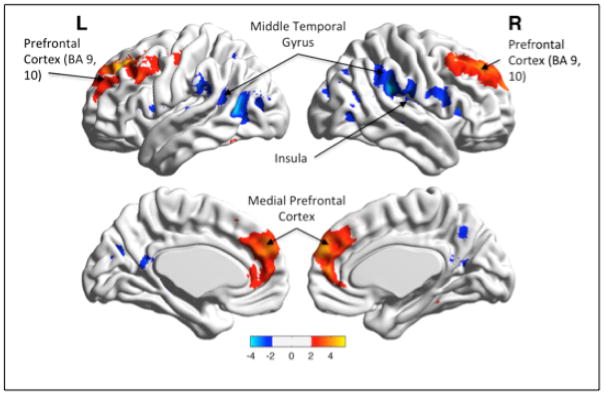
Main effect of Age.
Legend: Young participants had significantly greater functional connectivity between the PCC seed and multiple prefrontal medial prefrontal regions (BA 9,10, 32) [in red], while older participants had greater functional connectivity between the PCC seed and temporo-parietal regions, including the right insula (BA 13) [in blue]. Color bar presents t-values.
*Visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv).
Anxious vs. non-anxious: Non-anxious participants had greater functional connectivity in the ventro-lateral PFC (t=3.18, df = 51, p=0.001 uncorected, x/y/z=−35,54,1). The anxiety vs. comparison analysis results did not survive multiple comparison correction.
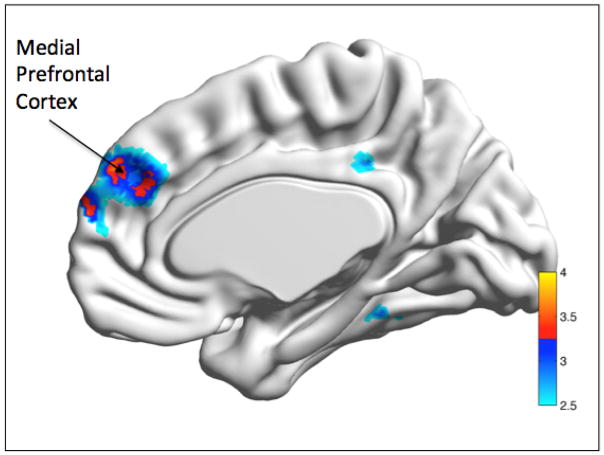
The age by anxiety interaction was set up as described in the Methods section: younger anxious - younger controls versus older anxious - older controls [YA-YC vs. OA-OC]. The interaction showed significantly greater functional connectivity in the ventromedial prefrontal cortex (bilateral) for the younger group (YA-YC) relative to the older participants (OA-OC) (t=3.60, df= 51, p<0.05 FWE-ROI, x/y/z= +/−4, 52,34) (see Fig 2).
Fig. 2.
Age x Anxiety interaction.
Legend: The interaction showed that younger and less anxious participants had greater connectivity between the PCC seed and the medial prefrontal cortex.
YA-YC>OA-OC: bilateral mPFC. YA= young anxious, YC= young controls, OA=old anxious, OC=old controls.
Color bar presents t-values.
*Visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv).
Voxelwise Regression Analysis
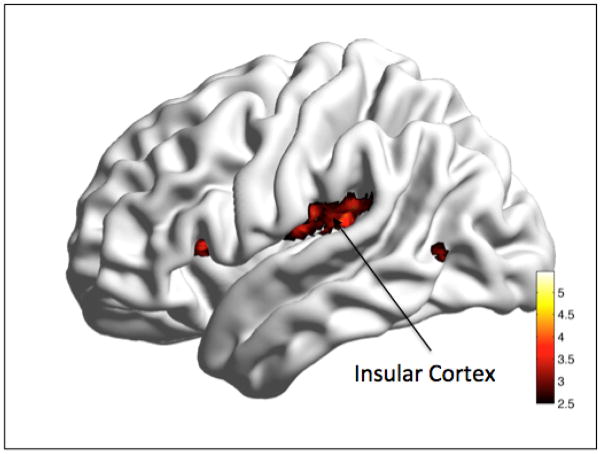
The duration of illness regression analysis showed a significant positive correlation between increased duration of illness and functional connectivity in the bilateral insula (t=6.11/4.79, df = 21, p<0.05 whole brain FWE, x/y/z=−33/20, 6/43, −8,20) (see Fig 3).
Fig. 3.
Regression analysis. Correlation with Duration of Illness.
Legend: Longer duration of illness was significantly correlated with increased functional connectivity between the PCC seed and bilateral Insular cortex. Color bar presents t-values.
*Visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv.
The HARS regression analysis showed an association between higher HARS scores and functional connectivity in the pregenual cingulate cortex (t=3.6, df = 21, p=0.001 uncorr, cluster size=32, x/y/z=2/34/14), but these results did not survive the multiple comparison correction.
The PSWQ regression analysis showed a significant negative correlation between PSWQ scores and functional connectivity in the mPFC (t=4.45, df= 21, p<0.05 whole brain FDR, x/y/z=3/51/4.) (see Fig 4).
Fig. 4.
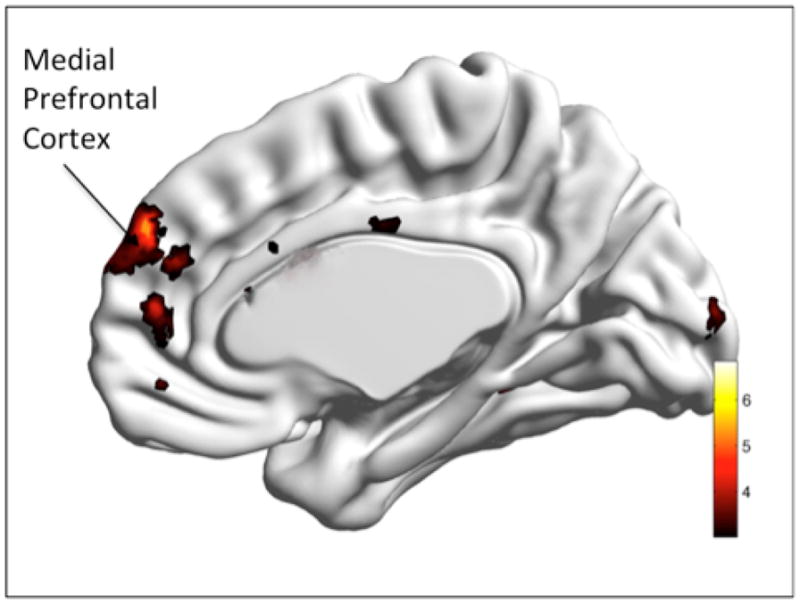
Regression Analysis. Correlation with Penn State Worry Questionnaire (PSWQ).
Legend: PSWQ scores were negatively correlated with greater functional connectivity between the PCC seed and medial prefrontal cortex. Color bar presents t-values.
*Visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv.)
The GADSS regression analysis showed an association between higher GADSS scores and functional connectivity in the frontal lobe (subgyral, x/y/z=−26/27/26); it did not survive multiple comparison correction.
DISCUSSION
To our knowledge, this is the first attempt to compare head-to-head the neural changes in the DMN functional connectivity in Generalized Anxiety Disorder across the lifespan. Our results show a significantly different pattern of connectivity in young versus older GAD participants. Also, we report a significant age x anxiety interaction in the frontal branch of the DMN. Moreover, duration of illness and worry severity (as measured by PSWQ) have a significant effect on the connectivity patterns in the DMN in GAD.
Other investigators have advocated for a different biological basis for the observed clinical differences between young and older subjects diagnosed with the same mental disorder, arguing that the neural network abnormalities described in younger adults might not be entirely translatable into the elderly, given the various anatomical and pathophysiological changes observed in the aging brain (9). While previous studies have reported a posterior-to-anterior shift in aging during cognitive tasks (35), very recent reports described a different effect of aging on functional brain networks at rest (36). Tomasi and Volkow hare reported in 2012 that long-range connections are more vulnerable to aging effects than short-range connections (36).
The vmPFC is regarded as an integrative center, that projects directly to nuclei involved in affect regulation, including the amygdala, ventral striatum, and periaqueductal gray (37). The vmPFC – amygdala interactions can be seen as flexibly manipulating and integrating mental representations of one’s interaction with the environment (37). Our results, which show a negative association between worry severity and vmPFC connectivity, leads us to speculate that pathologic worry, especially in the elderly, may be related to decreased flexibility in the prefrontal structures at rest. Thus, pathologic worriers may be “stuck” in a stereotypical task related activity (e.g. persistent worry) while at rest, a perspective that suggests they are unable to modify the mental representations in a flexible way based on both environmental and internal input (interoception) (38). Consequently, the ability to flexible respond to cognitive modifications such as those imbedded in CBT may be diminishes, which would explain the poor CBT response rates in late-life GAD (15). These hypotheses will require further testing, using designs that include both resting state and worry-induction in-scanner tasks.
It is worth noting that neither the HARS nor the GADSS regression analysis survived multiple comparisons. These results support the hypothesis of different neural substrates for discrete forms of anxiety such as phasic fear and sustained apprehension (39). Moreover, PSWQ explores a specific clinical construct (worry), while HARS measures general anxiety symptoms, offering a useful but less focused composite score. Thus, it is possible that the association between the worry severity and the DMN is rendered stronger due to the specificity of the instrument. The lack of significance related to illness severity as measured by GADSS is more difficult to interpret and it will probably need further replication on larger samples.
Our results show that the longer the illness, the greater the PCC-Insula connectivity. The contribution of the hyperactive insula in anxiety is well documented (3, 40). As the insular cortex is critically involved in integrating stimuli salience and in generating anticipatory signals regarding aversive outcomes, the persistent misperception of interoception [= “interoceptive hijacking”(41)] may play a crucial role in the chronicity of GAD, suggesting a worsening interoceptive hijacking with progression of illness. Future studies will be needed to test whether this feature may be used as a biomarker of treatment resistance.
In conclusion, our results indicate that the presence of GAD as well as the severity of worry worsens the effect of age on the functional connectivity in the DMN. We may speculate that increased worry and a diagnosis of GAD contribute to accelerate aging. It is worth noting that while the age-by-anxiety analysis was significant, the anxiety group analysis did not reach significance. These results may indicate that that anxiety impairs the intrinsic functional connectivity especially in the context of increased age. Moreover, the significant results obtained when exploring worry severity (but not a diagnosis of GAD), point toward pathologic worry as the clinical correlate of disrupted DMN connectivity in GAD. These findings support the use of a dimensional approach using severity of worry as outcome measure.
However, given the cross-sectional design of our study we cannot infer the direction of the anxiety-age correlation. As GAD is notably the only anxiety disorder in which new cases arise in later life (12), it is possible that aging increases the susceptibility to anxiety through changes in processes such as negative attentional bias and decreased prefrontal executive control (42).
Overall, our results confirm the role of age in healthy volunteers and expanded to it to GAD, one of the most prevalent mental disorders in the elderly (13). These results add weight to the approach that advocates for tailored research in late-life mental disorders such as late-life anxiety and depression (17).
Strength and limitations
Our study has several strengths: it analyzes a relatively large sample of GAD participants, it uses a uniform design to compare young versus older GAD participants, and it uses a “clean” sample (e.g. psychotropic free, with no other comorbid psychiatric illnesses at the time of scan except for other anxiety disorders). Additionally, the use of robust WLS in extracting Level 1 time-series allowed for a stronger detection of motion-related artifacts. Some limitations are worth noting. While the overall GAD sample is fairly large, the individual cells (especially for the younger group) are relatively small and may have reduced our ability to detect significant effects in the anxiety group analysis. The analysis presented in this study is cross-sectional. Thus, we cannot make direct inferences regarding the longitudinal burden of pathologic anxiety in the same cohort. The duration of illness has been obtained from participants’ report, a method weighted down by retrospective bias. Previous studies have used motion, whole-brain, cerebrospinal fluid, white-matter, and physiologic measures to remove unwanted signal. We chose to use motion and cerebrospinal fluid to limit the effects of motion and physiologic noise, while minimizing difficult-to-interpret anti-correlations (43). Regarding the age-related vulnerability of long-range connections, it is worth mentioning that two recent studies reported that weaker long-range connections might be related to motion artifacts (44). These artifacts were strongest in children and weakest in adults (44). It is unlikely that such artifacts were responsible for the results reported in this study as we supplemented the regular motion correction with the use of robust WLS to ensure that motion-induced outliers would not drive our results (32).
As our previous work strongly indicates a role for white matter lesions in the DMN changes in late-life depression (45), further directions of research include the exploration of the role of white matter structural lesions in eroding the long-range connections in the DMN of anxious subjects. Other further directions of research involve exploring the behavior of other functional networks (e.g. salience network, executive control network) during resting state, and using resting state fMRI to identify biomarkers of treatment response and to contribute to the development of tailored treatment options of individuals with generalized anxiety (46).
Acknowledgments
Supported by NIMH MH 086686, MH 071944, UL1 RR024153 and UL1TR000005, the Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (Dr. Andreescu)
The authors would like to thank Dr. John Ryan for his comments on a previous version of the manuscript, as well as the Geriatric Psychiatry Neuroimaging lab staff for their help with recruitment and data processing.
Footnotes
Conflict of Interest: Carmen Andreescu, Lei K. Sheu, Dana Tudorascu and Sarah Walker do not have any potential conflict of interest to acknowledge. Howard Aizenstein has received research support from Novartis Pharmaceuticals.
References
- 1.Joffe H, Chang Y, Dhaliwal S, et al. Lifetime history of depression and anxiety disorders as a predictor of quality of life in midlife women in the absence of current illness episodes. Arch Gen Psychiatry. 2012;69:484–492. doi: 10.1001/archgenpsychiatry.2011.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wager TD, van Ast VA, Hughes BL, et al. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etkin A, Prater KE, Hoeft F, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulesu E, Sambugaro E, Torti T, et al. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med. 2010;40:117–124. doi: 10.1017/S0033291709005649. [DOI] [PubMed] [Google Scholar]
- 6.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitschke JB, Sarinopoulos I, Oathes DJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etkin A, Prater KE, Schatzberg AF, et al. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GS, Gunning-Dixon FM, Lotrich FE, et al. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32:1857–1875. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 10.Mohlman J, Price RB, Eldreth DA, et al. The relation of worry to prefrontal cortex volume in older adults with and without generalized anxiety disorder. Psychiatry Res. 2009;173:121–127. doi: 10.1016/j.pscychresns.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreescu C, Lenze EJ, Mulsant BH, et al. High worry severity is associated with poorer acute and maintenance efficacy of antidepressants in late-life depression. Depress Anxiety. 2009;26:266–272. doi: 10.1002/da.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Lenze EJ, Rollman BL, Shear MK, et al. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009;301:295–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71:31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Thorp SR, Ayers CR, Nuevo R, et al. Meta-analysis Comparing Different Behavioral Treatments for Late-Life Anxiety. Am J Geriatr Psychiatry. 2008 doi: 10.1097/JGP.0b013e31818b3f7e. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenze EJ, Wetherell JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry. 2009;24:1–14. doi: 10.1002/gps.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gusnard DA, Akbudak E, Shulman GL, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentili C, Ricciardi E, Gobbini MI, et al. Beyond amygdala: Default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79:409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Ding J, Chen H, Qiu C, et al. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Reson Imaging. 2011;29:701–711. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Andreescu C, Wu M, Butters MA, et al. The default mode network in late-life anxious depression. Am J Geriatr Psychiatry. 2011;19:980–983. doi: 10.1097/JGP.0b013e318227f4f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:290–299. e292. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen AC, Etkin A. Hippocampal Network Connectivity and Activation Differentiates Post-Traumatic Stress Disorder From Generalized Anxiety Disorder. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MSR, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P), 2.0 ed. 1995 [Google Scholar]
- 28.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 30.Andreescu C, Belnap BH, Rollman BL, et al. Generalized anxiety disorder severity scale validation in older adults. Am J Geriatr Psychiatry. 2008;16:813–818. doi: 10.1097/JGP.0b013e31817c6aab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. Neuroimage. 2005;27:624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Brett MAJ-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Sendai, Japan: 2002. [Google Scholar]
- 35.Davis SW, Dennis NA, Daselaar SM, et al. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andor T, Gerlach AL, Rist F. Superior perception of phasic physiological arousal and the detrimental consequences of the conviction to be aroused on worrying and metacognitions in GAD. J Abnorm Psychol. 2008;117:193–205. doi: 10.1037/0021-843X.117.1.193. [DOI] [PubMed] [Google Scholar]
- 39.Davis M, Walker DL, Miles L, et al. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein MB, Simmons AN, Feinstein JS, et al. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 41.Mennin DS, McLaughlin KA, Flanagan TJ. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. J Anxiety Disord. 2009;23:866–871. doi: 10.1016/j.janxdis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Van Dijk KR, Hedden T, Venkataraman A, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M, Andreescu C, Butters MA, et al. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etkin A. Neurobiology of anxiety: from neural circuits to novel solutions? Depress Anxiety. 2012;29:355–358. doi: 10.1002/da.21957. [DOI] [PubMed] [Google Scholar]