Abstract
Polybrominated diphenyl ethers (PBDEs), extensively used in the past few decades as flame retardants in a variety of consumer products, have become world-wide persistent environmental pollutants. Levels in North America are usually higher than those in Europe and Asia, and body burden is 3 to 9-fold higher in infants and toddlers than in adults. The latter has raised concern for potential developmental toxicity and neurotoxicity of PBDEs. Experimental studies in animals and epidemiological observations in humans suggest that PBDEs may be developmental neurotoxicants. Pre- and/or post-natal exposure to PBDEs may cause long-lasting behavioral abnormalities, particularly in the domains of motor activity and cognition. The mechanisms underlying the developmental neurotoxic effects of PBDEs are not known, though several hypotheses have been put forward. One general mode of action relates to the ability of PBDEs to impair thyroid hormone homeostasis, thus indirectly affecting the developing brain. An alternative or additional mode of action involves a direct effect of PBDEs on nervous system cells; PBDEs can cause oxidative stress-related damage (DNA damage, mitochondrial dysfunction, apoptosis), and interfere with signal transduction (particularly calcium signaling), and with neurotransmitter systems. Important issues such as bioavailability and metabolism of PBDEs, extrapolation of results to low level of exposures, and the potential effects of interactions among PBDE congeners and between PBDEs and other contaminants also need to be taken into account.
Keywords: Polybrominated diphenyl ethers, Flame retardants, Hydroxylated PBDEs, Developmental neurotoxicity, Oxidative stress, Thyroid hormone homeostasis, Mechanisms, Calcium signaling, Apoptosis
PBDEs: occurrence and body burden
Polybrominated diphenyl ethers (PBDEs) are flame retardants, widely used in recent years in a variety of consumer products. Since PBDEs are not chemically bound to the polymer product, they leach out into the environment and have become persistent organic pollutants. Indeed, PBDEs have been detected in most biota, as well as in human blood, adipose tissue, and breast milk (Sjodin et al. 2003; Law et al. 2006). Levels of PBDEs in biota and in human tissues have significantly increased in the past three decades (Schecter et al. 2005), and levels in North America are one to two orders of magnitude higher than those reported in Europe and Asia (Lorber, 2008; USEPA, 2010: EFSA, 2011). PBDEs have been marketed as one of three mixtures, though there are 209 possible types of congeners. PentaBDE and octaBDE have been banned in the E.U. and in several states in the U.S.A., and are no longer produced in these countries. DecaBDE remains the most widely used PBDE globally, and is still produced in the U.S.A., though producers have agreed to cease production and commercialization by 2013 (USEPA, 2010). Five congeners (BDE-47, -99, -100, -153, -154) predominate in human tissues, usually accounting for 90% of the total body burden (McDonald, 2005; Costa et al. 2008), and BDE-47 (2, 2′, 4, 4′-tetrabrominated diphenyl ether) alone accounts for 50% (USEPA, 2010). In addition, BDE-47 is the dominant PBDE congener in the atmosphere in two remote sites as the Tibetan plateau and the Canadian High Arctic, making it a true global contaminant (Xiao et al. 2012). Fig. 1 shows the structure of some PBDE congeners (the tetraBDE BDE-47, the pentaBDE BDE-99, the hexaBDE BDE-153, and decaBDE BDE-209).
Fig. 1.
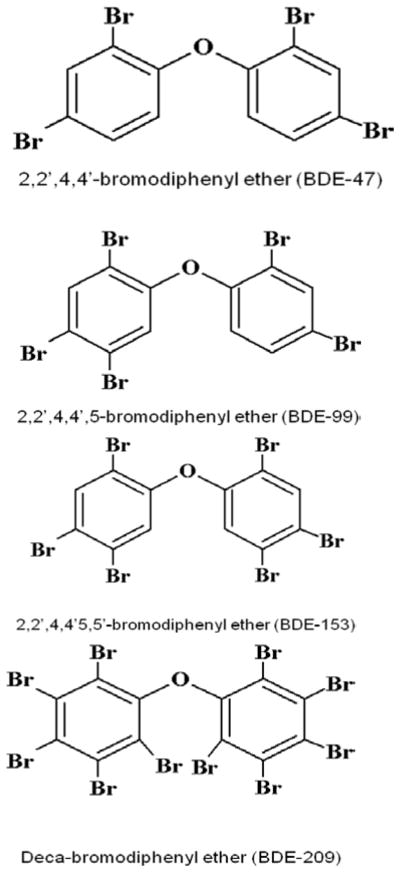
Structures of some major PBDE congeners.
Blood levels of PBDEs in adults in the USA are in the nanomolar range (30–100 ng/g lipid; Costa and Giordano, 2007), but body burden is much higher in infants (because of exposure through breast milk), and in toddlers (because of exposure through house dust and the diet) (Schecter et al. 2005; Fischer et al. 2006; Toms et al. 2009; Rose et al. 2010; USEPA, 2010). Indeed, blood levels of PBDEs in toddlers and children have been reported to be 3 to 9-fold higher than in adults (Fischer et al. 2006; Toms et al. 2009; Rose et al. 2010). In addition to higher exposures, differences in toxicokinetics may also be relevant; for example, young mice have been shown to have a reduced ability to excrete BDE-47, possibly due to a lower elimination (Staskal et al. 2006). Furthermore, PBDEs can also cross the placenta, and similar concentrations are found in maternal and fetal blood (Mazdai et al. 2003; Antignac et al. 2008).
Developmental neurotoxicity of PBDEs
The high body burden of PBDEs in infants and toddlers has raised concerns for their potential developmental toxicity and neurotoxicity (Eriksson et al. 2001; Branchi et al. 2003; Birnbaum and Staskal, 2004; McDonald, 2005; Costa and Giordano, 2007; Talsness, 2008; Costa et al. 2008; Williams and DeSesso, 2010; Dingemans et al. 2011; EFSA, 2011). Animal studies suggest that exposure to different PBDEs during the prenatal and/or postnatal periods causes long-lasting behavioral abnormalities, particularly in the domains of motor activity and cognition, though the results are not always perfectly consistent (Branchi et al. 2002; Dufault et al. 2005; Gee and Moser, 2008; Viberg et al. 2003; 2004; Suvorov et al. 2009; Cheng et al. 2009; Driscoll et al. 2009; Kodavanti et al. 2010; Ta et al. 2011). Evidence in humans is also suggestive of developmental effects of PBDEs, though findings are not always univocal. In Taiwan, elevated PBDE levels in breast milk were correlated with lower birth weight and length, lower head and chest circumference, and decreased Quetelet’s (body mass) index, and a follow-up study reported developmental delays in cognition (Chao et al. 2007; 2011). In contrast, a study in a Hispanic population in California found no association between prenatal exposure to PBDEs and birth length, head circumference and duration of gestation (Harley et al. 2011). However, the same authors reported that children of 5 and 7 years of age exposed to PBDEs prenatally and in early childhood had poorer attention, fine motor coordination and cognition (Eskenazi et al. 2013). Prenatal PBDE exposure (mostly to BDE-153) was found to be associated with altered cognitive skills and adaptive behavior in a population from Taiwan (Shy et al. 2011). In a study in the Netherlands, an association was found between blood PBDE levels in the mother at the 35th week of pregnancy and altered motor function, cognition and behavior of the child up to age six (Roze et al. 2009). In an additional cohort in New York City, prenatal PBDE exposure (as indicated by cord blood PBDE levels) was associated with lower scores on tests of mental and physical development at the ages of 1–4 and 6 years (Herbstman et al. 2010). In a North Carolina cohort, lactational PBDE exposure was associated with increased activity/impulsivity behaviors in early childhood (Hoffman et al. 2012), though a study by Gascon et al. (2011) in Spain found no associations between body burden of PBDEs in 4-year old children and any motor or cognitive alteration. In summary, though results in animals and humans are not always perfectly consistent, a strong pattern is emerging which indicates that PBDE exposure may affect neurodevelopment.
Considerations on PBDE metabolism and structure-activity relationship
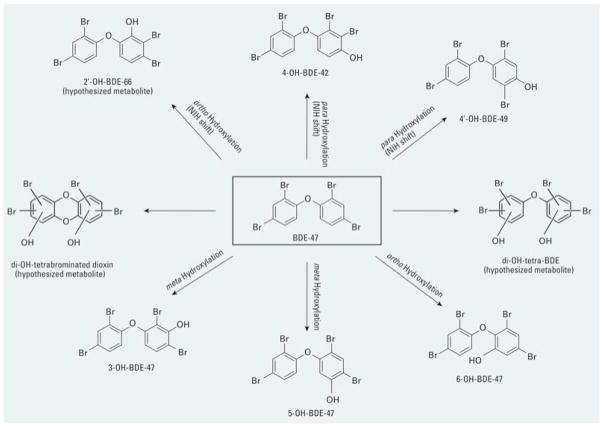
Substantial differences exist among PBDE congeners in the rate of absorption; for example, tetra- and penta-BDEs are better absorbed than decaBDE (BDE-209) (Hakk and Letcher, 2003; Costa and Giordano, 2007; 2011). In addition, lower brominated PBDEs have a much longer half-life (20–120 days) than BDE-209 (~15 days). The number and position of bromines on the PBDE molecule also influences their ability to enter cells; for example, in an in vitro study, BDE-100 (a pentaBDE) was found to have the highest accumulation in mouse cerebellar granule cells; BDE-209, with its bulky configuration, had the lowest (Huang et al. 2010). DecaBDE is believed to be debrominated to lower brominated congeners, though the extent of these metabolic reactions in mammals including humans is still unclear (Stapleton et al. 2009; Costa and Giordano, 2011). In contrast, there is substantial evidence for an oxidative metabolism of lower brominated BDEs (Hakk and Letcher, 2003; Malmberg et al. 2005; Lupton et al. 2009), which may play significant roles in their developmental neurotoxicity. Formation of hydroxylated metabolites of a number of PBDEs (e.g. BDE-47, BDE-99, and BDE-153) has been reported, and CYP2B6 is emerging as the main metabolic enzyme in this regard (Erratico et al. 2012; 2013; Feo et al. 2013). For example, Fig. 2 shows that metabolic products of BDE-47 formed by CYP2B6 include BDE-47 hydroxylated at the 3, 5 or 6 position, as well as different hydroxylated congeners (e.g. 4-OH-BDE-42, 4′-OH-BDE-49) (Erratico et al. 2013; Feo et al. 2013).
Fig. 2.
Oxidative metabolism of BDE-47 by human CYP2B6. From Erratico et al. (2013) with permission.
Most of the hydroxylated PBDE metabolites identified in in vitro studies or in animals, have also been found in humans (Athanasiadou et al. 2008; Qiu et al. 2009), and their levels are usually higher in cord serum than in maternal blood (Chen et al. 2013). Most importantly, as discussed in the following sections, OH-BDEs have been shown to have stronger and/or unique biological activities on a number of relevant end-points such as binding to thyroid hormone transport proteins (Marchesini et al. 2008), interference with calcium homeostasis (Dingemans et al. 2008; 2011; Kim et al. 2011), interaction with neurotransmitter receptors (Hendriks et al. 2010), or inhibition of stem cell differentiation (Li et al. 2013). Two additional aspects in this regard are that CYP2B6 is also present in human brain tissue (Miksys and Tyndale, 2004), thus allowing in situ formation of OH-BDEs, and that there is an almost 100-fold variability in the level of CYPB26 expression in human liver, due to regulatory phenomena and genetic polymorphisms (Zanger et al. 2007), which may contribute to individual differences in OH-BDE formation and susceptibility to PBDE neurotoxicity.
Potential mechanisms of PBDE developmental neurotoxicity
The exact mechanisms of PBDEs’ developmental neurotoxicity are still elusive, though two general, and not mutually exclusive, modes of action are emerging: one indirect, related to effects of PBDEs on thyroid hormones, and the other involving possible direct effects of PBDEs on the developing brain (Costa and Giordano, 2007; Alm and Scholz, 2010). Below we review the current knowledge on mechanisms which may underlie PBDE developmental neurotoxicity, deriving from animal, in vitro, and when possible, human studies. In addition, attention is devoted to the potential role of PBDE metabolites in the neurotoxic effects.
Alterations of thyroid hormone homeostasis
Thyroid hormones are known to play a relevant role in brain development (Chan and Rovet, 2003; LaFranchi et al. 2005), and hypothyroidism has been associated with a large number of neuroanatomical and behavioral effects (Haddow et al. 1999; Zoeller and Crofton, 2005). Since the structurally similar polychlorinated biphenyls (PCBs) had been found to perturb thyroid hormone homeostasis, the possibility that PBDEs may act through similar mechanisms has been extensively investigated. Zhou et al. (2001) first observed that treatment of weanling female rats with DE-71 (a tetra-pentaBDE mixture) or DE-79 (an octaBDE mixture), caused a reduction of serum thyroxine (T4) levels, without altering those of triiodotyronine (T3). Several subsequent studies in rats, exposed to DE-71 during gestation, found significant decreases of serum T4 in the dam, the fetuses and the pups, with no changes in T3 levels, and an increase of tyrotropin (TSH) level (Zhou et al. 2002; Ellis-Hutchings et al. 2006; Szabo et al. 2009; Kodavanti et al. 2010; Miller et al. 2012). An additional study in rats reported decreased T4 levels in pups upon postnatal exposure to DE-71 (Driscoll et al. 2009). Postnatal exposure of rats to BDE-209 was reported to decrease the serum levels of T4 (Rice et al. 2007), while gestational exposure to the same congener reduced T3, but not T4 levels (Tseng et al. 2008). A single exposure of rats to BDE-47 or BDE-99 on gestational day 6 was found to decrease T4 levels in dams and pups, but not at all time points (Kuriyama et al. 2004; 2007; Talsness et al. 2004), and decreases of T4 and T3 levels in lambs prenatally exposed to low doses of BDE-47 have also been reported (Abdelouahab et al. 2009). A few studies have reported changes in thyroid hormone homeostasis in pregnant women or their offspring, associated with PBDE exposure. Herbstman et al. (2008) found decreased serum T4 levels in newborns; in contrast, at different times of pregnancy, increased T4 levels (Stapleton et al. 2011), decreased TSH levels (Chevrier et al. 2010), or increased TSH with no changes in T4 levels (Zota et al. 2011) have been reported. In the largest study so far (n=380), Abdelouahab et al. (2013) reported an association between maternal and cord blood PBDE levels and decreased total T3 and T4, but increased free T3 and T4. Thus, human studies have not provided, so far, consisted findings, possibly because of a number of methodological issues, as recently discussed (Chevrier, 2013).
The findings of decreased thyroid hormone levels in animals following developmental exposures to PBDEs prompted investigations on possible underlying mechanisms. Hypotheses tested relate to an enhanced metabolism and excretion of T4 as a result of exposure to PBDEs, and interactions of PBDEs (or their metabolites) with the thyroid hormone transport systems or with thyroid receptors. Zhou et al. (2002) found that the decrease in T4 was associated with induction of uridine-5-diphospho-glucuronosyltransferase (UDPGT), a key phase II metabolizing enzyme involved in conjugation of T4. Such increased metabolism would result in enhanced excretion and hence in reduced circulating levels of T4 (Barter and Klaassen, 1992). However, induction of UDPGT cannot explain the reduced T4 levels induced by PBDEs, as decreases in T4 levels were seen at doses that did not induce UDPGT (Zhou et al. 2002; Hallgren et al. 2001; Kuriyama et al. 2004; Tseng et al. 2008). Richardson et al. (2008) concluded that while induction of UDPGT may be partly responsible for T4 decreases, other mechanisms should be considered; for example, Szabo et al. (2009) indicate that other phase I and II enzymes, as well as diodinase, may also be involved in the modulation of T4 levels by PBDEs.
An alternative/complementary hypothesis is that PBDEs may interfere with thyroid hormone transport. Two proteins are important in this regard: thyroxin-binding globulin (TBG) and transthyretin (TTR) which transport almost all T4 in human plasma. TBG is believed to facilitate iodine supply to the fetus (Schussler, 2000), while TTR delivers thyroid hormones to the fetus and to the brain (Schreiber, 2002). Meerts et al. (2000) first reported that several PBDEs could interact with TTR, thereby displacing T4. However, such interaction only occurred in the presence of phenobarbital-treated microsomes (enriched in CYP 1A, 2B and 4A3), implicating one or more PBDE metabolites. Several hydroxylated PBDEs, which have a structural resemblance to thyroid hormones, were most potent in displacing T4 from TTR, and to a minor extent, from TBG (Hamers et al. 2006; 2008; Marchesini et al. 2008; Cao et al. 2010). Displacement of T4 from TTR may lead to its increased glucuronidation and a consequent lower level of T4; in addition, because of the complicated feed-back regulation systems of thyroid hormone production, TSH levels may increase (Kodavanti et al. 2011). An additional mechanism of perturbation of thyroid hormone homeostasis may be related to a direct interaction of PBDEs and/or their metabolites with thyroid hormone receptors. Thyroid receptors (THα and THβ, the latter most widely expressed) are located in the cell nucleus and heterodimerize with a retinoid X receptor (RXR) (Ren and Guo, 2013). Certain PBDEs (e.g. BDE-47, BDE-99) do not appear to directly interact with TH receptors (Suvorov et al. 2011), while others (e.g. BDE-154, BDE-209) have been reported to inhibit the effect of T3 at THβ (Ibhazehiebo et al. 2011). In contrast, several OH-BDEs have been shown to act as agonists or antagonists at TH receptors (Hamers et al. 2006; Kojima et al. 2009; Li et al. 2010; Ren et al. 2013). For example, lower brominated hydroxy-BDEs (e.g. 5-OH-BDE-47 and 6-OH-BDE-47) were found to be TH receptor agonists, while higher brominated hydroxy-BDEs (e.g. 3′-OH-BDE-154 or 4-OH-BDE-188) acted as antagonists (Ren et al. 2013).
Independent of the specific underlying mechanisms, the effect of PBDEs on thyroid hormone homeostasis may be relevant in the context of developmental neurotoxicity. Behavioral studies in hypothyroidism (induced by developmental exposure to propyl thiouracyl (PTU)) have evidenced decreases in learning and habituation in maze tests, changes in anxiety-like behavior, and increases in locomotor activity in rats (Negishi et al. 2005). Some of these effects are seen following developmental exposure to PBDEs. Furthermore, thyroid hormone deficiency has been found to cause structural abnormalities in the hippocampus and the cerebellum (Zoeller and Crofton, 2005), and to increase apoptosis in the cerebellum (Singh et al. 2003). PTU treatment causes T4 level to fall below the limit of detection in offspring (Negishi et al. 2005), while decreases of T4 following developmental exposure to PBDEs are less pronounced (10–60%). Nevertheless, decrements in neurological development in children of mothers with 25% decrease in T4 have been reported (Haddow et al. 1999), particularly when hypothyroxemia occurs in early pregnancy (Pop et al. 2003; Chevrier et al. 2011a), suggesting that effects of PBDEs on thyroid hormones may contribute to their developmental neurotoxicity. However, it is still unclear whether exposure of humans to PBDEs leads to changes in thyroid hormones, and whether adverse developmental effects are always accompanied by disruption of hormone homeostasis. Though PBDE exposure during pregnancy has been associated with alterations in thyroid hormone status, the findings have been somewhat contradictory (Herbstman et al. 2008; Chevrier et al. 2010; Zota et al. 2011; Stapleton et al. 2011). Mazdai et al. (2003) and Kim et al. (2012a; 2012b) found no correlation between total PBDE and OH-BDE levels in fetal or maternal serum, and T4 or T3 concentrations. A lack of correlation between PBDE exposure and T4 or TSH blood levels was also reported by Chevrier et al. (2011b), Eggesbo et al. (2011), Gascon et al. 2011), Shy et al. (2012), and Kim et al. (2012a; 2012b). In addition, adverse developmental effects of PBDEs in animals have also been observed in the absence of significant thyroid hormone alterations (Branchi et al. 2005; Gee et al. 2008; He et al. 2011; Costa, Giordano, et al., unpublished; Table 1). Thus, overall, there is some evidence that PBDEs, mostly through their hydroxylated metabolites, can alter thyroid hormone homeostasis; however, whether this occurs in humans, and whether this is an important contributor to their developmental neurotoxicity remains to be determined. Alternative mechanisms relate to possible direct effects of PBDEs on brain cells, as shown by a large number of in vitro studies, and by a few in vivo ones, which are discussed below.
Table 1.
Effects of in vivo administration of BDE-47 in mice
| End-point | Control | BDE-47 |
|---|---|---|
| Oxidative stress in cerebellum | ||
| Lipid peroxidation (MDA, nmol/mg protein) | 3.5 ± 0.2 | 6.1 ± 0.3* |
| Reactive carbonyls (nmol/mg protein) | 0.7 ± 0.1 | 1.3 ± 0.2* |
| Apoptosis in cerebellum | ||
| Cleaved caspase-3 (OD, % of control) | 100 ± 5 | 195 ± 20* |
| Thyroid hormones in plasma | ||
| tT3 (ng/ml) | 4.3 ± 0.7 | 4.6 ± 0.6 |
| tT4 (ng/ml) | 84.9 ± 3.5 | 80.5 ± 2.6 |
Male mice were treated with BDE-47 (10 mg/kg in corn-oil) by gavage on post-natal day 10, as described elsewhere (Viberg et al. 2003; Gee and Moser, 2008). After 24 h, one mouse per litter (n =3) was chosen for measurements of lipid peroxidation, protein carbonyl levels and caspase-3 activation in cerebellum, and of total T3 and T4 in plasma. Administration of BDE-47 caused an increase in oxidative stress and apoptotic cell death, without any changes in thyroid hormone levels. Results represent the mean (±SD) of three separate determinations;
Significantly different from control, p<0.05. (Costa, Giordano et al., unpublished results).
Oxidative stress-induced DNA damage and apoptosis
Oxidative stress refers to the cytotoxic consequences of reactive oxygen species (ROS), which are generated as by-products of normal and aberrant metabolic processes that use molecular oxygen. When ROS production exceeds the antioxidant defense capacity of the cell, oxidative stress ensues, leading to damage of DNA, proteins, and membrane lipids. The tripeptide glutathione (GSH) is one of the most abundant cellular thiols, and a major player in cellular defense against ROS.
There is substantial evidence indicating that PBDEs can induce oxidative stress (Costa et al. 2010). Reistad and Mariussen (2005) first reported that DE-71 and BDE-47 increased the production of ROS in human neutrophil granulocytes; in contrast, and surprisingly, DE-71 did not appear to increase ROS levels in rat cerebellar granule cells, though its neurotoxicity was antagonized by an antioxidant (Reistad et al. 2006). BDE-47 and other PBDEs have been reported to cause oxidative stress in human neuroblastoma cells (Zhang et al. 2007; He et al. 2008a; Tagliaferri et al. 2010), hippocampal neurons (He et al. 2008b), and cerebellar granule cells (Huang et al. 2010; Blanco et al. 2011). In contrast to previous findings (Reistad et al. 2006), DE-71 was found to cause oxidative stress in mouse cerebellar granule neurons, as evidenced by an increase in ROS and in lipid peroxidation (Giordano et al. 2008). Induction of oxidative stress by DE-71 was more pronounced in neurons from Gclm (−/−) mice, which lack the modifier subunit of glutamate-cysteine ligase, the first and rate limiting enzyme in the synthesis of GSH, and therefore, display very low GSH levels. In addition, increasing intracellular GSH levels, or the presence of various antioxidants (e.g. melatonin, N-acetylcysteine, N-t-phenyl-α-butylnitrone), significantly decreased DE-71 neurotoxicity (Giordano et al. 2008). PBDE-induced oxidative stress has also been observed in non-nervous tissue cell types, such as human fetal liver hematopoietic cells (Shao et al. 2008), human hepatoma cells HepG2 (Hu et al. 2007; 2009), Jurkat T cells (Yan et al. 2011), and human umbilical vein endothelial cells (Kawashiro et al. 2009). Certain hydroxylated PBDEs have also been shown to induce oxidative stress in human liver L02 cells (Zhong et al. 2011).
There is also limited evidence that PBDEs may induce oxidative stress upon in vivo administration. Increases in lipid peroxidation and in the levels of oxidized glutathione were found in the liver of American kestrels (Falco sparverius) treated in ovo with a mixture of BDE-47, -99, -100, and -153 (Fernie et al. 2005). Furthermore, prenatal exposure to BDE-99 has been shown to increase levels of nitric oxide, possibly secondarily to an increase in calmodulin, which may lead to increased nitrosylation of proteins (Llansola et al. 2007). In vivo exposure of mice to BDE-47 (10 mg/kg, per os, on postnatal day 10) causes an increase of oxidative stress (evidenced by changes in lipid peroxidation and isoprostane and protein carbonyl levels) in cerebellum and hippocampus, without altering serum thyroid hormone levels (Costa, Giordano, et al., unpublished results; Table 1). Administration of BDE-99 to adult rats was found to induce oxidative stress in various brain regions, kidney and liver (Belles et al. 2010; Albina et al. 2010), and the same PBDE congener caused oxidative stress in fetal liver upon gestational exposure in rats (Blanco et al. 2012). When given to rats during gestation and postnatally at doses leading to a body burden only slightly higher that what present in humans, BDE-99 was found to cause neurobehavioral alteration accompanied by increased oxidative stress in various brain regions, particularly in the hippocampus (Cheng et al. 2009). Hydroxylated PBDEs have been shown to cause oxidative stress in zebrafish (Usenko et al. 2012). It is also of interest that hypothyroidism may induce oxidative stress, as evidenced by increased hydroxyl radicals, lipid peroxidation and protein carbonyl levels in PTU-treated rats (Rahaman et al. 2001). Thus, both thyroid hormone-mediated, and direct effects of PBDEs may occur, leading to a combined increase in oxidative stress in brain tissue.
Two consequences of PBDE-induced oxidative stress are DNA damage and apoptotic cell death. Madia et al. (2004) first reported that BDE-99 caused apoptotic cell death in human astrocytoma cells. Similar findings were later shown with DE-71 in rat cerebellar granule cells (Reistad et al. 2006), in mouse cerebellar granule cells and cerebellar astrocytes, and in hippocampal and cortical neurons and astrocytes (Giordano et al. 2008; 2009a). BDE-47 was shown to cause apoptotic cell death in hippocampal neurons, cerebellar granule neurons, and human neuroblastoma cells (He et al. 2008a; 2008b; Huang et al. 2010; Tagliaferri et al. 2010; de Laat et al., unpublished results). The cellular GSH content plays a primary role in modulating susceptibility to PBDE-induced apoptosis. For example, mouse hippocampal neurons were 5-fold more susceptible to DE-71-induced apoptosis than cortical neurons, which had a 3.5-fold higher GSH content (Giordano et al. 2008). Astrocytes were found to protect neurons from neurotoxicity of DE-71, by providing GSH (Giordano et al. 2009a). Table 2 shows similar results with regard to BDE-47: apoptosis is much more pronounced in cerebellar granule neurons from Gclm−/− mice because of their low GSH content, and protection by astrocytes is observed primarily in co-cultures of Gclm+/+ neurons and astrocytes. In vivo exposure to BDE-47 (10 mg/kg, per os, on postnatal day 10) was found to increase apoptotic cell death (evidenced by TUNEL staining and increased caspase-3 levels) in various brain regions of mice (Costa, Giordano et al., unpublished results; Table 1).
Table 2.
BDE-47-induced apoptotic cell death is modulated by intracellular glutathione
| Mice | Culture conditions | IC50 | GSH in neurons |
|---|---|---|---|
| Gclm+/+ | CGNs alone | 12.3 ± 3.1 | 7.7 ± 1.3 |
| CGNs + Astrocytes | 50.8 ± 5.1** | 13.2 ± 1.5* | |
| Gclm−/− | CGNs alone | 2.5 ± 0.8 | 0.9 ± 0.2 |
| CGNs + Astrocytes | 5.7 ± 1.3* | 1.3 ± 0.3 |
Cerebellar granule neurons (CGNs) from either Gclm+/+ or Gclm−/− mice were incubated for 24 hours with BDE-47 alone or in the presence of cerebellar astrocytes, in a “sandwich” co-culture system (Giordano et al. 2009a). Apoptosis was assessed by the Hoechst method, and IC50 values (μM) were calculated from concentration-response curves utilizing 4–5 concentrations of BDE-47. Neuronal glutathione (GSH) levels are expressed as nmol/mg proteins. Results represent the mean (± SD) of three separate determinations. CGNs from Gclm−/− mice are more susceptible to BDE-47 toxicity because of their low GSH content. Astrocytes provide a significant degree of protection in Gclm+/+ mice as they are capable on increasing GSH levels in neurons, while protection is much lower in Gclm−/− mice. Significantly different from CGNs alone,
p<0.05;
p<0.01. (Costa and Giordano, unpublished results).
The developing brain is particularly sensitive to oxidative stress, possibly due to its richness in free iron and a limited antioxidant capacity (Sola et al. 2007). The relevant role of oxidative stress in contributing to pathological apoptosis in the developing brain, and in general to developmental brain disorders, has also been underscored (Blomgren et al. 2007; Hayashi, 2009). DNA is a cellular target of oxidation processes, and modified DNA bases constitute one of the major classes of oxidative DNA damage. BDE-47 was shown to cause DNA damage, micronuclei, and nucleoplasmatic bridges increases in human neuroblastoma cells and in rat hippocampal neurons (He et al. 2008a; 2008b; 2010). Pellacani et al. (2012) further showed that both BDE-47 and BDE-209 caused DNA damage in human neuroblastoma cells; they also showed that DNA damage was mostly secondary to oxidative stress, as noted also by Gao et al. (2009). Two BDE-47 metabolites (6-OH-BDE-47 and 6-MeO-BDE-47) were found to cause DNA damage and to increase micronuclei in HepG2 human hepatoma cells (An et al. 2011). Oxidative stress-induced DNA damage may affect neurodevelopmental processes through several mechanisms, such as alterations in gene transcription (Wells et al. 2009; 2010).
The exact mechanisms involved in PBDE-induced oxidative stress and ensuing apoptotic cell death, have not been fully elucidated. However, several lines of evidence point at the mitochondria as the main site of PBDE neurotoxicity. First, PBDEs, which greatly accumulate into neurons (Mundy et al. 2004; Kodavanti et al. 2005; Wei et al. 2010; Huang et al. 2010; Napoli et al. 2013), show a preferential accumulation in mitochondria (Huang et al. 2010). In particular, for the most toxic congeners (e.g. BDE-47, BDE-100), the relative amount of PBDE associated with mitochondria was 30–43% of the total cellular PBDE content (Huang et al. 2010). Furthermore, the order of accumulation of PBDE congeners in neuronal mitochondria was identical to that of their relative potency in inducing oxidative stress and apoptosis (Huang et al. 2010). PBDEs have been shown to uncouple and inhibit electron transport leading to disrupted energy production and increased oxidative stress (van Boxtel et al. 2008; Napoli et al. 2013). BDE-47 and 6-OH-BDE-47 have been shown to inhibit mitochondrial respiration (Marcinek et al., unpublished observation). Mitochondria are a major contributor of cellular ROS; ROS produced in the mitochondria can also target the electron transport chain (e.g. complex I), resulting in a cycle generating more ROS, followed by ATP depletion and ultimately cell death (Ott et al. 2007). Furthermore, a number of studies have shown that PBDEs, and particularly OH-BDEs can alter calcium homeostasis (see following section); this latter effect may be of particular relevance, given the importance of calcium in triggering mitochondrial dysfunction and oxidative stress (Orrenius et al. 2011).
Interference with calcium signaling
Again, at least initially, because of similarities with PCBs, the ability of PBDEs to interfere with calcium homeostasis was investigated, as xenobiotic-induced increases in [Ca2+]i may underlie many of their toxic effects (Mariussen and Fonnum, 2006). In rat astrocytes and in PC12 cells, BDE-99 was found to increase intracellular Ca2+ levels (Smolnikar et al. 2001). Similar results were also obtained with BDE-47 in mouse cerebellar granule neurons (Costa, Tagliaferri, et al., unpublished). Kodavanti and Ward (2005) later showed that DE-71 (8–22 μM) inhibited Ca2+ uptake in microsomes, and particularly in mitochondria isolated from rat brain, and Coburn et al. (2008) confirmed these findings with BDE-47 and BDE-99.
In a subsequent series of studies, Dingemans et al. (2008; 2010a; 2010b) examined in detail the effects of BDE-47 and its hydroxylated metabolites on calcium homeostasis in PC12 cells. An initial finding was that 6-OH-BDE-47 was more potent than BDE-47 in disturbing calcium homeostasis. The hydroxylated metabolite caused an initial transient increase in [Ca2+]i, deriving from the emptying of the endoplasmic reticulum, which was followed by influx of extracellular calcium, and a late increase which originated primarily from the mitochondria (Dingemans et al. 2008). Calcium increase was accompanied by an increase release of catecholamines from PC12 cells. When testing various hydroxylated BDE-47 metabolites, 6-OH-BDE-47 was the most potent in releasing calcium from intracellular stores. Other hydoxylated metabolites were also effective, while a methoxylated metabolite (6-MeO-BDE-47) was ineffective. BDE-47 was the only PBDE, among those tested (BDE-49, -99, -100, -153), which altered calcium homeostasis, though with much less potency than its hydroxylated metabolites (Dingemans et al. 2010a). The hydroxlated metabolites of BDE-47, but not various parent PBDEs, were also capable of inhibiting depolarization-induced calcium release in PC12 cells (Dingemans et al. 2010b). Such rapid increases in [Ca2+]i in response to depolarization mainly originate from influx of calcium via voltage-gated Ca2+ channels in the cell membrane, which are regulated by protein kinases, some of which are also affected by PBDEs (see following section).
As ortho-substituted PCBs (the non-dioxin-like PCBs) disrupt calcium signaling by interfering with ryanodine receptors (Pessah et al. 2010), it was hypothesized that certain PBDEs and/or their metabolites, may have similar effects. Ryanodine receptors are calcium channels located in specialized regions of the sarcoplasmic/endoplasmic reticulum, where they contribute to, and regulate essential aspects of calcium signaling (Pessah et al. 2010). Kim et al. (2011) found that ortho- and para-substitutions were key determinants of PBDE interactions with ryanodine receptors in rodent neurons. Thus BDE-49, with two ortho-bromine and only one para-bromine, was much more potent in interacting with the ryanodine receptors than BDE-47 (which has two para-bromines). In contrast to its parent compound, 6-OH-BDE-47 was found to interact with ryanodine receptors (Kim et al. 2011). The identification of BDE-49 as a potent disruptor of calcium homeostasis may be significant, as this PBDE congener, already a major one in fish (Mariottini et al. 2008), has also been found in pregnant women at levels comparable to those of BDE-47 (Miller et al. 2009) (see also the findings with BDE-49 of Napoli et al. (2013).
Additional mechanisms
A variety of other potential mechanisms have been investigated in in vitro and in vivo studies with different PBDEs. Some relate to signal transduction, others to receptors and neurotransmission, others to more fundamental events in brain development (e.g. neuritogenesis, synaptogenesis).
In human astrocytoma cells, BDE-99 activated protein kinase C (PKC) α, ε and ζ (measured by assessing their translocation to the cell membrane), but only at high concentrations (100 μM) (Madia et al. 2004); this effect was not involved in cytotoxicity, as inhibition or down-regulation of PKCs did not affect the toxicity of BDE-99. Inhibition of mitogen activated protein kinase (MAPK) or tyrosine kinase also had no effect on BDE-99 toxicity, while inhibition of phosphatidylinositol 3-kinase significantly increased BDE-99 toxicity, suggesting that this PBDE may cause apoptosis (Madia et al. 2004). Indeed, as indicated earlier, this was the first demonstration that BDE-99 could cause apoptotic cell death. The lack of involvement of MAPK in PBDE neurotoxicity was confirmed by Fan et al. (2010), who reported that DE-71 could activate MAPK at concentrations below those required to elicit any cytotoxicity. The ability of DE-71, but not of DE-79 (an octaBDE mixture) to activate PKCs (assessed by binding of a phorbol ester) was confirmed in rat cerebellar granule cells (Kodavanti and Ward, 2005). In addition, Kodavanti et al. (2005) reported of similar effects on PKC by several PBDE congeners (BDE-47, -77, -99, -100, -153). Though not involved in cell survival (Madia et al. 2004), activation of PKC may play a role in the regulation of calcium homeostasis, as suggested by Dingemans et al. (2010b). Kodavanti and Derr-Yellin (2002) also reported that DE-71, but not DE-79, stimulated arachidonic acid release in rat cerebellar granule neurons, possibly by activating phospholipase A2. The significance of this interesting observation remains, however, unclear, as no additional studies have investigated this intracellular pathway.
A number of in vitro and in vivo studies have examined effects of PBDEs on receptors and neurotransmission, mostly related to the cholinergic, dopaminergic, GABAergic, and glutamatergic systems. Mariussen and Fonnum (2003) found that DE-71 was a very weak inhibitor of synaptosomal uptake of dopamine, glutamate of GABA (γ-aminobutyric acid), but a more potent inhibitor (IC50 = 8 μM) of vesicular dopamine uptake. In agreement with these in vitro findings, a study by Dreiem et al. (2010) reported that developmental exposure to DE-71 marginally reduced synaptosomal dopamine levels in the striatum. Recently, Bradner et al. (2013a) confirmed the ability of DE-71 to inhibit vesicular dopamine uptake (IC50 = 2.8 μM); these investigators also showed that repeated exposures of young mice to DE-71 decreased striatal dopamine levels and expression of the dopamine transporter and the vesicular monoamine transporter 2, which were accompanied by locomotor deficits. An increase of dopamine level were in contrast found in the cerebral cortex of mice exposed to a single 10 mg/kg does of BDE-47 on PND 10 (Gee et al. 2011). Increases and decreases in brain dopamine in animals exposed during early development or at a later age had been reported also with PCBs (Fonnum and Mariussen, 2009). Overall, effects on the dopaminergic system appear of relevance, given the widespread contamination with PBDEs and the possibility that these chemicals may contribute to the etiology of Parkinson’s disease.
Viberg et al. (2003; 2004; 2007) reported changes in cholinergic nicotinic receptors in the hippocampus of mice upon exposure to a single dose of BDE-99 or BDE-153 on postnatal day 10, or a single dose of BDE-209 on postnatal day 3. In contrast, Bull et al. (2007) found no changes in nicotinic receptors in the cerebral cortex of ranch mink (Mustela vison) following chronic developmental exposure to DE-71. The mechanism(s) of nicotinic receptor alterations, or their possible relevance, is unclear. More recent in vitro data, showing that 6-OH-BDE-47, but not BDE-47 itself, could act as antagonist at human α4β2 nicotinic receptors, do not shed any further light on this issue (Hendriks et al. 2010). This latter study also showed that 6-OH-BDE-47 could act as agonist at GABAA receptors (Hendriks et al. 2010). This may be of interest as, different from what observed in the adult nervous system where GABAA receptors mediate neuronal inhibition, in the developing brain activation of GABAA receptors leads to neuronal apoptotic cell death (Ikonomidou et al. 2001). Alterations in some components of the GABAergic system (e.g. decreases of the GABA-A 2α receptor subunit and the GABA synthesizing enzyme GAD67) have been reported in the cerebral cortex of young adult mice following a 30 day exposure to DE-71 (Bradner et al. 2013b).
A possible involvement of the glutamatergic system in PBDE developmental neurotoxicity has been suggested by various in vivo and in vitro studies. A single exposure of mice to BDE-47 on postnatal day 10 was found to decrease post-synaptic protein involved in glutamate receptor signaling (e.g. glutamate receptor subunits NR2B and GluR1), and to reduce hippocampal long-term potentiation and post-tetanic potentiation (Dingemans et al. 2007). Similar findings were obtained by Yan et al. (2012) in rats exposed for 30 days to BDE-47. They reported decreased levels of the NMDA receptor subunits NR1 and NR2B and of their mRNAs in the hippocampus, which was accompanied by decreased performance in the Morris water maze. In contrast, in young adult mice, a 30 day treatment with DE-71 did not alter NR2B levels, but increased expression of vesicular glutamate transporter (Bradner et al. 2013b). In another study in rats, prenatal exposure to BDE-99 was shown to increase the activity of the glutamate-nitric oxide-cGMP pathway, as assessed by microdialysis (Llansola et al. 2007), possibly as a result of an effect on calmodulin (see below). Reistad et al. (2006) showed that in vitro cytotoxicity of DE-71 was reduced by MK-801, an antagonist of the N-methyl-D-aspartate (NMDA) receptor. These findings were confirmed by Yu et al. (2008) in human neuroblastoma cells, and by Costa, Tagliaferri et al. (in preparation) in mouse cerebellar granule neurons exposed to BDE-47 (Table 3). These latter experiments show that BDE-47-induced increase in ROS levels and ensuing cytotoxicity were antagonized by the NMDA antagonist MK-801. In contrast, MK-801 did not affect the increase of extracellular glutamate induced by BDE-47, suggesting that PBDEs may increase glutamate extrusion and the latter would then stimulate ionotropic glutamate receptors (Table 3).
Table 3.
Effect of the NMDA-receptor antagonist on BDE-47 neurotoxicity in vitro
| End-point | BDE-47 (5 μM) | BDE-47 (5 μM) + MK-801 (10 μM) |
|---|---|---|
| Cell viability (% of control) | 55 ± 6* | 98 ± 8 |
| ROS (% of control) | 320 ± 65* | 135 ± 20 |
| Extracellular glutamate (% of control) | 210 ± 30* | 220 ±18* |
Mouse cerebellar granule neurons were treated with BDE-47 in the absence or presence of the NMDA receptor antagonist MK-801. MK-801 antagonized BDE-47-induced increase in reactive oxygen species and cytotoxicity. BDE-47-induced increase in extracellular glutamate was not antagonized by MK-801. See text for additional details. Results represent the mean (± SD) of at least three separate experiments.
Significantly different from control, p<0.05. (Costa, Tagliaferri et al., unpublished results).
A series of studies suggest that developmental PBDE exposure may interfere with neuritogenesis and/or synaptogenesis. Two proteomic studies reported that exposure of neonatal mice to BDE-99, and exposure to rat cortical cells to the same PBDE, altered the expression of GAP-43, stahmin, and neuromodulin (Alm et al. 2006; 2010). As these proteins are involved in neuritogenesis (Skene, 1989; Grenningloh et al. 2004), their alterations may suggest that BDE-99 interferes somehow with this developmental process. Another in vivo study with BDE-99 also reported alteration in some proteins (e.g. GAP-53) associated with the actin cytoskeleton, which plays a prominent role in neuronal development and differentiation (Alm et al. 2008). Neonatal exposure of mice to BDE-209, BDE-203 or BDE-206 altered the levels of GAP-53, and of CaMKII (calcium/calmodulin-dependent protein kinase II, a protein involved in synaptogenesis and synaptic plasticity) in the hippocampus (Viberg et al. 2008; Viberg, 2009). Changes in CaMKII were also reported by Dingemans et al. (2007) upon neonatal exposure of mice to BDE-47. An in vitro study in chicken neuronal cells showed that DE-71 altered the expression of teneurin, a protein also reported to be involved in neurite outgrowth (Crump et al. 2008). Upon developmental exposure to low doses of BDE-47, a microarray study revealed a down-regulation of genes (Ppfia3, Serpin1, Plxna2, Dscaml1, Dpysl3), involved in neurite growth and guidance (Suvorov and Takser, 2011). An in vivo study in zebrafish showed that exposure to BDE-47 disrupted axonal growth during the early developmental stages (Chen et al. 2012). These findings provide some initial suggestion that PBDEs may affect neuronal differentiation. In contrast, a study in PC12 cells reported that BDE-47 did not affect neurite outgrowth (Dishaw et al. 2011), though in follow-up work by the same investigators, BDE-99 did (Slotkin et al. 2013). A possibility that would be of interest to explore is that PBDEs may affect neuritogenesis “indirectly”, by interfering with astrocyte-neuron communications, as shown for example, in case of manganese (Giordano et al. 2009b). In an in vitro model of human brain development (primary fetal human neural progenitor cells cultured as neurospheres), Schreiber et al. (2010) found that BDE-47 and BDE-99 decreased migration and differentiation into neurons and oligodendrocytes. Of much interest is that addition of T3 rescued these PBDE effects on migration and differentiation, suggesting an involvement of endocrine disruption at thyroid hormone signaling.
Recently, the possibility that PBDEs may exert epigenetic effects upon developmental exposure has been investigated. Epigenetic changes involve a number of mechanisms including DNA methylation, histone modification, and micro RNAs (miRNAs) (Hou et al. 2012). DNA methylation (a covalent modification of primarily, but not exclusively, CG dinucleotide sequence) is the most studied with regard to environmental pollutants (Rusiecki et al. 2008; Baccarelli and Bollati, 2009; Lee et al. 2009; Hou et al. 2012), and limited evidence indicates that certain xenobiotics (e.g. metals, air pollutants) may cause global DNA hypomethylation (Rusiecki et al. 2008; Hou et al. 2012; Christensen and Marsit, 2011). DNA methylation plays an important role in neuronal development (van Bokhoven and Kramer, 2010; Schroeder et al. 2011), and two recent report have provided initial evidence that low doses of PBDEs may affect DNA methylation. In one study, perinatal exposure of mice to low levels of BDE-47 (yielding brain levels of ~20–40 ng/g lipids) was reported to decrease global DNA methylation in cerebral cortex (Woods et al. 2012). In another study, a similar low level perinatal exposure to BDE-47 caused a decrease of expression of a number of genes involved in DNA methylation and histone modification (Suvorov and Takser, 2011). Of much interest is that oxidative stress can suppress the DNA methylation cycle directly (Deth et al. 2008), or indirectly, by reducing intracellular GSH thereby shunting homocysteine into the GSH synthetic pathway rather than the methylation cycle (Lee et al. 2009).
An initial, so far in vitro study, has also started to address the novel hypothesis that PBDE exposure may affect neurogenesis. It is now apparent, that at difference with previous beliefs, functional neurons are generated throughout adult life. Adult neurogenesis, observed in hippocampus and olfactory bulb in rodents, and only in the former region in humans (Ming and Song, 2011; Bergman et al. 2012), is believed to be essential for cognitive processes and synaptic plasticity (Deng et al., 2010; Ming and Song, 2011; Freund et al. 2013). Li et al. (2013) found that 6-OH-BDE-47, but not its parent compound BDE-47, inhibited various aspects of adult neurogenesis in primary adult stem/progenitor cells isolated from the subventricular zone of adult mice.
The issues of dose/concentration and of potential interactions
As shown in the preceding sections, PBDEs and their metabolites can alter cell signaling, induce oxidative stress (which leads to DNA damage and apoptotic cell death), and cause a number of other adverse biological effects. Most of these in vitro mechanistic studies have, however, utilized micromolar concentrations of PBDEs. In contrast, levels of PBDEs in human blood or other tissues, even in the most exposed individuals, are in the nanomolar range. A recent study reports that average BDE-47 levels in human brain were 20 ng/g lipids (Woods et al. 2012). Various studies in animals have reported brain levels of different PBDEs ranging from 1 to 500 nM (Cheng et al. 2009; Reistad et al. 2006; Staskal et al. 2006; Voorspoels et al. 2006; Basu et al. 2007; Crump et al. 2008; Ta et al. 2011; Koenig et al. 2012). Furthermore, in all in vitro studies, the observed toxic effects have been directly correlated to the concentrations of PBDEs in the culture medium, rather than to PBDE burden in cells. PBDEs accumulate in cells over time; accumulation is concentration-dependent, and appears to be highest in cells of nervous system origin, where intracellular PBDE concentrations can be two to three orders of magnitude higher than the concentration of PBDE added to the culture medium (Mundy et al. 2004; Huang et al. 2010; Schreiber et al. 2010; Wei et al. 2010). Wei et al. (2010) concluded that in order for in vitro experiments to mimic realistic environmentally relevant exposures, concentrations of PBDEs should be in the low nanomolar range. Though it has been argued that findings obtained at higher concentrations may not be used to predict low-dose results (Myers et al. 2009), some findings (e.g. mitochondrial toxicity in neuronal progenitor striatal cells by 1 nM BDE-49; Napoli et al. 2013) are somewhat reassuring, as they resemble observations obtained utilizing higher concentrations of PBDEs.
Nevertheless, hormetic-type effects should also be considered upon exposure to low doses of PBDEs. Hormesis is generally defined as a dose-response phenomenon characterized by low-dose stimulation and high-dose inhibition, which may be graphically represented by a J- or U-shaped dose response (Mattson, 2008). Such biphasic dose-response has been shown to occur broadly in the biomedical sciences, being independent of biological model, end-point measured, and chemical class (Calabrese, 2011). Hormesis includes the phenomenon of pre-conditioning, in which “exposure to a low dose of an agent that is toxic at high doses, induces an adaptive, potentially beneficial effect on the cell or organism if exposed to a subsequent a more massive dose of the same or related stressor agent” (Calabrese et al. 2007). As said, one mechanism of PBDEs’ toxicity appears to be their ability to induce oxidative stress, and evidence is emerging to support hormetic roles for low increases in membrane oxidative stress (Calabrese et al. 2010). In particular, while high levels of oxidative stress are unquestionably detrimental to mitochondria, low levels of ROS may actually have a protective, hormetic effect, hence the term “mito-hormesis” (Tapia, 2006; Calabrese et al. 2010; Ristow and Schmeisser, 2011). Wang et al. (2012) reported that incubation of Hep2G human hepatoma cells with BDE-47 (0.1–10 nM) stimulated cell proliferation, while higher concentrations had an inhibitory effect. Similar effects was also observed in MCF-7 cells (a human breast cancer cell line) upon incubation with low (0.001–1.0 nM) or high (>1 μM) concentrations of various PBDE congeners (Barber et al. 2006; Llabjani et al. 2011). Increased cell proliferation at low concentrations has also been reported for BDE-209 (5–100 nM) in various human tumor cells (Li et al. 2012). Furthermore, pre-exposure of cells to BDE-47 (0.1–10.0 nM) appeared to decreased the effect of a higher concentration (50 uM) on DNA damage and apoptotic cell death (Wang et al. 2012).
Another important and practical issue to consider is that exposure to a certain PBDE does not occur in isolation; individuals are known to be exposed to dozens of PBDE congeners, as well to other pollutants, some of which, like the PCBs, may have similar mechanisms and end-points of toxicity. The possibility that interactions may occur is thus a realistic scenario. Tagliaferri et al. (2010) examined the interactions of BDE-47 and BDE-99 in human neuroblastoma cells using oxidative stress and cytotoxicity as endpoints. Results indicated that at relatively low concentrations of each compound (e.g. 1 μM BDE-47 and 5 μM BDE-99) the resulting effect was synergism, while at higher concentrations (15 and 50 μM, respectively) antagonism was observed. In HepG2 cells, BDE-47 and the flame retardant hexabromocyclododecane were found to induce additive toxicity (apoptosis) (Hu et al. 2009). Various studies have examined the interaction between PBDEs and PCBs. He and colleagues studied the interactions between BDE-47 and PCB-153 (both at low micromolar concentrations) in human neuroblastoma cells utilizing oxidative stress, DNA damage, and apoptosis as end-points (He et al. 2009; 2010; Gao et al. 2009). They found that the effects of the PCB were mostly additive to those of the PBDEs, though in some cases there was a synergistic interaction. More recently, Pellacani et al. (2013) investigated the interaction between two PBDEs (BDE-47 and BDE-99) and two PCBs (PCB-153 and PCB-126) in human neuroblastoma cells, with cytotoxicity as an end-point. The results showed that the nature of the interactions was related to the PCB structure. Mixtures of PCB-153 and both PBDEs had a prevalent synergistic effect; in contrast, mixtures of each PBDE congener with PCB-126 (a dioxin-like PCB) showed additive effects at threshold concentrations, and synergistic effects at higher concentrations. Available in vivo evidence is corroborative of the in vitro findings. Eriksson et al. (2006) found that combinations of PCB-52 (a non-dioxin-like PCB congener) and BDE-99, produced enhanced neurotoxicity (compared to each compound alone) in mice exposed on post-natal day ten. In a follow-up study (Fischer et al. 2008), exposure of mice to BDE-99 together with methylmercury enhanced the toxicity of the former in a more than additive manner. Another in vivo study by He et al. (2011) showed that concomitant exposures of rats to low doses of BDE-47 and PCB-153 on postnatal day 10 had a synergistic effect on serum T4 levels and behaviour in the Morris maze. Also in rats, exposure to DE-71 and to Fox River mix (a mixture of several Aroclor formulations containing multiple PCB congeners) during pregnancy, caused an additive effect on dopamine neurochemistry and on circulating T4 levels in developing pups (Dreiem et al. 2010; Miller et al. 2012). Altogether, these studies on mostly additive or synergistic interactions among PBDEs, or between PBDEs and other contaminants, reinforce the notion that the effects of multiple exposures should be taken into account in the risk assessment process.
Conclusions and perspectives
In a previous publication (Costa and Giordano, 2007), we had indicated that many issues needed to be investigated before a clearer picture on PBDE developmental neurotoxicity could emerge. The past six years have seen substantial progress in many areas; however, as it is often the case, more questions have been raised than answered. The fact that perinatal exposure to PBDEs may adversely affect central nervous system development has been confirmed by a number of animal studies, and in particular by the finding of associations between PBDE body burden and behavioral effects in infants and children. The mechanisms underlying such developmental effects remain, however, elusive. One aspect that is emerging is that some of the metabolites of PBDEs, particularly the hydroxylated ones, may play a significant role in developmental neurotoxicity. In some instances, hydroxylated metabolites are more potent than the parent compounds in affecting certain end-points (e.g. calcium homeostasis; Dingemans et al. 2010a; Kim et al. 2011), while in other situations they exert biological effects when the parent PBDE do not (e.g. inhibition of adult neurogenesis; Li et al. 2013).
As indicated previously (Costa and Giordano, 2007), two non-mutually exclusive modes of action are still being investigated. The first relates to an indirect effect of PBDEs which may be mediated by their interactions, at multiple levels, with thyroid hormone homeostasis. This mode of action remains reasonable and plausible, though the animal and human evidence is still contradictory, in that behavioral or biochemical changes have been reported in the absence of any thyroid hormone effect. More attention has been recently given to potential direct interactions of PBDEs with nerve cells. The fact that PBDEs may cause oxidative stress has been confirmed by multiple investigators in different cell systems in vitro, as well as in vivo. Responsible for such oxidative stress may be the ability of PBDEs to disrupt mitochondria, though the exact molecular mechanisms need further scrutiny. PBDE-induced oxidative stress may lead to important adverse effects such as DNA damage and apoptotic cell death, which have been observed in vitro, but whose in vivo significance remains to be established. Similarly, for some of the other interesting biological effects of PBDEs (interference with calcium homeostasis and with other signal transduction pathways, effects of neurotransmission), most reported findings have been obtained in vitro, and there is the need of in vivo confirmation.
One aspect that deserves much attention is that of the dose/concentration of PBDEs used in the studies. Most often, micromolar concentrations of PBDEs are utilized in vitro; these exceed those found in human biota, which are usual in the low to mid nanomolar range. Though PBDEs, particularly certain congeners, have been shown to significantly accumulate in brain cells (Mundy et al. 2004; Huang et al. 2010), there is the need to verify some of the findings utilizing lower, environmentally relevant concentrations. In addition, the issue of potential interactions needs to be taken into account. There are 209 PBDE congeners, and the same number of similar PCBs; in addition, there are several other contaminants known to be developmental neurotoxicants (Grandjean and Landrigan, 2006). The limited evidence available so far, deriving from both in vitro and in vivo studies, indicates that interactions between PBDEs or between PBDEs and other chemicals may result in additive if not synergistic effects.
Highlights.
The flame retardants PBDEs have become worldwide persistent environmental pollutants
Animal and human studies suggest that PBDEs may be developmental neurotoxicants
Underlying mechanisms may be indirect, e.g. disruption of thyroid hormones
Direct mechanism include oxidative stress leading to DNA damage and apoptotic cell death
PBDE also interfere with neurotransmission and signal transduction
Acknowledgments
Research by the authors was supported by grants from the National Institute of Environmental Health Sciences under awards numbers P30ES07033, P42ES04696, R21ES22611. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Gennaro Giordano for his participation in some of the work discussed here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelouahab N, Suvorov A, Pasquier JC, Langlois MF, Praud JP, Takser L. Thyroid hormone disruption by low-dose BDE-47 in prenatally exposed lambs. Neonatology. 2009;96:120–124. doi: 10.1159/000209316. [DOI] [PubMed] [Google Scholar]
- Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt141. in press. [DOI] [PubMed] [Google Scholar]
- Albina ML, Alonso V, Linares V, Belles M, Sirvent JJ, Domingo JL, Sanchez DJ. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology. 2010;271:51–56. doi: 10.1016/j.tox.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, Fischer C, Kultima K, Viberg H, Eriksson P, Dencker L, Stigson M. Proteomic evaluation of neonatal exposureto 2,2′,4,4′, 5 -pentabromodiphenyl ether. Environ Health Perspect. 2006;114:254–259. doi: 10.1289/ehp.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm H, Kultima K, Scholz B, Nilsson A, Andren PE, Fex-Svenningsen A, Dencker L, Stigson M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology. 2008;29:628–637. doi: 10.1016/j.neuro.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, Kultima K, Nilsson A, Andren PE, Savitski MM, Bergman A, Stigson M, Fex-Svenningsen A, Dencker L. In vitro neurotoxicity of PBDE-99: immediate and concentration-dependent effects on protein expression in cerebral cortex cells. J Prot Res. 2010;9:1226–1235. doi: 10.1021/pr900723c. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B. Developmental neurotoxicity of PBDEs: mechanisms and implications. In: Merlani PB, editor. Flame Retardants: Functions, Properties and Safety. Nova Science Publishers; New York: 2010. pp. 1–40. [Google Scholar]
- An J, Li S, Zhing Y, Wang Y, Zhen K, Zhang X, Wang Y, Wu M, Yu Z, Shang G, Fu J, Huang Y. The cytotoxic effects of synthetic 6-hydroxylated and 6-methoxylated polybrominated diphenyl ether 47 (BDE47) Environ Toxicol. 2011;26:591–599. doi: 10.1002/tox.20582. [DOI] [PubMed] [Google Scholar]
- Antignac JP, Cariou R, Maume D, Marchand P, Monteau F, Zalko D, Berrebi A, Cravedi JP, Andre F, Le Bizec B. Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Mol Nutr Food Res. 2008;52:258–265. doi: 10.1002/mnfr.200700077. [DOI] [PubMed] [Google Scholar]
- Athanasiadou M, Cuadra SN, Marsh G, Bergman A, Jacobsson K. Polybrominated diphenyl ethers (PBDEs) and bioaccumulative hydroxylated PBDE metabolite in young humans in Managua, Nicaragua. Environ Health Perspect. 2008;116:400–408. doi: 10.1289/ehp.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Op Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (PBDEs) induce altered characteristics in MCF-7 cells. Mutagenesis. 2006;21:351–360. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Barter RA, Klaassen CD. UDP-glucuronosyltransferase inducers reduce thyroid hormone levels in rats by an extrathyroidal mechanism. Toxicol Appl Pharmacol. 1992;113:36–42. doi: 10.1016/0041-008x(92)90006-e. [DOI] [PubMed] [Google Scholar]
- Basu N, Scheuhammer AM, O’Brien M. Polychlorinated biphenyls, organochlorinated pesticides, and polybrominated diphenyl ethers in the cerebral cortex of wild river otters (Lontra canadensis) Environ Pollut. 2007;149:25–30. doi: 10.1016/j.envpol.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Belles M, Alonso V, Linares V, Albina ML, Sirvent JJ, Domingo JL, Sanchez DJ. Behavioral effects and oxidative status in brain regions of adult rats exposed to BDE-99. Toxicol Lett. 2010;194:1–7. doi: 10.1016/j.toxlet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MSY, Steier P, Kutschera W, Johnson L, Landen M, Druid H, Spalding KL, Frisen J. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J, Mulero M, Lopez M, Domingo JL, Sanchez DJ. BDE-99 deregulates BDNF, Bcl-2 and the mRNA expression of thyroid receptor isoforms in rat cerebellar granular neurons. Toxicology. 2011;290:305–311. doi: 10.1016/j.tox.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Blanco J, Mulero M, Domingo JL, Sanchez DJ. Gestational exposure to BDE-99 produces toxicity through upregulation of CYP isoforms and ROS production in the fetal rat liver. Toxicol Sci. 2012;127:296–302. doi: 10.1093/toxsci/kfs082. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Wilson WW, Lazo CR, Stout KA, Kim HM, Wang MZ, Walker DI, Pennell KD, Richardson JR, Miller GW, Caudle WM. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system; role of dopamine handling and neurotoxicity. Exp Neurol. 2013a;241:138–147. doi: 10.1016/j.expneurol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Caudle WM. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture DE-71. Toxicology. 2013b doi: 10.1016/j.tox.2013.07.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioral development. Neurotoxicology. 2002;23:375–384. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Vitalone A, Madia F, Santucci D, Alleva E, Costa LG. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioral profile: interference from the administration route. Neurotoxicology. 2005;26:183–192. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bull K, Basu N, Zhang S, Martin JW, Bursian S, Martin P, Chan LH. Dietary and in utero exposure to a pentabrominated diphenyl ether mixture did not affect cholinergic parameters in the cerebral cortex of ranch mink (Mustela vison) Toxicol Sci. 2007;96:115–122. doi: 10.1093/toxsci/kfl179. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Toxicology rewrites its history and rethinks its future: giving equal focus to both harmful and beneficial effects. Environ Toxicol Chem. 2011;30:2658–2673. doi: 10.1002/etc.687. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, et al. Biological stress response terminology: integrating the concepts of adaptive response and pre-conditioning stress with hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antiox Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lin Y, Guo LH, Zhang AQ, Wei Y, Yang Y. Structure-based investigation on the binding interaction of hydroxylated polybrominated diphenyl ethers with thyroxine transport proteins. Toxicology. 2010;277:20–28. doi: 10.1016/j.tox.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Chan S, Rovet J. Thyroid hormones in fetal central nervous system development. Fetal Matern Med Rev. 2003;14:177–208. [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Intern. 2007;33:239–245. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Chao HR, Tsou TC, Huang HL, Chang-Chien GP. Levels of breast milk PBDEs from Southern Taiwan and their potential impact on neurodevelopment. Pediatr Res. 2011;70:596–600. doi: 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Chen X, Hunag C, Wang X, Chen J, Bai C, Chen Y, Chen X, Dong Q, Yang D. BDE-47 disrupts axonal growth and motor behaviorin developing zebrafish. Aquat Toxicol. 2012;120–121:35–44. doi: 10.1016/j.aquatox.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Chen A, Park JS, Linderholm L, Rhee A, Petreas M, DeFranco EA, Dietrich KN, Ho SM. Hydrpxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol. 2013;47:3902–3908. doi: 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Gu J, Ma J, Chen X, Zhang M, Wang W. Neurobehavioral effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99. Chemosphere. 2009;75:963–968. doi: 10.1016/j.chemosphere.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Chevrier J. Invited commentary: Maternal plasma polybrominated diphenyl ethers and thyroid hormones-challenges and opportunities. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt138. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harly KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118:1444–1449. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Kogut K, Holland N, Johnson C, Eskenazi B. Maternal thyroid function during the second half of pregnancy and child neurodevelopment at 6, 12, 24 and 60 months of age. J Thyroid Res ID 426427. 2011a:13. doi: 10.4061/2011/426427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Sjodin A, Eskenazi B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol. 2011b;174:1166–1174. doi: 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BC, Marsit CJ. Epigenomics in environmental health. Front Genet. 2011;2:1–10. doi: 10.3389/fgene.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, Kodavanti PRS. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem Res. 2008;33:355–364. doi: 10.1007/s11064-007-9430-x. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenylether flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- Costa LG, Huang SC, Giordano G. Oxidative stress as a potential mechanism for developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. In: Merlani PB, editor. Flame Retardants: Functions, Properties and Safety. Nova Science Publishers; New York: 2010. pp. 101–115. [Google Scholar]
- Costa LG, Giordano G. Isdecabromodiphenyl ether (BDE -209) a developmental neurotoxicant? Neurotoxicology. 2011;32:9–24. doi: 10.1016/j.neuro.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump D, Jagla MM, Chiu S, Kennedy SW. Detection of PBDE effects on mRNA expression in chicken (Gallus domesticus) neuronal cells using real-time RT-PCR and a new differential display method. Toxicol In Vitro. 2008;22:1337–1343. doi: 10.1016/j.tiv.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, Ramakers GMJ, Gardoni F, van Kleef GDM, Bergman A, Di Luca M, van den Berg M, Westerink RHS, Vijverberg HPM. Neonatal exposure to brominated flame retardants BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, de Groot A, ven Kleef RGDM, Bergman A, ven den Berg M, Vijverberg HPM, Westerink RHS. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ Health Perspect. 2008;116:637–643. doi: 10.1289/ehp.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, van den Berg M, Bergman A, Westerink RHS. Calcium-related processes involved in the inhibition of depolarization-evoked calcium increase by hydoxylated PBDEs in PC12 cells. Toxicol Sci. 2010a;114:302–309. doi: 10.1093/toxsci/kfp310. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, Heusinkveld HJ, Bergman A, van den Berg M, Westerink RHS. Bromination pattern of hydroxylated metabolites of BDE-47 affects their potency to release calcium from intracellular stores in PC-12 cells. Environ Health Perspect. 2010b;118:519–525. doi: 10.1289/ehp.0901339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, van den Berg M, Westerink RHS. Neurotoxicity of brominated flame retardants: (In)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM. Is the pentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol. 2011;256:281–289. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Okoniewski RJ, Brosch KO, Miller VM, Seegal RF. Polychlorinated biphenyls and polybrominated diphenyl ethers alter striatal dopamine neurochemistry in synaptosomes from developing rats in an additive manner. Toxicol Sci. 2010;118:150–159. doi: 10.1093/toxsci/kfq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: effects on learning, attention and tyroxine levels. Neurotoxicol Teratol. 2009;31:76–84. doi: 10.1016/j.ntt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci. 2005;88:172–180. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA J. 2011;9:2156, 274. doi: 10.2903/j.efsa.2024.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggesbø M, Thomsen C, Jørgensen JV, Becher G, Odland JØ, Longnecker MP. Associations between brominated flame retardants in human milk and thyroid-stimulating hormone (TSH) in neonates. Environ Res. 2011;111:737–743. doi: 10.1016/j.envres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Hutchings RG, Cherr GN, Hanna LA, Keen CL. Polybrominated diphenyl ether (PBDE)-induced alterations in vitamin A and thyroid hormone concentrations in the rat during lactation and early postnatal development. Toxicol Appl Pharmacol. 2006;215:135–145. doi: 10.1016/j.taap.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Fischer C, Fredriksson A. Polybrominated diphenyl ethers, a group of brominated flame retardants, can interact with polychlorinated biphenyls in enhancing developmental neurobehavioral defects. Toxicol Sci. 2006;94:302–309. doi: 10.1093/toxsci/kfl109. [DOI] [PubMed] [Google Scholar]
- Erratico CA, Szeitz A, Bandiera SM. Oxidative metabolism of BDE-99 by human liver microsomes: predominant role of CYP2B6. Toxicol Sci. 2012;129:280–292. doi: 10.1093/toxsci/kfs215. [DOI] [PubMed] [Google Scholar]
- Erratico CA, Szeitz A, Bandiera SM. Biotransformation of 2,2′,4,4′-tetrabroodiphenyl ether (BDE-47) by human liver microsomes: identification of cytochrome P450 2B6 as the major enzyme involved. Chem Res Toxicol. 2013;26:721–731. doi: 10.1021/tx300522u. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;12:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Besas J, Kodavanti PRS. Changes in mitogen-activated protein kinase in cerebellar granule neurons by polybrominated diphenyl ethers and polychlorinated biphenyls. Toxicol Appl Pharmacol. 2010;245:1–8. doi: 10.1016/j.taap.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Feo ML, Gross MS, McGarrigle BP, Eljarrat E, Barcelo D, Aga DS. Biotrasformation of BDE-47 to potentially toxic metabolites is predominantly mediated by human CYP2B6. Environ Health Perspect. 2013;121:440–446. doi: 10.1289/ehp.1205446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouilard KG, Ritchie IJ. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicol Sci. 2005;88:375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Fischer D, Hooper K, Athanasiadou M, Athanassiadis I, Bergman A. Children show highest levels of polybrominated diphenyl ethers in a California family of four: a case study. Environ Health Perspect. 2006;114:1581–1584. doi: 10.1289/ehp.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. Coexposure of neonatal mice to a flame retardant PBDE 99 (2,2′,4,4′,5-pentabromodiphenyl ether) and methylmercury enhances developmental neurotoxic effects. Toxicol Sci. 2008;101:275–285. doi: 10.1093/toxsci/kfm271. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J Neurochem. 2009;111:1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, Scahser N, Lindenberger U, Kempermann G. Emergence of individuality in genetically identical mice. Science. 2013;340:756–759. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- Gao P, He P, Wang A, Xia T, Xu B, Xu Z. Influence of PCB153 on oxidative DNA damage and DNA repair-related gene expression induced by PBDE-47 in human neuroblastoma cells in vitro. Toxicol Sci. 2009;107:165–170. doi: 10.1093/toxsci/kfn224. [DOI] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybrominated diphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Envir Int. 2011;37:605–611. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol Teratol. 2008;30:79–87. doi: 10.1016/j.ntt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Gee JR, Hedge JM, Moser VC. Lack of alterations in thyroid hormones following exposure to polybrominated diphenyl ether 47 during a period of rapid brain development in mice. Drug Chem Toxicol. 2008;31:245–254. doi: 10.1080/01480540701873194. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC, McDaniel KL, Herr DW. Neurochemical changes following a single dose of polybrominated diphenyl ether 47 in mice. Drug Chem Toxicol. 2011;34:213–219. doi: 10.3109/01480545.2010.536768. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG. Mouse cerebellar astrocytes protect cerebellar granule neurons against toxicity of the polybrominated diphenyl ether (PBDE) mixture DE-71. Neurotoxicology. 2009a;30:326–329. doi: 10.1016/j.neuro.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Pizzurro D, VanDeMark K, Guizzetti M, Costa LG. Manganese inhibits the ability of astrocytes to promote neuronal differentiation. Toxicol Appl Pharmacol. 2009b;240:226–235. doi: 10.1016/j.taap.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H. Role of microtubule destabilizing proteins SCG10 and stathmin in neuronal growth. J Neurobiol. 2004;58:60–69. doi: 10.1002/neu.10279. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New Eng J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hakk H, Letcher RJ. Metabolism, toxicokinetics and fate of brominated flame retardants-a review. Environ Internat. 2003;29:801–828. doi: 10.1016/S0160-4120(03)00109-0. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75:200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- Hamers TJ, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andrsson PL, legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hamers TJ, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJM, Brouwer A, Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Mol Nutr Food Res. 2008;52:284–298. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- Harley KG, Chevrier J, Schall RA, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174:885–892. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. Oxidative stress in developmental brain disorders. Neuropathology. 2009;25:1–8. doi: 10.1111/j.1440-1789.2008.00888.x. [DOI] [PubMed] [Google Scholar]
- He W, He P, Wang A, Xia T, Xu B, Chen X. Effects of PBDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro. Mutat Res. 2008a;649:62–70. doi: 10.1016/j.mrgentox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Chen X. PBDE-47 induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008b;29:124–129. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, Xu BY, Chen XM. Mechanism of the neurotoxic effect of PBDE-47 and interaction of PBDE-47 and PCB153 in enhancing toxicity in SH-SY5Y cells. Neurotoxicology. 2009;30:10–15. doi: 10.1016/j.neuro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- He W, Wang A, Xia T, Gao P, Xu B, Xu Z, He P, Chen X. Cytogenotoxicity induced by PBDE-47 combined with PCB153 treatment in SH-SY5Y cells. Environ Toxicol. 2010;25:564–572. doi: 10.1002/tox.20517. [DOI] [PubMed] [Google Scholar]
- He P, Wang A, Niu Q, Guo L, Xia T, Chen X. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB 153 that enhances toxicity in rats. Toxicol Ind Health. 2011;27:279–288. doi: 10.1177/0748233710387002. [DOI] [PubMed] [Google Scholar]
- Hendriks HS, Antunes Fernandes EC, Bergman A, van den Berg M, Westerink RH. PCB-47, PBDE-47, and 6-OH-PBDE-47 differentially modulate human GABAA and α4β2 nicotinic acetylcholine receptors. Toxicol Sci. 2010;118:635–642. doi: 10.1093/toxsci/kfq284. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect. 2008;116:1376–1382. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Adgent M, Goldman BD, Sjodin A, Daniels JL. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect. 2012;120:1438–1442. doi: 10.1289/ehp.1205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int J Epidemiol. 2012;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Xu Y, Hu DC, Hui Y, Yang FX. Apoptosis induction on human hepatoma cells HepG2 of decabrominated diphenyl ether (PBDE-209) Toxicol Lett. 2007;171:19–28. doi: 10.1016/j.toxlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Hu X, Hu D, Xu Y. Effects of tetrabrominated diphenyl ether and hexabromocyclododecanes in single and complex exposure to hepatoma HepG2 cells. Environ Toxicol Pharmacol. 2009;27:327–337. doi: 10.1016/j.etap.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Huang SC, Giordano G, Costa LG. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol Sci. 2010;114:124–132. doi: 10.1093/toxsci/kfp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhazehiebo K, Iwasaki T, Kimura-Koroda J, Miyazaki W, Shimokawa N, Kolbuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 2011;119:168–175. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Kawashiro Y, Fukata H, Sato K, Aburatani H, Takigami H, Mori C. Polybrominated diphenyl ethers cause oxidative stress in human umbilical vein endothelial cells. Hum Exp Toxicol. 2009;28:703–713. doi: 10.1177/0960327109350669. [DOI] [PubMed] [Google Scholar]
- Kim KH, Bose DD, Ghoga A, Riehl J, Zhang R, Barnhart CD, Lein PJ, Pessah IN. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect. 2011;119:519–526. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim UJ, Kim MY, Hong YH, Lee DH, Oh JE. Assessment of impact of internal exposure to PBDEs on human thyroid function-comparison between congenital hypothyroidism and normal paired blood. Environ Sci Technol. 2012a;46:6261–6268. doi: 10.1021/es2038678. [DOI] [PubMed] [Google Scholar]
- Kim TH, Bang DY, Lim HJ, Won AJ, Ahn MY, Patra N, Chung KK, Kwack SJ, Park KL, Han SY, Choi WS, Han JY, Lee BM, Oh JE, Yoon JH, Lee J, Kim HS. Comparisons of polybrominated diphenyl ethers levels in paired South Korean cord blood, maternal blood, and breast milk samples. Chemosphere. 2012b;87:97–104. doi: 10.1016/j.chemosphere.2011.11.074. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Derr-Yellin EC. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol Sci. 2002;68:451–457. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationship. Toxicol Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, Rayner JL, Kannan K, Birnbaum LS. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Szabo DT, Stoker TE, Fenton SE. Brominated flame retardants. In: Gupta RC, editor. Reproductive and Developmental Toxicology. Elvevier; New York: 2011. pp. 523–541. [Google Scholar]
- Koenig CM, Lango J, Pessah IN, Berman RF. Maternal transfer of BDE-47 to offspring and neurobehavioral development in C57BL/6J mice. Neurotoxicol Teratol. 2012;34:571–580. doi: 10.1016/j.ntt.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays involving Chinese hamster ovary cells. Environ Health Perspect. 2009;117:1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama SN, Fidalgo-Nieto AA, Grande SW, Akkoc Z, de Souza CAM, Chahoud I. Thyroid hormone levels and hepatic enzyme activity in lactating dams after gestational exposure to low dose of PBDE 47. Organohalogen Compd. 2004;66:3901–3906. [Google Scholar]
- Kuriyama SN, Wanner A, Fidalgo-Nieto AA, Talsness CE, Koerner W, Chahoud I. Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology. 2007;242:80–90. doi: 10.1016/j.tox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. 2005;15:60–71. doi: 10.1089/thy.2005.15.60. [DOI] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, deBoer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR, Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNS hypomethylation. Environ Health Perspect. 2009;117:1799–1802. doi: 10.1289/ehp.0900741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Xie Q, Li X, Li N, Chi P, Chen J, Wang Z, Hao C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: in vitro and in silico investigations. Environ Health Perspect. 2010;118:602–606. doi: 10.1289/ehp.0901457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang W, Pan YW, Xu L, Xia Z. A hydroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol Sci. 2013;134:111–124. doi: 10.1093/toxsci/kft083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Liu XY, Wang N, Chen JS, Chen YH, Huang JT, Su CH, Xie F, Yu B, Chen DJ. Effects of decabrominated diphenyl ether (PBDE-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environ Health Prespect. 2012;120:541–546. doi: 10.1289/ehp.1104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabjani V, Trevisan J, Jones KC, Shore RF, Martin FL. Derivation by infrared spectroscopy with multivariate analysis of bimodal contaminant-induced dose-response effects in MCF-7 cells. Environ Sci Technol. 2011;45:6129–6135. doi: 10.1021/es200383a. [DOI] [PubMed] [Google Scholar]
- Llansola M, Erceg S, Monfort P, Montoliu C, Felipo V. Prenatal exposure to polybrominated diphenylether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurons. Eur J Neurosci. 2007;25:373–379. doi: 10.1111/j.1460-9568.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Exp Sci Environ Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Lupton SJ, McGarrigle BP, Olson JR, Wood TD, Aga DS. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chem Res Toxicol. 2009;122:1802–1809. doi: 10.1021/tx900215u. [DOI] [PubMed] [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Malmberg T, Athanasiadou M, Marsh G, Brandt I, Bergman A. Identification of hydroxylated polybrominated diphenyl ether metabolites in blood plasma from polybrominated diphenyl ether exposed rats. Environ Health Technol. 2005;39:5342–5348. doi: 10.1021/es050574+. [DOI] [PubMed] [Google Scholar]
- Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Minuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008;23:150–160. doi: 10.1016/j.taap.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Mariottini M, Corsi I, Della Torre C, Caruso T, Bianchini A, Nesi I, Focardi S. Biomonitoring of polybrominated diphenyl ether (PBDE) pollution: a field study. Comp Biochem Physiol C Pharmacol Toxicol. 2008;148:80–86. doi: 10.1016/j.cbpc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Int. 2003;43:533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Crit Rev Toxicol. 2006;36:253–289. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Aging Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1:343–354. [PubMed] [Google Scholar]
- Meerts IATM, van Zanden JJ, Luijkis EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some polybrominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. The unique regulation of brain cytochrome P450 2 (CYP2) family of enzymes by drugs and genetics. Drug Metab Rev. 2004;36:313–333. doi: 10.1081/dmr-120034149. [DOI] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast Michigan. Environ Sci Technol. 2009;43:3042–3046. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Sanchez-Morrissey S, Brosch KO, Seegal RF. Developmental coexpsoure to polychlorinated biphenyls and polybrominated diphenyl ethers has additive effects on circulating thyroxine levels in rats. Toxicol Sci. 2012;127:76–83. doi: 10.1093/toxsci/kfs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of PBDE-47 in primary cultures of rat neocortical cells. Toxicol Sci. 2004;82:164–169. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 2009;117:1652–1655. doi: 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E, Hung C, Wong S, Giulivi C. Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuroal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicol Sci. 2013;132:196–210. doi: 10.1093/toxsci/kfs339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Sekiguchi S, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Attention-deficit and hyperactive neurobehavioral characteristics induced by perinatal hypothyroidism in rats. Behav Brain Res. 2005;159:323–331. doi: 10.1016/j.bbr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Pellacani C, Buschini A, Galati S, Mussi F, Franzoni S, Costa LG. Evaluation of DNA damage induced by two polybrominated diphenyl ether flame ratardants (BDE-47 and BDE-209) in SK-N-MC cells. Int J Toxicol. 2012;31:372–379. doi: 10.1177/1091581812449663. [DOI] [PubMed] [Google Scholar]
- Pellacani C, Tagliaferri S, Caglieri A, Goldoni M, Giordano G, Mutti A, Costa LG. Synergistic interactions betweenPBDEs and PCBs in human neuroblastoma cells. Environ Toxicol. 2013 doi: 10.1002/tox.21768. in press. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: ryanodine receptor activation as a convergent mechanismof PCB toxicity. Pharmacol Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, Van Baar AL, De Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol. 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect. 2009;117:93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman SO, Ghosh S, Mohanakumar KP, Das S, Sarkar PK. Hypothyroidism in the developing rat brain is associated with marked oxidative stress and aberrant intraneuronal accumulation of neurofilaments. Neurosci Res. 2001;40:273–279. doi: 10.1016/s0168-0102(01)00237-1. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (DE-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicol Sci. 2005;87:57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch Toxicol. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH. Molecular toxicology of polybrominated diphenyl ethers: nuclear hormone receptor mediated pathways. Environ Sci Process Impacts. 2013;15:702–708. doi: 10.1039/c3em00023k. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH, Gao Y, Zhang BT, Wan B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol Appl Pharmacol. 2013;268:256–263. doi: 10.1016/j.taap.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008;226:244–250. doi: 10.1016/j.taap.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending lifespan by increasing oxidative stress. Free Rad Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Rose M, Bennett DH, Bergman A, Fangstrom B, Pessah IC, Hertz-Picciotto I. PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol. 2010;44:2648–2653. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influence motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgreen J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schreiber G. The evolutionary and integrative roles of transthyretin in thyroid hormone homeostasis. J Endocrinol. 2002;175:61–73. doi: 10.1677/joe.0.1750061. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, Götz C, Hübenthal U, moors M, Krause G, Merk HF, Nguyen NH, Scanlan TS, Abel J, Rose CR, Fritsche E. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118:572–578. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder DI, Lott P, Korf I, LaSalle JM. Large-scale methylation domains mark functional subset of neuronally expressed genes. Genome Res. 2011;21:1583–1591. doi: 10.1101/gr.119131.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler GC. The tyroxine-binding proteins. Thyroid. 2000;10:141–149. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol Sci. 2008;101:81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- Shy CG, Huang HL, Cang-Chien GP, Chao HR, Tsou TC. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87:643–648. doi: 10.1007/s00128-011-0422-9. [DOI] [PubMed] [Google Scholar]
- Shy CG, Huang HL, Chao HR, Chang-Chien GP. Cord blood levels of thyroid hormones and IGF-1 weakly correlated with breast milk levels of PBDEs in Taiwan. Int J Hyg Environ Health. 2012;215:345–351. doi: 10.1016/j.ijheh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Singh R, Upadhyay G, Kumar S, Kapoor A, Kumar A, Tiwari M, Godbole MM. Hypothyroidism alters the expression of Bcl-2 family genes to induce enhanced apoptosis in the developing cerebellum. J Endocrinol. 2003;176:39–46. doi: 10.1677/joe.0.1760039. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Patterson DG, Bergman A. A review of human exposure to brominated flame retardants-particularly polybrominated diphenyl ethers. Environ Int. 2003;29:829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Infante A, Seidler FJ. BDE99 (2,2′,4,4′,5-pentabromodiphenyl ether) suppresses differentiation into neurotransmitter phenotypes in PC12 cells. Neurotoxicol Teratol. 2013;37C:13–17. doi: 10.1016/j.ntt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolnikar K, Dehnhardt M, Wiegand H. Perturbation by PBDE99 of calcium homeostasis after in vitro treatment. BFR abstracts. 2001 May 14–16;:189. 2001. [Google Scholar]
- Sola A, Rogido MR, Deulofeut R. Oxygen as a neonatal health hazard: call for detente in clinical practice. Acta Paediatrica. 2007;96:801–812. doi: 10.1111/j.1651-2227.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunch C. Metabolism of polybrominated diphenyl ethers by human hepatocytes in vitro. Environ Health Perspect. 2009;117:197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119:1454–1459. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Birnbaum LS. Disposition of BDE 47 in developing mice. Toxicol Sci. 2006;90:309–316. doi: 10.1093/toxsci/kfj098. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Girard S, Lapachelle S, Abdelouahab N, Sebire G, Takser L. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology. 2009;95:203–209. doi: 10.1159/000155651. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Takser L. Delayed response in the rat frontal lobe transcriptome to perinatal exposure to the flame retardant BDE-47. J Appl Toxicol. 2011;31:477–483. doi: 10.1002/jat.1667. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Bissonnette C, Takser L, Langlis MF. Does 2,2′,4,4′-tetrabromodiphenyl ether interact directly with thyroid receptor? J Appl Toxicol. 2011;31:179–184. doi: 10.1002/jat.1580. [DOI] [PubMed] [Google Scholar]
- Szabo DT, Richardson VM, Ros DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TA, Koenig CM, Golub MS, Pessah IN, Qi L, Aronov PA, Berman RF. Bioaccumulation and behavioral effects of 2,2′,4,4′,-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol Teratol. 2011;33:393–404. doi: 10.1016/j.ntt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG. Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol In Vitro. 2010;24:116–122. doi: 10.1016/j.tiv.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Talsness CE. Overview of toxicological aspects of polybrominated diphenyl ethers: a flame retardant additive in several consumer products. Environ Res. 2008;108:158–167. doi: 10.1016/j.envres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Kuriyama SN, Grande WS, Andrade A, Sterner-Kock A, Schnitker P, et al. Low dose effect on the rat female reproductive system following exposure to a single administration of PBDE-47. Organohalogen Compd. 2004;66:407–409. [Google Scholar]
- Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “mitohormesis” for health and vitality. Med Hyp. 2006;66:832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) then in infants and adults. Environ Health Perspect. 2009;117:1461–1465. doi: 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng LH, Li MH, Tsai SS, Lee CW, Pan MH, Yao WJ, Hsu PC. Developmental exposure to decabromodiphenyl ether (PBDE 209): effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere. 2008;70:640–647. doi: 10.1016/j.chemosphere.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Hopkins DC, Trumble SJ, Bruce ED. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol Appl Pharmacol. 2012;262:43–51. doi: 10.1016/j.taap.2012.04.017. [DOI] [PubMed] [Google Scholar]
- USEPA (United States Environmental Protection Agency) An Exposure Assessment of Polybrominated Diphenyl Ethers. USEPA; Washington DC: 2010. p. 378. [Google Scholar]
- van Bokhoven H, Kramer JM. Disruption of the epigenetic code: an emerging mechanism in mental retardation. Neurobiol Dis. 2010;39:3–12. doi: 10.1016/j.nbd.2010.03.010. [DOI] [PubMed] [Google Scholar]
- van Boxtel AL, Kamstra JH, Cenijin PH, Pieterse B, Wagner JM, Antink M, Krab K, van der Burg B, Marsh G, Brouwer A, Legler J. Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish. Environ Sci Technol. 2008;42:1773–1779. doi: 10.1021/es0720863. [DOI] [PubMed] [Google Scholar]
- Viberg H. Exposure to polybrominated diphenyl ethers 203 and 206 during the neonatal brian growth spurt affects proteins important for normal neurodevelopment in mice. Toxicol Sci. 2009;109:306–311. doi: 10.1093/toxsci/kfp074. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behavior, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2′,4,4′5-pentabromodiphenyl ether decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behavior in the adult mouse. Environ Toxicol Pharmacol. 2004;17:61–65. doi: 10.1016/j.etap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Changes in spontaneous behavior and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209) Neurotoxicology. 2007;28:136–142. doi: 10.1016/j.neuro.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Viberg H, Mundy W, Eriksson P. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29:152–159. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Voorspoels S, Covaci A, Lepom P, Jaspers VL, Schepens P. Levels and distribution of polybrominated diphenyl ethers in various tissues of birds of prey. Environ Pollut. 2006;144:218–227. doi: 10.1016/j.envpol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Wang L, Zou W, Zhong Y, An J, Zhang X, Wu M, Yu Z. The hormesis effect of BDE-47 in HepG2 cells and the potential molecular mechanism. Toxicol Lett. 2012;209:193–201. doi: 10.1016/j.toxlet.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Wei RG, Zhao YX, Liu PY, Qin ZF, Yan SS, Li Y, Qin XF, Xia XJ, Xu XB, Yan MC. Determination of environmentally relevant exposure concentrations of polybrominated diphenyl ethers for in vitro toxicological studies. Toxicol In Vitro. 2010;24:1078–1085. doi: 10.1016/j.tiv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJ, Perstin TJ, Wiley MJ, Wong AW. Oxidative stress in developmental origin of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Lam KC, Henderson JT, Ondovcik SL. Oxidative DNA damage and repair in teratogenesis and neurodevelopmental deficits. Birth Def Res. 2010;90:103–109. doi: 10.1002/bdrc.20177. [DOI] [PubMed] [Google Scholar]
- Williams AL, DeSesso JM. The potential of selected brominated flame retardants to affect neurological development. J Toxicol Environ Health, pt B. 2010;13:411–448. doi: 10.1080/10937401003751630. [DOI] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH, Kostyniak PJ, Pessah IN, Berman RF, LaSalle J. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2302 mutation. Hum Mol Genet. 2012;21:2399–2411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Shan L, Su Y, Barresi E, DeJong M, Hung H, Lei YD, Wania F, Reiner EJ, Sverko E, Kang SC. Atmospheric concentrations of halogenated flame retardants at two remote locations: the Canadian High Arctica and the Tibetan Plateau. Environ Pollut. 2012;161:154–161. doi: 10.1016/j.envpol.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Yan C, Huang D, Zhang Y. The involvement of ROS overproduction and mitochondrial dysfunction in PBDE-47-induced apoptosis on Jurkat cells. Exp Toxicol Pathol. 2011;63:413–417. doi: 10.1016/j.etp.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Yan T, Xiang L, Xuejun J, Chengzhi C, Youbin Q, Xuelan Y, Yang L, Changyan P, Hui C. Spatial learning and memory deficit of low level polybrominated diphenyl ether-47 in male adult rat is modulated by intracellular glutamate receptors. J Toxicol Sci. 2012;37:223–233. doi: 10.2131/jts.37.223. [DOI] [PubMed] [Google Scholar]
- Yu K, he Y, Yeung LWY, Lam PKS, Wu RSS, Zhou B. DE-71-induced apoptosis involving intracellular calcium and the Bax-mitochondria-caspase protease pathway in human neuroblastoma cells in vitro. Toxicol Sci. 2008;104:341–351. doi: 10.1093/toxsci/kfn088. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M. Polymorphic CYP2B6 molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–758. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]
- Zhang M, He WM, He P, Xia T, Chen XM, Wang AG. Effects of PBDE-47 on oxidative stress and apoptosis in SH-SY5Y cells. Zhongua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25:145–147. [PubMed] [Google Scholar]
- Zhong YF, Wang LL, Yin LL, An J, Hou ML, Zheng KW, Zhang XY, Wu MH, Yu ZQ, Sheng GY, Fu JM. Cytotoxic effects and oxidative stress response of six PBDE metabolites on human L02 cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2011;46:1320–1327. doi: 10.1080/10934529.2011.602937. [DOI] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Crofton KM. Mode of action: developmental thyroid hormone insufficiency - Neurological abnormalities resulting from exposure to propylthiouracil. Crit Rev Toxicol. 2005;35:771–781. doi: 10.1080/10408440591007313. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–7905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]