Abstract
Objective
The aim of this study is to investigate the prognostic value of F-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography (PET)/computed tomography (CT) in gallbladder cancer patients.
Methods
From June 2004 to June 2010, a total of 50 patients with gallbladder cancer who underwent diagnostic staging with F-18 FDG PET/CT following curative or palliative treatments were retrospectively evaluated. For the analysis, all patients were classified by age, sex, maximum standardized uptake value (SUVmax), lymph node (LN) or distant metastasis, serum level of CA19-9 and CEA, type of treatment and American Joint Committee on Cancer (AJCC) stage.
Results
The median survival for the 50 patients was 245 days and the median SUVmax in PET/CT was 8.3 (range, 0-19.7). Patients with SUVmax < 6 survived significantly longer than patients with SUVmax ≥ 6 (median 405 days vs 203 days, p = 0.0400). On Kaplan-Meier analysis, SUVmax (p = 0.0400), stage (p = 0.0001), CA19-9 (p = 0.013), CEA (p = 0.006), LN metastasis (p = 0.0001), distant metastasis (p = 0.0020), type of treatment (p = 0.0001) were significantly associated with overall survival. Multivariate analysis study revealed that the patients with lower SUVmax measured from initial staging PET/CT (p = 0.0380), no LN metastasis (p = 0.0260), a lower stage (p = 0.026) and curative treatment (p = 0.0005) had longer survivals.
Conclusions
The present study shows that SUVmax on F-18 FDG PET/CT can provide prognostic information in patients with gallbladder cancer.
Keywords: Survival, FDG, PET, Gallbladder cancer
Introduction
Gallbladder cancer is a highly aggressive malignancy with poor prognostic results because of its anatomical location, lack of typical symptoms and aggressive biologic nature. The 5-year survival rate of gallbladder cancer is reported at less than 15 % [1]. Because of the lack of obvious symptoms, most patients with gallbladder cancer are diagnosed at an advanced stage with clinically evident disease and when curative surgical resection is not possible. In a recent study, 80 % of the patients had metastatic disease and only 20 % had potentially resectable disease at time of the diagnosis [2].
Surgical resection is the only current therapeutic approach that is potentially curative, but tumor recurrence is common after curative resection and the benefits of adjuvant chemotherapy and radiotherapy are dubious.
Survival of this malignancy is related to the disease stage, depth of tumor invasion, lymph node (LN) and distant metastasis [3]. Because gallbladder cancer spreads early, an extensive workup is important in order to accurately define the tumor stage, especially LNs and distant metastases.
Multiple studies have evaluated the extent of the disease, with computed tomography (CT) and magnetic resonance imaging (MRI) being the most frequently utilized.
Fluorine-18 (F-18) fluorodeoxyglucose (FDG) positron emission tomography (PET) plus computed tomography (CT) has been recently reported to improve the sensitivity to detect primary or metastatic gallbladder malignancies [4]. F-18 FDG PET can detect tumors earlier than conventional imaging, and can evaluate the aggressiveness of tumor and predict a prognosis based on the increased glucose uptake by malignant cells [5]. As gallbladder cancer is a malignant tumor with a tendency to early systemic spread, this imaging tool could prove to be useful in identifying and selecting patients with disseminated disease not amenable to curative resection [6].
Recent studies using F-18 FDG PET/CT were limited to evaluation of staging and detection of distant metastasis or recurrence [2, 7], but there have been no studies to assess FDG uptake of F-18 FDG PET/CT as a prognostic predictor in patients with gallbladder cancer.
Therefore, the aim of this study was to investigate the prognostic value of F-18 FDG PET/CT in patients with gallbladder cancer.
Materials and Methods
Patient Eligibility
We obtained Institutional review board (IRB) approval. From June 2004 to June 2010, 50 consecutive patients with gallbladder cancer who underwent F-18 FDG PET as a pre-treatment workup were enrolled in our retrospective study. Informed consent for the procedure was obtained from each patient. In 45 of 50 patients, the presence of adenocarcinoma was histologically proved with specimens obtained by surgery, biopsy or cytologic analysis. And the rest of patients were clinically diagnosed based on imaging modalities of CT, endoscopic ultrasonography (EUS), cholangiography and hematological findings before treatment. Pathologic diagnosis and classification of the tumors were made according to the American Joint Committee on Cancer (AJCC) staging system. The clinical and pathologic records of each patient were reviewed, and the following information was gathered: age, sex, maximum standardized uptake value (SUVmax), serum level of carbohydrate antigen (CA)19-9 and carcinoembryonic antigen (CEA) before treatment, type of treatment (surgery, chemotherapy and or supportive care) and AJCC staging.
F-18 FDG PET/CT Acquisition
PET/CT scans were performed on Biograph6 (Siemens Medical Solution, Knoxville, TN, USA) and Discovery LS (General Electric Medical Systems, Milwaukee, USA) PET/CT scanners. All patients fasted for at least 6 h before the administration of F-18 FDG and FDG was injected intravenously with 10-12 mCi (370-444 MBq) 1 h prior to imaging. Blood sugar levels of all patients were measured prior to injection of F-18 FDG. A non-enhanced low-dose CT scan was obtained for attenuation correction because all patients had already undergone contrast-enhanced abdominopelvic CT before the F-18 FDG PET/CT scan. The CT portion of the Discovery LS consists of a multidetector helical scanner (LightSpeed Plus; General Electric Medical Systems) and Biograph 6 consists of a six-slice CT. Imaging parameters were as follows for an acquisition at five to seven bed positions: 140 kV, 80 mA, 0.8 s per CT rotation, a pitch of 6, a table speed of 22.5 mm/s, 722.5-1,011.5 mm coverage, a 31.9-37 s acquisition time for Discovery LS and 130 kV, 30 mA, 0.6 s per CT rotation, a pitch of 1.5, 20.89 s acquisition time for Biograph 6. The CT scan was performed before emission PET scans. The current of the CT tube was adjusted according to patient weight. The CT data were resized from a 512 × 512 matrix to a 128 × 128 matrix to match the PET data in order to generate a CT transmission map and to fuse images. PET emission data were acquired for five to seven bed positions, typically from the base of the skull through the upper thigh. Emission data were acquired for 6 min for each bed position. Each bed had 35 (for Discovery LS) or 39 (for Biograph 6) scanning planes with a 14.6 cm (for Discovery LS) or 16.2 cm (for Biograph 6) longitudinal field of view and a one-slice overlap. PET images were reconstructed using CT for attenuation correction with the ordered-subsets expectation maximization algorithm (two iterations, eight subsets) and a 5-mm Gaussian filter using a 128 × 128 matrix.
In the evaluation of inter-scanner comparison of SUV value, the measurement of radioactivity is most important, because SUV value has a linear relationship with the radioactivity. Therefore, we performed an experiment to evaluate the variability in radioactivity measurement from the both PET/CT cameras, using a pie-shaped phantom as previously described [21]. In this experiment, we used the same parameters for acquisition and reconstruction of image used in human study. We obtained pie-shaped images with six sectors reflecting different radioactivities (0, 7,000, 14,000, 28,000, 57,000, and 114,000 MBq/ml) of F-18, and placed six small circular regions of interest on the each sector to get pixel value in MBq/ml unit. The values of radioactivity from images were compared and showed the variable difference less than 5 % in quantitative measurements between two PET/CT cameras (estimated regression slope = 0.98, r2 = 0.99, p = 0.001).
FDG PET/CT Image Interpretation
All PET/CT scans were examined retrospectively by three observers on an interactive computer display using fusion software (Xeleris; General Electric Medical Systems and Syngo; Siemens Medical Solutions). This software allows review of PET, CT, and fused data using transaxial, sagittal, and coronal displays. To perform a quantitative analysis, the SUV was calculated in the suspected neoplastic foci (SUV tissue tracer concentration/injected dose/body weight). For the SUV analysis, a circular region of interest was placed over the area of maximal focal FDG uptake suspected to be a tumorous focus, and the maximal values were obtained.
Statistical Analysis
Statistical analysis was performed using Medcalc software v. 11.3. All values are expressed as means ± standard deviation (SD). The following statistical analyses of potential prognostic factors were performed in all 50 patients with gallbladder cancer. Patients were stratified and analyzed by univariate analysis, using age, sex, pre-treatment serum CA 19-9 level, serum CEA level, tumor size, tumor location, type of treatment, AJCC stage and the SUVmax of the primary lesion. Patients were classified into low SUVmax and high SUVmax subgroups by ROC analysis. Survival time was measured from the date of the pre treatment FDG-PET study until the date of death. Overall cumulative survival was analyzed by the Kaplan-Meier method and differences in survival between subgroups were compared using the log-rank test. A p value of <0.05 was considered to be statistically significant. Variables with p < 0.05 in univariate analysis of factors affecting survival were included in a subsequent multivariate analysis, using a Cox proportional hazard model.
Results
Patients Characteristics
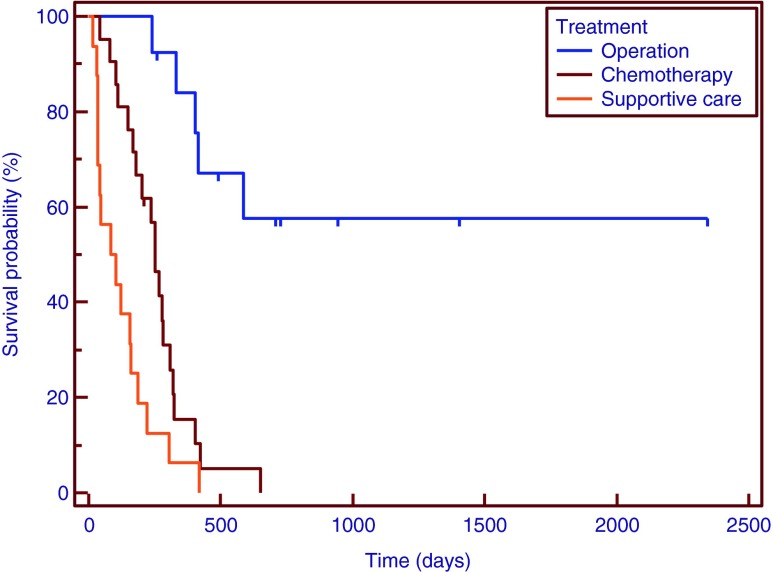
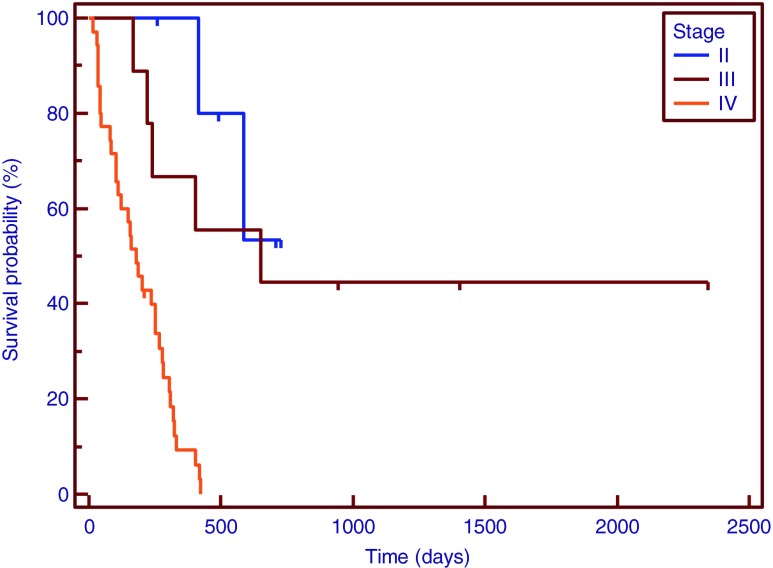
Patients’ characteristics are detailed in Table 1. The mean age of the patients in our study was 67 ± 8 years (range, 48–83 years; 21 men and 29 women). Overall median survival in the patients included in our study was 245 days, mean SUVmax was 8.3 (range, 0–19.7). The median CA19-9 and CEA level for the all patients was 69.9 U/ml and 14.3 U/ml. Number of patients by stage were: 6 in stage I and II, 9 in stage III, 35 in stage IV and type of treatment were 11 in surgery, 21 in chemotherapy, 18 in supportive care. The median survival time for patients with surgery was 753 days, 253 days in chemotherapy and 93 days in supportive care.
Table 1.
Characteristics of patients
| Low SUV (≤6.0) | High SUV (>6.0) | p value | |
|---|---|---|---|
| SUVmax (mean ± SD) | 4.6 ± 1.7 | 8.9 ± 3.6 | |
| Median survival (days) | 404 | 212 | |
| Age (mean ± SD, years) | 62 ± 6.1 | 69 ± 7.7 | 0.0200 |
| Sex | |||
| Male | 6 | 15 | 0.2800 |
| Female | 4 | 25 | |
| Stage | |||
| I | 0 | 0 | |
| II | 3 | 3 | 0.2000 |
| III | 1 | 8 | |
| IV | 6 | 29 | |
| Pathologic proven | 9 | 36 | |
| Clinical diagnosis | 1 | 4 | |
| CA19-9 (mean ± SD, U/ml) | 99 ± 2,931 | 64 ± 5,394 | 0.7800 |
| CEA (mean ± SD, U/ml) | 3.8 ± 1.44 | 3.6 ± 278 | 0.7800 |
| Treatment | |||
| Operation | 4 | 7 | |
| Chemotherapy | 4 | 17 | 0.2500 |
| Supportive care | 2 | 16 | |
SUVmax maximum standardized uptake value
Comparison of Survival by SUVmax
Cutoff value of the SUVmax for the 50 patients was 6.0 (10 patients had SUVmax of 6.0 and 40 had a SUVmax of ≥6.0) by ROC analysis. The median survival for patients with a SUVmax of <6.0 was 405 days versus 203 days for patients with a SUVmax of ≥6.0 (p < 0.0400). These two groups differ statistically with regard to age (p = 0.0200). Patient groups with stage III-IV had a higher SUVmax than those with stage II. We were able to find a statistical difference in survival and SUVmax between surgical and non-surgical groups (p = 0.0001). In addition, patient groups with higher SUVmax had a rising tendency to medical and or supportive care.
Survival Analysis
A Kaplan-Meier curve was drawn for patients with SUVmax of <6.0 or ≥6.0. In the univariate analysis, SUVmax (p = 0.0400), tumor stage (p = 0.0001), serum level of CA19-9 (p = 0.0130) and CEA (p < 0.0060), type of treatment (p = 0.0001), LN metastasis (p = 0.0001), distant metastasis (p = 0.0020) showed a significant relationship with overall survival (Table 2). In multivariate analysis, SUVmax (p = 0.0380), LN metastasis (p = 0.0260), stage (p = 0.0260) and type of treatment (p = 0.0005) were found to be independent predictors of overall survival. In particular, the hazard ratio of SUVmax 3.05 (95 % CI, 1.06–8.71), of LN metastasis was 3.35 (95 % CI, 1.16–9.66), and stage was 3.45 (95 % CI, 1.18–10.23) (Table 3 and Figs. 1, 2, 3, and 4).
Table 2.
Univariate analysis
| Variable | No. of patients | Median survival (days) | p value |
|---|---|---|---|
| Age | |||
| <67 | 25 | 306 | 0.5300 |
| ≥67 | 25 | 203 | |
| Sex | |||
| Male | 21 | 280 | 0.1500 |
| Female | 29 | 221 | |
| SUVmax | |||
| <6.0 | 10 | 405 | 0.0400 |
| ≥6.0 | 40 | 203 | |
| Stage | |||
| I | 0 | 0 | |
| II | 6 | 650 | 0.0001 |
| III | 9 | 530 | |
| IV | 35 | 180 | |
| CA19-9 | |||
| <69.9 | 25 | 323 | 0.0100 |
| ≥69.9 | 25 | 235 | |
| CEA | |||
| <14.3 | 25 | 309 | 0.0060 |
| ≥14.3 | 25 | 180 | |
| LN metastasis | |||
| N0 | 11 | 747 | 0.0001 |
| N1 | 7 | 330 | |
| N2 | 32 | 170 | |
| Treatment | |||
| Operation | 13 | 753 | |
| Chemotherapy | 21 | 253 | 0.0001 |
| Supportive | 16 | 93 | |
| Distant metastasis | |||
| No | 28 | 280 | 0.0020 |
| Yes | 22 | 155 | |
SUVmax maximum standardized uptake value
Table 3.
Multivariate analysis
| Covariable | Hazard ratio | 95 % confidence interval | p value |
|---|---|---|---|
| SUVmax | 3.0506 | 1.06–8.71 | 0.0383 |
| LN metastasis | 3.3503 | 1.16–9.66 | 0.0260 |
| Stage | 3.4576 | 1.18–10.23 | 0.0259 |
| Treatment | 3.1721 | 1.66–6.05 | 0.0005 |
| Distant metastasis | 2.0010 | 0.88–4.52 | 0.0973 |
| CA19-9 | 2.0501 | 0.69–3.21 | 0.3061 |
| CEA | 1.4954 | 0.90–4.64 | 0.2010 |
SUVmax maximum standardized uptake value
Fig. 1.
Survival curves of patients with maximum standardized uptake values (SUVmax) of >6.0 (40 patients, broken line) or ≤6.0 (10 patients, unbroken line)
Fig. 2.
Survival curves for patients of N stage 0 (11 patients, unbroken line), 1 and 2 (39 patients, dotted line)
Fig. 3.
Survival curves by treatment type: operation (11 patients, unbroken line), chemotherapy (21 patients, broken line), and other treatments (18 patients, dotted line)
Fig. 4.
Survival curves by tumor stage: II (6 patients, unbroken line), III (9 patients, broken line), and IV (35 patients, dotted line)
Discussion
F-18 FDG PET is a relatively recent, noninvasive imaging technique that is based on the principle of specific tissue metabolism because of selective F-18 FDG uptake and retention by malignant cells. F-18 FDG PET has the advantage of providing scans of the whole body in one session, and allowing initial staging (including LN and distant metastasis) and early detection. Petrowsky et al. [8] reported that PET-CT was superior to conventional imaging modality, such as contrast-enhanced CT scan in the diagnosis of LN and distant metastases in patients with gallbladder cancer. F-18 FDG PET has been proposed for diagnosis, staging, effectiveness of treatment and the prediction of long-term survival in different malignancies [9–12].
Several studies involving detection of recurrent gallbladder tumors [13], staging [14], and differentiating [15] have been conducted.
Various studies have assessed whether the tumor SUVmax can be used to predict the survival of patients with biliary tract malignancies. Furugawa et al. [16] reported that patients with high SUVmax of biliary tract carcinoma had a poorer survival rate than those with lower SUVs on univariate analysis, but multivariate analysis showed that the pN, pM, pTNM stage were independent factors, and SUVmax was not.
Kitamura et al. [17] reported a similar relation between prognosis and SUVmax at a cutoff of 5.7, and their multivariate analysis revealed that SUVmax, tumor stage, treatment and LN metastasis were an independent predictors of survival.
Although gallbladder cancer was classified under extrabile duct cancer, studies separated from extrabile duct cancer have not been done for prognostic parameters in patients with gallbladder cancer.
In addition, Shibata et al. [18] reported that LN metastasis, stage T3 were independent predictors of survival, and Chan et al. [19] reported that significantly better survival was associated with only curative treatment compared with palliative treatment in patients with gallbladder cancer. In these studies, independent prognostic factors were analyzed without using FDG PET.
Our study evaluates the association between FDG uptake of primary tumor and prognosis in patients with gallbladder cancer and showed that the SUVmax of the primary tumor was an independent prognostic factor.
Survival analysis showed that survival was significantly influenced also by stage, CA19-9, CEA, LN metastasis, distant metastasis and type of treatment in univariate analysis. In multivariate analysis, SUVmax, LN metastasis, stage and type of treatment were significantly associated with survival time. The hazard ratio for higher SUVmax scores was over 3.1-times that of lower SUVmax groups and was independent of other prognostic factors. The hazard ratio of SUVmax was similar to the LN metastasis and stage, well-known prognostic factors for gallbladder cancer [20].
According to our results, the SUVmax calculated with F-18 FDG-PET can be used as a strong independent prognostic parameter of gallbladder cancer, allowing accurate identification of those patients who will benefit from intensive anticancer treatment at different stages of the disease.
In previous study, Yoo et al. [5] reported that TLG was independent prognostic factors for predicting overall survival, but SUVmax was not a statistically significant prognostic factor. Although it is argued that SUVmax may not represent the heterogenous character of the whole tumor, the SUVmax may represent the higher glucose metabolism of tumor. And SUVmax is a widely used semi-quantitative value that can be easily assessed by a formula that uses the amount of FDG injected and the patient’s weight, this simplicity is useful in the clinical setting and contrasts with the complexity of full quantitative assessment or additional program settings to measure metabolic volume. In addition, measurement of metabolic tumor volume are under controversy because of methods of measuring of threshold (percentage of SUV or standard deviation). So results of our study is different from previous one, SUVmax may additional or supportive prognostic factor for predicting overall survival at least.
In addition, we verified that LN metastasis, stage and type of treatment significantly predict survival in pretreatment F-18 FDG PET/CT. Distant metastasis had a significant association with survival in univariate analysis, but did not have an independent effect in multivariate analysis (p < 0.058). Because F-18 FDG PET/CT has advantages of being able to detect early incidental or unexpected distant metastasis in initial staging and lower exposure to radiation due to one session scans compared with conventional imaging modalities, we suggest that tumor stage, LN and distant metastases in pretreatment F-18 FDG PET/CT can influence decisions of resectability and prognostic predictions in patients with gallbladder cancer. Meng et al. [21] reported that radical resection might result in a reasonable prognosis for gallbladder cancer patients with local metastasis of the LNs, but was not effective when distant LNs were involved.
An early diagnosis for gallbladder cancer is difficult because of lack of typical symptoms and aggressive biologic characteristics. There is a tendency for early LN metastasis and direct invasion into the liver, and spreading to abdominal cavity, biopsy tract at diagnosis. For this reason, a number of non-resectable patients in advanced gallbladder cancer received chemotherapy or supportive care more than other gastrointestinal malignancies [22]. Efficacy of non-operative therapy was doubtful and there are no established therapeutic options with improved prognosis [23, 24]. Therefore, we divided into three groups (operation, chemotherapy and supportive care) and overall survival time in resected patients was longer than that in non-resected patients in univariate analysis. This was most likely because that resected patients had a little local invasion and distant metastasis.
Our study had a several limitations. First, the use of ROC curve analysis in the prediction of binary events has achieved attractiveness, as the test characteristics of sensitivity and specificity are relevant to discriminating high-risk subjects from low-risk subjects. Analyzing differences in the ROC curve analysis is a common method of comparing two models for prognostic risk prediction. We used a simple ROC curve for calculation of the ideal cutoff point of SUVmax. However, there may be controversy regarding the correlation between prognosis and SUVmax due to possibility of bias by variation of follow-up period, but the important point is that of the F-18 FDG PET/CT parameters, SUVmax is also a predictor of prognosis with metabolic tumor volume. Second, we used two different types of PET scanners.
In conclusion, SUVmax by measured F-18 FDG PET/CT was found to be significantly related to survival and it could be useful to predict prognosis in patients with gallbladder cancer.
Acknowledgments
This work was partly supported by the Nuclear R & D Program (2010-0017587) of the Korea Science and Engineering Foundation funded by the Korean Ministry of Education, Science, and Technology and was partly supported by grants from the National Research Foundation (2012013722) of Korea.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Donohue JH. Present status of the diagnosis and treatment of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:530–534. doi: 10.1007/s005340100021. [DOI] [PubMed] [Google Scholar]
- 2.Butte JM, Redondo F, Waugh E, Meneses M, Pruzzo R, Parada H, et al. The role of PET-CT in patients with incidental gallbladder cancer. HPB (Oxford) 2009;11:585–591. doi: 10.1111/j.1477-2574.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery. 1987;101:731–737. [PubMed] [Google Scholar]
- 4.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. AJR Am J Roentgenol. 2011;197:260–265. doi: 10.2214/AJR.11.6995. [DOI] [PubMed] [Google Scholar]
- 5.Yoo J, Choi JY, Lee KT, Heo JS, Park SB, Moon SH, et al. Prognostic significance of volume-based metabolic parameters by 18 F-FDG PET/CT in gallbladder carcinoma. Nucl Med Mol Imaging. 2012;46:201–206. doi: 10.1007/s13139-012-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y, et al. 18 F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2007;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Clinical usefulness of 18 F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45(5):560–566. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- 8.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Maldonado A, Gonzalez-Alenda FJ, Alonso M, Sierra JM. PET-CT in clinical oncology. Clin Transl Oncol. 2007;9:494–505. doi: 10.1007/s12094-007-0093-5. [DOI] [PubMed] [Google Scholar]
- 10.Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oquchi M, et al. 18 F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small-cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- 11.Kim JH, Yoo SW, Kang SR, Cho SG, Oh JR, Chong A, et al. Prognostic significance of metabolic tumor volume measured by 18 F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–285. doi: 10.1007/s13139-012-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Won KS, Choi BW, Jo I, Zeon SK, Chung WJ, et al. Usefulness of F-18 FDG PET/CT in the evaluation of early treatment response after interventional therapy for hepatocellular carcinoma. Nucl Med Mol Imaging. 2012;46:102–110. doi: 10.1007/s13139-012-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuma R, Sharma P, Kumari A, Halanaik D, Malhotra A. Role of 18 F-FDG PET/CT in detecting recurrent gallbladder carcinoma. Clin Nucl Med. 2012;37(5):431–435. doi: 10.1097/RLU.0b013e31824d24c4. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8(1):90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Oe A, Kawabe J, Torii K, Kawamura E, Hiqashiyama S, Kotani J, et al. Distingushing benign from malignant gallbladder wall thickening using FDG-PET. Ann Nucl Med. 2006;20(10):699–703. doi: 10.1007/BF02984683. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa H, Ikuma H, Asakura K, Uesaka K. Prognostic importance of standardized uptake value on F-18 fluorodeoxyglucose-positron emission tomography in biliary tract carcinoma. J Surg Oncol. 2009;100:494–499. doi: 10.1002/jso.21356. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Narita M, et al. Prognostic value of 18 F-fluorodeoxyglucose positron emission tomography in patients with extrahepatic bile duct cancer. J Hepatobiliary Pancreat Sci. 2011;18:39–46. doi: 10.1007/s00534-010-0293-1. [DOI] [PubMed] [Google Scholar]
- 18.Shibata K, Uchida H, Iwaki K, Kai S, Ohta M, Kitano S. Lymphatic invasion : An important prognostic factor fot stages T1b-T3 gallbladder cancer and and indication for additional radical resection of incidental gallbladder cancer. World J Surg. 2009;33(5):1035–1041. doi: 10.1007/s00268-009-9950-4. [DOI] [PubMed] [Google Scholar]
- 19.Chan SY, Poon RT, Lo CM, Nq KK, Fan ST. Management of carcinoma of the gallbladder : a single-institute experience in 16 years. J Surg Oncol. 2008;97(2):156–164. doi: 10.1002/jso.20885. [DOI] [PubMed] [Google Scholar]
- 20.Kurosaki I, Hatakeyama K, Tsukada K. Long-term survival of patients with biliary tract cancer with lymph node involvement. J Hepatobiliary Pancreat Surg. 1999;6(4):399–404. doi: 10.1007/s005340050139. [DOI] [PubMed] [Google Scholar]
- 21.Meng H, Wang X, Fonq Y, Wang ZH, Wang Y, Zhang ZT. Outcomes of radical surgery for gallbladder cancer patients with lymphatic metastases. Jpn J Clin Oncol. 2011;41(8):992–998. doi: 10.1093/jjco/hyr072. [DOI] [PubMed] [Google Scholar]
- 22.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13(4):415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 23.Cho SY, Kim SH, Park SJ, Han SS, Kim YK, Lee KW, et al. Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol. 2010;102–1:87–93. doi: 10.1002/jso.21544. [DOI] [PubMed] [Google Scholar]
- 24.Tuqba Kos F, Aksoy S, Odabas H, Ozdemir N, Oksuzoglu B, Uncu D, et al. Adjuvant therapy for gallbladder and bile duct cancers: retrospective comparative study. J Buon. 2011;16(3):464–468. [PubMed] [Google Scholar]