Abstract
Objectives
Although diffuse splenic 18F-fluorodeoxyglucose (F-18 FDG) uptake exceeding hepatic activity, is considered abnormal, its clinical significance is rarely discussed in the literature. The aim of this study was to determine the contributing factors causing diffusely increased splenic FDG uptake in patients with cholangiocarcinoma.
Methods
From January 2010 to March 2013, 140 patients (84 men, 56 women) were enrolled in this study. All patients had been diagnosed with cholangiocarcinoma and underwent F-18 FDG positron emission tomography/computed tomography (PET/CT) for the pretreatment staging work up. Clinical records were reviewed retrospectively. Various hematological parameters, C-reactive protein (CRP) level, CEA, CA19-9, pancreatic enzymes and liver function tests were conducted within 2 days after the F-18 FDG PET/CT study.
Results
Diffuse splenic uptake was observed in 23 patients (16.4%). Of those, 19 patients (82.6%) underwent endoscopic retrograde cholangiopancreastography (ERCP) 7 days before F-18 FDG PET/CT. The CRP level (p < 0.001) and white blood cell count (p = 0.023) were significantly higher in the group of patients with diffuse splenic FDG uptake. The hemoglobin (p < 0.001) and the hematocrit (p < 0.001) were significantly lower in patients with diffuse splenic FDG uptake. Pancreatic enzymes, liver function test results, and tumor markers were not significantly different between the patients who did or did not have diffusely increased splenic FDG uptake. The significant factors for diffuse splenic F-18 FDG uptake exceeding hepatic F-18 FDG uptake on multivariate analysis included: performing ERCP before F-18 FDG PET-CT (odds ratio [OR], 77.510; 95% CI, 7.624-132.105), and the presence of leukocytosis (OR, 12.436; 95% CI, 2.438-63.445) or anemia (OR, 1.211; 95% CI, 1.051-1.871).
Conclusion
In conclusion, our study demonstrated that concurrent inflammation could be associated with diffusely increased splenic FDG uptake. We suggest that performing ERCP before F-18 FDG PET/CT could cause acute inflammation which may induce splenic FDG activity.
Keywords: Cholangiocarcinoma, ERCP, FDG uptake, PET/CT, Spleen
Introduction
The spleen, the body’s largest lymphoid organ, combines the innate and adaptive immune response [1]. In normal individuals, hepatic and splenic 18F-fluorodeoxyglucose (F-18 FDG) uptakes are generally low grade and diffuse on F-18 FDG positron emission tomography/computed tomography (PET/CT), and splenic FDG uptake is less than hepatic uptake [2]. Splenic FDG uptake that exceeds hepatic FDG uptake is considered abnormal [2, 3]. Focal uptake in the spleen may indicate lymphoma, a primary splenic neoplasm, metastasis, or infection [4, 5]. However, primary malignant lymphoma of the spleen and splenic metastases from nonlymphomatous malignancies are uncommon [4, 6]. In addition, primary nonhematopoietic tumors arise from the spleen extremely rarely [4]. Temporary focal FDG uptake in the spleen can also be due to splenic hypervascularity, mimicking metastasis or splenic malignancy [7].
Diffusely increased splenic FDG uptake is usually an incidental finding on whole-body F-18 FDG-PET/CT, but its clinical implication is still unclear. Only a few studies, including two from our institution, have reported that diffusely increased splenic F-18 FDG uptake frequently occurs in patients due to inflammatory processes [8–13].
Endoscopic retrograde cholangiopancreatography (ERCP) is the widely accepted method to safely and directly evaluate biliary and pancreatic disease [10]. ERCP permits direct visualization of the biliary tree and pancreatic ducts, which plays a role as a diagnostic tool and therapeutic procedure [10]. Tissue can be directly obtained via ERCP biopsy in pancreaticobiliary malignancies [10]. In addition, ERCP is used to relieve obstruction due to a primary pancreaticobiliary malignancy [10]. Compared with other endoscopic examinations, however, ERCP carries a higher potential for complications such as bleeding, cholangitis, and pancreatitis [11–14].
Therefore, this study was designed to determine the cause of increased splenic glucose metabolism in patients with cholangiocarcinoma, including performing ERCP before F-18 FDG PET/CT.
Methods
Study Population
We enrolled 140 patients who had been diagnosed with cholangiocarcinoma between January 2010 and March 2013. All patients had undergone F-18 FDG PET/CT for pretreatment staging workup. No patient had prior histories of treatment with granulocyte colony-stimulating factor or granulocyte–macrophage colony-stimulating factor and was suspected of having any primary malignancy in the spleen. ERCP was conducted in 57 patients 7 days before undergoing F-18 FDG PET/CT. Eighty-three patients received ERCP after F-18 FDG PET/CT or did not receive ERCP. The hematological data, C-reactive protein (CRP) level, liver function tests, pancreatic enzymes and tumor markers were obtained from laboratory studies within 2 days after performing F-18 FDG PET/CT. All the patients received antibiotic prophylaxis before and after ERCP. ERCP-related complications were defined according to consensus criteria [15]. Pancreatitis was based on serum amylase at least three times the normal level after ERCP. Acute cholangitis was defined as a febrile or septic illness occurring in patients with jaundice.
This study was approved by the clinical research ethics committee of our institution and all subjects provided written informed consent prior to participating.
F-18 FDG PET-CT Imaging
Fasting for at least 6 h, the patients were injected with 5.2 MBq of F-18 FDG per kilogram of body weight. Scanning was performed when the plasma glucose level before administration of F-18 FDG was <130 mg/dl. F-18 FDG PET/CT imaging studies were performed 60 min after intravenous injection of F-18 FDG. A low-dose non-contrast CT scan (30mAs, 120 kV, 512 × 512 matrix) was obtained for attenuation correction. PET studies were performed with a dedicated PET/CT scanner (Gemini; Philips, Milpitas, CA, USA) with a spatial resolution of 5.3 mm in the center of field of view. PET data were obtained for 30 min using a high-resolution whole body scanner with an axial field of view of 18 cm. The average axial resolution varied between 4.2 mm full width at half maximum in the center and 5.6 mm at 10 cm. Data were acquired in a three-dimensional (3D) mode after an intravenous administration of F-18 FDG. The obtained PET images were reconstructed using the 3D raw-action maximum likelihood iterative reconstruction algorithm.
SUV Measurement
To obtain a maximum standard uptake of value (SUVmax) and SUVmean of the bone marrow (BM), the spleen and the liver, the region of interest (ROI) was placed as follows. The ROI was drawn manually on the axial image of the F-18 FDG PET/CT.
The BM activity was measured using a circular ROI which was placed within the center of L1 to L5 lumbar vertebral bodies. The ROI did not contain cortical and sclerotic bone area. Then, the greatest value among the SUVs of L-spines was chosen as a SUVmax and average of SUVs of L-spines considered as a SUVmean of BM activity. The average diameter of circular ROI was 20 mm. To measure the activity of the spleen, a single round ROI was drawn on the center of the spleen. In addition, an elliptical ROI was placed in the middle of the right hepatic lobe to avoid mismatch between the CT and PET images and artifacts caused by respiratory movements. The average longitudinal diameter of ROI was 50 mm.
The spleen SUV/liverSUV (S/L ratio), spleen SUV/BM SUV (S/B ratio) and liver SUV/BM SUV (L/B ratio) were calculated. Both SUVmax and SUVmean were used to analyze the ratios described as above.
Statistical Analysis
A Mann–Whitney U test was used to compare continuous variables for the two groups. The X2-test was used to compare the two groups of categorical data. Univariate analysis (Χ2-test or Fisher’s exact test) was used to determine the relationship between the factors described and diffuse splenic uptake. A binary logistic regression analysis was used for multivariate analysis of independent variables with p < 0.05 in the univariate analysis, applying a stepwise approach. The statistical analyses were performed using the MedCalc software (MedCalc, Mariakerke, Belgium), version 12.5.0.0 and a p value less than 0.05 was regarded as indicative of significance.
Results
Patients’ Characteristics
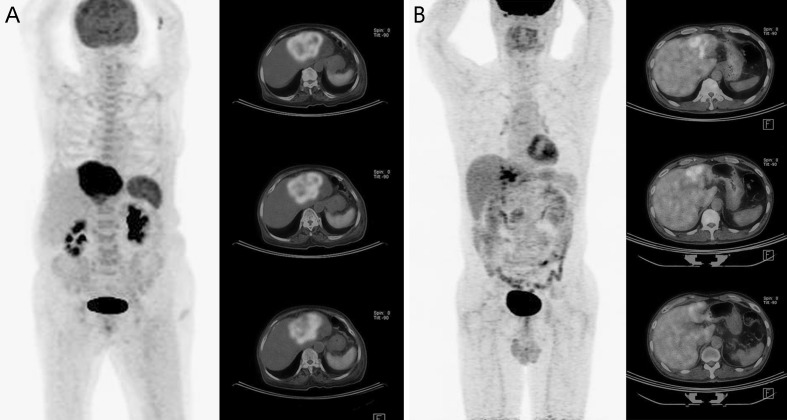
The characteristics of the patients are shown in Table 1. One hundred and forty-eight patients had histologically confirmed cholangiocarcinoma of the perihilar bile duct (n = 63), intrahepatic bile duct (n = 64), or distal bile duct (n = 13). Patients were divided into two groups according to the presence of diffusely increased FDG uptake in the spleen on MIP F-18 FDG PET images (Fig. 1). Twenty-three patients who had F-18 FDG PET uptake that exceeded hepatic uptake were included in the spleen group (Fig. 1a). One hundred and twenty-five patients who had lower splenic F-18 FDG uptake than hepatic uptake were included in the control group (Fig. 1b). Sites and stages of disease did not differ significantly between the two groups. Acute pancreatitis occurred in four patients. Two patients were in the spleen group and two patients were in the control group. Other complication was not found.
Table 1.
Baseline characteristics of the study cohort
| Characteristics | No. of patients | |||
|---|---|---|---|---|
| Spleen group (n = 23) | Control group (n = 117) | p value | ||
| No. of patients | ||||
| Gender | M | 10 | 74 | 0.124 |
| F | 13 | 43 | ||
| Mean age | 74 (35–85) | 69 (37–92) | 0.026 | |
| Disease | Cholangiocarcinoma | |||
| Perihilar | 13 | 50 | 0.527 | |
| Intrahepatic | 7 | 57 | ||
| Distal | 3 | 10 | ||
| AJCC stage | ||||
| Perihilar | Stage I/II | 5 | 17 | 0.941 |
| Stage III/IV | 8 | 32 | ||
| Intrahepatic | Stage I/II | 0 | 9 | 0.070 |
| Stage III/IV | 7 | 48 | ||
| Distal | Stage I/II | 0 | 3 | 0.821 |
| Stage III/IV | 3 | 8 | ||
AJCC American Joint Committee on Cancer, F female, M male
Fig. 1.
F-18 FDG PET/CT images. a Increased splenic FDG uptake (SUVmax 3.8) exceeding hepatic FDG uptake (SUVmax 2.2) in a 74-year-old woman with intrahepatic cholangiocarcinoma. The spleen-to-liver ratio was 2.45. The SUVmax of the primary mass was 7.5. b Splenic FDG uptake (SUVmax 2.7) lower than hepatic FDG uptake (SUVmax 3.8) in a 57-year-old man with intrahepatic cholangiocarcinoma. The spleen to liver ratio was 0.71. The SUVmax of the primary mass was 13.5
Comparison of SUVs and Laboratory Findings
The SUVmax and SUVmean of the spleen, the S/L ratios, and the S/B ratios were significantly higher in the spleen group than those in the control group. Patients in the spleen group showed a lower L/B ratio using both SUVmax and SUVmean than in the control group. BM glucose metabolism was higher in the diffusely increased splenic uptake group; however, there was no statistical significance. The SUVmax and SUVmean of the liver were not significantly different between the two groups.
Among the several hematological indices, patients in the spleen group showed significantly higher white blood cell counts (p = 0.023) and CRP levels (p < 0.001). Red blood cell counts (p = 0.003), hemoglobin (p < 0.001) and hematocrit (p < 0.001) were significantly lower in the spleen group. Platelet count (p = 0.818) was not significantly different between the two groups. The liver function tests, pancreatic enzymes, and tumor markers were also not statistically different between the two groups (Table 2).
Table 2.
Comparison of SUVs and laboratory findings
| Variable | Spleen group (n = 23) | Control group (n = 117) | p-value |
|---|---|---|---|
| Spleen SUVmax | 3.64 (1.70-15.50) | 2.37 (0.60-8.78) | <0.001 |
| Spleen SUVmean | 2.25 (1.10-13.00) | 1.50 (1.00-5.10) | <0.001 |
| Liver SUVmax | 2.40 (1.55-6.60) | 2.77 (1.30-4.70) | 0.938 |
| Liver SUVmean | 2.15 (1.40-4.80) | 2.10 (1.10-3.81) | 0.867 |
| BM SUVmax | 2.71 (1.10-5.10) | 2.16 (1.00-4.40) | 0.053 |
| BM SUVmean | 2.05 (1.10-4.81) | 1.83 (1.00-3.12) | 0.070 |
| Spleen SUVmax/Liver SUVmax | 1.35 (0.63-2.45) | 0.81 (0.30-2.26) | <0.001 |
| Spleen SUVmean/Liver SUVmean | 0.97 (0.41-2.71) | 0.70 (0.35-1.46) | <0.001 |
| Spleen SUVmax /BM SUVmax | 1.53 (0.58-4.70) | 1.06 (0.40-2.63) | <0.001 |
| Spleen SUVmean /BM SUVmean | 1.10 (0.39-6.19) | 0.98 (0.50-2.08) | 0.040 |
| Liver SUVmax /BM SUVmax | 1.05 (0.55-2.08) | 1.29 (0.84-2.10) | 0.007 |
| Liver SUVmean/BM SUVmean | 1.14 (0.58-2.29) | 1.28 (0.76-2.47) | 0.098 |
| WBC (x103/μl) | 9.13 (4.29-26.48) | 7.23 (3.51-22.13) | 0.023 |
| RBC (×103/μl) | 3.65 (2.10-5.47) | 3.96 (1.10-6.34) | 0.003 |
| Hb (g/dl) | 10.25 (6.50-14.80) | 12.00 (7.60-17.3) | <0.001 |
| Hct (%) | 32.00 (22.20-45.30) | 35.70 (23.50-48.10) | <0.001 |
| Platelet (×103/μl) | 251.00 (73.00-347.00) | 244.00 (52.00-459.00) | 0.818 |
| CRP (mg/dl) | 4.98 (0.31-28.12) | 1.56 (0.03-39.10) | <0.001 |
| AST (IU/l) | 36.00 (12.00-157.00) | 50.00 (4.00-115.90) | 0.065 |
| ALT (IU/l) | 37.00 (9.00-897.00) | 38.00 (10.00-842.00) | 0.725 |
| ALP (IU/l) | 489.00 (64.00-2523.00) | 268.00 (12.65-3760.00) | 0.198 |
| Total bilirubin (mg/dl) | 2.48 (0.27-23.41) | 1.70 (0.18-27.50) | 0.890 |
| Direct bilirubin (mg/dl) | 0.130 (0.11 -13.01) | 0.87 (0.06-18.65) | 0.871 |
| Amylase (IU/l) | 63.00 (22.10-826.70) | 58.60 (17.10-1222.00) | 0.432 |
| Lipase (IU/l) | 52.10 (9.10 -1953.20) | 41.40 (6.90-9614.00) | 0.454 |
| CA 19–9 (ng/ml) | 261.80 (6.60-99820.00) | 161.40 (0.64-75034.00) | 0.447 |
| CEA (U/ml) | 2.91 (0.71-166.70) | 3.94 (0.51-761.30) | 0.312 |
SUVmax maximum standardized uptake value, BM bone marrow, WBC white blood cell, RBC red blood cell, Hct hematocrit, CRP C-reactive protein, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, CA 19-9 carbohydrate antigen 19–9, CEA carcinoembryonic antigen
Univariate and Multivariate Analysis of Clinical Features
Table 3 shows the results of univariate analyses of the various clinical factors. The splenic and control groups were similar in terms of age, gender, site of disease, and the presence of jaundice and cholelithiasis. In contrast, almost all patients (82.6 %) in the spleen group underwent ERCP before F-18 FDG PET-CT, while two-thirds of patients in the control group did not receive ERCP before F-18 FDG PET-CT. There were statistically significant differences in the CRP level (p = 0.003) and presence of leukocytosis (p = 0.022) or anemia (p = 0.031).
Table 3.
Univariate analysis of clinical features
| Characteristics | Spleen group (n = 23) | Control group (n = 117) | p value | |
|---|---|---|---|---|
| Age | < 65 | 2 | 37 | 0.122 |
| > 65 | 21 | 80 | ||
| Gender | M | 10 | 74 | 0.124 |
| F | 13 | 43 | ||
| Site of disease | Hilar | 13 | 50 | 0.493 |
| Intrahepatic | 7 | 57 | ||
| Distal | 3 | 10 | ||
| Jandice | Yes | 10 | 51 | 0.826 |
| No | 13 | 66 | ||
| GB stone or CBD stone | Yes | 8 | 23 | 0.186 |
| No | 15 | 94 | ||
| ERCP | After F-18 FDG PET/CT or No ERCP | 4 | 79 | < 0.001 |
| Before F-18 FDG PET/CT | 19 | 38 | ||
| Anemia | Yes | 18 | 60 | 0.031 |
| No | 5 | 57 | ||
| Leukocytosis | Yes | 8 | 15 | 0.022 |
| No | 15 | 102 | ||
| CRP level normal | Yes | 1 | 35 | 0.003 |
| No | 22 | 81 | ||
| CEA level normal | Yes | 4 | 17 | 0.921 |
| No | 19 | 100 | ||
| CA 19–9 level normal | Yes | 19 | 75 | 0.107 |
| No | 4 | 42 |
GB gall bladder, GBD common bile duct, ERCP endoscopic retrograde cholangiopancreatography, CRP C-reactive protein;
Performing ERCP before F-18 FDG PET-CT (odds ratio [OR], 77.510; 95 % CI, 7.624-132.105), and the presence of leukocytosis (OR, 12.436; 95 % CI, 2.438-63.445) or anemia (OR, 1.211; 95 % CI, 1.051-1.871) remained as significant causes of diffuse splenic F-18 FDG uptake that exceeded hepatic F-18 FDG uptake on multivariate analysis (Table 4).
Table 4.
Multivariated logistic regression analysis between diffuse splenic FDG uptake and clinical features (stepwise)
| Variable | SE | p value | OR | 95 % CI |
|---|---|---|---|---|
| ERCP before F-18 FDG PET-CT | 1.183 | <0.001 | 77.510 | 7.624-132.105 |
| Leukocytosis | 0.831 | 0.002 | 12.436 | 2.438-63.445 |
| Anemia | 0.723 | 0.032 | 1.211 | 1.051-1.871 |
SE standard error, OR odds ratio, CI confidence interval, ERCP endoscopic retrograde cholangiopancreatography
Discussion
In this study, we demonstrated that patients with diffusely increased splenic FDG uptake had a concurrent inflammatory condition, as indicated by leukocytosis and the increased CRP level in the spleen group. Two previous studies from our institution reported similar results [8, 16]. One reported that increased splenic FDG uptake frequently occurs in patients with infection, bacteremia and sepsis [17]. It has been demonstrated that diffuse splenic F-18 FDG uptake can be associated with various infections: varicella, HIV, and malaria [18–20]. In our study, however, there was no evidence that patients had any infectious disease as mentioned above. According to these reports, presumably, diffusely increased splenic activity reflects increased glucose usage by the spleen due to extrasplenic inflammation [3].
ERCP is an invasive technique that is performed for diagnostic and therapeutic purposes; however, it has a potential for complications that range from insignificant incidents with prompt resolution to major life-threatening problem such as severe septic condition [11–14].
Among the several post-ERCP complications, pancreatitis and infection are the most common [11–14]. Suspected sphincter of Oddi dysfunction and a history of post-ERCP pancreatitis or baseline pancreatitis have been reported to be independent predictors of post-ERCP pancreatitis [11]. The patients enrolled in this study did not have histories of such disease. A previous study identified jaundice, prior ERCP and location of biliary stones as risk factors for post-ERCP cholangitis [13]. In our study, however, no significant factor was associated with splenic FDG uptake. It is reported that age, liver function tests, and the amylase level are not significant related factors in post-ERCP cholangitis [13]. The present study also showed that age, jaundice, cholelithiasis, liver function test results, and the presence of tumor markers did not cause diffusely increased FDG uptake in the spleen.
ERCP can be considered an invasive intervention that may activate the immune system; however, it was not discussed in the previous study. In this respect, we postulated that ERCP could be a factor inducing an inflammatory condition in patients with cholangiocarcinoma, and post-ERCP inflammation could contribute to diffusely increased splenic F-18 FDG uptake. Though there was no significant post-ERCP complication, performing ERCP before F-18 FDG PET-CT was revealed as one of the factors associated most significantly with diffuse splenic FDG uptake.
One report stated that surgical procedures could cause diffusely increased splenic uptake on FDG-PET [3], likely due to concurrent inflammation induced by surgery. An acute phase reaction of inflammation and an increased release of inflammatory mediators are associated with an acute phase reaction after traumatic injury, including an invasive surgical procedure [16, 21]. Among 57 patients who received ERCP before F-18 FDG PET/CT, only four patients were diagnosed with post-ERCP pancreatitis in the present study. Although no clinically significant complication was noticed, CRP level was elevated and leukocytosis was found after ERCP. ERCP was more commonly performed in the spleen group. This suggests that subclinical inflammatory condition which was induced by recent invasive procedure such as ERCP could be a cause of diffuse splenic uptake. Endotoxin and the CRP level increase significantly with the severity of the surgical intervention [16]. Previous studies reported that postoperative leukotriene B4 production was significantly increased [21]. It is known that leukotriene B4 has a strong chemotactic activity for leukocytes [21] and the spleen is a reservoir of several kinds of immune cells, including leukocytes [3]. Leukocytosis in the splenic group of the present study may be associated with the acute-phase reaction after ERCP as an invasive procedure.
Conclusion
In conclusion, our study demonstrated that concurrent inflammation can be associated with diffusely increased splenic FDG uptake, and we suggest that this can be caused by invasive procedures such as ERCP. The acute inflammatory process is a complicated and highly coordinated sequence of incidents involving numerous physiological, molecular and cellular changes; therefore, further controlled prospective studies with a larger sample size and animal model research are needed to fully understand the pathophysiologic implications of diffusely increased splenic uptake. In addition, we recommend performing ERCP after F-18 FDG PET/CT to prevent diffuse splenic FDG uptake, which can mask other causes of increased splenic glucose consumption.
Acknowledgments
Conflicts of Interest
None.
References
- 1.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 2.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847–50. doi: 10.1148/radiology.189.3.8234714. [DOI] [PubMed] [Google Scholar]
- 3.Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–68. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 4.Metser U, Miller E, Kessler A, Lerman H, Lievshitz G, Oren R, et al. Solid splenic masses: evaluation with 18F-FDG PET/CT. J Nucl Med. 2005;46:52–9. [PubMed] [Google Scholar]
- 5.Lee Y, Hwang KH, Hong J, Park J, Lee JH, Ahn JY, et al. Usefulness of F-18 FDG PET/CT for the evaluation of bone marrow involvement in patients with high-grade non-Hodgkin’s lymphoma. Nucl Med Mol Imaging. 2012;46:269–77. doi: 10.1007/s13139-012-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutok JL, Fletcher CD. Splenic vascular tumors. Semin Diagn Pathol. 2003;20:128–39. doi: 10.1016/S0740-2570(03)00011-X. [DOI] [PubMed] [Google Scholar]
- 7.Park YJ, Lee JH, Jee KN, Hwan N. Incidental detection of temporary focal FDG retention in the spleen. Nucl Med Mol Imaging. 2011;45:158–60. doi: 10.1007/s13139-011-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam HY, Kim SJ, Kim IJ, Kim BS, Pak K, Kim K. The clinical implication and prediction of diffuse splenic FDG uptake during cancer surveillance. Clin Nucl Med. 2010;35:759–63. doi: 10.1097/RLU.0b013e3181ef0905. [DOI] [PubMed] [Google Scholar]
- 9.Pak K, Kim SJ, Kim IJ, Kim DU, Kim K, Kim H. Impact of cytokines on diffuse splenic 18F-fluorodeoxyglucose uptake during positron emission tomography/computed tomography. Nucl Med Commun. 2013;34:64–70. doi: 10.1097/MNM.0b013e3283595cac. [DOI] [PubMed] [Google Scholar]
- 10.Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1–8. doi: 10.1016/j.gie.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox CM, Phadnis M, Varadarajulu S. Biliary stent placement is associated with post-ERCP pancreatitis. Gastrointest Endosc. 2010;72:546–50. doi: 10.1016/j.gie.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Andriulli A, Loperfido S, Napolitano G, Niro G. Maria Rosa Valvano, Fulvio Spirito, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–88. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 13.Salehimarzijarani B, Dadvar Z, Mousavi M, Mirsattari D, Zali MR, Mohammad Alizadeh AH. Risk factors for post-ERCP cholangitis in patients with pancreatic cancer from a single referral center in Iran. Asian Pac J Cancer Prev. 2012;13:1539–41. doi: 10.7314/APJCP.2012.13.4.1539. [DOI] [PubMed] [Google Scholar]
- 14.Hu KC, Chang WH, Chu CH, Wang HY, Lin SC, Wang TE, et al. Findings and risk factors of early mortality of endoscopic retrograde cholangiopancreatography in different cohorts of elderly patients. J Am Geriatr Soc. 2009;57:1839–43. doi: 10.1111/j.1532-5415.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ, Sr, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14–16, 2002. Gastrointest Endosc. 2002;56:803–09. doi: 10.1016/S0016-5107(02)70351-9. [DOI] [PubMed] [Google Scholar]
- 16.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–59. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 17.Love C, Parithivel K, Bhargava KK, Pugliese PV, Palestro CJ. Splenic F-18 FDG uptake in patients with infection (abstract) Clin Nucl Med. 2005;30:375. doi: 10.1097/00003072-200505000-00044. [DOI] [Google Scholar]
- 18.Sheehy N, Israel DA. Acute varicella infection mimics recurrent Hodgkin’s disease on F-18 FDG PET/CT. Clin Nucl Med. 2007;32:820–21. doi: 10.1097/RLU.0b013e318148b48f. [DOI] [PubMed] [Google Scholar]
- 19.Brust D, Polis M, Davey R, Hahn B, Bacharach S, Whatley M, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS. 2006;20:495–503. doi: 10.1097/01.aids.0000210603.40267.29. [DOI] [PubMed] [Google Scholar]
- 20.Kawai S, Ikeda E, Sugiyama M, et al. Enhancement of splenic glucose metabolism during acute malarial infection: correlation of findings of FDG-PET imaging with pathological changes in a primate model of severe human malaria. Am J Trop Med Hyg. 2006;74:353–60. [PubMed] [Google Scholar]
- 21.Berger D, Bölke E, Huegel H, Seidelmann M, Hannekum A, Beger HG. New aspects concerning the regulation of the post-operative acute phase reaction during cardiac surgery. Clin Chim Acta. 1995;239:121–30. doi: 10.1016/0009-8981(95)06105-M. [DOI] [PubMed] [Google Scholar]