Abstract
Purpose
To determine whether persisting cervical fluorodeoxyglucose (FDG) uptake after concurrent chemoradiotherapy (CCRT) for cervical cancer can reflect residual malignancy.
Methods
F-FDG PET/CT was performed before and after CCRT in 136 patients with cervical cancer. The maximum and mean standardized uptake values (SUVmax and SUVmean) were recorded from PET/CT scans performed pre- and post-treatment. SUVs were correlated with treatment response after CCRT. Final treatment response was determined by MRI and further follow-up PET/CT. One hundred four of 136 patients underwent pelvic MRI, and 32 of 136 patients underwent further follow-up PET/CT. Patients were classified into two categories: patients with residual tumor or patients without residual tumor (complete responder). Pre- and post-treatment serum squamous cell carcinoma antigen (SCC) levels were also recorded for comparison. The optimal cutoff value of SUVmax for predicting residual cervical tumor was determined using receiver-operating characteristic (ROC) analysis.
Results
Of 136 patients, 124 showed complete response on further follow-up studies and 12 were confirmed to have residual tumor. The post-treatment SUVmax and pre-/post-treatment SUVmean of complete responders were significantly lower than those of patients with residual tumor: 2.5 ± 0.8 and 7.2 ± 4.2/1.9 ± 0.7 for complete responders and 5.7 ± 2.6 and 12.8 ± 6.9/3.7 ± 0.7 for patients with residual tumor (p < 0.05). The pre-treatment SUVmax and pre-/post-treatment serum SCC levels of the complete responders tended to be lower than those of patients with residual tumor, but this did not have statistical significance. Using ROC analysis, an optimal cutoff SUVmax of 4.0 on the post-treatment PET/CT yielded a sensitivity, specificity, positive predictive value, and negative predictive value of 92 %, 94 %, 61 %, and 99 %, respectively (p < 0.001).
Conclusions
Persistent cervical FDG uptake in18F-FDG PET/CT after CCRT for cervical cancer may be caused by residual tumor or post-therapy inflammation. A higher cutoff SUVmax than conventional criteria for cervical cancer in post-CCRT PET/CT might help to detect residual tumor.
Keywords: Cervical cancer, PET/CT, CCRT, Treatment response
Introduction
Cervical carcinoma is the third most common gynecologic malignancy [1]. Clinical staging of cervical cancer is based on the International Federation of Gynecology and Obstetrics (FIGO) system, which was revised in 2009 [2]. Although clinical staging is based on physical examination and limited radiographic evaluation, magnetic resonance image (MRI) and computed tomography (CT) are important to assess local extracervical spread and to determine lymph node involvement. Positron emission tomography (PET) using 18 F-fluorodeoxyglucose (FDG) is also important to evaluate the metabolic status of cervical cancer [3].
For locally advanced cervical cancer, concurrent chemoradiotherapy (CCRT) is currently the standard treatment modality [4]. Disease recurrences develop in approximately one-third of cervical cancers, and the majority of the recurrences occur within the first 2 years after completion of treatment [5]. Clinical stage and lymph node status at the time of initial diagnosis are predictors for disease recurrence [6, 7]. In addition, presence of persistent tumor lesions on clinical examination performed 1 to 3 months after treatment is associated with an elevated recurrence rate [8]. Thus, assessment of the residual tumor burden is an important factor in the clinical evaluation of cervical cancer therapeutics [9].
18 F-FDG PET and PET/CT have been accepted as valuable tools in the determination of the cervical cancer stage and detection of disease recurrence [10–13]. Moreover, PET/CT is useful for the assessment of treatment response of locally advanced disease [3]. Several studies have reported an association of post-treatment PET/CT with tumor response and survival in cervical cancer; persistent significant FDG uptake after treatment has been shown to be predictive of a poor survival outcome [8, 14, 15]. However, to the best of our knowledge, the optimal cutoff value of FDG uptake to predict residual tumor has not been reported. Therefore, the aim of the present study was to investigate the optimal cutoff value of FDG uptake to predict residual tumor after CCRT in cervical cancer.
Materials and Methods
Patients
From December 2005 to July 2012, 156 women with cervical cancer were treated with CCRT in a single institute. The inclusion criteria of this study were (1) biopsy-proven cervical squamous cell cancer, (2) PET/CT performed before and after treatment, and (3) no contraindications for CCRT. The exclusion criteria were (1) having PET/CT performed at outside hospitals, (2) history of prior surgery or chemotherapy, and (3) inability to undergo PET/CT. Accordingly, a total of 136 patients (median age 57.1 years, range 27-82 years) were enrolled in our study group. Five patients were excluded because PET/CT images were obtained from an outside hospital, and 15 patients were excluded because of inappropriate SUV measurement. All patients signed a written informed consent form for the 18 F-FDG PET/CT scan, and the present study was approved by the Institutional Review Board of our medical center.
All patients also underwent a PET/CT scan, serum squamous cell carcinoma antigen (SCC) level check, and PAP smear before and after CCRT. Final treatment response was determined by MRI and further follow-up PET/CT. One hundred four of 136 patients underwent pelvic MRI before and after CCRT, and median interval from post-treatment PET/CT to post-treatment MRI was 1.6 ± 1.4 weeks. Thirty-two of 136 patients underwent further follow-up PET/CT instead of MRI, and median interval from post-treatment PET/CT to further follow-up PET/CT was 4.1 ± 1.3 months. During this period, no therapy was performed. Patients were classified into two categories: patients with residual tumor and patients without residual tumor (complete responder). On MRI, the tumor was evaluated based on the T2-weighted image (WI): no high signal intensity in the cervix on T2WI was considered as no residual tumor; high signal intensity in the cervix on T2WI with enhancement was considered as residual tumor [16]. On further follow-up PET/CT, no discernible FDG uptake in the cervix was considered as no residual tumor, while discernible increased uptake in the cervix was considered as residual tumor by visual analysis.
PET/CT Images and Image Analysis
For PET/CT, patients fasted for at least 6 h before intravenous injection of 18 F-FDG. Approximately 5.5 MBq of 18 F-FDG per kilogram of body weight was administered intravenously, and the duration of the uptake phase was 60 min. FDG PET image acquisition was performed using a Biograph TruePoint 40 PET/CT scanner (Siemens Medical Systems, CTI, Knoxville, TN, USA), with an axial field of view of 21.6 cm and a spatial resolution of 4.2 mm in full width at half maximum at 1 cm from the center. A low-dose CT scan for attenuation correction was obtained, immediately followed by emission imaging from the neck to thigh in three-dimensional mode at 3 min per bed position. The patient then underwent a diagnostic CT scan with intravenous contrast. PET data were reconstructed iteratively using an ordered-subset expectation maximization algorithm, with low-dose CT data sets used for attenuation correction. Images were analyzed by two nuclear medicine physicians. For evaluation of cervical cancer, 18 F-FDG uptake of the tumor was measured before and after CCRT. The localization of cervical cancer on the PET/CT was determined based on a comparison of clinical findings, contrast-enhanced CT findings, and MRI findings. SUVmax and SUVmean of cervical cancer before and after CCRT were compared between patients with residual tumor and patients without residual tumor.
Statistical Analysis
Continuous variables are displayed as mean ± SD, and categorical variables are displayed as counts and percentages. The optimal cutoff value of SUV in predicting residual tumor was determined using ROC analysis. SUV and SCC levels were compared using the unpaired Student’s t-test. Statistical analyses were carried out with SPSS software (version 20.0, SPSS, Chicago, IL, USA), and a p-value of < 0.05 was considered statistically significant.
Results
Table 1 presents the patient characteristics. The median interval from the end of CCRT to post-treatment PET/CT was 47 ± 39 days. Before CCRT, the average SUVmax and SUVmean of cervical cancer were 13.5 ± 7.1 and 7.7 ± 4.7, respectively. After CCRT, the SUVmax and SUVmean of cervical cancer were 2.6 ± 1.4 and 2.1 ± 0.9, respectively. Before and after the CCRT, the average levels of serum SCC were 15.6 ± 34.3 and 1.8 ± 2.5, respectively.
Table 1.
Clinical characteristics of patients (n = 136)
| Characteristic | Number of patients (%) |
|---|---|
| FIGO stage | |
| IB1 | 2 (1.4) |
| IB2 | 1 (0.5) |
| IIA | 5 (3.7) |
| IIB | 97 (71.3) |
| IIIA | 2 (1.4) |
| IIIB | 15 (11) |
| IVA | 7 (5.1) |
| IVB | 7 (5.1) |
| Histology | |
| Squamous cell carcinoma | 124 (91.2) |
| Adenocarcinoma | 9 (6.6) |
| Other | 3 (2.2) |
| Age | Median 57.1 (range 27-82) |
Of the 136 patients, 124 showed complete response on the further follow-up studies; 12 of 136 patients showed residual tumor. The post-treatment average SUVmax, pre-/post-treatment average SUVmean, and serum SCC levels of the patients with complete response were significantly lower than those with residual tumor: 2.4 ± 0.9, 7.4 ± 4.3/1.9 ± 0.7, and 12.9 ± 28/1.7 ± 2.2, respectively, for patients with complete response; 5.3 ± 3.4, 11.9 ± 6.8/3.6 ± 1.9, and 46.8 ± 68/3.3 ± 4.2, respectively, for patients with residual tumor (p < 0.05) (Table 2). The pre-treatment average SUVmax and pre-/post-treatment serum SCC levels of the patients with complete response tended to be lower than those with residual tumor: 12.7 ± 6.1 and 14.2 ± 2.9/1.6 ± 2.2, respectively, for patients with complete response; 18.4 ± 1.1 and 30.0 ± 6.8/3.6 ± 4.2, respectively, for those with residual tumor (p < 0.5).
Table 2.
Results for SUVmax, SUVmean, and serum SCC level pre- and post-treatment
| Patients with complete response (n = 124) |
Patients with residual tumor (n = 12) |
|||
|---|---|---|---|---|
| Pre-treatment | SUVmax | 12.7 ± 6.1 | 18.4 ± 1.1 | NS |
| SUVmean | 7.2 ± 4.2 | 12.8 ± 6.9 | p < 0.001 | |
| SCC (ng/ml) | 14.2 ± 2.9 | 30.0 ± 6.8 | NS | |
| Post-treatment | SUVmax | 2.5 ± 0.8 | 5.7 ± 2.6 | p < 0.001 |
| SUVmean | 1.9 ± 0.7 | 3.7 ± 1.7 | p < 0.001 | |
| SCC (ng/ml) | 1.6 ± 2.2 | 3.6 ± 4.2 | NS |
Fifty-two of 136 patients showed persistent FDG uptake in the cervix equal to or higher than SUVmax 2.5, the usually accepted cutoff level for malignancy, on post-treatment PET/CT. Of 54 patients with persistent FDG uptake, 42 patients did not reveal any evidence of residual tumor in the further studies (Fig. 1). Only 12 of 54 patients revealed residual tumor in the further studies; the specificity and positive predictive value were low using a cutoff SUVmax of 2.5: 66 % and 22 %, respectively.
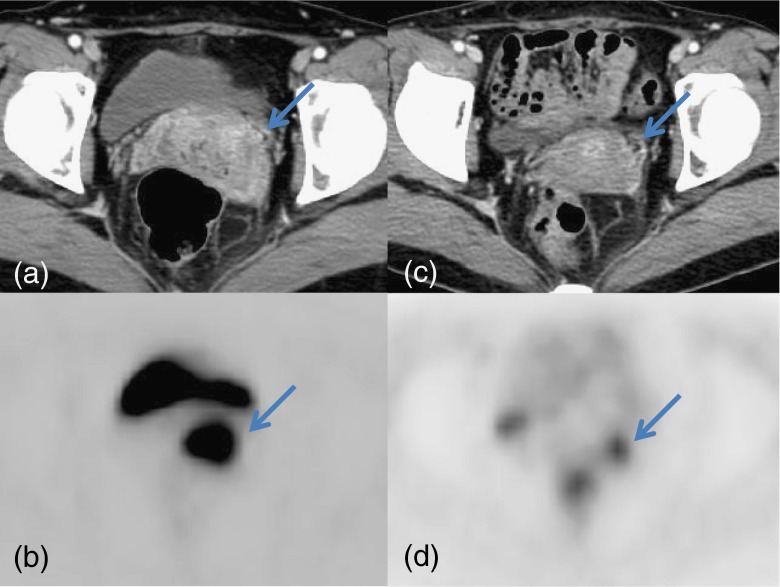
Fig. 1.

Axial images of contrast-enhanced abdominal CT (a) and PET (b) show an enhancing uterine cervical mass with FDG uptake (SUVmax 15.9). After CCRT, axial images of contrast-enhanced abdominal CT (c) and PET (d) show decreased size of the cervical mass with SUVmax 2.7. Sagittal T2-weighted image of MRI shows no mass in the cervix with low cervical signal intensity. Colposcopy and PAP smear results were negative for malignancy
In ROC analyses, an optimal cutoff value of SUVmax 4.0 on post-treatment PET/CT yielded sensitivity, specificity, positive predictive value, and negative predictive value of 92 %, 95 %, 61 %, and 99 %, respectively. The area under the curve was 0.974 (p < 0.001) (Fig. 2). Eighteen of 136 patients showed persistent FDG uptake above SUVmax 4.0 in the cervix in post-treatment PET/CT. Eleven of 18 patients with FDG uptake equal to or higher than 4.0 showed residual tumor in the further studies (Fig. 3). Seven of 18 patients showed no residual tumor in the cervix. The differing values between the 11 patients with residual tumor and 7 patients without residual tumor are presented in Table 3. Most of the 118 patients with FDG uptake below SUVmax 3.9 did not show residual tumor in the cervix, except for one patient; SUVmax of this patient was 3.5 (Fig. 4).
Fig. 2.
ROC curve for SUVmax and treatment response (area under curve, 0.974; p < 0.001)
Fig. 3.
Axial images of contrast-enhanced abdominal CT (a) and PET (b) show an enhancing uterine cervical mass (SUVmax 30.6). After CCRT, axial images of contrast-enhanced abdominal CT (c) and PET (d) show decreased size of the cervical mass (SUVmax 5.4). THe punch biopsy result was positive for malignancy
Table 3.
Results for SUVmax, SUVmean, and serum SCC level pre- and post-treatment for patients with FDG uptake equal to or higher than 4.0
| Patients with complete response (n = 7) |
Patients with residual tumor (n = 11) |
|||
|---|---|---|---|---|
| Pre-treatment | SUVmax | 12.0 ± 3.9 | 19.3 ± 10.9 | NS |
| SUVmean | 8.8 ± 3.2 | 13.3 ± 6.9 | NS | |
| SCC (ng/ml) | 7.7 ± 10.3 | 32.3 ± 71.0 | NS | |
| Post-treatment | SUVmax | 4.4 ± 0.4 | 5.9 ± 2.7 | NS |
| SUVmean | 2.9 ± 0.5 | 3.8 ± 1.8 | NS | |
| SCC (ng/ml) | 0.6 ± 0.3 | 3.9 ± 4.3 | p < 0.05 |
Fig. 4.

Axial images of contrast-enhanced abdominal CT (a) and PET (b) show an enhancing uterine cervical mass (SUVmax 8.5). After CCRT, axial images of contrast-enhanced abdominal CT (c) and PET (d) show decreased size of the cervical mass (SUVmax 3.5). Sagittal T2-weighted image of MRI shows a residual cervical mass with relatively high signal intensity. PAP smear results were positive for malignancy
Discussion
Predictors of disease recurrence in cervical cancer include not only the clinical stage and lymph node status at the time of initial diagnosis, but also tumor response after treatment [17, 18]. The 5-year survival outcome of patients with no appreciable tumor was 76 % compared to 42 % for those with findings suggestive of a tumor and 8 % for those with a persistent tumor [8]. Post-therapeutic tumor burden change is an important feature in cancer therapeutics. Current conventional radiologic imaging techniques rely on identifying a change in tumor dimension to evaluate therapeutic response [9]. However, biological and molecular changes that occur early in responders usually precede these anatomical size changes [19, 20]. For predicting therapeutic response in cervical cancer, several studies using 18 F-FDG PET imaging have shown promising results. Grigsby et al. reported three findings: (1) a complete metabolic response on PET was predictive of good survival (92 % 5-year survival); (2) a partial metabolic response (persistent, abnormal FDG uptake) was predictive of poor survival (46 % 5-year survival); (3) progressive disease (new sites of abnormal FDG uptake) was predictive of death from cervical cancer (p < 0.001) [14]. Schwarz et al. demonstrated in a prospective cohort study that post-treatment FDG uptake was predictive of survival; the 3-year progression-free survival rates of patients with metabolic complete response, persistent FDG uptake in the irradiated region, and new sites of FDG uptake outside of the initial irradiated region were 78, 33, and 0 %, respectively (p < 0.001) [15]. However, the authors of the aforementioned two studies only reported the survival rate. They did not compare the metabolic response with results obtained using other techniques, such as MRI or histology, to confirm treatment response, nor did they did present a definition of ‘residual FDG uptake’ for partial metabolic response.
In our experience, the majority of patients with mild to moderate FDG uptake after CCRT were revealed to have no residual tumor in the cervix. However, to our knowledge, no study has suggested a cutoff value of SUVmax on post-treatment PET/CT for predicting residual tumor. Therefore, in our study, an ROC curve was calculated for an optimal cutoff value of SUVmax in post-treatment PET/CT to predict residual cervical tumor. The results of the present study indicate that post-treatment PET/CT may provide an important and reliable method for the prediction of residual tumor in the cervix with a cutoff value of SUVmax 4.0. The specificity and positive predictive value of the cutoff value of SUVmax 4.0 were higher than those of the cutoff value of SUVmax 2.5, which is the usually accepted cutoff value for malignancy (95 % and 61 % versus 66 % and 22 %, respectively). Higher FDG uptake in the cervix after CCRT is likely due to radiation-related inflammation. FDG activity tends to show an initial increase followed by a steady decline after irradiation. Early increase of FDG uptake may be due to acute inflammation in normal tissue caused by irradiation or due to elevated metabolic activity in tumor cells. The later decrease in FDG uptake is caused by a decreased viable tumor cell number or reduced tumor metabolism [21]. Another study suggests that although tumors will show a complete metabolic response at the completion of chemoradiation, some tumors will show continued therapeutic response even after therapy has been completed [22].
Only 1 of 118 patients with FDG uptake less than 4.0 showed residual tumor on the MRI and PAP smear studies. On the post-treatment PET/CT, the residual tumor size was relatively small, which may result in a partial volume effect on the measurement of SUVmax. Change of the tumor shape may also result in a partial volume effect [23].
Seven of 18 patients with FDG uptake higher than 4.0 had no residual tumor on further studies. Table 3 shows that only the post-treatment serum SCC level of 11 patients with residual tumor was significantly higher than that of 7 patients without residual tumor. The results of several studies have indicated that serum SCC is potentially useful in monitoring therapeutic response of cervical cancer after primary therapy [24–26]. Persistently elevated SCC levels after completion of therapy represent persistent tumor or progressive disease [25]. Hong et al. showed that residual induration and/or high SCC concentration at 2-3 months after radiotherapy represents a high risk for treatment failure [27]. Thus, in patients with higher than SUVmax 4.0 in the cervix after CCRT, a post-treatment high serum SCC level may help predict residual tumor in the cervix.
There are several limitations to our study. First, because it was a retrospective study, the post-treatment PET/CT evaluations were performed at times varying from 1 to 5 months after treatment. Second, our study is a single-institution study. Thus, our findings may not be generalized across all centers. Third, since our patients did not undergo an operation after CCRT, we could not confirm the pathologic treatment response. Finally, we only measured the SUVmax and SUVmean, which may not be adequate markers to represent tumor biology. Further study is needed using other parameters that can represent tumor biology, such as metabolic tumor volume.
Conclusion
Persistent FDG uptake in 18 F-FDG PET/CT after CCRT is frequently encountered in cervical cancer, but only a few patients evincing it have residual tumors. A higher cutoff SUVmax than conventional criteria for cervical cancer in post-CCRT PET/CT might help to detect residual tumor.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Patel CN, Nazir SA, Khan Z, Gleeson FV, Bradley KM. 18 F-FDG PET/CT of cervical carcinoma. AJR Am J Roentgenol. 2011;196(5):1225–1233. doi: 10.2214/AJR.10.5084. [DOI] [PubMed] [Google Scholar]
- 4.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25:2952–2965. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 5.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22(5):872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 6.Sommers GM, Grigsby PW, Perez CA, Camel HM, Kao MS, Galakatos AE, et al. Outcome of recurrent cervical carcinoma following definitive irradiation. Gynecol Oncol. 1989;35(2):150–155. doi: 10.1016/0090-8258(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 7.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19(17):3745–3749. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs AJ, Faris C, Perez CA, Kao MS, Galakatos A, Camel HM. Short-term persistence of carcinoma of the uterine cervix after radiation: an indicator of longterm prognosis. Cancer. 1986;57(5):944–950. doi: 10.1002/1097-0142(19860301)57:5<944::AID-CNCR2820570512>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Lee Y, Hwang KH, Choe W, Park CY. Usefulness of F-18 FDG PET/CT in Assessment of Recurrence of Cervical Cancer After Treatment. Nucl Med Mol Imaging. 2011;45(2):111–116. doi: 10.1007/s13139-010-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjurberg M, Kjellén E, Ohlsson T, Ridderheim M, Brun E. FDG-PET in cervical cancer: staging, re-staging and follow-up. Acta Obstet Gynecol Scand. 2007;86:1385–1391. doi: 10.1080/00016340701625388. [DOI] [PubMed] [Google Scholar]
- 12.Bural GG, Shriaknthan S, Houseni M, Alavi A. FDG-PET is useful in staging and follow-up of primary uterine cervical lymphoma. Clin Nucl Med. 2007;32:748–750. doi: 10.1097/RLU.0b013e318124fd89. [DOI] [PubMed] [Google Scholar]
- 13.Loft A, Berthelsen AK, Roed H, Ottosen C, Lundvall L, Knudsen J, et al. The diagnostic value of PET/CT scanning in patients with cervical cancer: a prospective study. Gynecol Oncol. 2007;106:29–34. doi: 10.1016/j.ygyno.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Grigsby PW, Siegel BA, Dehdashti F, Rader J, Zoberi I. Posttherapy [18F] fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J Clin Oncol. 2004;22(11):2167–2171. doi: 10.1200/JCO.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–2295. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- 16.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717–740. doi: 10.1148/radiol.12120315. [DOI] [PubMed] [Google Scholar]
- 17.Hardt N, van Nagell JR, Hanson M, Donaldson E, Yoneda J, Maruyama Y. Radiation-induced tumor regression as a prognostic factor in patients with invasive cervical cancer. Cancer. 1982;49:35–39. doi: 10.1002/1097-0142(19820101)49:1<35::AID-CNCR2820490108>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Hong JH, Chen MS, Lin FJ, Tang SG. Prognostic assessment of tumor regression after external irradiation for cervical cancer. Int J Radiat Oncol Biol Phys. 1992;22:913–917. doi: 10.1016/0360-3016(92)90787-I. [DOI] [PubMed] [Google Scholar]
- 19.Lee KC, Moffat BA, Schott AF, Layman R, Ellingworth S, Juliar R, et al. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13:443–450. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 20.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–847. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Huh SJ, Nam H, Ju SG. Early response of patients undergoing concurrent chemoradiotherapy for cervical cancer: a comparison of PET/CT and MRI. Ann Nucl Med. 2013;27(1):37–45. doi: 10.1007/s12149-012-0659-3. [DOI] [PubMed] [Google Scholar]
- 22.Grigsby PW. PET/CT imaging to guide cervical cancer therapy. Future Oncol. 2009;5(7):953–958. doi: 10.2217/fon.09.70. [DOI] [PubMed] [Google Scholar]
- 23.Soret M, Bacharach SL, Buvat I. Parital –volume effect in PET tumor imaging. J Nucl Med. 2007;48;932-45. [DOI] [PubMed]
- 24.Bolli JN, Doering DL, Bosscher JR, Day TG, Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–173. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- 25.Bonfrer JMG, Gaarenstroom KN, Korse CM, Van Bunningen BNFM, Kenemans P. Cyfra 21–1 in monitoring cervical cancer: a comparison with tissue polypeptide antigen and squamous cell carcinoma antigen. Anticancer Res. 1997;17:2329–2334. [PubMed] [Google Scholar]
- 26.Brioschi PA, Bischof P, Delafosse C, Krauer F. Squamous cell carcinoma antigen (SCC-A) values related to clinical outcome of pre-invasive and invasive cervical carcinoma. Int J Cancer. 1991;47:376–379. doi: 10.1002/ijc.2910470311. [DOI] [PubMed] [Google Scholar]
- 27.Hong JH, Tsai CS, Chang JT, Wang CC, Lai CH, Lee SP, et al. The prognostic significance of pre-and post-treatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:823–830. doi: 10.1016/S0360-3016(98)00147-3. [DOI] [PubMed] [Google Scholar]