Abstract
Purpose
It is often difficult to differentiate parkinsonism, especially when patients show uncertain parkinsonian features. We investigated the usefulness of dopamine transporter (DAT) imaging for the differential diagnosis of inconclusive parkinsonism using [18F]FP-CIT PET.
Methods
Twenty-four patients with inconclusive parkinsonian features at initial clinical evaluation and nine healthy controls were studied. Patients consisted of three subgroups: nine patients whose diagnoses were unclear concerning whether they had idiopathic Parkinson’s disease or drug-induced parkinsonism (‘PD/DIP’), nine patients who fulfilled neither the diagnostic criteria of PD nor of essential tremor (‘PD/ET’), and six patients who were alleged to have either PD or atypical parkinsonian syndrome (‘PD/APS’). Brain PET images were obtained 120 min after injection of 185 MBq [18F]FP-CIT. Imaging results were quantified and compared with follow-up clinical diagnoses.
Results
Overall, 11 of 24 patients demonstrated abnormally decreased DAT availability on the PET scans, whereas 13 were normal. PET results could diagnose PD/DIP and PD/ET patients as having PD in six patients, DIP in seven, and ET in five; however, the diagnoses of all six PD/APS patients remained inconclusive. Among 15 patients who obtained a final follow-up diagnosis, the image-based diagnosis was congruent with the follow-up diagnosis in 11 patients. Four unsolved cases had normal DAT availability, but clinically progressed to PD during the follow-up period.
Conclusion
[18F]FP-CIT PET imaging is useful in the differential diagnosis of patients with inconclusive parkinsonian features, except in patients who show atypical features or who eventually progress to PD.
Keywords: Parkinsonism, Inconclusive parkinsonian features, Dopamine transporter, [18F]FP-CIT, Positron emission tomography
Introduction
The diagnosis of parkinsonism is based on clinical findings. Parkinsonism has various etiologies [1], and each has its own distinctive clinical features. In the early stages of the disease, however, it is often difficult to know whether a patient’s parkinsonism is due to idiopathic Parkinson’s disease (PD) or another condition that mimics it. Surprisingly, 10 to 25 % of patients are misdiagnosed even by movement disorder specialists [2–4]. It is more difficult to make a correct differential diagnosis when symptoms are overlapping and inconclusive parkinsonian features are present. To improve the diagnostic accuracy, imaging tests have been combined with clinical examinations. Dopamine transporter (DAT) imaging, transcranial ultrasound, functional magnetic resonance imaging, and cardiac metaiodobenzylguanidine scintigraphy are among these [5–7].
DAT imaging is the most widely and successfully used diagnostic test; it evaluates the striatal presynaptic dopaminergic function [5]. It has been demonstrated that DAT SPECT imaging is useful for the differential diagnosis of parkinsonism as well as assessment of the severity and progression of PD, and prediction of the development of PD in PD patients’ families [8–15]. DAT PET imaging is relatively new. It has several advantages over SPECT imaging and potential to better differentiate parkinsonism. [18F]FP-CIT has superior tracer kinetics than SPECT tracers, and PET has superior spatial resolution than SPECT. First, [18F]FP-CIT is better than [123I]FP-CIT for the quantification of the DAT because the metabolite of [18F]FP-CIT is not radioactive; in contrast, [123I]FP-CIT has demonstrated radiolabeled metabolites that may enter the brain [16]. Second, unlike β-CIT, FP-CIT is taken up more rapidly in the human striatum, and the equilibrium of specific to non-specific uptake of radioactivity is achieved earlier [17]. With [18F]FP-CIT, patients can be scanned on the same day, 2 to 3 h after injection. Third, FP-CIT also has relatively higher selectivity for DAT than β-CIT [17]. Furthermore, a newly developed radiochemistry method using a protic solvent system dramatically increased the radiochemical yields of [18F]FP-CIT [18]. Superior spatial resolution of PET is also useful in analyzing the DAT availability of small brain structures to explain non-motor symptoms of PD. The aim of this study was to investigate the usefulness of [18F]FP-CIT PET imaging for the differential diagnosis of patients with inconclusive parkinsonian features.
Materials and Methods
Subjects
Our institutional review board approved the protocol for this study. Patients with inconclusive parkinsonian features were eligible for the study. Clinical records and imaging data were retrospectively inspected from the [18F]FP-CIT PET registry between June 2009 and June 2010. First, patients who visited our neurology clinic with parkinsonian features were interviewed and examined by a movement disorder specialist (KWP). Diagnosis was made based on the United Kingdom Parkinson’s Disease Society Brain Bank diagnostic criteria for Parkinson’s disease. When the diagnosis was uncertain, they were categorized as patients with inconclusive parkinsonian features. All patients were evaluated with neurological tests, including the United Parkinson’s Disease Rating Scale (UPDRS), video scale, tremor scale, and minimal mental status examination. Patients with other history of or concomitant major neuropsychiatric diseases, such as stroke, dementia, head trauma, hydrocephalus, and depression, were excluded. Brain CT or MR was done when needed in order to rule out those conditions.
A total of 24 patients with inconclusive parkinsonian features were enrolled and divided into three groups. The first group (‘PD/DIP’) included nine patients taking antidopaminergic drugs whose initial clinical diagnoses were not clear concerning whether their parkinsonism was from idiopathic Parkinson’s disease (PD) or drug-induced parkinsonism (DIP). The second group (‘PD/ET’) was made up of nine patients who had both resting and postural tremors and did not fulfill the diagnostic criteria of either PD or essential tremor (ET). The last group (‘PD/APS’) included six patients who demonstrated atypical parkinsonian features and needed to be differentiated between PD and atypical parkinsonian syndrome (APS). A second clinical diagnosis was made by two movement disorder specialists (KWP and CNL) based on the follow-up results. The average follow-up period was 16 months (range 3–26 months).
Nine age-matched normal controls (NC) were enrolled using inclusion criteria of healthy adults older than 20 years with no present illness or past medical history of major neuropsychiatric and systemic diseases, including seizure, stroke, dementia, head trauma, hydrocephalus, malignancy, uncontrolled hypertension, metabolic and endocrinologic diseases, systemic infection, and drug intoxication. Table 1 demonstrates the subjects’ characteristics.
Table 1.
Subjects’ characteristics
| Control | PD/DIP | PD/ET | PD/APS | |
|---|---|---|---|---|
| Number | 9 | 9 | 9 | 6 |
| M:F | 2:7 | 2:7 | 5:4 | 4:2 |
| Mean age (years) | 64.4 ± 4.8 | 72.7 ± 8.0 | 68.8 ± 6.6 | 66.3 ± 7.8 |
| Onset age (years) | N/A | 71.1 ± 9.1 | 66.7 ± 5.9 | 54.4 ± 24.2 |
| Duration of illness (months) | N/A | 28.9 ± 22.1 | 26.5 ± 29.8 | 136.4 ± 246.7 |
| H&Y stage | N/A | 2.4 ± 0.5 | 2.1 ± 0.9 | 1.6 ± 0.8 |
| UPDRS-III score | N/A | 30.4 ± 12.0 | 33.3 ± 12.2 | 22.0 ± 9.7 |
Data are shown as mean ± SD
M male, F female, H&Y Stage Hoehn and Yahr Stage, UPDRS-III Score Unified Parkinson’s disease Rating Scale-Part III Score, PD idiopathic Parkinson’s disease, DIP drug-induced parkinsonism, ET essential tremor, APS atypical parkinsonian syndrome, N/A not applicable
[18F]FP-CIT PET Acquisition
Brain [18F]FP-CIT PET images were obtained 120 min after injection of 185 MBq [18F]FP-CIT using a PET/CT scanner (GEMINI TF, Philips Medical System, USA). Levodopa, catechol-O-methyltransferase-inhibitors, monoamine oxidase type B inhibitors, dopamine agonists, and NMDA antagonists were allowed, since they are known not to have a significant influence on DAT imaging [19]. Seven patients were using levodopa and one was taking amantadine. The rest of the patients were antiparkinsonian medication-naive at the time of imaging. None of the patients were taking drugs reported to influence DAT availability [19].
Visual Analysis of [18F]FP-CIT PET Images
The caudate nucleus and the putamen are the areas where the specific binding of [18F]FP-CIT occurs. Visual and semiquantitative analyses of the [18F]FP-CIT binding to these areas were performed without clinical information. Visual analysis was performed on summed images from 120 to 135 min post-injection by a nuclear medicine physician (EKP). First, each image was classified as normal or abnormal. Images were categorized as normal when they showed a normal pattern of DAT availability with no discernible reduction, whereas they were categorized as abnormal when the DAT availability of the striatal region was decreased. Further analyses were conducted on the images categorized as abnormal, including the symmetry/asymmetry of the DAT availability of the bilateral putamen, the degree of the DAT availability reduction, and the caudate nucleus involvement. First, the symmetry versus asymmetry of the DAT availability between the right and left putamen was determined according to the absence or presence of visually discernable asymmetry. Second, the degree of DAT availability reduction was evaluated by dividing the putamen into three equal parts along the long axis. When the DAT availability was reduced or absent within the posterior one third of the putamen, the availability was labeled ‘mild’ reduction. When it showed a decrease or absence up to the posterior two thirds of the putamen, it was labeled ‘moderate’ reduction. Finally, it was labeled ‘severe’ reduction when the anterior one third of the putamen was also involved. The head of the caudate nucleus was also examined to clarify its involvement.
Semiquantitative Analysis of DAT Availability
Semiquantitative analysis was performed using the region-of-interest (ROIs) method. Brain PET images were spatially normalized into an [18F]FP-CIT PET template made in house to remove individual anatomical variability. The template was made from images taken in nine normal controls using Statistical Parametric Mapping software (SPM2, Wellcome Trust Centre for Neuroimaging, London, UK) implemented in Matlab 6.5 (MathWorks Inc., Sherborn, MA, USA). Standard ROIs were constructed to measure the putaminal DAT availability using MRIcro v1.4 (www.cabiatl.com/mricro). Automated ROIs were created at the bilateral putamen on the three consecutive transaxial slices of the template image that showed the best resolution for those areas, with a cutoff of 50 % of the maximum count of the putamen. The number of voxels of the automatically created ROIs of the right and left sides were made in the same way by manual editing. The standard ROIs were applied to the normalized images of each subject, and the mean counts of the putamen were measured in each side. Finally, the non-displaceable binding potential of [18F]FP-CIT (BPND), a measure of DAT availability, was calculated in the putamen using the cerebellum as a reference region by equilibrium analysis method. Standard ROIs of the caudate nucleus and the striatum were made with the same process as for the putamen, and the BPND of these areas was also calculated. To diagnose normal versus abnormal DAT availability, the lower 2 standard deviation (−2 SD) values from the mean BPND of the bilateral caudate nucleus, putamen, and striatum in NC were used as cutoff values, and the diagnostic results were compared with those from visual analysis. In addition, the putamen-to-caudate nucleus ratio of BPND was calculated to analyze the relationship of DAT availability between those two regions. Asymmetry of the DAT availability between the right and the left sides of the putamen was evaluated using an asymmetry index (AI), which was calculated by [(better side BPND −worse side BPND)/average BPND of the two sides] × 100 (%).
Statistical Analysis
BPND values of the subjects were analyzed, and the mean values, putamen-to-caudate nucleus ratios, and asymmetry indices of each group were compared using the Kruskal-Wallis test. When the results showed a statistically significant difference, the Mann–Whitney U-test was additionally performed to test the significant difference between groups two by two. The difference was considered significant if the p value was less than 0.05.
Results
Overall, 11 of 24 patients (45.8 %) demonstrated abnormally decreased putaminal DAT availability on the [18F]FP-CIT PET images, whereas 13 (54.2 %) were normal by visual analysis. In all 11 patients with decreased DAT availability, there was bilateral putamen involvement; the reduction pattern was asymmetric in nine patients (81.8 %) and symmetric in two (18.2 %). Relatively more prominent reduction was observed in the posterior putaminal areas, demonstrating an anterior-to-posterior gradient. The degree of reduction in the putamen was mild in three patients (27.3 %), moderate in five (45.4 %), and severe in three (27.3 %). The head of the caudate nucleus was not affected in nine patients (81.8 %), whereas two (18.2 %) showed DAT availability reduction in the caudate nucleus as well as the putamen.
Patients in each group were categorized by analyzing the pattern of DAT distribution visually. In the PD/DIP group, two of nine patients (22.2 %) demonstrated decreased DAT availability and were categorized as having PD. The putaminal DAT availability was moderately reduced in these patients, symmetrically in one and asymmetrically in the other. The caudate nucleus was not involved. The other seven patients (77.8 %) were categorized as having DIP based on the normal DAT distribution patterns (Fig. 1a and b).
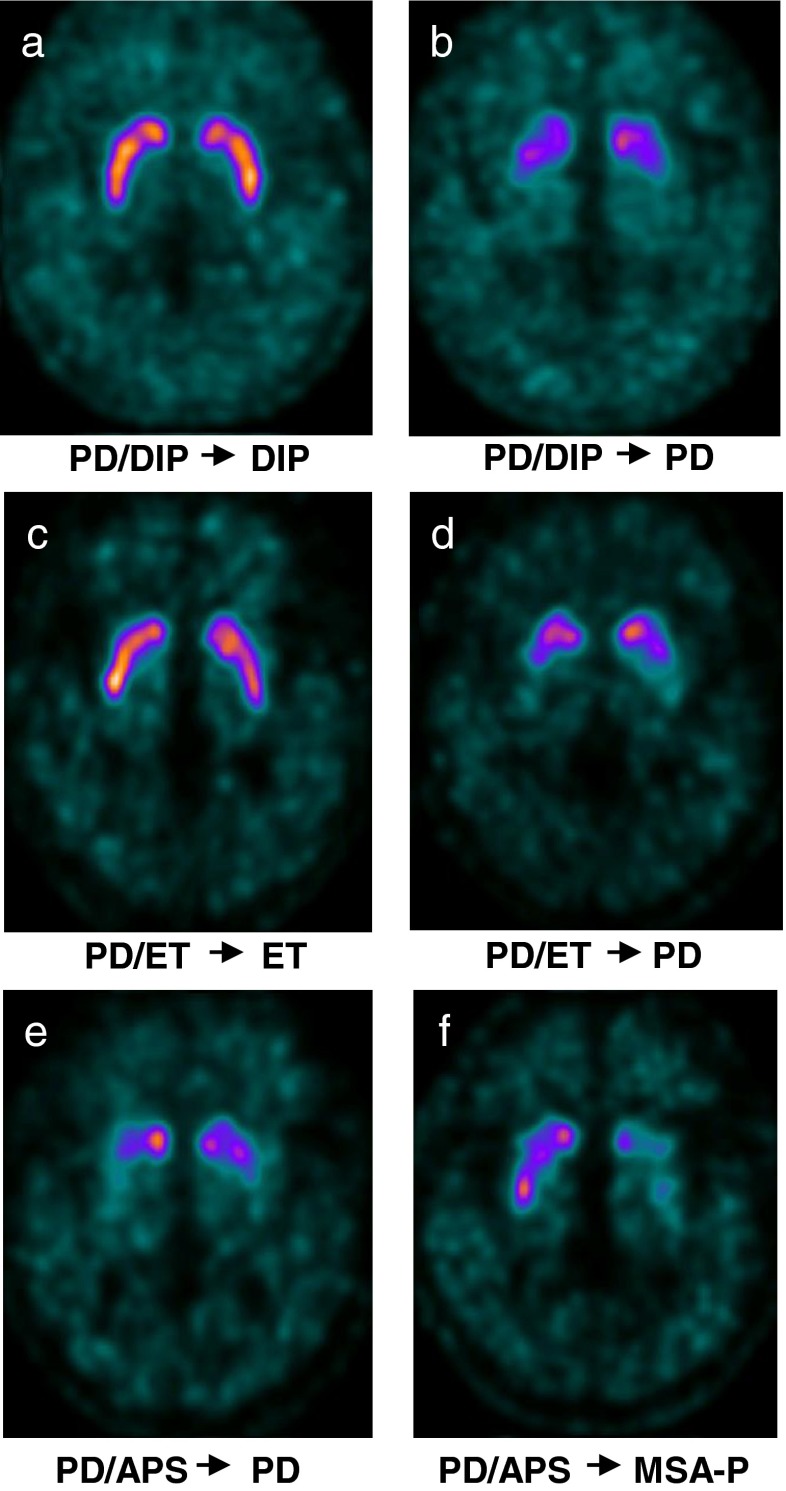
Fig. 1.
Brain [18F]FP-CIT PET/CT images in patients with inconclusive parkinsonian features. a A 67-year-old female patient presented with inconclusive parkinsonian features and levosulpiride medication history (‘PD/DIP’ group). Brain [18F]FP-CIT PET demonstrated normal DAT availability in the bilateral striatum, and the patient was categorized having as DIP. Three months later, clinical features became evident, and the final diagnosis of DIP was made. b Another 67-year-old female patient in the ‘PD/DIP’ group was categorized as having PD based on the imaging finding of reduced striatal DAT availability. The follow-up clinical diagnosis made at 16 months after imaging was PD, in agreement with the image-based diagnosis. c A 75-year-old male patient in the ‘PD/ET’ group had both resting and postural tremor. Normal DAT availability in the bilateral striatum categorized him as having ET. The follow-up diagnosis made at 11 months after imaging confirmed the diagnosis. d Another 67-year-old male patient in the ‘PD/ET’ group showed decreased striatal DAT availability and was categorized as having PD. The follow-up clinical diagnosis made 14 months after imaging was also PD. e and f Patients in the ‘PD/APS’ group could not be differentiated solely based on the [18F]FP-CIT PET imaging results. The striatal DAT availability was decreased in both PD and APS patients, without significantly distinctive features in their DAT reduction patterns. After follow-up for 21 and 22 months, respectively, they were clinically diagnosed as having PD (e) and MSA-P (f)
In the PD/ET group, four of nine patients (44.4 %) showed asymmetrically decreased DAT availability and were categorized as having PD. The degree of DAT reduction was mild in one patient, moderate in two, and severe in the remaining one. One patient demonstrated caudate nucleus involvement in the side ipsilateral to the more affected putamen. The other five patients (55.6 %) with normal findings were categorized as having ET (Fig. 1c and d).
In the PD/APS group, DAT availability was decreased in five of six patients (83.3 %). The degree of DAT reduction was mild in two patients, moderate in one, and severe in two. The patient with moderate reduction showed symmetric putaminal involvement, whereas the remainig four patients (80.0 %) demonstrated an asymmetric decrease. Bilateral caudate nuclei were affected in one patient with severe reduction of the putaminal DAT. These six PD/APS patients could not be differentiated solely based on the DAT distribution patterns on the PET images (Fig. 1e and f).
Semiquantitative analysis results are as follows. Mean BPND of the bilateral putamen in NC was 4.08 ± 0.62. When 2.83, the −2 SD value of the mean BPND of the bilateral putamen in NC was used as a cutoff value to define normality and abnormality; patients were divided into 11 abnormal and 13 normal DAT patterns, which accorded with the categorization results of the visual analysis. There was no overlap of the BPND values of individual patients between PD and DIP between either PD or ET.
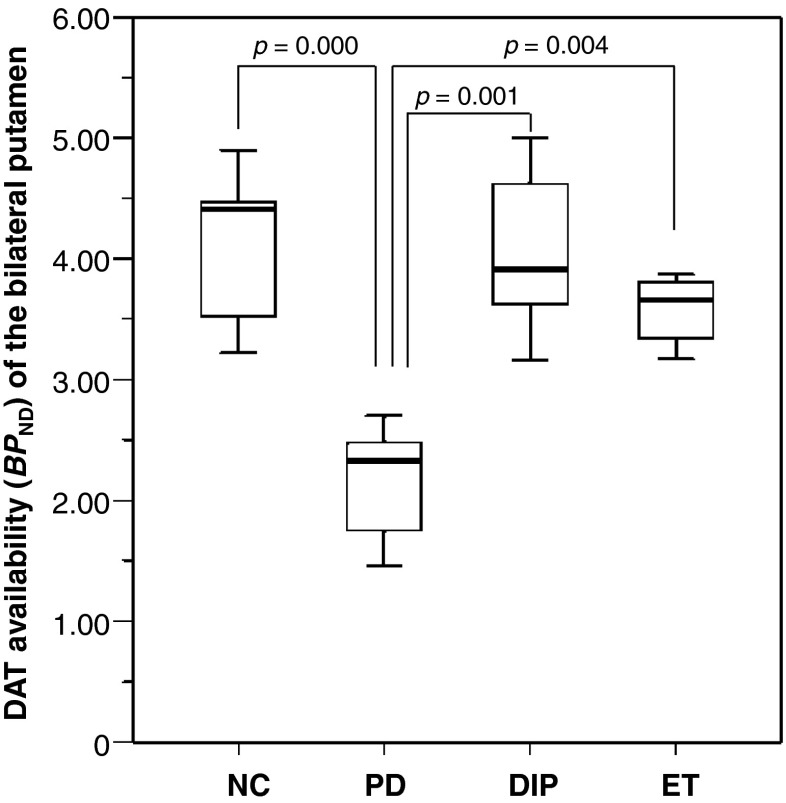
Mean BPND of the bilateral putamen in six patients categorized as having PD after imaging was 2.18 ± 0.47, which was significantly lower than those of DIP (4.08 ± 0.68) and ET patients (3.57 ± 0.30) (p = 0.002) (Fig. 2). The striatal BPND was also significantly lower in patients with PD (2.30 ± 0.49) than in those with DIP (4.00 ± 0.61) or ET (3.49 ± 0.33) (p = 0.002). There was no significant difference in the putaminal and striatal BPND among DIP, ET, and NC. However, the six PD/APS patients in the third group could not be differentiated solely based on the DAT availability quantification results, because all the patients but one demonstrated abnormally decreased BPND with no significant statistical difference (2.24 ± 1.32 for bilateral putamen; 2.23 ± 1.29 for bilateral striatum).
Fig. 2.
Comparative putaminal DAT availability. In patients with PD, mean DAT availability in the putamen was significantly lower than those of DIP, ET, and NC patients. No significant difference was observed among DIP, ET, and NC
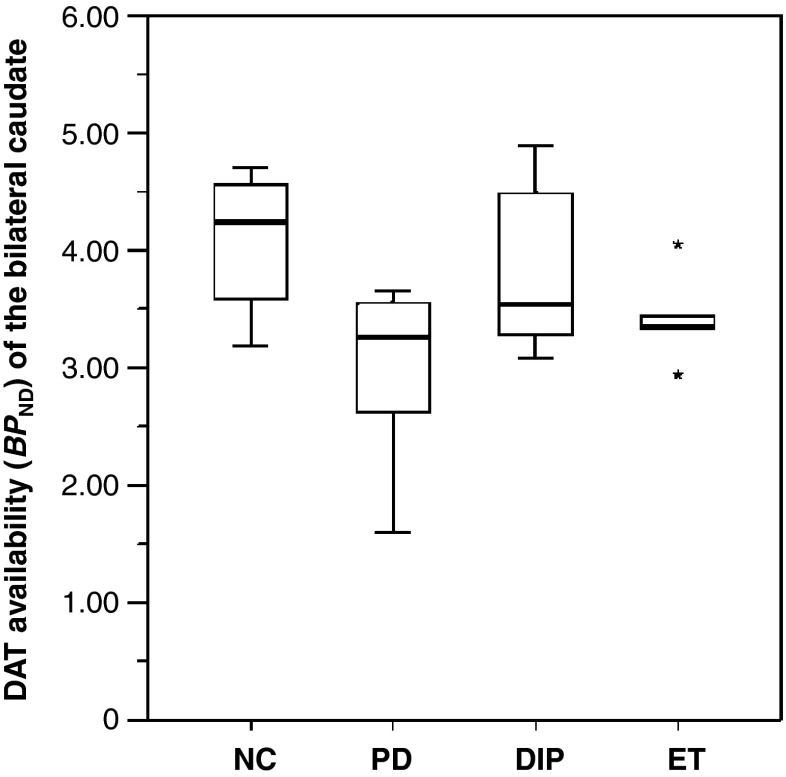
Semiquantitative analysis results on the caudate nucleus involvement were not in total agreement with those of the visual analysis. Semiquantitative analysis detected caudate nucleus involvement more sensitively than visual analysis. The caudate nucleus was revealed to be affected in 6 of 11 patients (54.5 %), while only 2 patients appeared to have caudate nucleus involvement by visual analysis. In the other five patients (49.5 %), the BPND of the bilateral caudate nucleus was lower than the mean value of NC (4.04), but higher than −2 SD (2.84). There was no significant difference in the mean BPND values among NC, PD, DIP, and ET patients (Fig. 3).
Fig. 3.
Comparative caudate nucleus DAT availability. In patients with PD, mean DAT availability in the caudate nucleus was not significantly different from those of DIP, ET, or NC patients
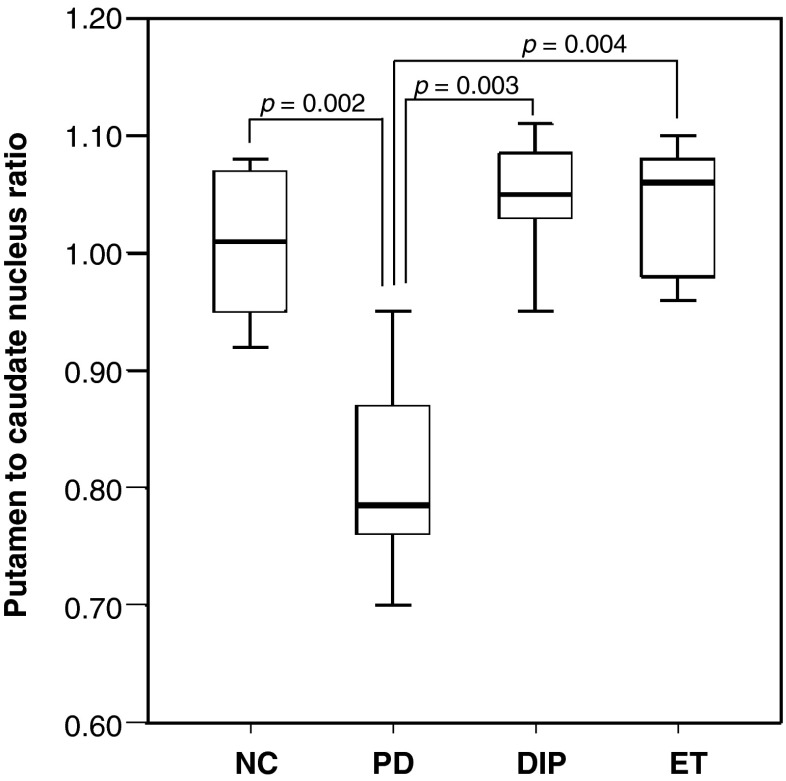
The BPND ratio of the putamen to the caudate nucleus in PD patients (0.81 ± 0.09) was significantly lower than those of DIP (1.05 ± 0.05), ET (1.04 ± 0.06), and NC patients (1.01 ± 0.06) (p = 0.001, p = 0.002, and p = 0.002, respectively; Fig. 4), indicating the preferential loss of DAT availability in the putamen of PD patients. There was no overlap of the putamen-to-caudate nucleus ratio between individual PD versus DIP and ET patients. All patients in the PD/APS group, however, showed ratios lower than the −2 SD value of NC (0.83 ± 0.14) and could not be differentiated solely by this ratio.
Fig. 4.
Comparative bilateral putamen-to-caudate nucleus ratio. The ratio of putamen-to-caudate nucleus DAT availability of PD patients was significantly lower than those of DIP, ET, and NC patients, indicating that the putamen is more affected than the caudate nucleus in PD patients
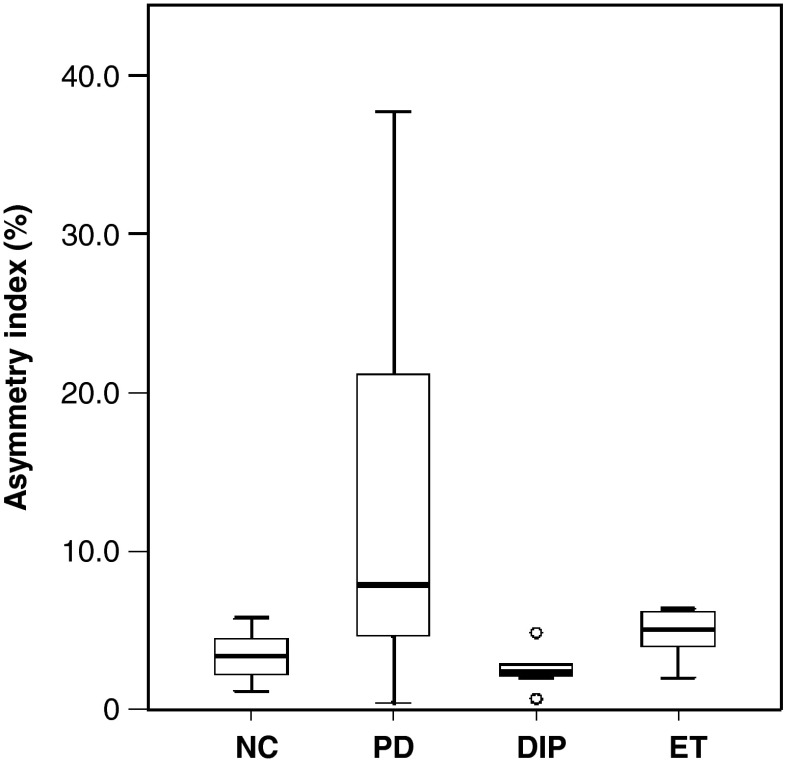
The asymmetry index (AI) reflects the difference in DAT reduction between the right and left putamen. The mean AI of NC was 3.4 ± 1.6 % (Fig. 5). All patients with DIP and ET had AIs within the normal range, whereas three of six patients with PD showed values higher than +2 SD (6.75 %), which indicates asymmetric DAT availability in the bilateral putamen. In the PD/APS group, AIs were above the +2 SD value in four of five patients having decreased putaminal DAT availability (32.3 ± 17.7). AI could not differentiate patients with PD from those with APS.
Fig. 5.
Comparative asymmetry index. The asymmetry index of PD patients was not significantly different from those of DIP, ET, and NC patients
Clinical follow-up diagnosis was compared with the [18F]FP-CIT PET imaging result in each patient. Figure 6 shows the follow-up diagram. Image-based diagnosis was available for all 18 patients in the PD/DIP and PD/ET groups. Two of them had normal DAT availability but were lost to follow-up before a definite clinical diagnosis was reached. The other 16 were followed up for 3 to 26 months, and the final follow-up diagnosis was reached in 15 patients with 1 remaining clinically inconclusive case. The image-based diagnosis was congruent with the follow-up diagnosis in 11 patients and incongruent in 4. The accuracy of image-based diagnosis was 75 % in the PD/DIP group and 67 % in the PD/ET group. Notably, all of the four incongruent cases had normal DAT availability and were diagnosed as having DIP (n = 2) and ET (n = 2) by imaging, but clinically progressed to PD during the follow-up period. Patients in the PD/APS group for whom the image-based diagnosis could not be made were finally diagnosed as having PD in three, progressive supranuclear palsy in two, and multisystem atrophy with predominant parkinsonism in one.
Fig. 6.
Follow-up clinical diagnosis and its comparison with image-based diagnosis. Image-based diagnosis was congruent with the follow-up clinical diagnosis in 73.3 %. Abbreviation: Dx diagnosis, F/U follow-up
Discussion
[18F]FP-CIT PET imaging could successfully demonstrate the striatal DAT availability and be used in sorting out patients with Parkinson’s disease (PD) from the patients with clinically inconclusive parkinsonian features. To the best of our knowledge, this is the first study using [18F]FP-CIT PET imaging in the differential diagnosis of patients with inconclusive parkinsonian features. In this study, patients with inconclusive parkinsonian features were diagnosed as having PD, drug-induced parkinsonism (DIP), or essential tremor (ET) by analysis of the DAT availability pattern on the brain [18F]FP-CIT PET imaging. DIP is a common syndrome induced by dopamine receptor antagonist drugs and has been reported to be the cause of parkinsonism in 24–35 % of parkinsonian patients. According to a prospective study, DIP accounted for 51 % of hospital admissions due to parkinsonism [20]. ET is characteristic of symmetric postural tremor and is known to be the most common diagnostic error in patients with the initial diagnosis of PD in the community setting [21].
[18F]FP-CIT PET imaging failed to lead to a differential diagnosis in patients whose initial diagnosis was not certain concerning whether it was PD or APS. There are contradictory results concerning the differential diagnosis of PD from APS using DAT imaging. In early studies, researchers argued that relatively more severe DAT reduction in the posterolateral putamen of patients with PD was characteristic, unlike in APS. However, the following studies reported that they could not differentiate PD from APS based on this anterior-posterior gradient and therefore denied that it is a characteristic feature in the differential diagnosis of PD from APS [22]. We could not find any significant difference in the DAT reduction patterns of patients with PD and APS.
Even when the clinical diagnosis of a patient showing parkinsonism is evident, such as PD, DIP, or ET, DAT imaging can be useful. DAT imaging can reveal underlying PD pathology in DIP patients. Two interesting studies have been performed regarding this issue. Lorberboym et al. [12] studied patients who developed parkinsonism while taking neuroleptic medications. Nine of 20 patients demonstrated normal [123I]FP-CIT SPECT findings, consistent with the clinical diagnosis of DIP, whereas 11 of 20 patients showed significantly decreased DAT availability in the SPECT, and they were diagnosed as having DIP plus underlying PD. There were no differences in clinical features between patients with normal and abnormal scans, and DAT imaging helped to determine whether DIP was entirely drug-induced or an exacerbation of subclinical PD. Hambye et al. [23] investigated 22 patients who showed parkinsonism during amiodarone therapy. Their clinical diagnoses were all DIP. However, when patients were evaluated with [123I]FP-CIT SPECT, 50 % of them demonstrated abnormal results. This means that they found patients with underlying PD in 50 % of the study population. Furthermore, they proved that in patients who were revealed to have underlying PD, treating PD had more impact on motor changes than modifying the antiarrhythmic drug.
Another advantage of DAT imaging is that it can predict the progression of DIP or ET patients to PD. Chaudhuri et al. [24] analyzed patients with asymmetrical postural tremor who subsequently developed rest tremor and parkinsonism with a mean tremor duration of 19.2 years. Initial [123I]β-CIT SPECT imaging revealed reduced DAT availability in the striatum contralateral to the dominantly affected arm. They argued DAT imaging was useful for predicting which patients would develop PD, whereas alcohol sensitivity of tremor, family history of tremor, or responsiveness to beta-blockers may not be helpful in patients presenting with late-onset asymmetrical postural tremor, even if there is no rest tremor.
Meanwhile, one thing that should be noted is that in the early stages of parkinsonism, a normal baseline DAT SPECT or PET imaging may neither completely exclude PD nor preclude unnecessary clinical follow-up [25]. There are patients who progress to PD. Marshall et al. [26] followed up patients who demonstrated normal baseline DAT SPECT results for an average of 2 years and revealed that 3 % of the patients’ parkinsonism had degenerative origins. We had DIP and ET patients who had normal DAT availability initially, but clinically progressed to PD during the follow-up period. Diagnostic accuracy can be significantly improved when DAT imaging results are combined with the follow-up clinical information [27].
Our study has some potential limitations. The number of normal controls was relatively small and might not be sufficient to represent the −2 SD cutoff value of the healthy population. It is possible that this induced the low sensitivity of image-based diagnosis in this study. The follow-up period of patients was relatively short, as some other studies adopted up to 3 years as follow-up periods [14, 28]. However, those studies were multicenter studies, and in other studies, as little as 6 months could have been used successfully [29, 30]. In our study, the follow-up period of 3 months to 2 years was sufficient for our experienced neurologists to establish a diagnosis except in one patient. It could also be considered a limitation that men and women were mixed in the study population, since the DAT availability appears to be influenced by gender, i.e., higher DAT availability in women than in men [31]. Nevertheless, gender was not limited in any of the reviewed studies, probably for the practical reason of increasing the study population.
Evaluation of the striatal DAT availability with [18F]FP-CIT PET imaging was feasible and useful in the differential diagnosis of patients with inconclusive parkinsonian features. [18F]FP-CIT PET imaging could successfully, easily, and clearly differentiate and categorize patients with inconclusive parkinsonian features into PD, DIP, and ET, with some limitation in the differential diagnosis of patients who were presumed to have either PD or APS. Meanwhile, caution is needed when the imaging results show normal DAT availability, because there may be patients who progress to PD after some period of time. The DAT imaging using [18F]FP-CIT PET can be useful when clinicians have diagnostic uncertainty and could significantly reduce the tediousness and anxiety during the follow-up period.
Acknowledgments
We thank Prof. Jae Sung Lee for providing FMItool; Hyerina Kim for assistance; Mi-Ok Kim, Jae-Hoon Baek, Ji-Han Kim, and the rest of the staff at the Korea University Anam Hospital PET Center for excellent technical assistance.
This study was supported by Korea University Grants (K0931131 and K0932081).
Financial Support
Korea University Grants (K0931131 and K0932081)
Conflict of Interest
Eunkyung Park, Yu Mi Hwang, Chan-Nyoung Lee, Sujin Kim, Sun Young Oh, Young Chul Kim, Jae Gol Choe, and Kun Woo Park declare that they have no conflict of interest.
References
- 1.Koller W, Minagan A. Parkinson’s disease management guide. Montvale: Medical Economics Company Inc; 2001. Treatment strategies for the management of Parkinson’s disease; pp. 101–133. [Google Scholar]
- 2.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearnley JM, Lees AJ. Striatonigral degeneration. A clinicopathological study. Brain. 1990;113(Pt 6):1823–1842. doi: 10.1093/brain/113.6.1823. [DOI] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 5.Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord. 2003;18(12):1415–1423. doi: 10.1002/mds.10592. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson R, Siderowf A, Stern MB. Premotor Parkinson’s disease: clinical features and detection strategies. Mov Disord. 2009;24(Suppl 2):S665–S670. doi: 10.1002/mds.22403. [DOI] [PubMed] [Google Scholar]
- 7.Michell AW, Lewis SJ, Foltynie T, Barker RA. Biomarkers and Parkinson’s disease. Brain. 2004;127(Pt 8):1693–1705. doi: 10.1093/brain/awh198. [DOI] [PubMed] [Google Scholar]
- 8.Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med. 1998;39(11):1879–1884. [PubMed] [Google Scholar]
- 9.Asenbaum S, Pirker W, Angelberger P, Bencsits G, Pruckmayer M, Brucke T. [123I]beta-CIT and SPECT in essential tremor and Parkinson’s disease. J Neural Transm. 1998;105:1213–1228. doi: 10.1007/s007020050124. [DOI] [PubMed] [Google Scholar]
- 10.Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15(4):692–698. doi: 10.1002/1531-8257(200007)15:4<692::AID-MDS1014>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Catafau AM, Tolosa E. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord. 2004;19(10):1175–1182. doi: 10.1002/mds.20112. [DOI] [PubMed] [Google Scholar]
- 12.Lorberboym M, Treves TA, Melamed E, Lampl Y, Hellmann M, Djaldetti R. [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson’s disease. Mov Disord. 2006;21(4):510–514. doi: 10.1002/mds.20748. [DOI] [PubMed] [Google Scholar]
- 13.Zijlmans J, Evans A, Fontes F, Katzenschlager R, Gacinovic S, Lees AJ, et al. [123I] FP-CIT spect study in vascular parkinsonism and Parkinson’s disease. Mov Disord. 2007;22(9):1278–1285. doi: 10.1002/mds.21479. [DOI] [PubMed] [Google Scholar]
- 14.Tolosa E, Borght TV, Moreno E. Accuracy of DaTSCAN (123I-Ioflupane) SPECT in diagnosis of patients with clinically uncertain parkinsonism: 2-year follow-up of an open-label study. Mov Disord. 2007;22(16):2346–2351. doi: 10.1002/mds.21710. [DOI] [PubMed] [Google Scholar]
- 15.Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ. The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol. 1999;45(5):577–582. doi: 10.1002/1531-8249(199905)45:5<577::AID-ANA5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Bergstrom KA, Halldin C, Lundkvist C, Swahn CG, Akerman KK, Kuikka JT, et al. Characterization of C-11 or I-123 labelled beta-CIT-FP and beta-CIT-FE metabolism measured in monkey and human plasma. Identification of two labelled metabolites with HPLC. Hum Psychopharmacol. 1996;11(6):483–490. doi: 10.1002/(SICI)1099-1077(199611)11:6<483::AID-HUP818>3.0.CO;2-9. [DOI] [Google Scholar]
- 17.Abi-Dargham A, Gandelman MS, DeErausquin GA, Zea-Ponce Y, Zoghbi SS, Baldwin RM, et al. SPECT imaging of dopamine transporters in human brain with iodine-123-fluoroalkyl analogs of beta-CIT. J Nucl Med. 1996;37(7):1129–1133. [PubMed] [Google Scholar]
- 18.Lee SJ, Oh SJ, Chi DY, Kang SH, Kil HS, Kim JS, et al. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol. 2007;34(4):345–351. doi: 10.1016/j.nucmedbio.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Booij J, Kemp P. Dopamine transporter imaging with [123I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging. 2008;35(2):424–438. doi: 10.1007/s00259-007-0621-0. [DOI] [PubMed] [Google Scholar]
- 20.Tolosa E, Coelho M, Gallardo M. DAT imaging in drug-induced and psychogenic parkinsonism. Mov Disord. 2003;18(Suppl 7):S28–S33. doi: 10.1002/mds.10575. [DOI] [PubMed] [Google Scholar]
- 21.Newman EJ, Breen K, Patterson J, Hadley DM, Grosset KA, Grosset DG. Accuracy of Parkinson’s disease diagnosis in 610 general practice patients in the West of Scotland. Mov Disord. 2009;24(16):2379–2385. doi: 10.1002/mds.22829. [DOI] [PubMed] [Google Scholar]
- 22.Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord. 2010;25(3):357–362. doi: 10.1002/mds.22977. [DOI] [PubMed] [Google Scholar]
- 23.Hambye AS, Vervaet A, Dethy S. FP-CIT SPECT in clinically inconclusive Parkinsonian syndrome during amiodarone treatment: a study with follow-up. Nucl Med Commun. 2010;31(6):583–589. [PubMed] [Google Scholar]
- 24.Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long duration asymmetrical postural tremor is likely to predict development of Parkinson’s disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005;76(1):115–117. doi: 10.1136/jnnp.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felicio AC, Shih MC, Godeiro-Junior C, Andrade LA, Bressan RA, Ferraz HB. Molecular imaging studies in Parkinson disease: reducing diagnostic uncertainty. Neurologist. 2009;15(1):6–16. doi: 10.1097/NRL.0b013e318183fdd8. [DOI] [PubMed] [Google Scholar]
- 26.Marshall VL, Patterson J, Hadley DM, Grosset KA, Grosset DG. Two-year follow-up in 150 consecutive cases with normal dopamine transporter imaging. Nucl Med Commun. 2006;27(12):933–937. doi: 10.1097/01.mnm.0000243374.11260.5b. [DOI] [PubMed] [Google Scholar]
- 27.Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med. 2001;28(3):266–272. doi: 10.1007/s002590000460. [DOI] [PubMed] [Google Scholar]
- 28.Marshall VL, Reininger CB, Marquardt M, Patterson J, Hadley DM, Oertel WH, et al. Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord. 2009;24(4):500–508. doi: 10.1002/mds.22108. [DOI] [PubMed] [Google Scholar]
- 29.Bairactaris C, Demakopoulos N, Tripsianis G, Sioka C, Farmakiotis D, Vadikolias K, et al. Impact of dopamine transporter single photon emission computed tomography imaging using I-123 ioflupane on diagnoses of patients with parkinsonian syndromes. J Clin Neurosci. 2009;16(2):246–252. doi: 10.1016/j.jocn.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Jennings DL, Seibyl JP, Oakes D, Eberly S, Murphy J, Marek K. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. 2004;61(8):1224–1229. doi: 10.1001/archneur.61.8.1224. [DOI] [PubMed] [Google Scholar]
- 31.Varrone A, Halldin C. Molecular imaging of the dopamine transporter. J Nucl Med. 2010;51(9):1331–1334. doi: 10.2967/jnumed.109.065656. [DOI] [PubMed] [Google Scholar]