Abstract
Thyroid incidentalomas are common findings during imaging studies including 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) for cancer evaluation. Although the overall incidence of incidental thyroid uptake detected on PET imaging is low, clinical attention should be warranted owing to the high incidence of harboring primary thyroid malignancy. We retrospectively reviewed 2,368 dual-time-point 18F-FDG PET/CT cases that were undertaken for cancer evaluation from November 2007 to February 2009, to determine the clinical impact of dual-time-point imaging in the differential diagnosis of thyroid incidentalomas. Focal thyroid uptake was identified in 64 PET cases and final diagnosis was clarified with cytology/histology in a total of 27 patients with 18F-FDG-avid incidental thyroid lesion. The maximum standardized uptake value (SUVmax) of the initial image (SUV1) and SUVmax of the delayed image (SUV2) were determined, and the retention index (RI) was calculated by dividing the difference between SUV2 and SUV1 by SUV1 (i.e., RI = [SUV2 - SUV1]/SUV1 × 100). These indices were compared between patient groups that were proven to have pathologically benign or malignant thyroid lesions. There was no statistically significant difference in SUV1 between benign and malignant lesions. SUV2 and RI of the malignant lesions were significantly higher than the benign lesions. The areas under the ROC curves showed that SUV2 and RI have the ability to discriminate between benign and malignant thyroid lesions. The predictability of dual-time-point PET parameters for thyroid malignancy was assessed by ROC curve analyses. When SUV2 of 3.9 was used as cut-off threshold, malignancy on the pathology could be predicted with a sensitivity of 87.5 % and specificity of 75 %. A thyroid lesion that shows RI greater than 12.5 % could be expected to be malignant (sensitivity 88.9 %, specificity 66.3 %). All malignant lesions showed an increase in SUVmax on the delayed images compared with the initial images. But in the group of benign lesions, 37.5 % (6/16) showed a decrease or no change in SUVmax. Dual-time-point 18F-FDG PET/CT, obtaining additional images 2 h after injection, seems to be a complementary method for the differentiation between malignancy and benignity of incidental thyroid lesions.
Keywords: 18F-FDG PET/CT, Thyroid incidentaloma, Retention index, Delay scan, Dual-phase PET, Dual-time-point imaging, SUVmax
Introduction
Thyroid incidentaloma is defined as an unsuspected, asymptomatic thyroid lesion initially discovered during an operation or on imaging modality—including ultrasonography (USG), computed tomography (CT) and magnetic resonance imaging (MRI)—in a patient without a history of thyroid disease [1]. Recently, positron emission tomography (PET) is widely used as an imaging tool for characterization, staging and surveillance of patients with malignancies. Several retrospective studies have reported that thyroid incidentalomas were found in 1.2–4.3 % as a focal uptake pattern on 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) [2, 3]. Although the overall incidence of thyroid incidentalomas detected on PET imaging is low, the chance of malignancy among the incidentalomas detected with 18F-FDG PET is higher than incidental nodules discovered on the other imaging modalities, with a chance for malignancy ranging from 14 to 59 % [4–7].
Differentiation between malignant and benign thyroid lesion is very important to avoid unnecessary operation and to improve the quality of life of the patient. In a large scale study of a total of 1,921 patients, Ishimori et al. [8] reported that 18F-FDG PET/CT detected another primary malignant lesion in at least 1.2 % of patients with underlying cancer. And thyroid lesions showed the highest frequency of false-positive PET finding after pathologic assessment among the unexpectedly detected additional primary malignancies. Many researchers postulated that malignant lesions tend to show higher 18F-FDG uptake than that of benign lesions [9]. The most reliable criterion for differential diagnosis of malignant from benign is to use SUVmax. However, it is not easy to differentiate malignancy from benignity using SUVmax only. 18F-FDG, itself, is not a tumor-specific tracer and that results in low specificity in differential diagnosis of malignant tumors from benign lesions [10]. One of the methods to overcome this low specificity is to use dual time point PET imaging in prediction of malignant lesions [11]. Zhuang et al. [12] suggested that dual-time-point 18F-FDG PET imaging has a potential role in distinguishing malignancy from benign process, on the basis of the different time-activity responses in 18F-FDG uptake patterns in both in vitro and in vivo studies. Other investigators have found controversial results on using dual-time-point acquisition of 18F-FDG PET/CT for various malignant diseases [13–17].
The aim of this study was to investigate the clinical value of dual-time-point imaging for the differentiation of malignancy from benignity in incidentally detected 18F-FDG-avid thyroid lesions by elucidating the relationship between the semi-quantitative indices of dual-time-point 18F-FDG PET/CT.
Materials and Methods
Patient Population
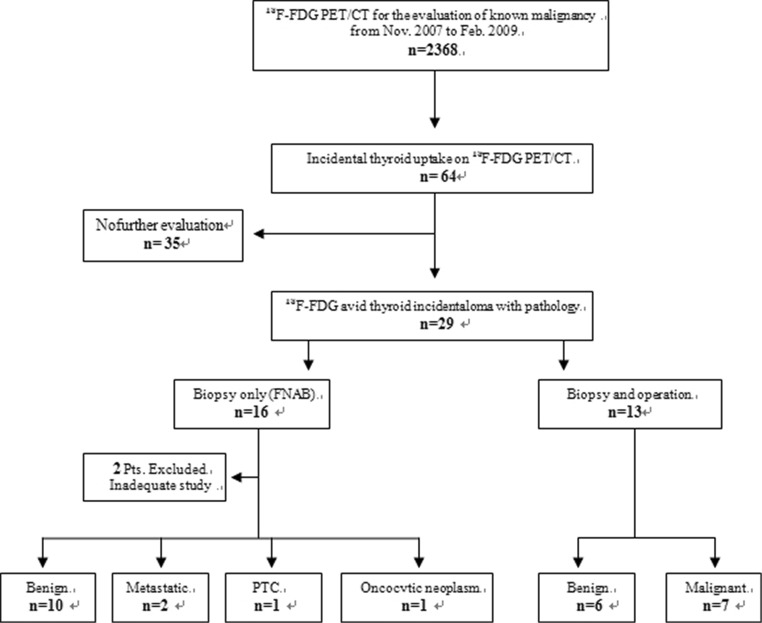
A total of 2,368 patients who had undergone 18F-FDG PET/CT for cancer evaluation from November 2007 to February 2009 were retrospectively reviewed. Figure 1 shows a flow chart detailing the process of the current study. There were 64 cases of 18F-FDG PET/CT in which focal thyroid uptakes were identified. Thirty-five patients were excluded because there was no further evaluation on this thyroid lesion due to the patients’ poor general condition or the advanced stage of underlying primary malignancies of the patients. Among these 64 cases, a total of 29 patients (24 women and 5 men, mean age 58.8 ± 6.2 years) with pathologically confirmed 18F-FDG-avid incidental thyroid lesion were selected. Those patients were evaluated for malignancies of head and neck (n = 2), gastrointestinal tract (n = 10), lung (n = 8), breast (n = 5), carcinoma of the cervix (n = 1), melanoma (n = 1) and malignancy of double primary origin (n = 2; one stomach cancer with lung cancer and one breast cancer with colon cancer, respectively). All the patients underwent dual-time-point PET/CT at 60 and 120 min after administration of 18F-FDG. Aspiration biopsy and/or operation were performed and pathologic reports were obtained.
Fig. 1.
Flow chart illustration detailing the process of current study
The protocol for the current study was reviewed and approved by institutional review board (KUGH13014) of Korea University Guro Hospital. The requirement for informed consent was waived as this study was a minimal risk retrospective study using a review of considered patient records.
Image Acquisition
18F-FDG PET/CT was performed using the Gemini TF 16-Slice PET/CT scanner (Philips Medical Systems, Cleveland, Ohio). Dual-time-point PET/CT images were routinely obtained at 60 and 120 min (early and delayed scan, respectively) after intravenous injection of 370–550 MBq 18F-FDG according to the body weight. All patients fasted at least 6 h before undergoing PET/CT. Blood glucose level was measured and confirmed to be less than 200 mg/dl before the administration of 18F-FDG. Emission PET scan was acquired from the level of skull base to the thigh for 10 min (1 min per bed position) and a low-dose CT scan was obtained for attenuation correction. Image analysis was performed on a dedicated workstation (Extended Brilliance Workspace 3.5 with PET/CT viewer for automated image registration, Philips). Maximum intensity projection (MIP), cross-sectional views and fusion images were generated and reviewed.
Image Interpretation and Analysis
A spherical region of interest (ROI) was placed on the identical areas in the same axial slice levels in both early and delayed 18F-FDG PET/CT of the thyroid incidentaloma. The maximum standardized uptake values (SUVmax) of early images (SUV1) and delayed images (SUV2) at each time point were recorded over the most intense slice of the thyroid lesion visible. SUVs were automatically calculated by normalization of regional radioactivity concentration to injected dose and body weight by using the following equation:
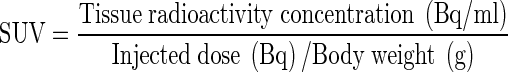 |
From these semi-quantitative indices (SUV1 and SUV2), the retention index (RI) was calculated using the following equation:
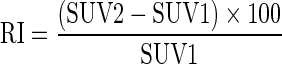 |
Statistical Analysis
Semi-quantitative PET parameters (SUV1, SUV2 and RI) and pathologic results were reviewed and correlated. Each PET parameter between malignant and benign thyroid lesions was compared using the nonparametric Mann–Whitney U test and Wilcoxon signed rank test because distribution of the analyzed data was significantly different from the normal distribution.
Receiver-operating-characteristic (ROC) curves were derived and the areas under the curves (AUCs) were compared. Optimal cut-off values were determined, and the sensitivity and specificity of each parameter were derived.
All statistical analyses were done using SPSS Software (version 13.0; SPSS, Chicago, IL, USA) for Windows and the statistically significant level was set at 0.05.
Results
Patient Characteristics
We have retrospectively reviewed 2,368 cases where 18F-FDG PET/CT were undertaken for oncological purpose. Incidental focal thyroid uptake was observed in 64 cases. Out of these 64, no pathologic assessment was possible in 35 patients due to patient refusal or highly palliative situations of the underlying disease. A total 29 patients underwent further investigation to determine the nature of the thyroid lesion. All the 29 patients got a fine-needle-aspiration biopsy (FNAB) and two of them were excluded because the aspirated specimen was inadequate for diagnosis but further diagnostic confirmation was not done (Fig. 1). Of 27 incidentally identified focal thyroid lesions on PET/CT with pathologic verification, 14 patients underwent FNAB only and the other 13 patients underwent FNAB and operation subsequently.
Of the 14 patients who underwent FNAB only, 10 thyroid lesions were diagnosed as benign disease (6 nodular hyperplasias, 2 benign cystic lesions and 2 follicular adenomas). Four were reported to be malignant lesions as two metastatic cancers (metastatic malignant melanoma and metastatic adenocarcinoma, respectively), one oncocytic carcinoma, and one papillary thyroid cancer.
Thirteen patients underwent thyroidectomy (nine total thyroidectomies, two near-total thyroidectomies, and two bilateral subtotal thyroidectomies) after cytolopathologic diagnosis with FNAB. Preoperative FNAB diagnostic results of these patients were: eight suspicious for malignancy, two atypias of undetermined significance (AUS), and three benign lesions (one adenomatous goiter, one nodular hyperplasia, and one cystic lesion). Three patients who were diagnosed to have benign thyroid lesion underwent thyroidectomy subsequently for either curative (unresolved thyrotoxicosis after antithyroid drug treatment which caused atrial tachyarrhythmia and recurrent parathyroid carcinoma, respectively) or prophylactic purpose (patient had a strong familial history of thyroid cancer). Two patients who were reported as AUS underwent bilateral subtotal thyroidectomy because of highly suspicious preoperative neck USG findings; however, all the lesions were finally proven to be benign.
A total seven out of the 13 resected thyroid specimens proved to be malignant lesion with final diagnosis. Among these malignant thyroid lesions, five were papillary thyroid cancer, one follicular thyroid carcinoma, and one anaplastic carcinoma. The clinical and pathologic characteristics of thyroid incidentalomas in this study were demonstrated in Table 1. Among 27 incidentally identified focal thyroid lesions on 18F-FDG PET/CT with histological confirmation being done, a total of 11 thyroid lesions were histologically proven to be malignant (40.7 %).
Table 1.
Clinical characteristics of the thyroid incidentaloma
| Thyroid incidentaloma | p value | ||
|---|---|---|---|
| Malignant (n = 11) | Benign (n = 16) | ||
| Male/female | 1/10 | 4/12 | |
| Age (years) | 59.1 ± 11.2 | 57.7 ± 10.6 | 0.635 |
| Nodule size(cm) | 2.4 ± 1.6 | 1.9 ± 1.3 | 0.73 |
| Pathology | PTC (n = 6) | Nodular hyperplasia (n = 8) | |
| FTC (n = 1) | Cyst (n = 5) | ||
| Metastatic (n = 2) | Follicular adenoma (n = 2) | ||
| Anaplastic carcinoma (n = 1) | AG (n = 1) | ||
| Oncocytic carcinoma (n = 1) | |||
| SUV1 | 5.47 ± 1.15 | 3.62 ± 0.66 | 0.126 |
| SUV2 | 6.74 ± 1.57 | 4.16 ± 0.83 | 0.032 |
| RI (%) | 28.4 ± 16.6 | 15.3 ± 10.5 | 0.007 |
PTC papillary thyroid cancer, FTC follicular thyroid cancer, AG adenomatous goiter
PET Data Comparisons Between Malignant and Benign Thyroid Lesions
The comparisons of semi-quantitative indices of dual-time-point 18F-FDG PET/CT between benign and malignant thyroid lesions were done and results are shown in Figs. 2, 3 and 4.
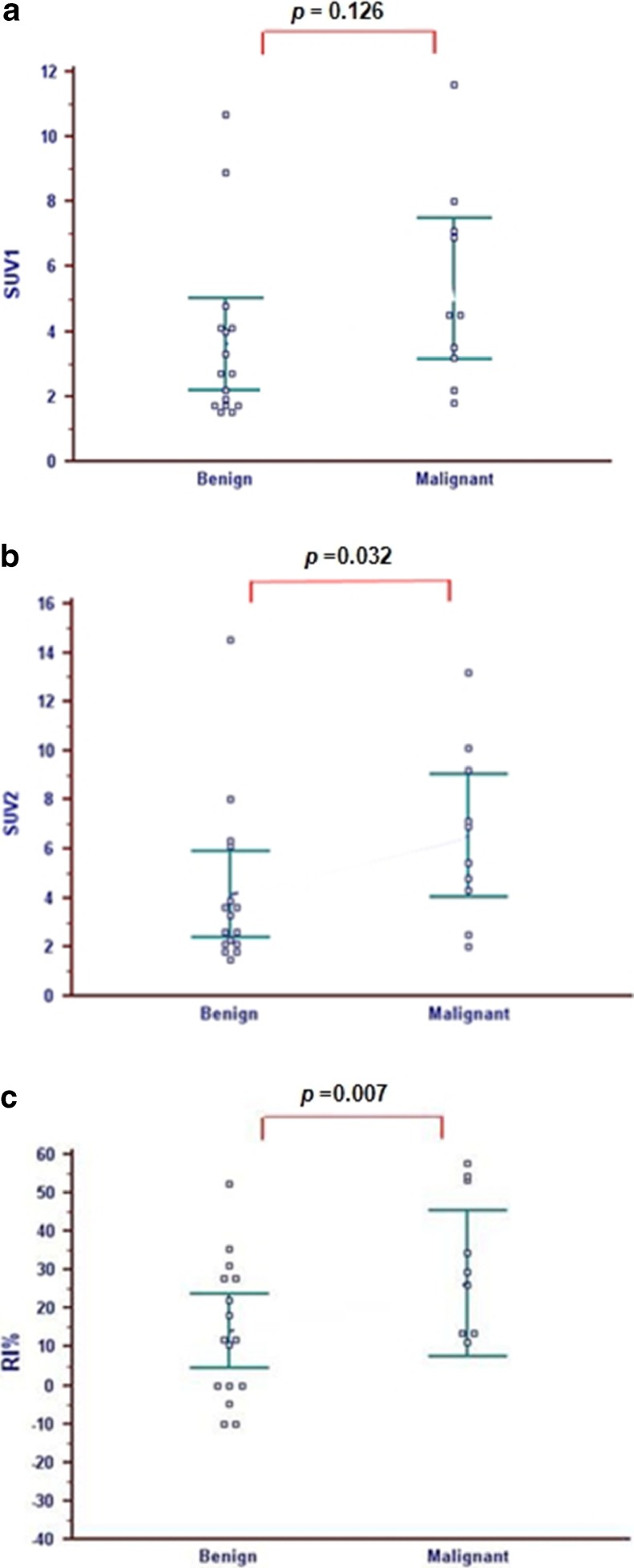
Fig. 2.
Data comparison of semi-quantitative indices (SUV1, SUV2, and RI) of dual-time-point 18F-FDG PET/CT between malignant and benign thyroid lesions. a There was no statistical difference in SUVmax on the initial PET images (SUV1) between the groups with malignant (5.47 ± 1.15) and benign thyroid lesions (3.62 ± 0.66), (p = 0.126). b SUVmax on the delayed PET images (SUV2) of the malignant patient group (6.74 ± 1.57) was statistically significantly higher than the benign group (4.16 ± 0.83) (p = 0.032). c RI of the malignant thyroid lesion (28.4 ± 16.6 %) was also higher than the benign lesions (15.3 ± 10.5 %) with statistical significance (p = 0.007)
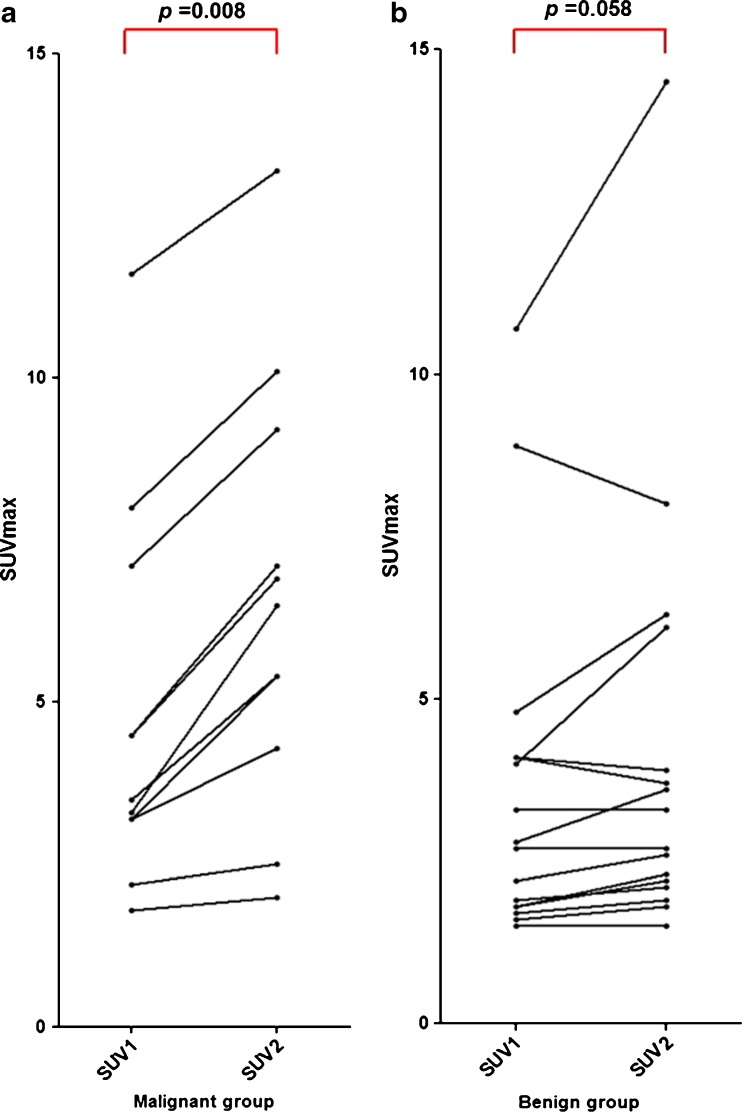
Fig. 3.
Alterations of SUVmax on dual-time-point 18F-FDG PET/CT a SUVmax values of all malignant lesions increased on the delayed image compared with the initial images. Wilcoxon signed rank test showed that in the group of malignant thyroid lesions, SUV2 was significantly higher than SUV1 (p = 0.008). b On the other hand, in the group of benign thyroid lesions, there was no significant difference between SUV1 and SUV2 (p = 0.058).Of the benign lesions, 37.5 % (6/16) showed decreased SUVmax values or no changes on the delayed images
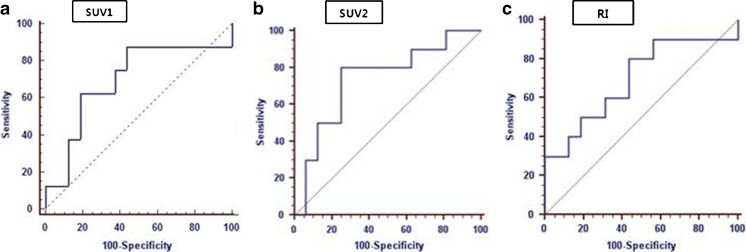
Fig. 4.
ROC curves for comparing overall diagnostic performance and determined cut-off values with sensitivity and specificity of PET parameters. a The AUC value from the ROC curve of SUV1 was 0.691 (95 % CI = 0.476–0.858). b SUV2 cut-off of 3.9 for dual-time-point PET/CT yielded an AUC value of 0.736 (95 % CI = 0.581–0.890) with sensitivity 87.5 % and specificity 75.0 % in distinguishing malignant thyroid lesion from benign. c An RI cut-off of 12.5 % yielded an AUC value of 0.771 (95 % CI = 0.613–0.915) with sensitivity 88.9 % and specificity 66.3 %
SUV1 of the malignant thyroid lesions was higher than that of benign lesions but no significant difference between two groups was found (malignant, 5.47 ± 1.15 vs benign, 3.62 ± 0.66; p = 0.126) (Fig. 2a). On the other hand, SUV2 of the malignant lesions was significantly higher than that of benign lesions (malignant, 6.74 ± 1.57 vs benign, 4.16 ± 0.83; p = 0.032) (Fig. 2b). RI of malignant lesions was also statistically significantly higher than that of benign lesions (malignant, 28.4 ± 16.6 % vs benign, 15.3 ± 10.5 %; p = 0.007) (Fig. 2c).
All malignant lesions showed higher SUVmax values on a delayed PET/CT (SUV2), compared with the values on a initial image (SUV1), i.e., RI > 0 (Figs. 2c and 3a). However, in the group of benign lesions, three had no change in SUVmax (RI = 0) and another three showed lower SUVmax values on a delayed image (RI < 0), those were 37.5 % (6/16) of the benign lesions (Figs. 2c and 3b). Wilcoxon signed rank test showed SUV2 of the malignant group was significantly higher than SUV1 (p = 0.008), but in the benign group there was no statistical difference (p = 0.058) (Fig. 3).
We generated ROC curves to assess the potential usefulness of SUV1, SUV2, and RI for the differentiation of malignant thyroid lesion from benign. The AUC values from the ROC curves of SUV1 was 0.691 (95 % confidence interval [CI] = 0.476–0.858), SUV2 was 0.736 (95 % CI = 0.581–0.890), and RI was 0.771 (95 % CI = 0.613–0.915) (Fig. 4).
The optimal cut-off value of SUVmax was determined by ROC curve analysis to predict the risk for a malignant process of the thyroid incidentaloma on 18F-FDG PET/CT. When an SUVmax of 3.9 on the delay image (SUV2) is used as a cut-off threshold, malignancy could be predicted with sensitivity of 87.5 % and specificity of 75.0 %. With a retention index (RI) cut-off of 12.5 %, focal thyroid lesion on PET/CT could be expected to be malignant with a sensitivity of 88.9 % and specificity of 66.3 %.
Discussion
Detection of unanticipated malignant lesions other than primary lesion has a clinical impact on patients with known malignant disease because most diagnostic work-ups mostly focus on the primary disease. This tends to impede proper management of another coexisting primary malignant lesion. As 18F-FDG PET/CT is used to survey the entire body, incidental findings are more common than with the other imaging modalities. Previous reports have documented that PET/CT could identify second primary malignant tumors with non-negligible incidence ranging up to 4.8 % [18].
As regards to incidentally detected 18F-FDG-avid thyroid lesion, diffuse 18F-FDG uptake in thyroid glands has been reported to be an indicator of chronic thyroiditis, particularly Hashimoto’s thyroiditis [19–21]. Kurata et al. [20] previously reported that all the 25 subjects with diffuse 18F-FDG uptake were diagnosed as having Hashimoto’s thyroiditis through a retrospective review of 1,626 subjects. On the contrary, focal 18F-FDG-avid thyroid lesions generally require clinical attentions due to a considerable risk of harboring malignancy, reported ranging to 50 % [3, 5, 6, 22]. With these backgrounds, we evaluated incidental PET findings with focal thyroid uptake in the present study. Focal thyroid lesion incidentally identified by 18F-FDG PET/CT occurred with a prevalence of 2.7 % of the cancer patients (64/2368) in our study, which is well correlated with previous studies reporting the prevalence as 1.2–4.3 % [2, 3]. Also, 40.7 % (11/27) of the patients with focal thyroid 18F-FDG uptake had malignant lesions in the thyroid by pathologic verification, similar to previous studies [2, 3, 6, 22].
Some recent systematic reviews concerning the usefulness of 18F-FDG PET in thyroid incidentalomas postulated that incidentally found thyroid nodules are at high risk of harboring malignancy if uptake is focal and SUVmax is high [23]. Are et al. [4] has also stated that further investigation and particular clinical attention should be considered if there is a focal thyroid uptake on PET/CT because of the high malignancy risk, even though there was no definitive PET criteria to help diagnose malignancy in thyroid incidentalomas.
Although SUV is a semi-quantitative parameter that reflects metabolic activity of the malignant lesion, but it is not a tumor-specific marker [24]. Many criteria have been proposed to distinguish benign from malignant lesions in literature, but no reliable cut-off has been clarified yet and the real significance of SUV and its role in discriminating malignancy is far from being definitely established. Furthermore, a different grade of inflammation in a lesion could mimic, contribute, or be a confounding factor in discriminating benignity from malignancy [25].
Zhuang et al. [9] suggested a potential role of dual-time-point 18F-FDG PET imaging for differentiating malignant from inflammatory processes in 2001, dual-phase 18F-FDG PET or PET/CT acquisition is currently used as an added tool for the differentiation of malignant lesion from benign. Yamada et al. [26] suggested that 18F-FDG uptake in inflammatory tissue showed a gradual increase until 60 min after 18F-FDG injection and then decreased gradually. However, in cases of malignant lesions, the uptake of 18F-FDG continues to increase in tumors for several hours after injection [27, 28]. Dual-time-point 18F-FDG PET imaging is likely to significantly improve the sensitivity and specificity in differentiating malignant tumors from benign, especially in lung and colorectal cancers [15, 17, 29–31], despite some limitations and controversies [32, 33].
Thyroid incidentalomas are commonly found on imaging modalities and it is important to identify which lesions are at risk for containing malignancy. We retrospectively reviewed dual-time-point 18F-FDG PET/CT for the characterization of the thyroid incidentaloma. The purpose of the present study is to investigate the clinical role of dual-phase 18F-FDG PET/CT for the differentiation of malignancy from benignity in incidentally detected 18F-FDG-avid thyroid lesion by elucidating the relationship between the semi-quantitative indices of dual-time point PET/CT. The results suggested significant advantages of dual time point 18F-FDG PET/CT imaging for differentiation of malignant from benign lesions. SUVmax on the delayed image (SUV2) and retention index (RI) of the malignant thyroid lesions were significantly higher than those of benign lesions. But there was no significant difference in SUVmax of the initial image (SUV1) between the two groups. Kim et al. [34] previously reported that dual-time-point 18F-FDG PET/CT was not a useful method for differentiating malignant and benign nodules through an analysis of a total of 50 patients. They found no statistical differences of dual-phase PET parameters (SUV1, SUV2, and RI) between benign and malignant thyroid lesions. In the previous study, all of the incidentally detected thyroid lesions showed positive RI values, irrespective of the pathologic characteristics. In contrast, the SUVmax of all malignant thyroid lesions increased over time in the current study. But in the cases of benign lesions, three lesions showed no changes in SUVmax (RI = 0) and another three lesions showed a decreased value (RI < 0) as shown in Fig. 3b. Therefore, we suggest that an incidental focal thyroid lesion on PET scan which shows decreased SUVmax on delayed scan could be predicted as a benign lesion. And that means further invasive diagnostic procedures such as biopsy could be avoided with those features. This is important because thyroid incidentalomas are discovered at a high rate, often leading to unnecessary testing and procedures as well as increasing patient anxiety. Differentiation between malignant and benign thyroid lesions prevents unnecessary operation and improves the quality of life of the patients, especially those with a coexisting other primary malignancy.
Our ROC analyses revealed that RI showed the highest AUC, SUV2 was in the middle, and SUV1 showed a lower value. RI has better in overall diagnostic performance than SUV2 [35]. On the other hand, the lower band of the 95 % CI of SUV1 was under the practical lower limit of AUC with regard to diagnostic performance (0.5). Hence, when SUVmax of the single-phase 18F-FDG PET/CT alone is used for differential diagnosis of incidentally detected 18F-FDG-avid thyroid lesion, there is a chance of making the diagnostic decision based on pure chance.
There are some limitations of the present study. First, this is a highly selected and retrospectively reviewed study with a small number of subjects and had a limited period of follow-up. Because of this retrospective design, final diagnosis with cytopathologic verification was not done in about half of the subjects who had incidental focal thyroid lesions found on 18F-FDG PET/CT. Second, PET parameters using dual-time-point imaging seemed to be more helpful to suggest benign thyroid diseases than to differentiate malignancy from benign lesions in this study. Until now, the role of the dual-time-point method in differential diagnosis of incidentally detected focal thyroid lesion on 18F-FDG PET/CT is complementary and further studies are needed to validate the result of this pilot study. Further prospective study is in progress with a larger number of subjects to verify the usefulness of dual-time-point 18F-FDG PET/CT and PET indices in the differential diagnosis of thyroid incidentaloma in our institute.
Conclusion
In conclusion, the major finding of the present study was that we could predict benignity when the incidentally detected thyroid lesion showed a decrease or no change in SUVmax on the delayed image. SUV2 and RI of the malignant lesions were significantly higher than those of benign lesions. With the current study, we suggest that dual-time-point 18F-FDG PET/CT imaging, obtaining additional images 2 h after injection, seems to have a complementary role to distinguish between malignancy and benignity of the incidental thyroid lesions. Further studies are needed to confirm these results and improve statistical accuracy.
Acknowledgments
This study was supported by the Seoul National University Hospital Research Fund (grant number 03-2004-013-0) and a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A070001) and a grant from Korea University (grant number K1132631 and K0714991).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jin J, McHenry CR. Thyroid incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26(1):83–96. doi: 10.1016/j.beem.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Kang KW, Kim SK, Kang HS, Lee ES, Sim JS, Lee IG, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab. 2003;88(9):4100–4104. doi: 10.1210/jc.2003-030465. [DOI] [PubMed] [Google Scholar]
- 3.Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47(4):609–615. [PubMed] [Google Scholar]
- 4.Are C, Hsu JF, Schoder H, Shah JP, Larson SM, Shaha AR. FDG-PET detected thyroid incidentalomas: need for further investigation? Ann Surg Oncol. 2007;14(1):239–247. doi: 10.1245/s10434-006-9181-y. [DOI] [PubMed] [Google Scholar]
- 5.Pagano L, Samà MT, Morani F, Prodam F, Rudoni M, Boldorini R, et al. Thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography with CT (FDG-PET/CT): clinical and pathological relevance. Clin Endocrinol (Oxf). 2011;75(4):528–534. doi: 10.1111/j.1365-2265.2011.04107.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Arslan N, Dehdashti F, Doherty GM, Lairmore TC, Brunt LM, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery. 2001;130(6):941–946. doi: 10.1067/msy.2001.118265. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson IL, Arnberg F, Zedenius J, Sundin A. Thyroid incidentaloma detected by fluorodeoxyglucose positron emission tomography/computed tomography: practical management algorithm. World J Surg. 2011;35(12):2691–2697. doi: 10.1007/s00268-011-1291-4. [DOI] [PubMed] [Google Scholar]
- 8.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46(5):752–757. [PubMed] [Google Scholar]
- 9.Soelberg KK, Bonnema SJ, Brix TH, Hegedüs L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012;22(9):918–925. doi: 10.1089/thy.2012.0005. [DOI] [PubMed] [Google Scholar]
- 10.Suga K, Kawakami Y, Hiyama A, Sugi K, Okabe K, Matsumoto T, et al. Differential diagnosis between 18F- FDG-avid metastatic lymph nodes in non-small cell lung cancer and benign nodes on dual-time point PET/CT scan. Ann Nucl Med. 2009;23(6):523–531. doi: 10.1007/s12149-009-0268-y. [DOI] [PubMed] [Google Scholar]
- 11.Costantini DL, Vali R, Chan J, McQuattie S, Charron M. Dual-time-point FDG PET/CT for the evaluation of pediatric tumors. AJR Am J Roentgenol. 2013;200(2):408–413. doi: 10.2214/AJR.12.8930. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42(9):1412–1417. [PubMed] [Google Scholar]
- 13.Caprio MG, Cangiano A, Imbriaco M, Soscia F, Di Martino G, Farina A, et al. Dual-time-point 18F-FDG PET/CT in the diagnostic evaluation of suspicious breast lesions. Radiol Med. 2010;115(2):215–224. doi: 10.1007/s11547-009-0491-6. [DOI] [PubMed] [Google Scholar]
- 14.Mavi A, Urhan M, Yu JQ, Zhuang H, Houseni M, Cermik TF, et al. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med. 2006;47(9):1440–1446. [PubMed] [Google Scholar]
- 15.Hu M, Han A, Xing L, Yang W, Fu Z, Huang C, et al. Value of dual-time-point FDG PET/CT for mediastinal nodal staging in non-small-cell lung cancer patients with lung comorbidity. Clin Nucl Med. 2011;36(6):429–433. doi: 10.1097/RLU.0b013e3182173810. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK. Limited prognostic value of dual time point 18F- FDG PET/CT in patients with early stage (stage I & II) non-small cell lung cancer (NSCLC) Radiother Oncol. 2011;98(1):105–108. doi: 10.1016/j.radonc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Shinya T, Fujii S, Asakura S, Taniguchi T, Yoshio K, Alafate A, et al. Dual-time-point 18F-FDG PET/CT for evaluation in patients with malignant lymphoma. Ann Nucl Med. 2012;26(8):616–621. doi: 10.1007/s12149-012-0619-y. [DOI] [PubMed] [Google Scholar]
- 18.Stokkel MP, Moons KG, ten Broek FW, van Rijk PP, Hordijk GJ. 18F-Fluorodeoxyglucose dual-head positron emission tomography as a procedure for detecting simultaneous primary tumors in cases of head and neck cancer. Cancer. 1999;86(11):2370–2377. doi: 10.1002/(SICI)1097-0142(19991201)86:11<2370::AID-CNCR27>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, et al. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48(6):896–901. doi: 10.2967/jnumed.106.039024. [DOI] [PubMed] [Google Scholar]
- 20.Kurata S, Ishibashi M, Hiromatsu Y, Kaida H, Miyake I, Uchida M, et al. Diffuse and diffuse-plus-focal uptake in the thyroid gland identified by using FDG-PET: prevalence of thyroid cancer and Hashimoto’s thyroiditis. Ann Nucl Med. 2007;21(6):325–330. doi: 10.1007/s12149-007-0030-2. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, et al. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207(3):775–778. doi: 10.1148/radiology.207.3.9609903. [DOI] [PubMed] [Google Scholar]
- 22.Kim TY, Kim WB, Ryu JS, Gong G, Hong SJ, Shong YK. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope. 2005;115(6):1074–1078. doi: 10.1097/01.MLG.0000163098.01398.79. [DOI] [PubMed] [Google Scholar]
- 23.Shie P, Cardarelli R, Sprawls K, Fulda KG, Taur A. Systematic review: prevalence of malignant incidental thyroid nodules identified on 18F-fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2009;30(9):742–748. doi: 10.1097/MNM.0b013e32832ee09d. [DOI] [PubMed] [Google Scholar]
- 24.Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23:1409–1415. doi: 10.1007/BF01367602. [DOI] [PubMed] [Google Scholar]
- 25.Albes JM, Dohmen BM, Schott U, Schülen E, Wehrmann M, Ziemer G. Value of positron emission tomography for lung cancer staging. Eur J Surg Oncol. 2002;28(1):55–62. doi: 10.1053/ejso.2001.1144. [DOI] [PubMed] [Google Scholar]
- 26.Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995;36(7):1301–1306. [PubMed] [Google Scholar]
- 27.Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43(7):871–875. [PubMed] [Google Scholar]
- 28.Lodge MA, Lucas JD, Marsden PK, Cronin BF, O'Doherty MJ, Smith MA. A PET study of 18F-FDG uptake in soft tissue masses. Eur J Nucl Med. 1999;26(1):22–30. doi: 10.1007/s002590050355. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Kim SK, Lee SM, Moon SH, Kim TS. Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol. 2011;13(3):565–572. doi: 10.1007/s11307-010-0394-x. [DOI] [PubMed] [Google Scholar]
- 30.Lin YY, Chen JH, Ding HJ, Liang JA, Yeh JJ, Kao CH. Potential value of dual-time-point 18F-FDG PET compared with initial single-time-point imaging in differentiating malignant from benign pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun. 2012;33(10):1011–1018. doi: 10.1097/MNM.0b013e32835710d6. [DOI] [PubMed] [Google Scholar]
- 31.Choi EK, Yoo IER, Kim SH, O JH, Choi WH, Na SJ, et al. The clinical value of dual-time point 18F-FDG PET/CT for differentiating extrahepatic cholangiocarcinoma from benign disease. Clin Nucl Med. 2013;38(3):106–111. doi: 10.1097/RLU.0b013e318266f402. [DOI] [PubMed] [Google Scholar]
- 32.Barger RL, Jr, Nandalur KR. Diagnostic performance of dual-time 18F-FDG PET in the diagnosis of pulmonary nodules: a meta-analysis. Acad Radiol. 2012;19(2):153–158. doi: 10.1016/j.acra.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Döbert N, Hamscho N, Menzel C, Neuss L, Kovács AF, Grünwald F. Limitations of dual time point FDG-PET imaging in the evaluation of focal abdominal lesions. Nuklearmedizin. 2004;43(5):143–149. doi: 10.1267/nukl04050143. [DOI] [PubMed] [Google Scholar]
- 34.Kim SJ, Kim BH, Jeon YK, Kim SS, Kim IJ. Limited diagnostic and predictive values of dual-time-point 18F FDG PET/CT for differentiation of incidentally detected thyroid nodules. Ann Nucl Med. 2011;25(5):347–353. doi: 10.1007/s12149-011-0468-0. [DOI] [PubMed] [Google Scholar]
- 35.Park SH, Goo JM, Jo CH. Receiver operating characteristic (ROC) curve: practical review for radiologists. Korean J Radiol. 2004;5(1):11–18. doi: 10.3348/kjr.2004.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]