Abstract
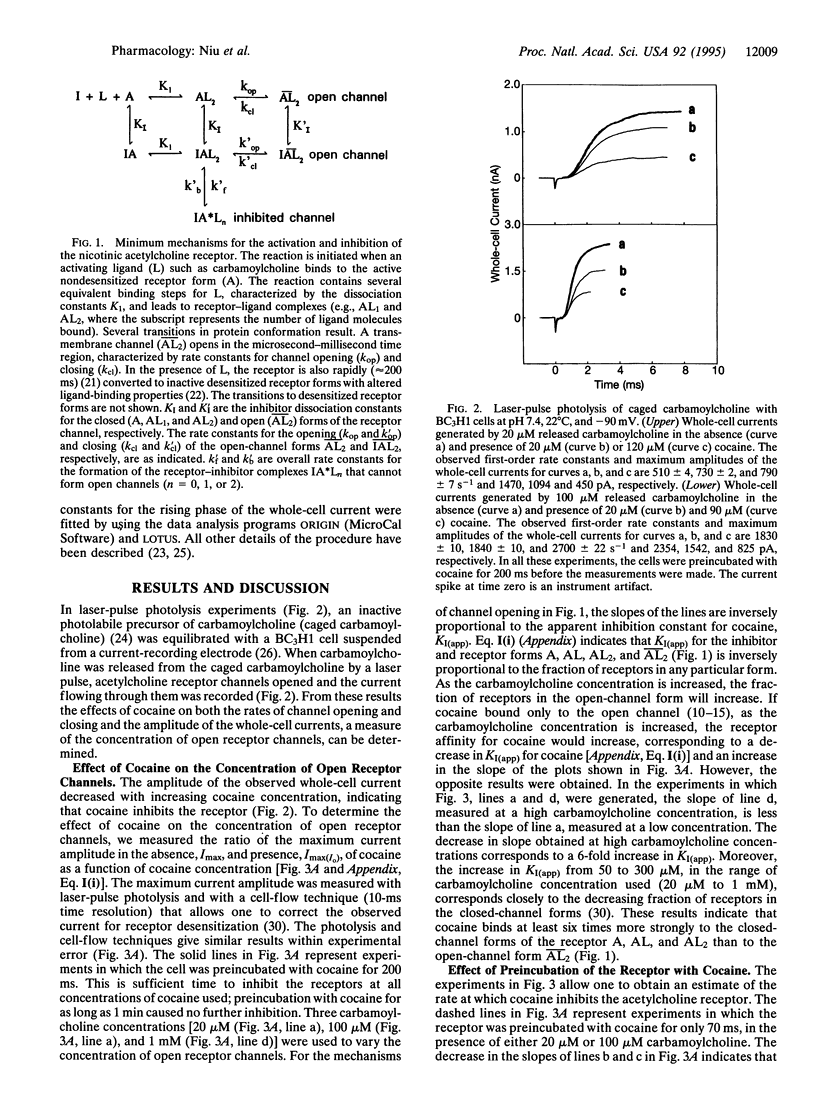
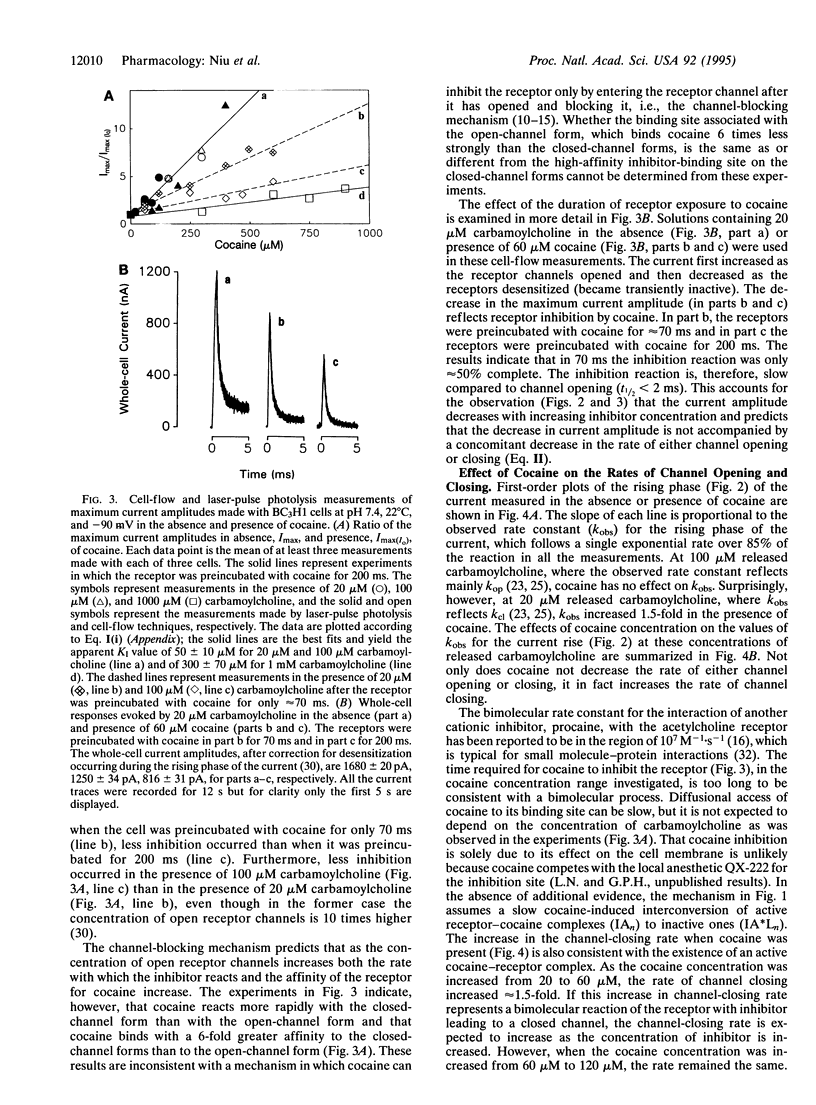
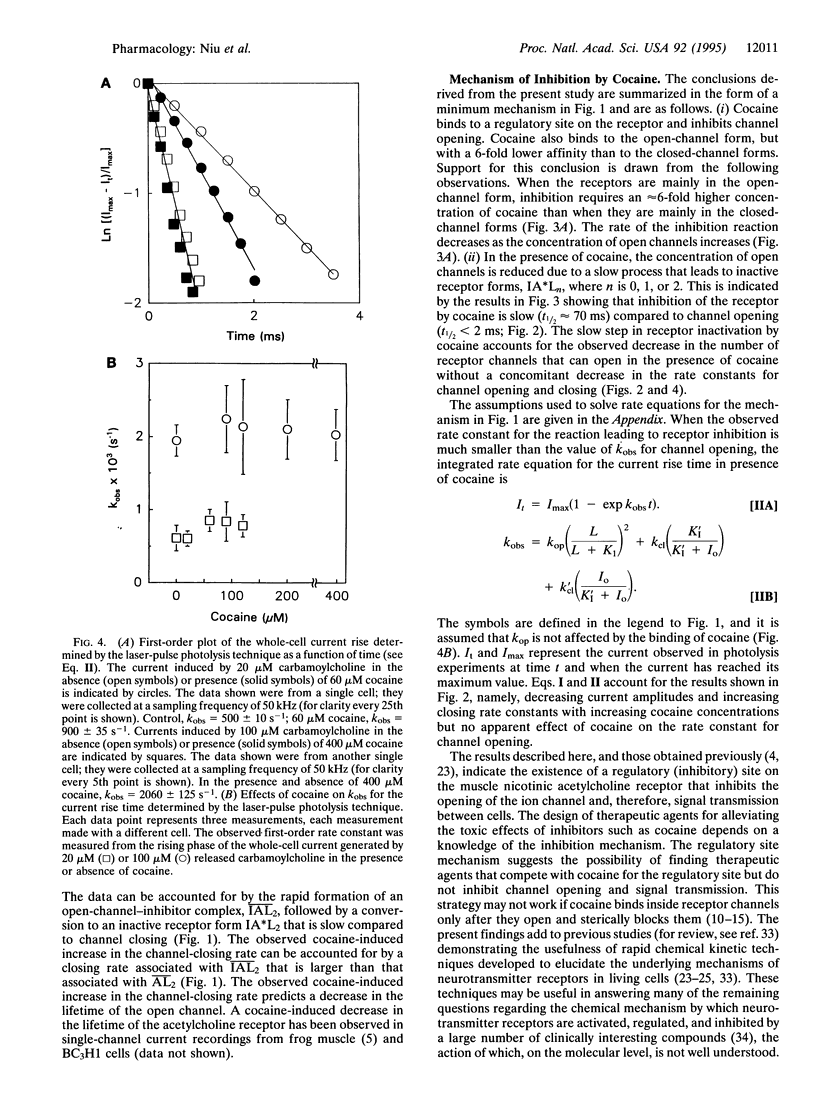
Effects of cocaine on the muscle nicotinic acetylcholine receptor were investigated by using a chemical kinetic technique with a microsecond time resolution. This membrane-bound receptor regulates signal transmission between nerve and muscle cells, initiates muscle contraction, and is inhibited by cocaine, an abused drug. The inhibition mechanism is not well understood because of the lack of chemical kinetic techniques with the appropriate (microsecond) time resolution. Such a technique, utilizing laser-pulse photolysis, was recently developed; by using it the following results were obtained. (i) The apparent cocaine dissociation constant of the closed-channel receptor form is approximately 50 microM. High carbamoylcholine concentration and, therefore, increased concentrations of the open-channel receptor form, decrease receptor affinity for cocaine approximately 6-fold. (ii) The rate of the receptor reaction with cocaine is at least approximately 30-fold slower than the channel-opening rate, resulting in a cocaine-induced decrease in the concentration of open receptor channels without a concomitant decrease in the channel-opening or -closing rates. (iii) The channel-closing rate increases approximately 1.5-fold as the cocaine concentration is increased from 20 to 60 microM but then remains constant as the concentration is increased further. The results are consistent with a mechanism in which cocaine first binds rapidly to a regulatory site of the receptor, which can still form transmembrane channels. Subsequently, a slow step (t1/2 approximately 70 ms) leads to a receptor form that cannot form transmembrane channels, and acetylcholine receptor-mediated signal transmission is, therefore, blocked. Implications for the search for therapeutic agents that alleviate cocaine poisoning are mentioned.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Flynn D. D., Vaishnav A. A., Mash D. C. Interactions of cocaine with primary and secondary recognition sites on muscarinic receptors. Mol Pharmacol. 1992 Apr;41(4):736–742. [PubMed] [Google Scholar]
- Gage P. W., Wachtel R. E. Some effects of procaine at the toad end-plate are not consistent with a simple channel-blocking model. J Physiol. 1984 Jan;346:331–339. doi: 10.1113/jphysiol.1984.sp015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bessis A., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Gawin F. H. Cocaine addiction: psychology and neurophysiology. Science. 1991 Mar 29;251(5001):1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion fluxes in membrane vesicles investigated by fast reaction techniques. Nature. 1979 Nov 15;282(5736):329–331. doi: 10.1038/282329a0. [DOI] [PubMed] [Google Scholar]
- Hess G. P. Determination of the chemical mechanism of neurotransmitter receptor-mediated reactions by rapid chemical kinetic techniques. Biochemistry. 1993 Feb 2;32(4):989–1000. doi: 10.1021/bi00055a001. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Hess G. P. Cocaine, phencyclidine, and procaine inhibition of the acetylcholine receptor: characterization of the binding site by stopped-flow measurements of receptor-controlled ion flux in membrane vesicles. Biochemistry. 1986 Apr 8;25(7):1777–1785. doi: 10.1021/bi00355a049. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Lathers C. M., Tyau L. S., Spino M. M., Agarwal I. Cocaine-induced seizures, arrhythmias and sudden death. J Clin Pharmacol. 1988 Jul;28(7):584–593. doi: 10.1002/j.1552-4604.1988.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Lester H. A. The permeation pathway of neurotransmitter-gated ion channels. Annu Rev Biophys Biomol Struct. 1992;21:267–292. doi: 10.1146/annurev.bb.21.060192.001411. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Billington A. P., Hess G. P. How fast does an acetylcholine receptor channel open? Laser-pulse photolysis of an inactive precursor of carbamoylcholine in the microsecond time region with BC3H1 cells. Biochemistry. 1992 Jun 23;31(24):5507–5514. doi: 10.1021/bi00139a012. [DOI] [PubMed] [Google Scholar]
- Milburn T., Matsubara N., Billington A. P., Udgaonkar J. B., Walker J. W., Carpenter B. K., Webb W. W., Marque J., Denk W., McCray J. A. Synthesis, photochemistry, and biological activity of a caged photolabile acetylcholine receptor ligand. Biochemistry. 1989 Jan 10;28(1):49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L., Hess G. P. An acetylcholine receptor regulatory site in BC3H1 cells: characterized by laser-pulse photolysis in the microsecond-to-millisecond time region. Biochemistry. 1993 Apr 20;32(15):3831–3835. doi: 10.1021/bi00066a001. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Papke R. L., Oswald R. E. Mechanisms of noncompetitive inhibition of acetylcholine-induced single-channel currents. J Gen Physiol. 1989 May;93(5):785–811. doi: 10.1085/jgp.93.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey J., Ritz M. C., Schenden J. A., Hanson R. C., Kuhar M. J. Cocaine inhibits muscarinic cholinergic receptors in heart and brain. J Pharmacol Exp Ther. 1988 Sep;246(3):1048–1052. [PubMed] [Google Scholar]
- Sine S., Taylor P. Functional consequences of agonist-mediated state transitions in the cholinergic receptor. Studies in cultured muscle cells. J Biol Chem. 1979 May 10;254(9):3315–3325. [PubMed] [Google Scholar]
- Swanson K. L., Albuquerque E. X. Nicotinic acetylcholine receptor ion channel blockade by cocaine: the mechanism of synaptic action. J Pharmacol Exp Ther. 1987 Dec;243(3):1202–1210. [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Bakry N. M., Eldefrawi M. E., Eldefrawi A. T. Voltage- and time-dependent actions of piperocaine on the ion channel of the acetylcholine receptor. Mol Pharmacol. 1979 Nov;16(3):909–921. [PubMed] [Google Scholar]
- Udgaonkar J. B., Hess G. P. Chemical kinetic measurements of a mammalian acetylcholine receptor by a fast-reaction technique. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8758–8762. doi: 10.1073/pnas.84.24.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]



