Abstract
Transcranial alternating current stimulation (tACS) is used in clinical applications and basic neuroscience research. Although its behavioral effects are evident from prior reports, current understanding of the mechanisms that underlie these effects is limited. We used motion perception, a percept with relatively well known properties and underlying neural mechanisms to investigate tACS mechanisms. Healthy human volunteers showed a surprising improvement in motion sensitivity when visual stimuli were paired with 10 Hz tACS. In addition, tACS reduced the motion-after effect, and this reduction was correlated with the improvement in motion sensitivity. Electrical stimulation had no consistent effect when applied before presenting a visual stimulus or during recovery from motion adaptation. Together, these findings suggest that perceptual effects of tACS result from an attenuation of adaptation. Important consequences for the practical use of tACS follow from our work. First, because this mechanism interferes only with adaptation, this suggests that tACS can be targeted at subsets of neurons (by adapting them), even when the applied currents spread widely throughout the brain. Second, by interfering with adaptation, this mechanism provides a means by which electrical stimulation can generate behavioral effects that outlast the stimulation.
Keywords: discrimination sensitivity, mechanisms, motion adaptation, motion after effect, transcranial alternating current stimulation
Introduction
There is rapidly growing interest in using transcranial alternating current stimulation (tACS) to modulate brain activity in both clinical applications and cognitive neuroscience research (Utz et al., 2010; Zaghi et al., 2010). For instance, tACS has been claimed to suppress parkinsonian tremors (Brittain et al., 2013), entrain motor performance (Joundi et al., 2012), aid recovery after stroke (Schlaug et al., 2008; Fedorov et al., 2010), and improve learning and memory (Kirov et al., 2009), to name just a few. The mechanisms that underlie these long-term effects, however, remain poorly understood (Reato et al., 2013).
Even though applied fields clearly modulate membrane polarization (Radman et al., 2009), the long-term effects of electrical stimulation may not be the direct consequence of this polarization, but the indirect consequence of changes in plasticity induced by the stimulation (Rosenkranz et al., 2000; Antal et al., 2004, 2012; Fricke et al., 2011). We used visual motion discrimination in humans to investigate this view. This model system has the advantage that its neural mechanisms are relatively well understood (Krekelberg, 2008), that a specific cortical area (hMT+) has been identified to play a critical role (Sunaert et al., 1999), and that a large arsenal of objective measures for behavioral report are available for its study (for review, see Nishida, 2011).
We first hypothesized that direct, tACS-induced perturbations should generate impairments in motion discrimination, because such perturbations are uninformative with respect to the direction of visual motion. Our experiments rejected this hypothesis; instead, we found that subjects were better at motion direction discrimination during the application of tACS. Puzzled by this unexpected improvement in performance we hypothesized that tACS could have prevented the reduction in motion discrimination performance that was previously reported to occur for prolonged stimulus presentations (Van Wezel and Britten, 2002).
In a second set of experiments we tested this hypothesis using a standard motion adaptation paradigm (Hiris and Blake, 1992; Blake and Hiris, 1993). In such paradigms, a few seconds of exposure to, for instance, an upward moving pattern generates the illusory percept of downward motion in a subsequent stationary or random motion stimulus. As alluded to above, adaptation also typically reduces motion discrimination performance (Van Wezel and Britten, 2002). The behavioral effects of motion adaptation have been linked to neural adaptation in the middle temporal area (Kohn and Movshon, 2004; Krekelberg et al., 2006a), and the time scale at which these effects persist (tens of seconds) suggest that they rely on plastic changes, such as synaptic depression, or long-term after hyperpolarization (Kohn, 2007). Consistent with our hypothesis, the experiments confirmed that tACS during the presentation of the visual motion stimulus (i.e., during the induction of adaptation) attenuated motion adaptation.
Together our experiments suggest a novel mode of action of tACS; the attenuation of adaptation. In the discussion, we address the implications of these findings for using tACS and speculate about the underlying neural mechanisms.
Materials and Methods
Electrode placement
One electrode was placed above the canonical location of left hMT+; PO7-PO3 in the 10-20 system. The other electrode was placed on the vertex (Cz). In the main experiments, the parietal electrode was contralateral to the visual stimuli. In the ipsilateral control experiments, the electrode was placed above the hMT+ that was ipsilateral to the visual stimuli.
Subjects
Fifteen subjects participated in the experiments (8 female; 14 naive and 1 experimenter in total; 9 subjects for the motion discrimination task, 10 subjects for the motion adaptation task, 10 subjects for the recovery task, and 8 subjects for the pre stimulus tACS task). They gave written consent and had normal or corrected to normal vision. This study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Institutional Review Board of Rutgers University.
Apparatus
tACS was delivered through a STG4002 stimulus generator (Multi Channel Systems). The stimulating electrodes were prepared as saline soaked sponges attached to conductive rubber electrodes (3″ diameter). We used a sinusoidal current (1 mA peak to peak) at a frequency of 10 Hz. For safety reasons, the maximum voltage to produce the transcranial current was limited to 20 V. The maximum current intensity was 0.5 mA and the electrode surface area was 45.6 cm2. All eye movements were recorded using an eye tracker (Eyelink II V 2.2) at 500 Hz. Stimulus presentations and the triggering of stimulation were under the control of Neurostim (http://neurostim.sourceforge.net).
Visual stimuli
Stimuli were presented on a CRT monitor (Sony FD Trinitron) with a resolution of 1024 × 768 pixels at a refresh rate of 120 Hz. The main motion stimulus was a dynamic random dot kinematogram (RDK) consisting of 700 dots with an infinite lifetime and an effective diameter of 1.5 pixels using spatial dithering (OpenGL point size of 1.5). The dots were restricted inside a circular aperture of radius 5° centered 7° to the left or right of the center of the screen. The luminance of the dots was 30 cd/m2, and the background 0.4 cd/m2. The dots moved at a constant speed of 3°/s except during the speed change detection task (used to control for attention during adaptation; see below) when they moved at 6°/s for a brief (200 ms) period of time. We refer to the percentage of dots moving in the same direction (positive coherence: up; negative coherence: down) as the coherence. The remainder of the dots moved in randomly chosen directions. Stimuli such as these are commonly used to quantify motion perception (Newsome and Pare, 1988; Scase et al., 1996).
The RDK was used to construct the following five types of motion stimuli: (1) Long Adapter: RDK with dots moving upward with a coherence of 100% for 40 s. (2) Top-up Adapter: RDK with dots moving upward with a coherence of 100% for 4 s. (3) Test: RDK with different levels of coherence, presented for 1 s. (4) Long Test: RDK with different levels of coherence, presented for 4 s. (5)Random: RDK with all dots moving in a randomly chosen direction (0% coherence).
Experimental procedures
Subjects were seated in a dark room at a distance of 57 cm from the center of the monitor. Head movements were restricted by a molded bite bar. The subjects indicated their response using the keyboard. Fixation of a central red dot was monitored and trials in which the eye strayed beyond a virtual window of 2° were discarded.
Because transcranial electrical stimulation has been shown to have long lived effects (Nitsche and Paulus, 2001; Bolzoni et al., 2013), experimental conditions with and without stimulation could not be interleaved. The minimal time to start blocks of trials without stimulation after tACS had been administered for any paradigm was 24 h.
Behavioral tasks
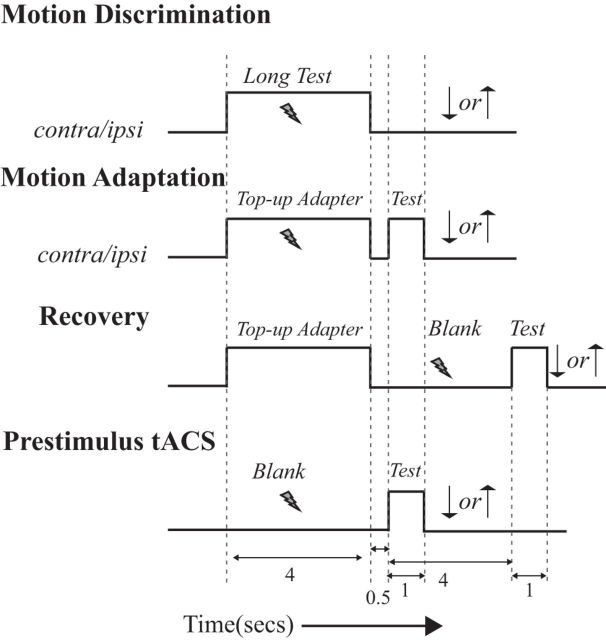
In each of the experiments, the subjects' task was to indicate the perceived global direction of motion of the test stimulus: up or down (Fig. 1).
Figure 1.
Summary of experimental paradigms (see Materials and Methods). The lightning bolt represents the application of tACS. In each paradigm subjects indicated the perceived direction of motion of the Test stimulus by pressing the up (↑) or down (↓) button.
Paradigm 1: motion discrimination.
This paradigm served to measure the instantaneous influence of tACS on coarse motion discrimination. Eight subjects participated in the experiment. We presented the Long Test stimuli and the subjects indicated the perceived global direction of motion (up or down). The coherence of the Long Test stimuli ranged from −100% (all dots moving down) to +100% (all dots moving up). Stimulation was applied over the left hMT+ only during the presentation of the Long Test stimuli. In separate sessions, the visual stimulus was either presented in the right hemifield (contralateral condition) or left hemifield (ipsilateral condition).
Paradigm 2: motion adaptation.
This paradigm measured the influence of tACS on the induction of adaptation using a standard top-up design. Each experimental session started with a single, 40 s presentation of the Long Adapter stimulus. In all subsequent trials the Top-up Adapter stimulus (4 s) was followed by a blank period (500 ms) and then by the Test stimulus (1 s). The subject's task was to indicate the coherent motion direction of the Test stimulus.
In the stimulation conditions, we applied tACS only when the Long Adapter or Top-up Adapter stimulus was on the screen. In the no-tACS conditions, no stimulation was applied. For the contralateral and ipsilateral experiments, we stimulated the left hemisphere while showing the stimulus on the right hemifield and left hemifield, respectively.
To monitor and control the allocation of attention, subjects were instructed to attend to the adapter stimulus and press a key when a brief (200 ms) doubling of speed occurred (at an unpredictable time). As a secondary benefit, this attention to the adapter also increases the strength of adaptation (Rezec et al., 2004). Trials in which the subjects failed to detect the speed changes were removed from the analysis.
Paradigm 3: recovery.
This paradigm probed the influence of tACS on recovery from adaptation. In this experiment, the time between adapter and test (during which the screen was blank) was 4 s; in most subjects this still produces a residual aftereffect (Spigel, 1964). In separate sessions, either no tACS was ever applied, or tACS was applied during each 4 s blank period.
Paradigm 4: prestimulus tACS.
This paradigm investigated whether behavioral effects of tACS require the neural changes induced by adaptation. Each trial started with a 4 s blank period, followed by an interval of 500 ms and then by the Test stimulus. In separate sessions, stimulation was either always off or on during every 4 s prestimulus blank period.
Data analysis
Curve fitting.
We used Probit Analysis (Finney, 1947) to evaluate the data. We fit the behavioral choice data (proportion of upward choice) with cumulative Gaussians using MATLAB (MathWorks). We assumed binomial noise on the proportion of up/down responses. The fitted curves all had R2 values >0.7. The curve fits provided us with two dependent measures; the point of subjective equality (PSE) and the sensitivity. The PSE was defined as the coherence level at which the fitted curve reached 0.5 and the sensitivity as the slope of the fitted curve at the PSE. We quantified the motion after effect (MAE) as the difference between the PSE of the adapted and unadapted conditions (both in the absence of tACS; Hiris and Blake, 1992; Castet et al., 2002).
Statistical analysis.
At the single-subject level we used nonparametric permutation tests (Efron and Tibshirani, 1994) to determine whether PSEs and sensitivities were significantly different between two conditions (e.g., adapted without tACS and adapted with tACS). In this procedure, we combined the responses from all trials in both conditions, drew (with replacement) two complete datasets from this distribution, and determined the difference in the PSE or sensitivity. We repeated this resampling process 1000 times to obtain a null distribution of the differences. We then determined the p value of the test as the fraction of values in the null distribution that were larger than the actual difference between the two conditions. Unlike the methods that are derived from asymptotic theory, the bootstrap method is ideal for analyzing psychophysical data because its accuracy does not depend on large numbers of trials, or assumptions (such as normality) about the underlying distributions (Hinkley, 1988).
At the group level, we performed a paired Wilcoxon signed rank test separately for the motion discrimination, motion adaptation, recovery from adaptation, and prestimulus tACS experiments. For the motion adaptation and the motion discrimination experiments we also used a two-sided Wilcoxon rank sum test to compare the differences in the changes (sensitivity and PSE) induced by tACS during the contralateral versus the ipsilateral condition. All statistical conclusions remained the same even after the exclusion of the data collected from the non-naive subject.
Analysis of the relation between adaptation strength and tACS-induced effects.
To investigate whether the influence of tACS (on the PSE or the slope) increased with the strength of adaptation, we calculated the Pearson correlation coefficient (ρ) between the tACS-induced change and the MAE. Specifically, for the change in PSE:
We used a permutation test to test the null hypothesis that this correlation was larger for contralateral than for ipsilateral tACS stimulation. We created a null distribution of differences in correlation by randomly sampling PSEs from the ipsilateral and contralateral conditions, and calculating the difference in ρ for 1000 shuffled datasets. A statistically significant difference in correlation between contralateral and ipsilateral tACS was defined as a difference in ρ that was larger than the 95th percentile of this null distribution. The analogous analysis was performed for the sensitivity data.
Results
We measured the influence of tACS (±0.5 mA, 10 Hz) on motion sensitivity and adaptation by applying it at various times during a standard motion discrimination task; during discrimination, before discrimination, during adaptation, and during recovery from adaptation.
tACS improved motion sensitivity
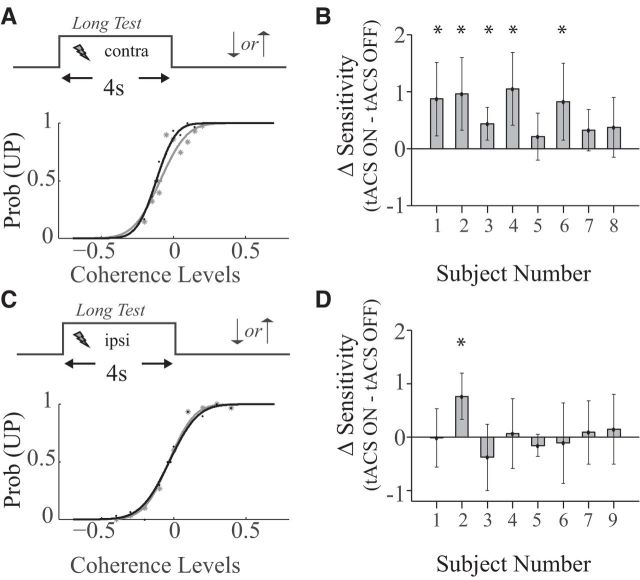
We first tested the hypothesis that tACS injects nuisance perturbations in the motion direction discrimination system. This hypothesis predicts a decrease in the subjects' sensitivity when tACS is applied over hMT+ during a motion discrimination task (see Materials and Methods, Paradigm 1). Figure 2A (bottom) shows the performance of one of the subjects with (thick black curve) and without (thin gray curve) stimulation. From such curves, we extracted the two measures of interest; the PSE and the sensitivity (see Materials and Methods). Contrary to our expectation, transcranial stimulation improved discrimination sensitivity (Fig. 2B; p < 0.05, Wilcoxon signed rank test; Cohen's d = 0.79; effect size (r) = 0.36).
Figure 2.
Motion discrimination task. A, Top, Task design. Bottom, Psychometric functions computed for an example subject with (thick black curve) and without (thin gray curve) tACS. B, Change in sensitivity after application of tACS (for all 8 subjects). Error bars indicate bootstrapped SDs of the sensitivity estimate. C, D, Same as A, B for the ipsilateral motion discrimination task. *Indicates a significant change in sensitivity for an individual subject. These data show that tACS improved motion sensitivity in the contralateral, but not in the ipsilateral hemifield.
The functioning of area hMT+ is lateralized, that is, the right hemisphere responds primarily to stimuli presented in the left visual field and vice versa (Dukelow et al., 2001). This allowed us to perform control experiments to assess the selectivity of tACS and exclude a number of potential confounds. In these experiments, the parietal electrode was placed ipsilateral to the visual stimulus. Assuming that the tACS-induced fields are at least coarsely localized (i.e., within a hemisphere), this should not affect motion processing, hence, these experiments control for general changes in arousal or attention induced by tACS (see Discussion).
Stimulating the ipsilateral hemisphere did not induce any consistent change in performance (Fig. 2C,D; p > 0.05). Moreover, the sensitivity during contralateral stimulation was significantly larger than during ipsilateral stimulation (two sided Wilcoxon rank sum test, p < 0.05; Cohen's d = 1.76; effect size (r) = 0.66).
tACS attenuated the motion after effect
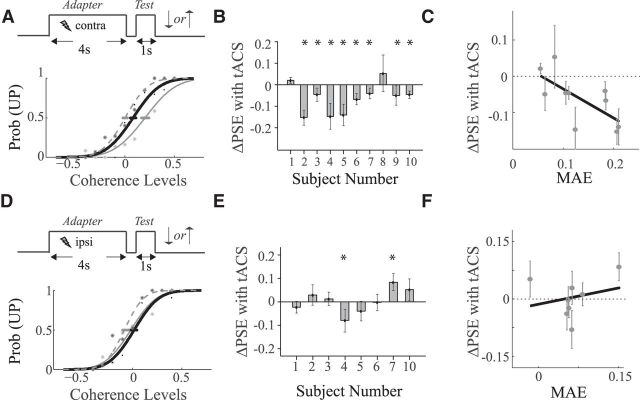
In a second set of experiments we tested the hypothesis that tACS affected a form of plasticity that is reflected in the behavioral changes occurring after prolonged exposure to a moving stimulus. Specifically, we determined psychometric curves for motion discrimination before and after motion adaptation, with and without contralateral or ipsilateral tACS during the adaptation phase (see Materials and Methods, Paradigm 2).Figure 3A (bottom) shows the results for one subject: the dashed curve is the psychometric curve in the unadapted condition. The PSE was at −0.08, which means that this subject reported upward and downward motion equally often when the fraction of downward moving dots was 8% (indicating an upward bias). After adaptation, the (thin solid) psychometric curve was shifted rightward to a PSE of +0.13. Hence, after adaptation a pattern in which 13% of the dots moved upward was reported to move upward or downward equally often. This is the MAE, which we quantified as the difference in the PSE between the adapted and unadapted condition (Castet et al., 2002). For this subject the MAE size was PSEadapt − PSEunadapt = 13% − (−8%) = 21%. The thick solid psychometric curve shows the results when tACS was applied during the adaptation phase, this curve is shifted less compared with the unadapted curve, which shows that tACS reduced the MAE. We quantified the tACS effect as the difference in PSE between the stimulated and not-stimulated adaptation condition: PSEadapt,tACS − PSEadapt = −2 − 13% = −15%.
Figure 3.
Motion adaptation task. A, Top, Task design for contralateral condition. Bottom, Psychometric functions computed for an example subject with (thick black curve) and without (thin gray curve) tACS. The dashed psychometric curve represents the performance in the unadapted condition. The horizontal error bars refer to the bootstrapped SD of the PSE estimate. B, Change in PSE after application of contralateral tACS (for all 10 subjects). Error bars indicate bootstrapped SDs of the PSE estimate. C, Changes in PSE with tACS during adaptation (PSEadapt,tACS − PSEadapt) as a function of MAE induced by adaptation without tACS (PSEadapt − PSEunadapt). The black solid line is a linear orthonormal fit to the data points. D–F, Same as A–C but for the ipsilateral condition. *Indicates a significant change in PSE for an individual subject. Contralateral but not ipsilateral tACS reduced motion adaptation proportional to the amount of adaptation induced without tACS.
Across the group of subjects the contralateral application of tACS during motion adaptation significantly reduced the MAE (Fig. 3A,B; p < 0.05, Wilcoxon signed rank test; Cohen's d = 0.93, effect size (r) = 0.42). By comparison, ipsilateral stimulation did not yield a significant change in MAE (Fig. 3D,E; p > 0.05), and a direct comparison showed that the effect of contralateral tACS was significantly larger than ipsilateral tACS (p < 0.05; Cohen's d = 0.90, effect size (r) = 0.41).
Subjects with a large MAE in the absence of tACS typically had a larger reduction in MAE when tACS was applied (Fig. 3C). This negative correlation supports the idea that tACS interferes with the mechanisms of adaptation (Fig. 3C; Pearson correlation coefficient = −0.63). Such a correlation was not found for ipsilateral stimulation (Fig. 3F), and a permutation test (see Materials and Methods) confirmed that the correlation induced by contralateral stimulation was significantly larger than that induced by ipsilateral tACS.
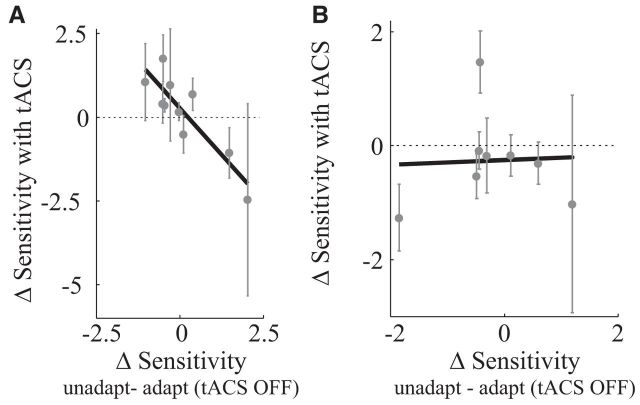
tACS attenuated sensitivity changes during adaptation
Adaptation not only shifted the psychometric curve, it also changed its slope, a measure of subjects' sensitivity to motion. This is consistent with the results of Van Wezel and Britten (2002), who demonstrated that adaptation reduces motion sensitivity. We found a similar reduction in sensitivity (a shallower slope) in most of our subjects. For each of those subjects, tACS increased sensitivity. For two of our subjects adaptation significantly increased sensitivity; for those subjects tACS decreased sensitivity. This negative correlation is further evidence that tACS attenuates adaptation (Fig. 4A; Pearson correlation is −0.68). This relationship was not found during ipsilateral stimulation (Fig. 4B) and the difference between the contralateral and ipsilateral condition was statistically significant (permutation test; p < 0.05; see Materials and Methods).
Figure 4.
Sensitivity changes during motion adaptation. A, Changes in sensitivity with contralateral tACS during adaptation (Sensitivityadapt,tACS − Sensitivityadapt) as a function of sensitivity changes induced by adaptation without tACS (Sensitivityunadapt − Sensitivityadapt). B, Changes in sensitivity with ipsilateral tACS. The black solid lines are linear orthonormal fits to the data points. Sensitivity changes induced by adaptation were attenuated by contralateral tACS, but unaffected by ipsilateral tACS.
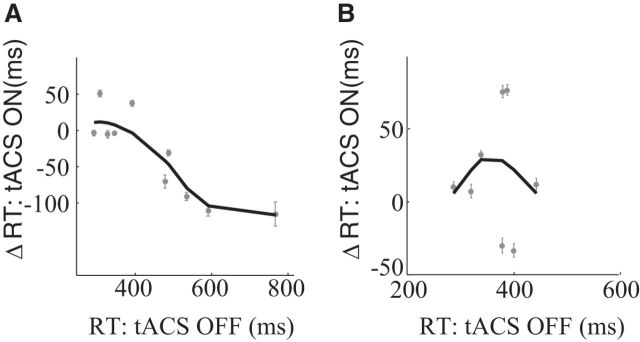
To control and monitor the allocation of attention, the subjects performed a speed detection task during the adaptation phase. This provided us with an additional and independent measure of motion sensitivity. We found that contralateral stimulation reduced subjects' reaction time on this task (Fig. 5A). This was mainly driven by subjects whose reaction times were long in the absence of tACS. Ipsilateral stimulation, on the other hand, did not affect the reaction time systematically (Fig. 5B).
Figure 5.
Reaction time (RT) changes during tACS. A, Changes in RT (ΔRT) in the speed detection task induced by tACS as a function of reaction times without tACS. The bold line is a robust locally weighted polynomial regression fit to the data. The vertical error bars represent the SE. B, Same as in A, now for ipsilateral stimulation. tACS reduced RTs, but only for contralateral visual stimuli.
tACS did not affect recovery from adaptation
In our adaptation paradigm, we can distinguish between an induction phase (the time when the adapter was on the screen) and a recovery phase (defined here as the time between the adapter and the test stimulus, when the screen was blank). The previous experiment showed that tACS during the induction phase reduced the MAE. Here we investigated whether tACS during recovery could also change the MAE.
We increased the duration of the recovery phase (the time between adapter and test) to 4 s, and applied tACS only during recovery. In this phase, the subjects had already been adapted to the prior visual stimuli (Top-up Adapter) but they did not receive visual motion input (see Materials and Methods, Paradigm 3). Stimulation in the recovery phase had no significant effect on the subsequent MAE nor did it change the slope of the psychometric curves (p > 0.05; average ΔPSE = 0.01, SD = 0.05), average ΔSensitivity = −0.0067, SD = 0.82). In other words, tACS affected the induction of adaptation (Fig. 3), but not the recovery from adaptation.
tACS effects required motion adaptation
In the motion adaptation experiments above, tACS was always applied well before the test stimulus (together with the adapter), hence it is possible that simply preceding a test stimulus by tACS induced a behavioral effect and that adaptation was not required per se. To test this hypothesis, we performed experiments in which each test stimulus was preceded by 4 s of a blank screen. tACS was applied only during this blank period (see Materials and Methods, Paradigm 4). Under these conditions, there was no significant effect of tACS on the PSE or sensitivity (p > 0.05; average ΔPSE = 0.02, SD = 0.06, average ΔSensitivity = −0.19, SD = 1.33). In other words, when applied outside the adaptation context, tACS had no effect, supporting our interpretation that tACS interfered with adaptation.
Discussion
We investigated how transcranial alternating currents affect human motion perception. We found that tACS reduced motion adaptation and improved motion discrimination sensitivity. Electrical stimulation did not affect motion perception when applied before visual stimulus presentation, or during the recovery phase of adaptation. Together, these findings can be summarized succinctly as demonstrating that tACS attenuates the induction of adaptation.
We first address some of the confounding factors and limitations in the interpretation of our data. Then we speculate on the neural mechanisms that could be involved in this and conclude with a brief discussion of the implications of our findings for the practical usage of tACS.
Confounds
tACS at 10 Hz can generate phosphenes due to current spread to the retina (Kar and Krekelberg, 2012). As an additional “visual” stimulus that is only present in the tACS conditions, these retinal phosphenes could, in principle, interfere with adaptation. Several arguments, however, speak against this. First, phosphenes occur in the periphery (Kar and Krekelberg, 2012), and given the receptive field locations (Hartmann et al., 2011) of neurons in motion areas, the visual stimulation induced by tACS phosphenes and the motion stimulus affect nonoverlapping populations of neurons. Second, tACS induces phosphenes in both hemifields, with no obvious patterns of lateralization (Kar and Krekelberg, 2012). Hence, if tACS reduced adaptation by drawing attention away from the adapter (Chaudhuri, 1990) one would expect to find it in both ipsilateral and contralateral stimulation conditions. Our control experiment (Fig. 3D), however, shows that only contralateral stimulation reduced adaptation. The specificity of the effect for contralateral tACS also argues that the action of tACS is significantly more pronounced in the cortical hemisphere over which it is applied and is incompatible with a general change in arousal induced directly or indirectly via the generation of phosphenes.
Limitations
The amplitude of tACS in our experiments was 0.5 mA, and its temporal frequency was 10 Hz. Of course many other patterns of stimulation are possible (different frequencies, or nonsinusoidal patterns, different electrode montages) and potentially worth exploring. We note, however, that this is an extremely large search space, and more insight into the underlying mechanisms may be required to perform an informed search.
Under our particular experimental conditions, we found that tACS increased motion sensitivity. This is incompatible with the view that tACS injects neural noise or perturbations. Of course, one cannot extrapolate such a finding to higher currents, other temporal frequencies, or other stimulation patterns. In fact, it is inevitably the case that at high enough currents, tACS would impact behavioral performance negatively and therefore be behaviorally equivalent to the injection of “noise.”
Comparison with tDCS
Antal et al. (2004) have shown that transcranial direct current stimulation (tDCS) over hMT+ reduces the subjective duration of the motion after effect. The goal of the Antal et al. (2004) study, however, was not to investigate which aspects of motion adaptation tDCS interferes with, but to provide support for the causal involvement of hMT+ in the MAE. Presumably for this reason, tDCS was applied continuously both during adaptation induction, recovery, and the subsequent motion detection task. Hence, the reduction in MAE duration could have been the consequence of tDCS' interference with any of these processes; this prevents a direct comparison with our findings. Nevertheless, it is of interest to note that Antal et al. (2004) found that tDCS reduced the MAE regardless of whether the anode or the cathode was placed over hMT+. This is compatible with our finding that tACS, which also generates current flow of both polarities, attenuates adaptation. Our behavioral data cannot address the question whether the same mechanisms underlie the influence of tACS and tDCS, but for tDCS we can speculate that the underlying mechanism is likely different from the (polarity-dependent) modulation of excitability reported in motor cortex (Nitsche et al., 2005).
Mechanism
Our experiments show that tACS attenuates motion adaptation. Hence, one would expect that tACS attenuates any of the consequences of adaptation. Together with the finding that motion adaptation reduces performance on a coarse motion detection task (Van Wezel and Britten, 2002) this provides a succinct explanation of the behavioral changes we observed. For instance, tACS increased sensitivity and reduced reaction times most for those subjects who showed a large adaptation effect (Figs. 4A, 5A). Importantly, this also accounts for the tACS-induced increase in sensitivity during the presentation of a single RDK (Fig. 2B). Even though this experiment did not involve a separate adaptation stimulus, the 4 s long RDK likely triggered adaptation. Our data support the view that this adaptation was attenuated by tACS, and this led to an increase in sensitivity.
At the circuit level, prolonged exposure to moving stimuli is known to result in firing rate changes throughout visual cortex. Individual neurons can increase or decrease their firing rate with adaptation and this depends critically on the relationship between the tuning of the neuron and the properties of the adapter and test stimuli. For instance, the speed of the moving stimulus (Krekelberg et al., 2006a), the direction of motion (Kohn and Movshon, 2004), as well as its size and duration (Wissig and Kohn, 2012; Duijnhouwer et al., 2013; Patterson et al., 2013), all affect firing rate changes induced in an adaptation protocol (Krekelberg et al., 2006b). This shows that the consequences of adaptation depend critically on the circuit in which neurons are embedded (Richert et al., 2013) and implies that the consequences of tACS for a single neuron will also depend strongly on its connections within the local circuit. In other words, based on our behavioral observations and the known properties of adaptation at the single neuron level, it seems unlikely that tACS would generally increase or decrease firing in a population of neurons. We plan to test this in the future using extracellular recording in the middle temporal area of the macaque during transcranial stimulation.
Linking behavioral data with cellular mechanisms requires many assumptions, and is inevitably speculative. Nevertheless, we believe it is valuable to put forward a novel and testable hypothesis that aims to do so. We start from the observation that small membrane voltage fluctuations reduce spike frequency adaptation, as shown by in vitro recordings of rat hippocampal CA1 neurons using direct somatic current injection (Fernandez et al., 2011). We speculate that tACS could induce such membrane fluctuations in the soma or dendrites and thereby interfere with adaptation.
Two major questions need to be answered before this can be accepted as a viable mechanism. First, can tACS generate membrane fluctuations that are large enough? Current finite element current flow models predict membrane voltage changes at pyramidal cell somas on the order of 0.2 mV (Radman et al., 2009; Reato et al., 2013). Although it should be noted that considerable uncertainty is associated with these predictions, this is an order of magnitude smaller than the somatic voltage fluctuations (∼2 mV) used by Fernandez et al. (2011). Second, do small membrane fluctuations generate effects that last long enough? Fernandez et al. (2011) argue that voltage fluctuations reduce spike frequency adaptation through Na+ de-inactivation. Given the rapid time course of de-inactivation, this mechanism alone is unlikely to lead to behavioral effects that last several seconds. Slice recordings could address these questions. The first by measuring whether smaller somatic voltage fluctuations suffice to reduce adaptation, or by investigating the role of dendritic voltage fluctuations. The second by determining whether the slower voltage or Na+-dependent K-channels known to be involved in adaptation (Sanchez-Vives et al., 2000), are also affected by membrane fluctuations.
Implications for tACS usage
Current understanding of tDCS is that the orientation of a neuron in the applied field determines whether its excitability will be increased or decreased through a net depolarization or hyperpolarization of the soma (Radman et al., 2009). If, however, tACS influences neurons through the interaction of subthreshold oscillations with adaptation, then it would be affected less by the orientation of the neuron in the field. Given that field orientation in a target area is highly idiosyncratic and difficult to predict (Datta et al., 2009), this could be a considerable practical advantage of tACS over tDCS.
Footnotes
This work was supported by Eye Institute of the National Institutes of Health Grant EY017605, and the Charles and Johanna Busch Memorial Fund at Rutgers, The State University of New Jersey. We thank Denis Paré for helpful discussions, and Minal Patel and Jasmine Siegel for their excellent technical support.
A patent application has been filed on subject matter disclosed in this paper.
References
- Antal A, Varga ET, Nitsche MA, Chadaide Z, Paulus W, Kovács G, Vidnyánszky Z. Direct current stimulation over MT+/V5 modulates motion aftereffect in humans. Neuroreport. 2004;15:2491–2494. doi: 10.1097/00001756-200411150-00012. [DOI] [PubMed] [Google Scholar]
- Antal A, Kovács G, Chaieb L, Cziraki C, Paulus W, Greenlee MW. Cathodal stimulation of human MT+ leads to elevated fMRI signal: a tDCS-fMRI study. Restor Neurol Neurosci. 2012;30:255–263. doi: 10.3233/RNN-2012-110208. [DOI] [PubMed] [Google Scholar]
- Blake R, Hiris E. Another means for measuring the motion aftereffect. Vision Res. 1993;33:1589–1592. doi: 10.1016/0042-6989(93)90152-M. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Pettersson LG, Jankowska E. Evidence for longlasting subcortical facilitation by transcranial direct current stimulation (tDCS) in the cat. J Physiol. 2013;591:3381–3399. doi: 10.1113/jphysiol.2012.244764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castet E, Keeble DR, Verstraten FA. Nulling the motion aftereffect with dynamic random-dot stimuli: limitations and implications. J Vis. 2002;2(4):3, 302–311. doi: 10.1167/2.4.3. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A. Modulation of the motion aftereffect by selective attention. Nature. 1990;344:60–62. doi: 10.1038/344060a0. [DOI] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207.e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijnhouwer J, Noest AJ, Lankheet MJ, van den Berg AV, van Wezel RJ. Speed and direction response profiles of neurons in macaque MT and MST show modest constraint line tuning. Front Behav Neurosci. 2013;7:22. doi: 10.3389/fnbeh.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukelow SP, DeSouza JF, Culham JC, van den Berg AV, Menon RS, Vilis T. Distinguishing subregions of the human MT+ complex using visual fields and pursuit eye movements. J Neurophysiol. 2001;86:1991–2000. doi: 10.1152/jn.2001.86.4.1991. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. Boca Raton, FL: Chapman and Hall/CRC; 1994. [Google Scholar]
- Fedorov A, Chibisova Y, Szymaszek A, Alexandrov M, Gall C, Sabel BA. Non-invasive alternating current stimulation induces recovery from stroke. Resto Neurol Neurosci. 2010;28:825–833. doi: 10.3233/RNN-2010-0580. [DOI] [PubMed] [Google Scholar]
- Fernandez FR, Broicher T, Truong A, White JA. Membrane voltage fluctuations reduce spike frequency adaptation and preserve output gain in CA1 pyramidal neurons in a high-conductance state. J Neurosci. 2011;31:3880–3893. doi: 10.1523/JNEUROSCI.5076-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge: Cambridge UP; 1947. [Google Scholar]
- Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–1149. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- Hartmann TS, Bremmer F, Albright TD, Krekelberg B. Receptive field positions in area MT during slow eye movements. J Neurosci. 2011;31:10437–10444. doi: 10.1523/JNEUROSCI.5590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley DV. Bootstrap methods. J R Stat Soc Series B Stat Methodol. 1988;50:321–337. [Google Scholar]
- Hiris E, Blake R. Another perspective on the visual motion aftereffect. Proc Natl Acad Sci U S A. 1992;89:9025–9028. doi: 10.1073/pnas.89.19.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Jenkinson N, Brittain JS, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol. 2012;22:403–407. doi: 10.1016/j.cub.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar K, Krekelberg B. Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. J Neurophysiol. 2012;108:2173–2178. doi: 10.1152/jn.00505.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov R, Weiss C, Siebner HR, Born J, Marshall L. Slow oscillation electrical brain stimulation during waking promotes EEG theta activity and memory encoding. Proc Natl Acad Sci U S A. 2009;106:15460–15465. doi: 10.1073/pnas.0904438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat Neurosci. 2004;7:764–772. doi: 10.1038/nn1267. [DOI] [PubMed] [Google Scholar]
- Krekelberg B. Motion detection mechanisms. In: Basbaum A, editor. The senses: a comprehensive reference. Oxford: Elsevier; 2008. [Google Scholar]
- Krekelberg B, van Wezel RJ, Albright TD. Adaptation in macaque MT reduces perceived speed and improves speed discrimination. J Neurophysiol. 2006a;95:255–270. doi: 10.1152/jn.00750.2005. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJ. Adaptation: from single cells to BOLD signals. Trends Neurosci. 2006b;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Paré EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S. Advancement of motion psychophysics: review 2001–2010. J Vis. 2011;11(5):11, 1–53. doi: 10.1167/11.5.11. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/WNL.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CA, Wissig SC, Kohn A. Distinct effects of brief and prolonged adaptation on orientation tuning in primary visual cortex. J Neurosci. 2013;33:532–543. doi: 10.1523/JNEUROSCI.3345-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2 doi: 10.1016/j.brs.2009.03.007. 215–228.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci. 2013;7:687. doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezec A, Krekelberg B, Dobkins KR. Attention enhances adaptability: evidence from motion adaptation experiments. Vision Res. 2004;44:3035–3044. doi: 10.1016/j.visres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Richert M, Albright TD, Krekelberg B. The complex structure of receptive fields in the middle temporal area. Front Syst Neurosci. 2013;7:2. doi: 10.3389/fnsys.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett. 2000;296:61–63. doi: 10.1016/S0304-3940(00)01621-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scase MO, Braddick OJ, Raymond JE. What is noise for the motion system? Vision Res. 1996;36:2579–2586. doi: 10.1016/0042-6989(95)00325-8. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel IM. The use of decay inhibition in an examination of central mediation in movement aftereffects. J Gen Psychol. 1964;70:241–247. doi: 10.1080/00221309.1964.9920594. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Exp Brain Res. 1999;127:355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- Utz KS, Dimova V, Oppenländer K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology–a review of current data and future implications. Neuropsychologia. 2010;48:2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Van Wezel RJ, Britten KH. Motion adaptation in area MT. J Neurophysiol. 2002;88:3469–3476. doi: 10.1152/jn.00276.2002. [DOI] [PubMed] [Google Scholar]
- Wissig SC, Kohn A. The influence of surround suppression on adaptation effects in primary visual cortex. J Neurophysiol. 2012;107:3370–3384. doi: 10.1152/jn.00739.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]