Abstract
Background/Aims
To investigate the rate of detection of monosodium urate (MSU) crystals in the synovial fluid (SF) of patients with acute gouty arthritis and factors associated with false-negative results.
Methods
A total of 179 patients with acute gouty arthritis who had undergone SF crystal examination were identified from the data warehouse of two university hospitals. Clinical and laboratory data were obtained from the medical records.
Results
The overall rate of detection of MSU crystals was 78.8%. In univariate analyses, the only significant differences between the variables of crystal-negative and crystal-positive patients were a lower C-reactive protein level (p = 0.040) and fewer patients undergoing emergent surgery in the crystal-positive group (p = 4.5 × 10-6). In logistic regression analyses, MSU crystal-negative results were significantly associated with the interval from arthritis onset to crystal examination (p = 0.042), and this was the most significant risk factor for arthroscopic surgery (p = 2.1 × 10-4). Seventeen patients who underwent arthroscopic surgery had a significantly longer hospital stay (p = 0.007) and a significant delay in gout treatment (p = 8.74 × 10-5). The distribution of crystal-negative patients differed significantly between the SF samples that were evaluated by both the laboratory medicine and the rheumatology departments (p = 1.2 × 10-14), and the κ value was 0.108.
Conclusions
Although several clinical features were associated with detection failure, SF MSU crystal identification was critically dependent on the observer. Considering the impact on the treatment outcomes, implementation of a quality control program is essential.
Keywords: Gout; Uric acid; Synovial fluid; Microscopy, polarization
INTRODUCTION
Gout is the most common form of crystal-induced arthritis and results from the deposition of monosodium urate (MSU) crystals in the joints. The global burden of this condition is substantial and is increasing [1]. The prevalence of gout in Korea was reported to be 0.4% in 2008 and increased by 2.3-fold from 2001 to 2008 [2]. As gout is a potentially curable disease, an appropriate diagnosis is essential to achieve a successful treatment outcome.
A clinical diagnosis of acute gouty arthritis can be made on the basis of a typical presentation, according to the 1977 criteria of the American College of Rheumatology (ACR) and recommendations from the European League Against Rheumatism (EULAR) [3,4]. However, episodic acute arthritis, a hallmark of gout, can also develop in patients with various other diseases [5]. Therefore, acute attacks with rapid development of severe pain, swelling, and overlying erythema are not specific for gout. Gouty tophus may be confused with rheumatoid nodules or tophaceous pseudogout. Furthermore, hyperuricemia, a major risk factor of gout, is not a key diagnostic sign. Only 16% of males with hyperuricemia had gout [6] and one quarter of patients with gout attacks showed normouricemia in Korea [7]. Especially, in cases with chronic or polyarticular gout, it may be difficult to establish a diagnosis of gout based on clinical findings. In this context, identification of MSU crystals is considered the gold standard for diagnosis of gout, although the sensitivity of detection of MSU crystals in the synovial fluid (SF) varies from 50% to 84% [3,8,9,10,11].
There have been no studies regarding the sensitivity of SF crystal examination and the factors associated with false-negative results in Korean patients with gout. In the present study, we investigated the detection rate of SF MSU crystals in patients with acute gouty arthritis and the clinical factors associated with false-negative results by comparing crystal-positive and crystal-negative patients.
METHODS
Data sources and study subjects
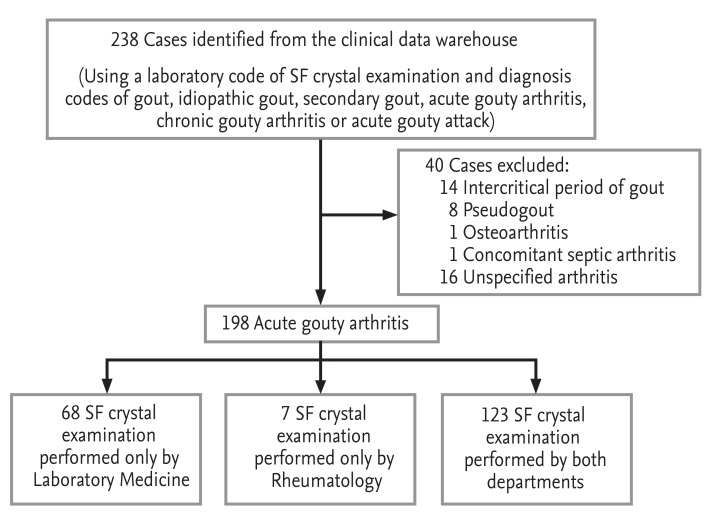
All data in the present retrospective study were collected from the clinical data warehouse of two university hospitals. We searched the data warehouse to identify patients with a diagnosis of gout, who had undergone SF examination from October 1999 to September 2011. After a review of the medical records of 217 patients (238 SF samples), 16 patients with unclassified acute arthritis (16 samples), 13 patients with intercritical gout without acute symptoms (14 samples), seven pseudogout patients (eight samples), one osteoarthritis patient, and one patient with concomitant septic and gouty arthritis were excluded. A total of 179 patients (198 SF samples) were classified as having acute gouty arthritis according to the 1977 ACR classification criteria (Fig. 1) [3]. The study was approved by the local ethics committees (IRB-No. B-0506/021-004 and H-1304-020-478).
Figure 1.
Sampling scheme of patients included in the study. Acute gouty arthritis was diagnosed based on the American College of Rheumatology criteria for classification of acute arthritis for primary gout [2]. SF, synovial fluid.
Clinical and laboratory variables
Demographic data and clinical features, including the number of involved joints, interval from onset of symptoms to SF analysis, associated systemic symptoms, and treatment, were collected. In addition, patient histories regarding medications, alcohol consumption, and comorbidities were obtained. Laboratory variables were acquired on the day of arthrocentesis.
MSU crystals in the SF samples were identified by the Department of Laboratory Medicine (LM) and/or Rheumatology (RH) in both hospitals. Polarized light microscopy (Eclipse E600POL, Nikon, Tokyo, Japan; Axioscope A1, Carl Zeiss, Jena, Germany) was used to identify needle-shaped crystals with strong negative birefringence. Of the 198 samples, 123 (62.1%) were examined by both the LM and RH departments, 68 (34.3%) were examined by only the LM department, and seven (3.5%) were examined by only the RH department (Fig. 1). Therefore, the department performing the analysis was considered a categorical variable. The presence of MSU crystals was defined as the qualitative identification of crystals by either the LM or RH department in a SF sample. The 198 cases were classified into crystal-positive (156 cases, 78.8%) and crystal-negative (42 cases, 21.2%) groups.
Statistical analysis
All continuous variables are expressed as means and standard deviations. Student t test was used for comparison of continuous variables between the MSU crystal-positive and MSU crystal-negative groups. The chi-square test, Fisher exact test, or McNemar test was used as applicable for comparison of categorical variables. Cohen's κ index was used for analysis of concordance between the results of crystal examinations performed by the LM and RH departments. Multivariate logistic regression analysis was performed to determine the variables independently associated with false-negative results for the SF crystal examination or surgical treatment. Variables with p < 0.1 in univariate analyses were subjected to all regression models. All statistical analyses were performed using PASW version 18 (SPSS Inc., Chicago, IL, USA), and p < 0.05 was taken to indicate statistical significance.
RESULTS
Clinical characteristics of study subjects
The clinical characteristics of the study subjects are summarized in Table 1. The subjects were predominantly male (169/179; 94.4%). The mean ± SD age at onset of gout was 62.6 ± 16.4 years and the mean disease duration was 11.3 ± 7.2 years. Eighty-seven patients (43.9%) had experienced their first gout attack and 40 patients (20.2%) had chronic tophaceous gout. Eighty-one patients (40.9%) had stage II to IV chronic kidney disease and 104 patients (52.5%) currently consumed alcohol. Fever was present in 26/196 cases (13.1%) and the mean number of involved joints was 2.2 ± 2.1. The time interval from the onset of acute arthritis symptoms to SF analysis was 3.3 ± 3.5 days. Hyperuricemia was noted in 86% (160/186) of male patients and 100% (12/12) of female patients. Peripheral blood leukocytosis (> 10,000/mm3) was observed in 89/185 cases (48.1%) at the time of SF analysis. Nineteen cases (9.0%) showed SF leukocytosis of ≥ 50,000/mm3.
Table 1.
Clinical and laboratory features of patients with acute gouty arthritis
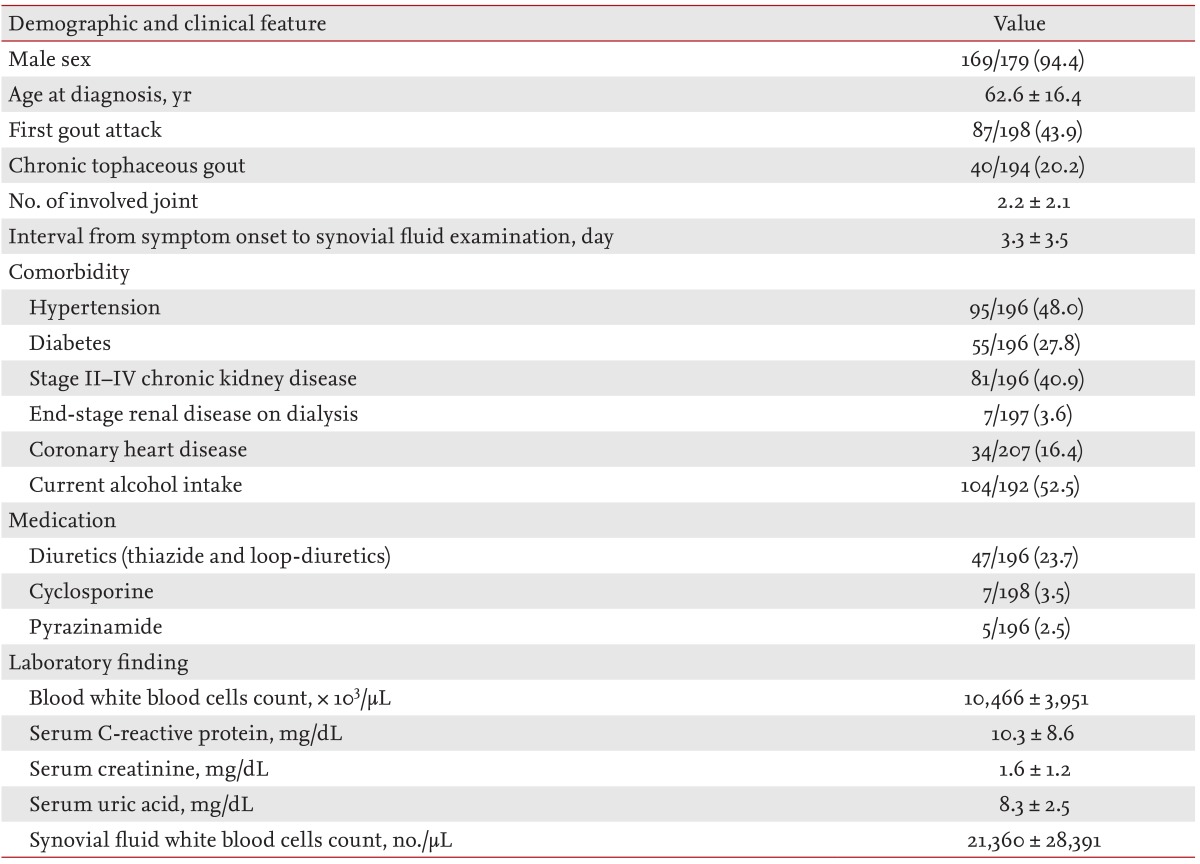
Values are presented as mean ± SD or number/total number (%).
When stratified by institutions, there were no differences in clinical characteristics between gout patients diagnosed by each hospital, with the exception of disease duration (13.5 ± 7.0 years vs. 9.9 ± 7.4 years; p = 0.001 by t test). The distributions of crystal-positive and crystal-negative cases were comparable between the two hospitals. When subgroups based on the departments that performed the crystal examination were compared, the number of cases with monoarticular involvement examined by the LM department was greater than that examined by both departments (74.6% vs. 48.4%, respectively; p = 0.001 by chi-square test).
Clinical factors associated with MSU crystal-negative gout
Overall, the detection rate of MSU crystals was 78.8% (Table 2). There were no differences in the distribution of comorbidities, current medications, patients currently consuming alcohol, SF or peripheral blood leukocyte counts, and levels of serum uric acid between crystal-positive and crystal-negative patients on univariate analyses. However, the crystal-negative group had significantly lower levels of serum C-reactive protein (CRP, 8.1 ± 6.5 mg/dL vs. 10.9 ± 9.1 mg/dL, respectively; p = 0.04 by t test), and tended to have a longer time interval between the onset of gout attacks and SF analysis (4.5 ± 5.1 days vs. 3.0 ± 2.8 days, respectively; p = 0.087) compared to the crystal-positive group. On multivariate regression logistic analysis, a longer interval from the onset of arthritis to SF analysis (odds ratio [OR], 1.105; 95% confidence interval [CI], 1.004 to 1.216; p = 0.042) and younger age (OR, 0.986; 95% CI, 0.957 to 1.016; p = 0.049) remained significantly associated with negative results for MSU crystals.
Table 2.
Clinical and laboratory variables in crystal-positive and crystal-negative patients
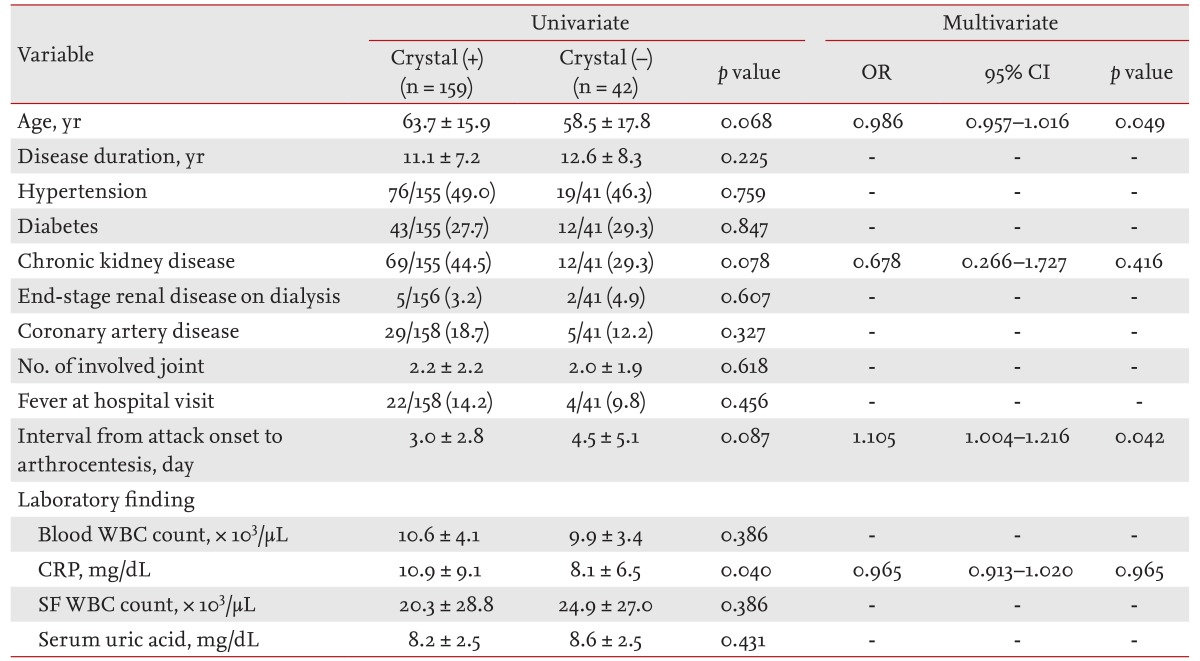
Values are presented as mean ± SD or number/total number (%).
OR, odds ratio; CI, confidence interval; WBC, white blood cells; CRP, C-reactive protein; SF, synovial fluid.
We repeated the analysis after stratification by testing department. In the samples examined by the LM department alone, crystal-negative gout had a significantly longer interval from onset of symptoms to SF crystal examination (4.7 ± 5.6 days vs. 2.3 ± 2.0 days, respectively; p = 0.036 by t test) and tended to have a greater number of involved joints (1.7 ± 1.2 vs. 1.2 ± 0.6, respectively; p = 0.05) compared to the crystal-positive patients.
Interobserver variability of the analysis of MSU crystals in SF
Although the detection rate of MSU crystals was 51.8% in samples examined by the LM department, it was 93.8% in those examined by the RH department. Due to this discrepancy, we evaluated interpretative agreement for MSU crystal identification using 123 samples, where polarized microscopic examination was conducted by both departments (Table 3). The detection rate of MSU crystals was significantly different between the two departments (93.5% by the RH department vs. 51.2% by the LM department; p = 1.19 × 10-14 by McNemar test) and the κ index was 0.108, which indicated a slight degree of agreement according to Landis and Koch [12]. In addition, on multivariate analysis using the testing department as a variable, crystal examination by the LM department alone was the most significant variable associated with crystal-negative gout (OR, 36.996; 95% CI, 9.731 to 140.648; p = 1.16 × 10-7).
Table 3.
Detection rates of monosodium urate crystals according to the department performing the examination
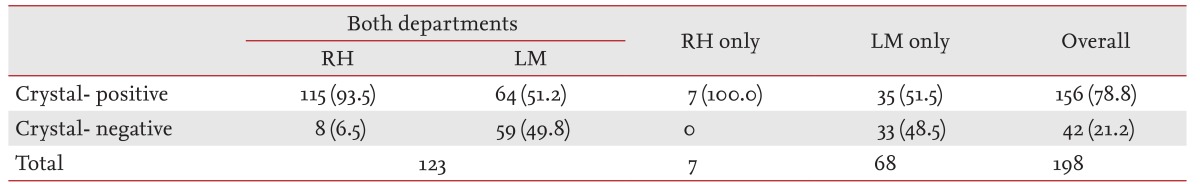
Values are presented as number (%).
RH, Department of Rheumatology; LM, Department of Laboratory Medicine.
Treatment and clinical course after SF crystal examination
Most patients with a diagnosis of acute gouty arthritis received nonsteroidal anti-inflammatory drugs (40.9%), colchicine (48.5%), or intraarticular glucocorticoid injection (18.7%). Intraarticular glucocorticoid injection was used less frequently in crystal-negative compared to crystal-positive patients (7.1% vs. 21.8%, respectively; p = 0.043 by Fisher exact test), whereas the rates of use of other drugs were comparable in both groups. The 17 patients who underwent emergent arthroscopic drainage consisted of more crystal-negative than crystal-positive patients (26.2% vs. 3.8%, respectively; p = 4.48 × 10-6 by chi-square test) (Table 4). Preoperatively, 58.8% (10/17) of the SF samples were reported to be crystal-negative by the LM department. The orthopedic surgeons had decided on emergent arthroscopic drainage based on suspected septic arthritis; however, all cases had clearly visible crystalline deposits in the joints (four ankle joints and 13 knee joints) and negative SF and/or blood culture results. In multivariate logistic regression analysis, negative results for MSU crystal examination (OR, 23.760; 95% CI, 4.451 to 126.843; p = 2.10 × 10-4), the presence of fever (OR, 15.123; 95% CI, 2.739 to 83.503; p = 0.002), and the first episode of acute gouty arthritis (OR, 6.954; 95% CI, 1.577 to 30.662; p = 0.01) were independent risk factors for emergent surgery.
Table 4.
Treatments used in crystal-positive and crystal-negative patients
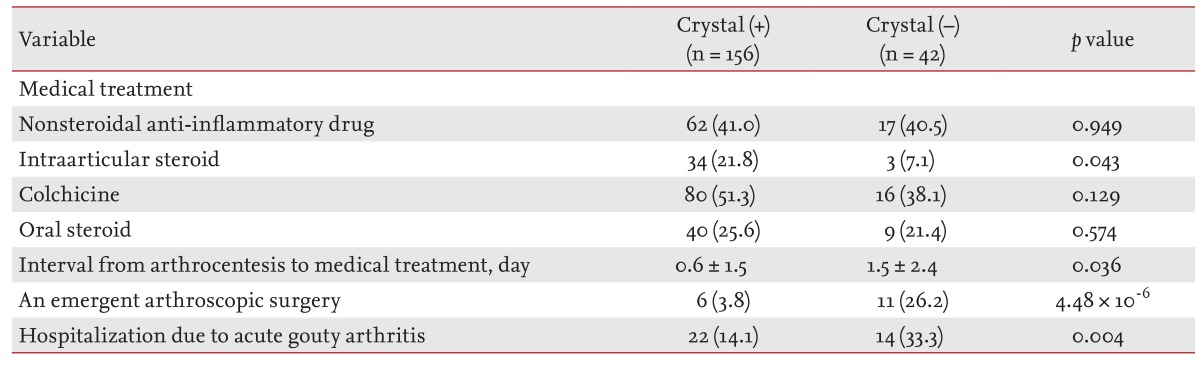
Patients undergoing arthroscopic surgery had a significantly longer hospital stay than the 19 patients (10.5%) hospitalized for medical treatment (18.4 ± 12.6 days vs. 8.9 ± 6.7 days, respectively; p = 0.007 by t test). Furthermore, anti-inflammatory drugs for gouty attacks were administered significantly later in patients undergoing surgical treatment than in those receiving medical treatment (5.0 ± 3.4 days vs. 0.4 ± 0.8 days, respectively; p = 8.74 × 10-5 by t test).
DISCUSSION
Detection of MSU crystals in SF or tophus aspirates has been the "gold standard" in the diagnosis of gouty arthritis since McCarty and Holander [13] identified the crystals in gouty SF. The recent EULAR recommendations indicated that MSU crystal identification has a specificity of 100% (95% CI, 99 to 100) and a likelihood ratio of 566.60 (95% CI, 35.5 to 9,053.5). However, SF MSU crystals may not be found in up to 25% of patients with acute gouty arthritis [14]. The suggested causes of MSU crystal detection failure include SF sampling error, the presence of only ultramicroscopic crystals (i.e., crystals too small to be observed by microscopy), the presence of only spherical "beach ball-like" crystals, and the dissolution of crystals [15,16].
In the present study, delay in visiting the hospital was the only significant clinical factor associated with crystal-negative gout on multivariate analysis. Spontaneous resolution within approximately 2 weeks is a characteristic feature of acute gouty arthritis. The mechanisms underlying the self-limiting nature of gouty inflammation may include a decrease in MSU crystal load, changes in crystal-coating proteins, clearance of apoptotic neutrophils, and induction of anti-inflammatory cytokines, including transforming growth factor (TGF)-β, from MSU crystal-stimulated macrophages [17,18]. With regard to the load of MSU crystals, myeloperoxidase and superoxide anion can digest or dissolve the crystals, and noninflammatory macrophages (differentiated from initially immature monocytes) can more avidly take up MSU crystals [19]. Pascual et al. [20] found that the number of MSU crystals per field decreased in the second SF samples that were aspirated 6 to 8 days after the first SF aspirate was obtained. Thus, the later the SF sample is aspirated from gout patients, the greater the decrease in MSU crystal load that can be expected. von Essen and Holtta [21] reported that the incidence of false-negative results was 0% to 38% when MSU crystals were abundant and up to 67% when they were rare. However, MSU crystals are detectable even in SF samples from patients with subclinical arthritis or intercritical gout [22]. In addition, the mean difference in interval from onset of symptoms to SF aspiration was only 1.6 days in the present study. Therefore, the delay in visiting the hospital could not be considered critical to the failure of MSU crystal detection.
In the present study, the number of cases examined by technicians from the LM department was higher than that examined by clinicians (192 samples vs. 134 samples, respectively). In a survey in the United Kingdom, crystal examination was performed by more technicians (69%) than clinicians (31%) [23]. This is partly because clinicians may not have sufficient time to examine SF samples immediately. In addition, non-rheumatologists who treat patients with acute arthritis lack experience in SF evaluation or have limited access to polarized microscopy equipment. Missing data in the present study may have another cause; rheumatologists performed SF crystal examination without a laboratory order because the Korean National Health Insurance Corporation reimburses the service only when it is performed by clinical pathologists. Regardless of the cause, sending the sample to a laboratory for crystal examination could increase the risk of a false-negative result due to the delay in SF analysis. Kerolus et al. [24] reported that the number of SF leukocytes decreased within a few hours after SF aspiration and the MSU crystals became smaller in size and number and less birefringent over time. As SF crystal examination is conducted only during regular hours in the LM departments of our hospitals, it is possible that the delay in examination contributed to the false-negative results.
More importantly, SF crystal examination is known to be observer-dependent even though the accuracy is high if the test is performed by experts. In a recent systematic review, Swan et al. [14] reported that the analytical sensitivity ranged from 63% to 78%. Interobserver reliability (κ) was reported to range from 0.35 to 0.63 [8]. The present study showed poor interobserver reliability (κ, 0.108) and a marked difference in detection rate between the LM and RH departments (51.8% vs. 93.8%). Pascual et al. [22] detected the crystals in the first microscopic field in 61% of samples and in the first 5 fields in 90% of samples. The number of fields examined by the RH and LM departments, before the absence of MSU crystals was decided, may be different. Although technicians at the LM departments are not aware of the clinical status of the patients, rheumatologists, who highly suspect gout in a patient, exert greater effort in detecting the crystals. Another explanation for the difference in detection rate between the departments may involve training process in SF analysis. In the present study, the LM department analyzed approximately seven to nine SF samples for gout per year (191 SF samples analyzed at two institutes over 12 years). Considering the change in the technician-in-charge at the LM department, the level of experience may not be adequate for accurate SF crystal analysis. In contrast, in the RH departments at each hospital, most SF crystal analyses were performed under the supervision of faculty. Lumbreras et al. [25] reported that inexperienced residents demonstrated a sensitivity of 95.3% and specificity of 97.2% (κ index, 0.93) for identification of MSU crystals after a 3-month training course. Therefore, an appropriate training program in crystal identification should be developed to reduce the occurrence of false-negative results.
Ho and DeNuccio [26] reported that 17 of 67 patients (25.3%) with acute gouty arthritis or pseudogout experienced delayed diagnosis or ineffective treatment before rheumatologic consultation. In the present study, 16 patients (8.9%) underwent emergent arthroscopic surgery due to suspected septic arthritis before rheumatology consultation. As clinical manifestations of septic and crystal-induced arthritis often overlap and the mortality rate of septic arthritis is high, it is recommended that patients with acute arthritis with overlying erythema should be treated as having septic arthritis until proven otherwise [27]. Moreover, the presence of MSU crystals in the SF aspirates does not conclusively exclude the possibility of an infectious process, as several groups have reported the coexistence of septic arthritis in patients with acute gout attacks (0.5% to 4% of crystal proven gout or 1.3 to 2 cases per year) [27,28,29,30,31]. However, our results demonstrated that failure to detect MSU crystals negatively affects the outcome in patients with acute gouty arthritis. Patients undergoing surgical drainage were hospitalized for a longer period and received the recommended treatment for acute gouty arthritis significantly later than those who received medical treatment alone.
In conclusion, a false-negative result can lead to a significant delay in proper diagnosis and treatment as MSU crystal examination has unique diagnostic value. As the identification of SF MSU crystals is largely observer-dependent, systematic efforts should be made to improve the diagnostic performance of microscopic examination for MSU crystals.
KEY MESSAGE
The overall rate of false-negative results for synovial fluid monosodium urate (SF MSU) crystal examination in the present study (the first conducted on this topic in Korea) was 21.2%.
False-negative results for MSU crystal examination were associated with surgical treatment, and led to significantly longer hospital stays and delays in adequate treatment for acute gouty arthritis.
As the detection of SF MSU crystals is highly observer-dependent, an appropriate quality-control program is required.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223. doi: 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CH, Sung NY. The prevalence and features of Korean gout patients using the National Health Insurance Corporation database. J Rheum Dis. 2011;18:94–100. [Google Scholar]
- 3.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1301–1311. doi: 10.1136/ard.2006.055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastner DL. Intermittent and periodic arthritis syndrome. In: Koopman WJ, editor. Arthritis and Allied Conditions: A Textbook of Rheumatology. 13th ed. Boltimore: Williams & Wilkins; 1997. pp. 1279–1306. [Google Scholar]
- 6.Kim EH, Jeon K, Park KW, et al. The prevalence of gout among hyperuricemic population. J Korean Rheum Assoc. 2004;11:7–13. [Google Scholar]
- 7.Baek HJ, Lee EB, Yoo CD, Kim HA, Song YW, Lim YS. The study on the clinical features of gouty arthritis. Korean J Med. 1997;52:727–736. [Google Scholar]
- 8.Schumacher HR, Jr, Sieck MS, Rothfuss S, et al. Reproducibility of synovial fluid analyses: a study among four laboratories. Arthritis Rheum. 1986;29:770–774. doi: 10.1002/art.1780290610. [DOI] [PubMed] [Google Scholar]
- 9.Gordon C, Swan A, Dieppe P. Detection of crystals in synovial fluids by light microscopy: sensitivity and reliability. Ann Rheum Dis. 1989;48:737–742. doi: 10.1136/ard.48.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Essen R, Holtta AM. Quality control of the laboratory diagnosis of gout by synovial fluid microscopy. Scand J Rheumatol. 1990;19:232–234. doi: 10.3109/03009749009095048. [DOI] [PubMed] [Google Scholar]
- 11.McGill NW, York HF. Reproducibility of synovial fluid examination for crystals. Aust N Z J Med. 1991;21:710–713. doi: 10.1111/j.1445-5994.1991.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 13.Mccarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med. 1961;54:452–460. doi: 10.7326/0003-4819-54-3-452. [DOI] [PubMed] [Google Scholar]
- 14.Swan A, Amer H, Dieppe P. The value of synovial fluid assays in the diagnosis of joint disease: a literature survey. Ann Rheum Dis. 2002;61:493–498. doi: 10.1136/ard.61.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agudelo CA. Gout and hyperuricemia. Curr Opin Rheumatol. 1989;1:286–293. doi: 10.1097/00002281-198901030-00009. [DOI] [PubMed] [Google Scholar]
- 16.Dieppe P, Hornby J, Swan A, Hutton C, Preece A. Laboratory handling of crystals. Ann Rheum Dis. 1983;42(Suppl 1):60–63. doi: 10.1136/ard.42.suppl_1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty DJ. Crystal-induced inflammation of the joints. Annu Rev Med. 1970;21:357–366. doi: 10.1146/annurev.me.21.020170.002041. [DOI] [PubMed] [Google Scholar]
- 18.Marcolongo R, Calabria AA, Lalumera M, Gerli R, Alessandrini C, Cavallo G. The "switch-off" mechanism of spontaneous resolution of acute gout attack. J Rheumatol. 1988;15:101–109. [PubMed] [Google Scholar]
- 19.Landis RC, Yagnik DR, Florey O, et al. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum. 2002;46:3026–3033. doi: 10.1002/art.10614. [DOI] [PubMed] [Google Scholar]
- 20.Pascual E. Persistence of monosodium urate crystals and low-grade inflammation in the synovial fluid of patients with untreated gout. Arthritis Rheum. 1991;34:141–145. doi: 10.1002/art.1780340203. [DOI] [PubMed] [Google Scholar]
- 21.von Essen R, Holtta AM, Pikkarainen R. Quality control of synovial fluid crystal identification. Ann Rheum Dis. 1998;57:107–109. doi: 10.1136/ard.57.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual E, Batlle-Gualda E, Martinez A, Rosas J, Vela P. Synovial fluid analysis for diagnosis of intercritical gout. Ann Intern Med. 1999;131:756–759. doi: 10.7326/0003-4819-131-10-199911160-00007. [DOI] [PubMed] [Google Scholar]
- 23.Amer H, Swan A, Dieppe P. The utilization of synovial fluid analysis in the UK. Rheumatology (Oxford) 2001;40:1060–1063. doi: 10.1093/rheumatology/40.9.1060. [DOI] [PubMed] [Google Scholar]
- 24.Kerolus G, Clayburne G, Schumacher HR., Jr Is it mandatory to examine synovial fluids promptly after arthrocentesis? Arthritis Rheum. 1989;32:271–278. doi: 10.1002/anr.1780320308. [DOI] [PubMed] [Google Scholar]
- 25.Lumbreras B, Pascual E, Frasquet J, Gonzalez-Salinas J, Rodriguez E, Hernandez-Aguado I. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis. 2005;64:612–615. doi: 10.1136/ard.2004.027268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho G, Jr, DeNuccio M. Gout and pseudogout in hospitalized patients. Arch Intern Med. 1993;153:2787–2790. [PubMed] [Google Scholar]
- 27.Coakley G, Mathews C, Field M, et al. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford) 2006;45:1039–1041. doi: 10.1093/rheumatology/kel163a. [DOI] [PubMed] [Google Scholar]
- 28.Yu KH, Luo SF, Liou LB, et al. Concomitant septic and gouty arthritis: an analysis of 30 cases. Rheumatology (Oxford) 2003;42:1062–1066. doi: 10.1093/rheumatology/keg297. [DOI] [PubMed] [Google Scholar]
- 29.Shah K, Spear J, Nathanson LA, McCauley J, Edlow JA. Does the presence of crystal arthritis rule out septic arthritis? J Emerg Med. 2007;32:23–26. doi: 10.1016/j.jemermed.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Weng CT, Liu MF, Lin LH, et al. Rare coexistence of gouty and septic arthritis: a report of 14 cases. Clin Exp Rheumatol. 2009;27:902–906. [PubMed] [Google Scholar]
- 31.Papanicolas LE, Hakendorf P, Gordon DL. Concomitant septic arthritis in crystal monoarthritis. J Rheumatol. 2012;39:157–160. doi: 10.3899/jrheum.110368. [DOI] [PubMed] [Google Scholar]