Abstract
Aims
To test recommended implantable cardioverter defibrillator (ICD) follow-up methods by ‘in-person evaluations’ (IPE) vs. ‘remote Home Monitoring’ (HM).
Methods and results
ICD patients were randomized 2:1 to automatic HM or to Conventional monitoring, with follow-up checks scheduled at 3, 6, 9, 12, and 15 months post-implant. Conventional patients were evaluated with IPE only. Home Monitoring patients were assessed remotely only for 1 year between 3 and 15 month evaluations. Adherence to follow-up was measured. HM and Conventional patients were similar (age 63 years, 72% male, left ventricular ejection fraction 29%, primary prevention 73%, DDD 57%). Conventional management suffered greater patient attrition during the trial (20.1 vs. 14.2% in HM, P = 0.007). Three month follow-up occurred in 84% in both groups. There was 100% adherence (5 of 5 checks) in 47.3% Conventional vs. 59.7% HM (P < 0.001). Between 3 and 15 months, HM exhibited superior (2.2×) adherence to scheduled follow-up [incidence of failed follow up was 146 of 2421 (6.0%) in HM vs. 145 of 1098 (13.2%) in Conventional, P < 0.001] and punctuality. In HM (daily transmission success rate median 91%), transmission loss caused only 22 of 2275 (0.97%) failed HM evaluations between 3 and 15 months; others resulted from clinic oversight. Overall IPE failure rate in Conventional [193 of 1841 (10.5%) exceeded that in HM [97 of 1484 (6.5%), P < 0.001] by 62%, i.e. HM patients remained more loyal to IPE when this was mandated.
Conclusion
Automatic remote monitoring better preserves patient retention and adherence to scheduled follow-up compared with IPE.
Clinical trial registration
Keywords: Defibrillators, Patient monitoring, Follow-up, Remote Monitoring' Guidelines
Introduction
Cardiac implantable electronic devices (CIEDs) are increasing in prevalence in response to widening indications. The implant—not an end goal in itself—initiates an indefinite commitment to manage both the device and patient condition being treated, and these needs may change with time. By definition, most CIED recipients constitute a high-risk population demanding close attention. Hence, post-implant monitoring is important. However, follow-up schedules vary according to facility, physician preference, and available resources.1 Analysis of Medicare beneficiaries from 2005 to 2009 showed that most patients receiving a new CIED were not seen at all within 3 months, and almost a quarter of patients not reviewed in the year after implant.2 The consequences of defaulting were vividly illustrated by the comparative survival advantage gained by those patients who did adhere to prescribed follow-up.3 To address inconsistent clinical practice, professional organizations outlined a minimum frequency of follow-up, in the form of regular periodic ‘in-person’ or ‘remote’ assessments.4 However, the efficacy of each of these methods, and their equivalency (implicit in this statement), had not been determined then. This has implications for patient security and clinic workflow organization, and for regulatory bodies seeking establishment and/or reimbursement for remote patient management.5 Potential limitations exist. Patient-dependent follow-up is notoriously subject to non-compliance.6 On the other hand, successful remote management may be determined by the technology (reliability and ease of use), and ability to reconfigure clinic workflows. Different operating characteristics among different proprietary technologies generate further complexity. Thus, although remote management is appealing, its role in clinical practice remains largely undefined, even more so as functional distinctions are drawn between remote follow-up and remote monitoring.7 Prolonged remote management without scheduled in-person evaluations (IPEs) potentially arouses concern, and remains unevaluated. Lack of these data significantly impedes wider acceptance and adoption of this innovative technology.
The TRUST trial previously reported clinic efficiencies ensuing from remote patient management.8 In the current analysis, we examined the comparative efficacy of in-person vs. remote management specifically regarding achievement of the core guideline objective of maintaining structured follow-up. Thus, we tested the trial hypothesis that remote management would more effectively achieve the key aims of patient retention, and adherence to and punctuality of regular periodic assessments.9
Methods
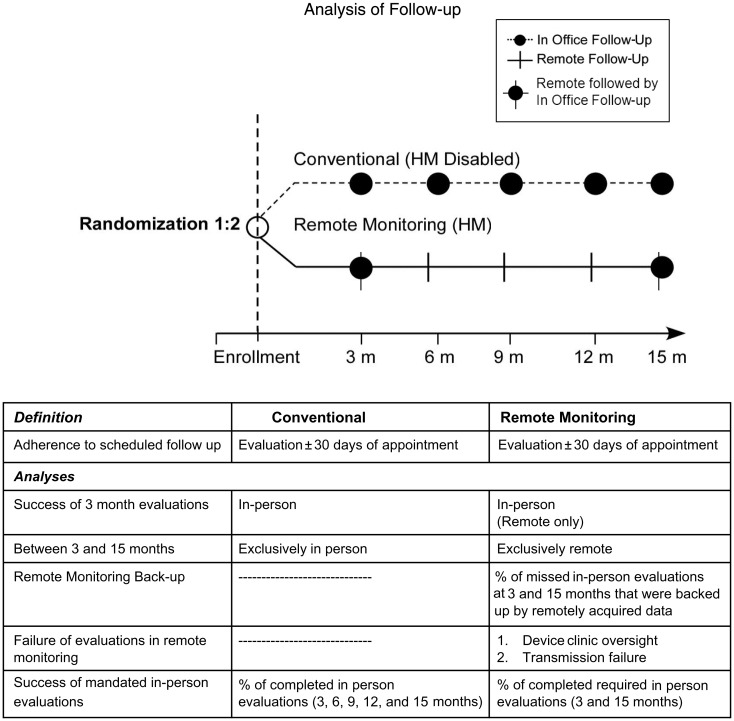
TRUST, a prospective randomized multi-centre clinical trial, compared the safety and utility of automatic remote Home Monitoring (HM) in implantable defibrillator recipients compared with Conventional in-person follow-up. Home Monitoring is a proprietary remote monitoring system, using an antenna within the pulse generator to wirelessly transmit stored data daily to a bedside transceiver for relay telephonically (cellular and/or landline) to a service centre for automatic processing and online review. High-fidelity transmission was demonstrated in pilot studies.10 Alert notifications were set for missed transmissions for >3 days. TRUST was an investigator-initiated clinical trial designed by a steering committee consisting of physicians (also serving as investigators) in collaboration with the sponsor.9 The protocol was written by the principal investigator and sponsor. All hypotheses and data queries were initiated by the principal investigator without sponsor involvement. The safety and efficacy of HM to reduce overall clinic burden (45% reduction of scheduled and unscheduled evaluations) relative to conventional care during 12 months of continuous monitoring have been reported previously.8 The objectives of the current analysis were to specifically test and compare efficacy and implementation of scheduled implantable cardioverter defibrillator (ICD) follow-up with either conventional or remote management strategies, according to recommendations, and to identify sources of failure. This was performed during 15 months of post-implant follow-up which, in HM-treated patients, included a full year of continuous monitoring without any scheduled IPE (Figure 1).
Figure 1.
TRUST trial: post-implant follow-up scheme for 15 months. Three monthly (total 5) guideline-based4 evaluations were prescribed in both study arms. All were in-person (IPE) in Conventional. In Home Monitoring, evaluations were always remote, but at 3 and 15 months were followed by in-person, i.e. remote management was exercised solely for the interim 12 months.
The trial design was reported previously.8,9 Briefly, patients were randomized post-implant 2:1 to either HM or conventional care with remote monitoring disabled. Follow-up evaluations were scheduled at 3, 6, 9, 12, and 15 months post-implant in both groups (Figure 1). Conventional patients were assessed in-person only. In this case, successful follow-up depended on coordination between patient and clinic. Home Monitoring patients were assessed remotely at identical time points. Three and 15 month checks in HM were followed by in-person visits, maintaining the first post-implant office visit and the minimum yearly face-to-face examinations according to recommendations.4 Six, 9, and 12 month checks were remotely accessed, and thus dependent on successful data delivery coupled to timely clinic download. Transmission reliability of the remote technology was assessed by the number of days during which remote transmissions were received per patient relative to total possible, i.e. daily until study exit. Familiarity with technology was assessed by site experience of HM prior to first enrolment. Site participation in TRUST did not include training in handling of patients assigned to remote management with HM. The ability to implement recommended scheduled follow-up was measured by adherence and punctuality to the protocol-specified evaluations in each study arm during the course of the trial. Failure (and its underlying reason) was reported as a protocol deviation. Adherence to the first 3 monthly post-implant check, common to both study arms, was determined.
Patients following up at least once during the trial (scheduled or unscheduled, including those who may have missed their 3 month evaluations) were then assessed for retention to follow-up and adherence (including punctuality) to all recommended scheduled checks. The impact of geographical constraints on patient retention was assessed by comparing proportions of exited subjects (excluding death) residing ≥50 miles or ≥60 min from the clinic. To directly compare the two follow-up methods of in-person vs. remote methods of scheduled evaluations, we measured the proportion of missed in-person visits in Conventional vs. number of failed remote evaluations in HM at 6, 9, and 12 months, i.e. during 1 year. Pre-specified reasons for unsuccessful HM check were transmission failure and clinic oversight. These proportions were analysed. For those resulting from transmission loss, the time taken for their correction was measured. The converse situation was tested by assessing the incidence of successful HM checks backing up appointed in-person failures at the 3 and 15 month time points, i.e. when protocol specified a combined HM and in-person check.
Finally, since the purpose of follow-up is to maintain patient engagement with clinic services, we assessed incidence of failure to attend mandated IPEs in HM (3 + 15 months) compared with Conventional (3, 6, 9, 12, and 15 months).
Analysis and statistics
Four patients with Sprint Fidelis lead crossed over from Conventional to HM on advisory notification, but were analysed as Conventional (intention-to-treat). Continuous variables were summarized as means and standard deviations, and categorical variables in frequency distributions. Group differences were compared with Student's t-tests.
Results
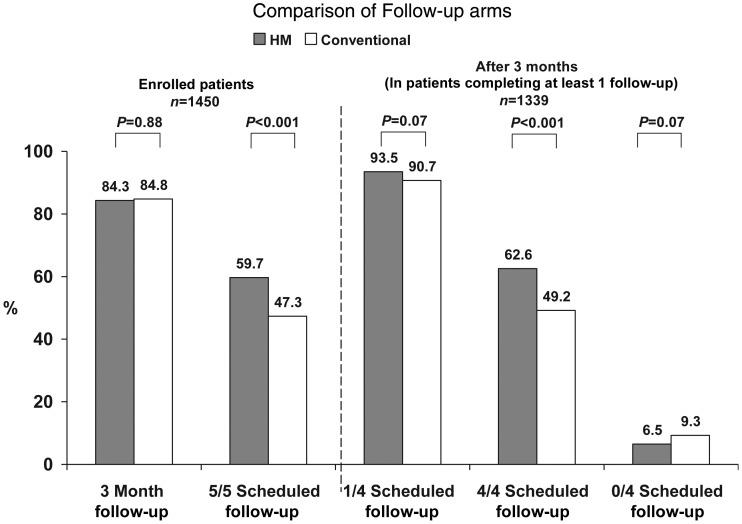
One hundred and two study sites enrolled 1450 patients from November 2005 to February 2008. 56.9% of sites had used HM for ≥1 year, and 25.5% were HM-naïve within 90 days of first enrolment. One hundred and eighty-five (12.8%) of patients were followed in academic centres and 1265 (87.2%) in community hospitals. Nine hundred and seventy-seven patients were enrolled in HM vs. 473 in Conventional care. Of these, 824 (84.3%) in HM received a 3 month follow-up evaluation compared with 401 (84.7%) in Conventional care (P = 0.88; Figure 2). In HM, 94.6% of these remote evaluations were followed by IPEs but, in 46 of 824 (5.6%) cases, only remote checks were performed since patients failed to show (protocol deviation). Eighty-four HM and 30 Conventional patients did not have a 3 month evaluation but had at least one follow-up during the course of the study (P = 0.15). 7.1% HM and 8.9% Conventional patients never followed after enrolment (P = 0.25).
Figure 2.
Conventional vs. Home Monitoring success rates compared.
Of enrolled patients, 908 of 977 (92.9%) HM and 431 of 473 (91.1%) Conventional patients completed at least one scheduled follow-up at some time point during the trial (P = 0.25). Patient characteristics in these groups were similar, though ischaemic heart disease was slightly more prevalent in Conventional (Table 1). Mean time from implant to first office visit was 104 ± 65 days in HM vs. 99 ± 44 days in Conventional (P = 0.21). Mean follow-up durations were 407 ± 103 (range 21–617) days for the HM group and 399 ± 111 (range 32–582) days for Conventional (P = 0.17). Mean follow-up times were <15 months because of the ±30 day allowable window around the 15 month visit and subjects who withdrew during the study. Mortality rate did not differ between groups over 15 months of follow-up [HM vs. Conventional: n = 36 (4.0%) vs. n = 21 (4.9%), P = 0.47]. Patient attrition (withdrawal and lost to follow-up) during this 15 month period with Conventional care was 87 of 431 (20.1%) compared with 129 of 908 in HM (14.2%, P = 0.007), i.e. HM improved patient retention significantly. This was not due to distance from the clinic: 15.9% (22 of 138) of HM patients residing ≥50 miles away exited compared with 22.1% (15 of 68) of Conventional (P = 0.34), and if ≥60 min from clinic 18.9% (18 of 95) HM vs. 21.2% (7 of 33) Conventional subjects exited the study (P = 0.80).
Table 1.
Patient Demographics
| HM (n = 908) | Conventional (n = 431) | P value | |
|---|---|---|---|
| Age at enrolment (years) | 63.3 ± 12.8 | 64.0 ± 12.1 | 0.365 |
| Gender: male | 72.0% | 73.1% | 0.695 |
| ICD indications: prophylactic | 72.2% | 73.8% | 0.599 |
| LVEF % (within last 12 months) | 29.0 ± 10.7 | 28.5 ± 9.8 | 0.497 |
| Patients with CAD | 64.8% | 71.7% | 0.013 |
| Dual-chamber implants | 57.8% | 56.6% | 0.679 |
| β-Blockers | 79.6% | 76.3% | 0.176 |
| ACE | 42.8% | 46.4% | 0.239 |
| ARBs | 7.7% | 9.5% | 0.289 |
P: statistical comparison between study arms.
LVEF, left ventricular ejection fraction; CAD, coronary artery disease; ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Scheduled checks were completed more often in HM. There was 100% adherence to the five appointed checks (3, 6, 9, 12, and 15 months) in 542 (59.7%) of HM vs. 204 (47.3%) in Conventional patients (P < 0.001). Similarly, when considering the year following the initial 3 month evaluation during which HM patients were completely remotely managed, there was 100% adherence to the four appointed checks (6, 9, 12, and 15 months) in 568 (62.6%) of HM vs. 212 (49.2%) in Conventional patients (P < 0.001; Figure 2). Thus, HM secured a >25% greater adherence to all recommended follow-up evaluations. 93.5% HM vs. 90.7% Conventional (P = 0.07) patients were evaluated at least once more until trial conclusion but 59 (6.5%) HM patients vs. 40 (9.3%) (P = 0.07) Conventional patients failed to have any further scheduled interrogations. When accounting for patient attrition due to death, withdrawal, and lost to follow-up, overall adherence to all five possible scheduled follow-up evaluations remained higher in HM (3759 of 4056, 92.7%) vs. Conventional (1648 of 1847, 89.2%) (P < 0.001). Moreover, 32% of follow-ups in HM vs. 29% in Conventional occurred within 7 days of the appointment (P = 0.028), and 54.0 vs. 50.3% (HM vs. Conventional) within 15 days (P = 0.012), i.e. punctuality was better maintained by HM relative to the ±30 day window of the assigned follow-up date.
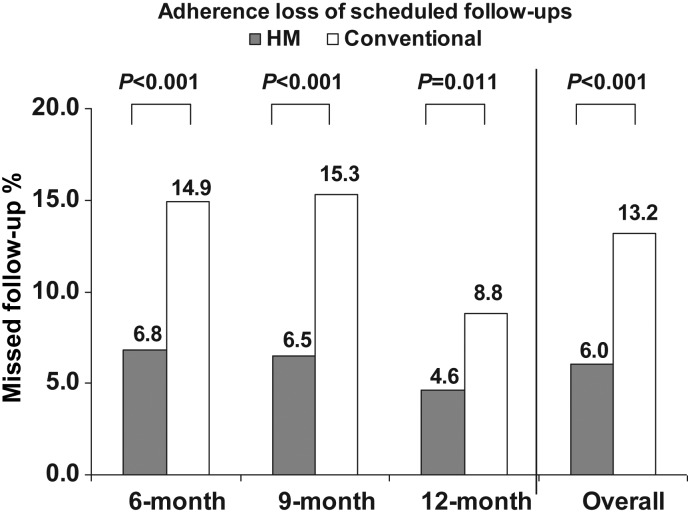
The two follow-up mechanisms were directly compared between 3 and 15 month time points when scheduled evaluations were exclusively in-person in Conventional and remote in HM for a full 1 year. The incidence of failed scheduled follow-ups at 6, 9, and 12 month time points in these respective study arms were 145 of 1098 (13.2%) in Conventional contrasting with 146 of 2421 (6.0%) in HM (P < 0.001). Home Monitoring advantage was observed consistently at each individual time point (Figure 3). Thus, Conventional management was associated with more than two-fold greater loss of adherence, indicating that patient-dependent mechanisms were more prone to failure.
Figure 3.
Rates of failed calendar-based evaluations in remote-only vs. conventional care between 3 and 15 months, i.e. at 3, 6, and 9 months, and total [right —146 of 2421 (6%) in Home Monitoring vs. 145 of 1098 (13.2%) with conventional].
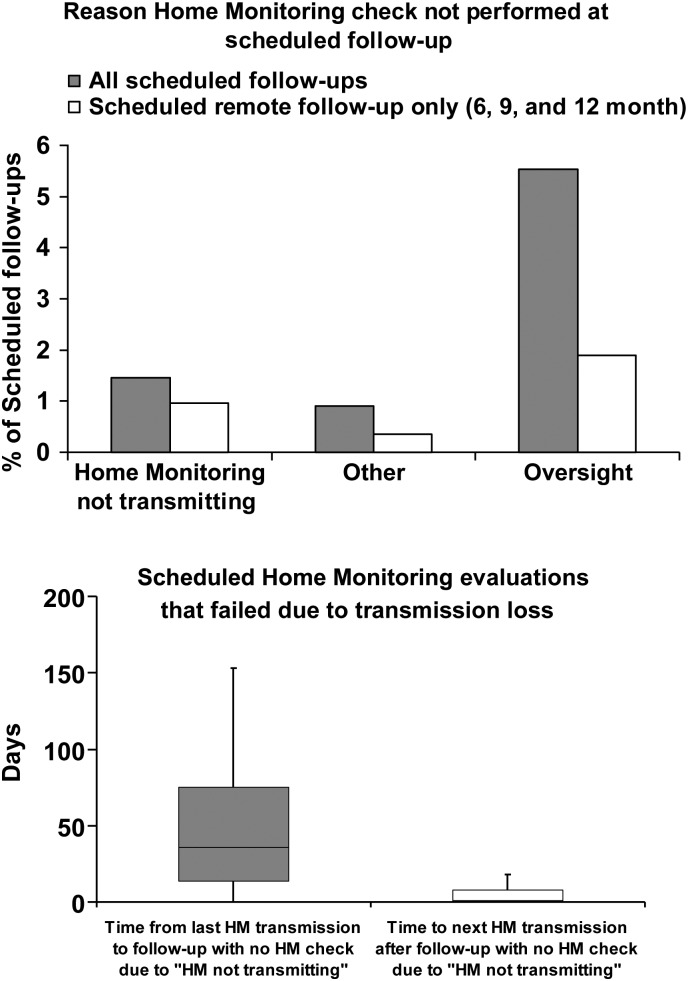
HM-based follow-up method was examined further. Daily transmission success was high. Time to first transmission was median 1 day after enrolment. Thereafter, overall success per patient was 91% during a median follow-up of 434 days, and daily HM transmissions were received in 315 795 of a potential 363 450 days (87%). The reason for failure of scheduled remote checks was most often due to oversight from the following facility (Figure 4, top). When accounting for this, transmission loss as a cause for failed 6, 9, and 12 month remote-only follow-ups (i.e. during the 1 year period when follow-up was completely dependent on technology reliability) was only 22 of 2275 (0.97%). Altogether, over the 15 months of the trial, 55 of 3759 (1.46%) of scheduled evaluations in HM were unsuccessful due to transmission loss. These occurred in 49 of 908 (5.4%) individuals, i.e. most losses occurred only once in any affected patient, in whom other scheduled transmissions remained successful. In these, last updated transmission occurred at a median time of 36 days prior to ‘failed’ scheduled check, i.e. relatively recently refreshed data still remained available for assessment. Transmission was reinstated within a median interval of 1 day after discovery (Figure 4, bottom).
Figure 4.
(Top) Reasons for failed Home Monitoring checks during all vs. remote-only (6, 9, and 12 month) appointments. Clinic oversight was dominant. Transmission loss was infrequent, but when responsible for ‘unsuccessful follow-up’, (Bottom) last transmission available occurred a median of 36 days prior (left), and (right) after discovery corrected promptly (median 1 day).
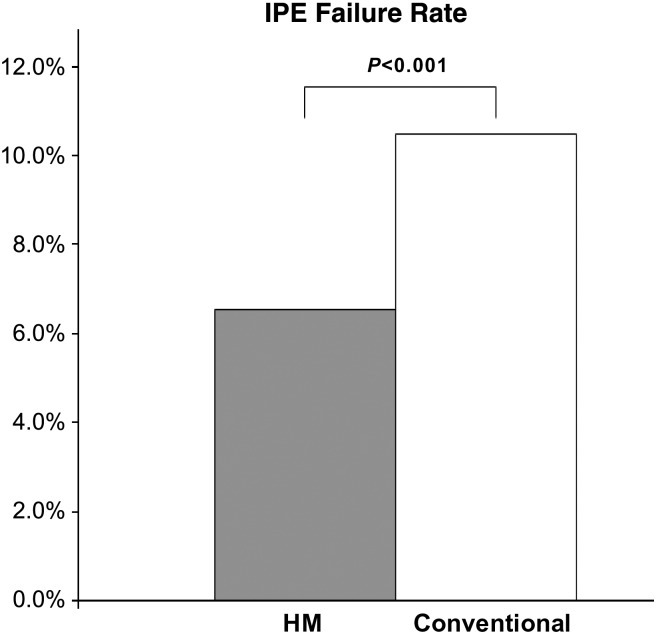
In HM, trial design specified that remote evaluation be performed prior to yearly scheduled in-person visit. However, protocol deviation occurred in 97 of 1484 (6.5%) of 3 and 15 month visits because in-person visits failed. In all of these cases, the availability of automatically acquired up-to-date HM data permitted satisfactory remote-only evaluation, i.e. HM supported 100% of these scheduled evaluations. Notably, compulsory scheduled IPEs failed more often in Conventional (193 of 1841 of 3, 6, 9, 12,+15 month time points, P < 0.001, Figure 5).
Figure 5.
Failure rate of mandated in person follow-up was 6.5% (97 of 1484 of 3 and 15 month time points) in Home Monitoring contrasting with 10.5% (193 of 1841) in Conventional (3, 6, 9, 12, and 15 months), i.e. 62% greater in Conventional.
Discussion
Here, we showed that automatic remote monitoring during extended periods more effectively and durably attained follow-up goals of punctual scheduled follow-up and patient retention compared with conventional methods. Patient-clinic linking improved. Technology reliability was excellent. These results support a preferred follow-up strategy based on remote patient management but full capitalization on its advantages demands organization of receiving facilities.
Post-implant follow-up is a necessity in patients receiving CIEDs.4 The 2008 transatlantic consensus recommendations advocated a regular calendar-based system of follow-up, although its success with regard to adherence and the comparative efficacy of ‘in-person or remote’ methods were unknown at the time of publication.4 CIED implant also carries regulatory implications. In the USA, a legal requirement exists for the registration and tracking of clinical devices (Safe Medical Devices Act of 1990, amended by the FDA Modernization Act of 1997). However, analysis of recent (2005–09) Medicare beneficiaries indicated significant shortfalls in prevailing practice—only 42% of patients were seen within 3 months and 22% not at all in a year post-CIED implant.2 The TRUST trial was conducted during this identical time period and tested two methods of post-CIED implant follow-up, i.e. a system based on the traditional face-to-face evaluations at regular intervals vs. a remote patient management strategy without scheduled IPEs over the extended time frame of 1 year. The current results demonstrate the positive effects of instituting either recommended follow-up schedule (success of patient evaluations at 3 months doubled and incidence of those remaining completely unevaluated more than halved), but when directly compared, the superiority of remote monitoring over conventional care. Potential barriers to implementation were also identified.
The Conventional arm of the TRUST trial represents the first test of in-person scheduled follow-up according to recommendations. Eighty-four per cent of patients were evaluated post-CIED implant for their first post-implant check. The proportion was identical in patients assigned to HM. This represents a 100% improvement over the contemporaneous standard of 42%.2 This result is important because several potential problems cluster in this early post-implant period and require direct IPE. For example symptomatic reactions to implantation (e.g. pacemaker syndrome, diaphragmatic pacing, and pocket infection), lead perforations, and imperfect wound healing.4 Final pacing parameters are set at this time point and permit assessment of erratic threshold changes. However, in HM, 5.6% of 3-monthly checks were conducted remotely without the mandated IPE. The practice of relying on HM check only at this specific time point, though effective for conveying electrical parameters, should be discouraged since this first check demands in-person assessment. Overall, these results indicate the significant positive effects of imposing the recommended follow-up protocol.
Results over the course of the trial were revealing. Despite the high adherence to the first post-implant 3 month visit and >90% adherence to at least one of four subsequent recommended evaluations, adherence to each and every recommended follow-up time point was not sustained, though better in HM. Less than one half of patients adhered to five of five mandated in-person checks with conventional follow-up (Figure 2). Adherence proportion was significantly improved by HM, and achieved with greater punctuality. These results indicate that a system of frequent periodic assessment is more successfully accomplished using remote management. The period of 1 year between the 3 and 15 month time points, when assessment was purely remote in HM and only in-person in Conventional, is instructive since adherence loss to specified follow-up protocol time points was more than two-fold higher with traditional follow-up system (Conventional 13.2 vs. HM 6.0%, P < 0.001).
HM patients were more compliant with compulsory scheduled IPEs, i.e. remained more engaged with clinical services (Figure 5). This illustrates patient fatigue during frequent routine follow-up, lending itself to drop-out (according with other studies6) but better loyalty to required IPEs if scheduled only yearly.11 In comparison, technology-based relay of routine data without any requirement for patient participation secured more durable follow-up and better preserved patient–clinic linking. This superiority of HM was largely due to improved patient retention compared with conventional care. However, when only those patients who remained in the trial were directly compared, adherence to recommended follow-up was still greater in HM.
Sustained fidelity of a transmission system is essential when using it for extended monitoring without IPE. This was rigorously tested here in HM patients in whom follow-up for a whole year completely depended on technology, itself programmed to transmit daily. Technology performance was excellent. A median of 91% of daily transmissions were transmitted successfully (translating into more than 27 of 30 possible updates per month), i.e. evaluations at appointed intervals could access up-to-date data reliably. This consistency, achieved without patient participation, permitted successful completion of >99% of all scheduled evaluations during 12 consecutive months of remote patient management (Figure 4, bottom). Action taken in response to alert notifications for missed transmissions for >3 days may have contributed to the high level of maintenance of remote transmission. Sustained transmission lapses were isolated and observed, on average, only once/year in affected patients, with remaining HM checks successful in those same patients. Their rectification within 1 day of discovery indicated that these were not due to significant technology problems but more likely from set-up error and/or travel without accompanying transceiver. The 3 and 15 month time points in this study are revealing, since they illustrate that when IPE failed, HM backed up consistently. This is because of robust delivery of refreshed data by HM, available for review even when patients failed to show. These are significant findings because transmission characteristics differ among available technologies yet few have been examined this closely in large clinical trials. Some attempt single periodic transmissions at 3 month intervals, i.e. four times per year. If vulnerable to high rates of transmission failure,12 this poses safety concerns making reliance on such a system inadvisable for remote management during extended periods as tested here. Thus, it is vital that all versions of remote technologies be tested for operational performance prior to application in clinical practice.
The current results show that when the traditional barrier of active patient participation for routine monitoring is removed by automatic mechanisms, and with the strength of technology performance demonstrated, follow-up lapses during remote management are generated at the point of handling by receiving facilities. Although few, there is opportunity for improvement. Most failed HM evaluations resulted from device clinic oversight. Even when successful, punctuality was modest (but still better than traditional methods). These results, in this first trial with a first-generation wireless automatic technology in largely HM-naïve sites, though unanticipated, illustrate the necessity for structured workflow patterns in receiving facilities practising remote patient management. Efficient and punctual handling may be aided by recent generation devices which trigger automatic notifications to review at appointed intervals (unavailable in TRUST), alerts for transmission discontinuation, and also website management improvements since trial completion. Clinic workflow routines may be pivotal. These enabled reliable data capture coupled to an astonishing manpower efficiency in HomeGuide.13 Critical elements were the universal adoption of a specific workflow programme with personnel and patients primed to process and expectations, and use of HM. In contrast, troubleshooting remote systems susceptible to transmission failure, or issuing reminders (phone calls and/or mails) to patients with inductive remote technology systems, exacts a heavy service burden with only moderate success.6,12,14
The patient is integral to the success of remote management. The absence of patient participation (or awareness) in the HM transmission process and reducing the frequency of routine non-actionable clinic attendance is important to patient and clinic alike. (All previous trials indicated that any reliance on patient participation erodes retention and compliance by ∼50% per year with non-implantable6 or even in some implantable wireless remote technologies12.) However, maintenance of regular patient–clinic interaction during the remote follow-up was vital.13 Patient reactions to remote management were tracked in TRUST. No remotely managed patient crossed over during the trial and 98% retained this form of follow-up on trial conclusion.15 Here, we show that remote management induced a behavioural change since patients became more loyal to mandated in-person assessment (i.e. they preferred to be seen when necessary), demonstrating increased patient engagement during follow-up (Figure 5). Patient satisfaction remains excellent with HM during chronic follow-up.16
In summary, the current results resolved a concern that a prolonged (1 year) remote monitoring strategy accelerates patient attrition: indeed, an opposite effect was observed. Technology reliability was excellent, backing up unsuccessful in-person appointments due to patient non-compliance. Paradoxically, patient-clinic linking improved during remote management. In comparison, frequent ‘routine’ IPE as a follow-up method promoted attrition. While remote monitoring has tended to be used adjunctively in current practice, our current findings, together with safety and clinic efficiencies noted previously, support a preferred follow-up strategy based on remote patient management.8
Strengths and limitations
The recommendations tested in this trial pertained to post-implant follow-up schedules. Their successful implementation correlated with survival gain.3 A possible mechanism may be that improved adherence enables timely clinical intervention thereby improving the outcome. In this regard, the current report showing that HM better attains long-term follow-up adherence are significant. This factor, when coupled to the early problem discovery capability of some remote technologies, may underlie survival advantages reported with remote management in the ALTITUDE17 and INTIME heart failure studies.18 (Conversely, reliance on patient participation only, which wanes rapidly over prolonged time periods, has failed to influence the course of heart failure6.) The ability to provide this remote ‘monitoring’ function varies among current remote monitoring platforms and even when available is often disabled depending on operator preference.4,5,7 Although not a follow-up objective stated in the 2008 guidelines (and not reported in this TRUST analysis), this additional functionality merits prospective evaluation.
The clear definition for the role of remote management in the practice model tested here, together with accompanying device clinic/manpower efficiencies,13 may address outstanding questions regarding clinical application, and aid specific health-economical analyses required for instituting reimbursement.5 The current results were achieved even with limited centre experience (most were HM-naïve at the time of enrolment) and >85% of patients were drawn from a community setting, reflecting national US practice patterns. These suggest that this innovative technology and practice may be adopted relatively easily in ‘real-world practice’ with due attention to clinic handling. However, attrition rates among enrolled patients, and their rate of missed follow-ups, were unexpectedly high in both conventionally and remotely managed patients (unrelated to distance from receiving facility), aligning with contemporary US practice.2 In Europe, a 17.4% attrition rate (‘withdrawal, moving, lost to follow-up’) over 27 months occurred under trial conditions.19 These observations point to a less-appreciated challenge in CIED patient management, common across health-care systems. Importantly, the current results indicate that remote management alleviates this problem. Causes for residual losses (e.g. economical concerns) require further elucidation.
In conclusion, this first test of consensus-based follow-up recommendations of CIED recipients demonstrated significant impact compared with prevailing practice. Furthermore, although stated scheduled follow-up could be ‘in-person’ or ‘remote’, we showed that automatic remote monitoring more effectively and durably attained follow-up goals. This forces a re-evaluation of the traditional gold standard built on IPE only.
Funding
This work was supported by Biotronik USA. Funding to pay the Open Access publication charges for this article was provided Biotronik, Berlin, Germany.
Conflict of interest: N.V.: Chair of TRUST trial 2005–2009 Modest research/consultancy/speaker fees. B.S.: Modest research/consultancy/speaker fees. J.M.: Scientist, Biotronik, salary support. B.P.: Modest research/consultancy/speaker fees.
References
- 1.Marinskis G, van Erven L, Bongiorni MG, Lip GY, Pison L, Blomstrom-Lundqvist C. Practices of cardiac implantable electronic device follow-up: results of the European Heart Rhythm Association survey. Europace. 2012;14:423–425. doi: 10.1093/europace/eus020. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khatib SM, Mi X, Wilkoff BL, Qualls LG, Frazier-Mills C, Setoguchi S, Hess PL, Curtis LH. Follow-up of patients with new cardiovascular implantable electronic devices: are experts’ recommendations implemented in routine clinical practice? Circ Arrhythm Electrophysiol. 2012;6:108–116. doi: 10.1161/CIRCEP.112.974337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess PL, Mi X, Curtis LH, Wilkoff BL, Hegland DD, Al-Khatib SM. Follow-up of patients with new cardiovascular implantable electronic devices: is adherence to the experts’ recommendations associated with improved outcomes? Heart Rhythm. 2013;10:1127–1133. doi: 10.1016/j.hrthm.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and European Heart Rhythm Association (EHRA); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), European Society of Cardiology (ESC), Heart Failure Association of ESC(HFA), and Heart Failure Society of America (HFSA) Europace. 2008;10:707–725. doi: 10.1093/europace/eun122. [DOI] [PubMed] [Google Scholar]
- 5.PricewaterhouseCoopers. Moving towards good practice in the reimbursement of CIED telemonitoring. Germany, Italy, Spain, Netherlands: UK: A study conducted in five European countries; 2012. http://www.eucomed.org/uploads/Modules/Publications/whitepaper_reimbursementciedtelemonitoring.pdf (10 February 2014, date last accessed). [Google Scholar]
- 6.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burri H. Remote follow-up and continuous remote monitoring, distinguished. Europace. 2013;15(Suppl 1):i14–i16. doi: 10.1093/europace/eut071. [DOI] [PubMed] [Google Scholar]
- 8.Varma N, Epstein A, Irimpen A, Schweikert R, Shah J, Love CJ. Efficacy and safety of automatic remote monitoring for ICD follow-up: the TRUST trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 9.Varma N. Rationale and design of a prospective study of the efficacy of a remote monitoring system used in ICD follow-up: the Lumos-T reduces routine office device follow-up study (TRUST) study. Am Heart J. 2007;154:1029–1034. doi: 10.1016/j.ahj.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Varma N, Stambler B, Chun S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S133–S136. doi: 10.1111/j.1540-8159.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 11.Varma N, Michalski J. Home monitored ICD patients are more loyal to follow up-the paradox of remote patient management in the TRUST trial. Eur Heart J. 2013;34(Suppl 1):263. Abstract. [Google Scholar]
- 12.Crossley G, Boyle A, Vitense H, Chang Y, Mead RH. The clinical evaluation of remote notification to reduce time to clinical decision (CONNECT) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Ricci RP, Morichelli L, D'Onofrio A, Calo L, Vaccari D, Zanotto G, Curnis A, Buja G, Rovai N, Gargaro A. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: The Homeguide registry. Europace. 2013;15:970–977. doi: 10.1093/europace/eus440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cronin E, Ching EA, Varma N, Martin DO, Wilkoff B, Lindsay BD. Remote monitoring of cardiovascular devices-a time and activity analysis. Heart Rhythm. 2012;9:1947–1951. doi: 10.1016/j.hrthm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Varma N, Stambler B. Patient aspects of remote home monitoring of ICDs—the TRUST trial. Circulation. 2010;123:e247. doi: 10.1161/CIRCULATIONAHA.110.983148. [DOI] [PubMed] [Google Scholar]
- 16.Ricci RP, Morichelli L, Quarta L, Sassi A, Porfili A, Laudadio MT, Gargaro A, Santini M. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace. 2010;12:674–679. doi: 10.1093/europace/euq046. [DOI] [PubMed] [Google Scholar]
- 17.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122:2359–2367. doi: 10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 18.Hindricks G. 2013. The INTIME trial http://www.escardio.org/about/press/esc-congress-2013/press-conferences/Documents/slides/hindricks.pdf ESC Hotline Session (November 2013, date last accessed)
- 19.Hindricks G, Elsner C, Piorkowski C, Taborsky M, Geller JC, Schumacher B, Bytesnik J, Kottkamp H. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial. Eur Heart J. 2014;35:98–105. doi: 10.1093/eurheartj/eht207. [DOI] [PMC free article] [PubMed] [Google Scholar]