Abstract
Aims
Using a large, contemporary primary care population we aimed to provide absolute long-term risks of cardiovascular death (CVD) based on the QTc interval and to test whether the QTc interval is of value in risk prediction of CVD on an individual level.
Methods and results
Digital electrocardiograms from 173 529 primary care patients aged 50–90 years were collected during 2001–11. The Framingham formula was used for heart rate-correction of the QT interval. Data on medication, comorbidity, and outcomes were retrieved from administrative registries. During a median follow-up period of 6.1 years, 6647 persons died from cardiovascular causes. Long-term risks of CVD were estimated for subgroups defined by age, gender, cardiovascular disease, and QTc interval categories. In general, we observed an increased risk of CVD for both very short and long QTc intervals. Prolongation of the QTc interval resulted in the worst prognosis for men whereas in women, a very short QTc interval was equivalent in risk to a borderline prolonged QTc interval. The effect of the QTc interval on the absolute risk of CVD was most pronounced in the elderly and in those with cardiovascular disease whereas the effect was negligible for middle-aged women without cardiovascular disease. The most important improvement in prediction accuracy was noted for women aged 70–90 years. In this subgroup, a total of 9.5% were reclassified (7.2% more accurately vs. 2.3% more inaccurately) within clinically relevant 5-year risk groups when the QTc interval was added to a conventional risk model for CVD.
Conclusion
Important differences were observed across subgroups when the absolute long-term risk of CVD was estimated based on QTc interval duration. The accuracy of the personalized CVD prognosis can be improved when the QTc interval is introduced to a conventional risk model for CVD.
Keywords: QTc interval, Gender, Marquette 12SL validation, Cardiovascular death, Risk prediction
See page 1303 for the editorial comment on this article (doi:10.1093/eurheartj/ehu120)
Introduction
The QT interval on the surface electrocardiogram (ECG) represents the time from the beginning of ventricular depolarization (beginning of the QRS complex) to the end of ventricular repolarization (end of the T wave). Both a shortened and a prolonged ventricular repolarization time, reflected in the ECG as a shortened or prolonged QT interval, respectively, are considered as substrates for lethal ventricular tachyarrhythmias. At least, this seems to be evident for persons with the rare congenital short or long QT syndromes, characterized by extreme QT intervals at each end of the spectrum together with a high risk of sudden cardiac death.1
On a general population level, several studies have associated the heart rate-corrected QT (QTc) interval with the risk of both all-cause and cardiovascular death (CVD).2 Despite this, there is a lack of knowledge on the absolute risks associated with varying degrees of QTc interval duration, particularly for community-based individuals with more extreme QTc intervals. Moreover, the extent to which QTc interval duration is of clinical value in long-term prediction of CVD on an individual level has not been investigated. This is important to examine because CVD is potentially preventable and identifying high-risk subpopulations are thus warranted.3
Using a large, contemporary primary care population, we aimed (i) to estimate absolute risks of CVD based on varying degrees of QTc interval duration within relevant subgroups and (ii) to evaluate whether QTc interval duration is of value in personalized long-term risk prediction of CVD. We studied this in middle-aged and elderly people; those with a reasonable absolute risk of CVD within our study period.
Methods
Study population
In the greater region of Copenhagen, Denmark, the vast majority of general practitioners refer their patients to one core facility (Copenhagen General Practitioners' Laboratory, CGPL) for clinical tests, such as biochemistry and ECG recordings. The Copenhagen ECG study comprises all persons who had an ECG recorded at the CGPL on behalf of their general practitioner during 2001–11, as described previously.4 For the current analysis, we excluded persons <50 or ≥90 years of age, persons with a pacemaker or an implantable cardioverter-defibrillator at inclusion,5 or persons with an ECG unsuitable for QT interval measurement (see Electrocardiography).
Because our study was register-based with no active participation from study subjects, no approval from an ethics committee was required according to Danish law. The use of register data was approved by the Danish Data Protection Agency.
Electrocardiography
All ECGs were recorded digitally and stored in the MUSE® Cardiology Information System (GE Healthcare, Wauwatosa, WI, USA) and were later processed using version 21 of the Marquette 12SL algorithm. Using the 12SL algorithm, we excluded ECGs with findings inconsistent with a valid measurement of the QTc interval (e.g. atrial fibrillation and bundle branch blocks). The QT interval was obtained as a representative median beat from all PQRST complexes in the 12 leads of the 10 s ECG tracing (see Supplementary material online, Appendix for details). QT intervals were corrected for heart rate using the Framingham linear regression formula (QTcFram = QT + 154[1–60/heart rate]) or, for sensitivity analysis, with the Bazett's formula (QTcBaz = QT/[RR interval]1/2). An evaluation of the 12SL algorithm for QT interval measurement compared with manual measurement is provided in the Supplementary material online, Appendix.
Clinical baseline variables and endpoints
Using Danish administrative registers and a unique personal identification number assigned to all persons with permanent residence in Denmark, it is possible to follow individuals with respect to death, emigration, the use of prescription medication, and any hospital, ambulatory, or emergency room discharge diagnoses. By using data in these registers, we identified individuals with the following baseline variables: heart failure, myocardial infarction, valvular heart disease, treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), beta-blockers, or calcium-antagonists prior to study inclusion, treatment with QTc-prolonging drugs (Supplementary material online, Table S1) or digoxin at the day of ECG recording, and finally, we constructed a modified Charlson co-morbidity index taking several comorbidities including diabetes, various cancer diseases, liver diseases, and vascular diseases into account (Supplementary material online, Table S2). The endpoints of interest were CVD and non-CVD. These data were retrieved from The Danish Register of Causes of Death. Detailed information on the identification of covariates and outcomes in the Danish registers are provided in the Supplementary material online, Appendix.
Statistical analyses
Women and men were separately divided into nine categories based on QTc interval distributions with cut-offs at the 1st, 5th, 20th, 40th, 60th, 80th, 95th, and 99th percentiles. Individual follow-up time began on the day of the first ECG recording (index ECG) and ended at death, emigration, or on 31 December 2011, which was the end of follow up. A two-sided P-value <0.05 was considered statistically significant. Analyses were conducted using the Stata 12.0 software package (StataCorp LP, College Station, Texas, USA) and R [R Foundation for Statistical Computing, Vienna, Austria (URL http://www.R-project.org/)].
Association analyses
We used Cox regression to investigate associations between QTc interval duration, obtained from the index ECG, and hazards of all-cause death, CVD, and non-CVD. Analyses were performed separately for women and men. Cox models were adjusted for conventional risk factors that were obtained on the day of inclusion. Age was used as timescale. For all outcomes, the QTc interval subgroup with the lowest hazard was chosen as reference group (denoted as optimal QTc intervals hereafter). The functional relationship between the non-categorized QTc interval and the risk of CVD was assessed using restricted cubic regression splines (see Supplementary material online, Appendix).
Risk prediction
Prediction analyses were performed independently for women and men in age groups 50–70 and 70–90 years. The personalized risk of CVD and non-CVD in the period t-years from index ECG were predicted in a competing risk framework by combining the two cause-specific Cox regression models.6 All risk factors were obtained at baseline. Time-on-study was used as timescale and age within the current age group was included as a risk factor.
To summarize the personalized risk, we report median 5-year risk of CVD in subgroups defined by age (≥50 to <70 and ≥70 to <90 years), gender, cardiovascular disease (myocardial infarction, heart failure, or valvular heart disease at baseline), and QTc interval categories.
The discriminative value of QTc interval duration for the purpose of CVD-specific risk prediction was evaluated using C-statistics at t-years.7 C-statistics were corrected for over-optimism using internal cross-validation based on 100 splits of the dataset into training sets for fitting Cox regression models and validation sets for estimating C-statistics.8 In the competing risk setting, the C-statistic is the ability of the model to correctly rank the CVD event times up to time t and to distinguish them from non-CVD event times.7 Model calibrations were evaluated by calculating Brier scores.9
To evaluate reclassification as a result of adding QTc interval to the Cox regression models, we defined the following risk categories for the predicted risk of CVD and non-CVD within 5 years from the index ECG: very low risk (≤5%), low risk (>5 to ≤15%), intermediate risk (>15 to ≤25%), high risk (>25 to ≤35%), and very high risk (>35%). Reclassification was considered appropriate for persons who had an event (CVD or non-CVD) within 5 years on study who moved up in risk category and for persons without events (5-years survivors) who moved down in risk category when the QTc interval was introduced to the model. Similarly, inappropriate reclassification was defined as persons with events who moved down in risk category and persons without events who moved up in risk category. We did not compute the net reclassification index as a summary of the reclassification table.10
Proportional hazard assumptions were checked graphically and accepted for all Cox models.
Results
Study population
The greater region of Copenhagen has a current population of 1.18 million citizens. Of these, 341 698 individuals (∼29%) had one or more ECGs recorded at CGPL during the 11-year period from 2001 to 2011. Of the individuals referred for ECG recording, a total of 173 529 (51%) were eligible for inclusion (Supplementary material online, Figure S1). Baseline clinical characteristics of the study population are shown in Table 1. The median follow-up time was 6.1 years [interquartile range (IQR) 3.3–8.8 years], corresponding to 1 037 198 person-years. During follow-up, a total of 27 153 died, of whom 6647 died due to cardiovascular causes. Follow-up was 100% with regard to clinical endpoints and emigration.
Table 1.
Baseline characteristics of the study population
| Women, age (years) |
Men, age (years) |
|||
|---|---|---|---|---|
| Characteristics | 50–70 (n = 63475) | 70–90 (n = 35650) | 50–70 (n = 56589) | 70–90 (n = 17815) |
| Age—years, median (IQR) | 59 (55–64) | 78 (74–82) | 59 (55–64) | 76 (73–81) |
| Medical history—no. (%) | ||||
| Myocardial infarction | 1012 (1.6) | 1716 (4.8) | 2689 (4.8) | 1760 (9.9) |
| Heart failure | 363 (0.6) | 1152 (3.2) | 495 (0.9) | 654 (3.7) |
| Valvular heart disease | 221 (0.4) | 446 (1.3) | 257 (0.5) | 250 (1.4) |
| Charlson co-morbidity score | ||||
| 0 Points | 50 114 (79) | 22 707 (64) | 45 555 (81) | 11 033 (62) |
| 1 Point | 6968 (11) | 6289 (18) | 6309 (11) | 3177 (18) |
| ≥2 Points | 6393 (10) | 6654 (19) | 4725 (8) | 3605 (20) |
| Medication history—no. (%) | ||||
| ACE inhibitors or ARBs | 10 702 (17) | 9812 (28) | 10 958 (19) | 4883 (27) |
| Beta-blockers | 11 569 (18) | 8767 (25) | 8846 (16) | 4026 (23) |
| Calcium antagonists | 8768 (14) | 9719 (27) | 8495 (15) | 4618 (26) |
| Medication on day of inclusion—no. (%) | ||||
| QTc-prolonging medicationa | 5913 (14) | 4456 (13) | 3744 (6.6) | 1844 (10) |
| Digoxin | 149 (0.2) | 637 (1.8) | 183 (0.3) | 311 (1.8) |
| Index ECG variables | ||||
| Heart rate—b.p.m.: median (IQR) | 70 (63–79) | 73 (65–82) | 68 (60–78) | 69 (406–431) |
| QTcFram interval—ms: median (IQR) | 420 (409–432) | 420 (408–433) | 415 (404–427) | 419 (406–431) |
| QTcFram interval—ms: mean (SD) | 421 (18) | 421 (20) | 416 (18) | 419 (20) |
| Left ventricular hypertrophy—no. (%) | 1191 (1.9) | 1649 (4.6) | 2945 (5) | 1134 (6.4) |
ms, milliseconds; CI, confidence interval; SD, standard deviation; IQR, inter-quartile range; b.p.m., beats per minute; ACE, angiotensin converting enzyme; ARBs, angiotensin receptor blockers.
aSee Supplementary material online, Table S2 for a comprehensive list.
Association analyses
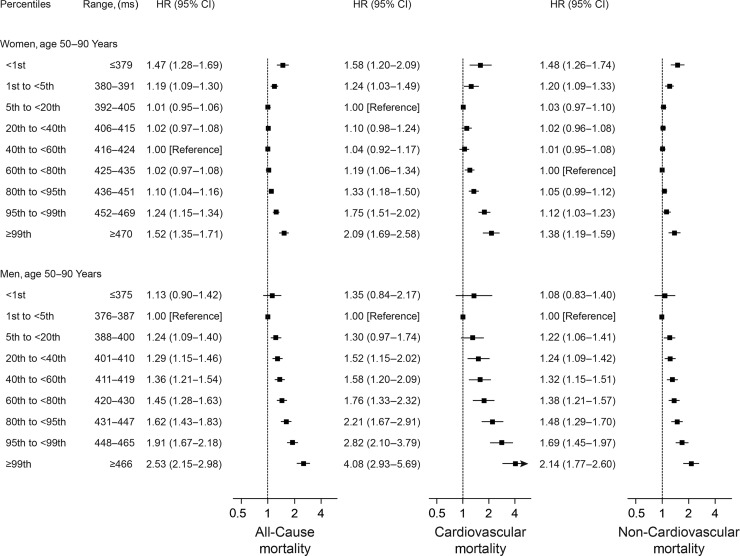
We observed a dose–response relationship between longer QTc intervals and the risk of both all-cause, cardiovascular, and non-CVD (Figure 1). The association was strongest for CVD in men where a QTcFram interval ≥99th percentile (≥466 ms) was associated with a hazard ratio (HR) of 4.08 [95% confidence interval (CI) 2.93–5.69, P < 0.001] for CVD compared with men with an optimal QTcFram interval (376–387 ms; defined as the reference group). We also observed an association between short QTc intervals and risk of death. This effect was strongest in women where a QTcFram interval <1st percentile (≤379 ms) was associated with a HR of 1.58 (95% CI 1.20–2.09, P = 0.001) for CVD compared with women with an optimal QTcFram interval (392–405 ms). The association between short QTc intervals and the risk of CVD was not statistically significant for men when using categorical analysis. However, the spline-based analysis indicated a statistically significant increased risk of CVD for men with extremely short QTc intervals (Supplementary material online, Figure S2). Results based on the Bazett's formula are provided in Supplementary material online, Figure S3.
Figure 1.
Multivariable-adjusted HRs for all-cause, cardiovascular, and non-CVD by categories of the QTcFram interval. All models were adjusted for heart failure, myocardial infarction, valvular heart disease, Charlson comorbidity index (0 points, 1 point, or ≥2 points), treatment with ACE-inhibitors or ARBs, beta-blockers, or calcium antagonists prior to inclusion, treatment with QTc-prolonging medications or digoxin on the day of ECG recording, left ventricular hypertrophy on the index ECG, and age was used as the timescale. The vertically dotted lines represent a HR of 1. The horizontal solid lines represent 95% CIs.
Absolute risk predictions
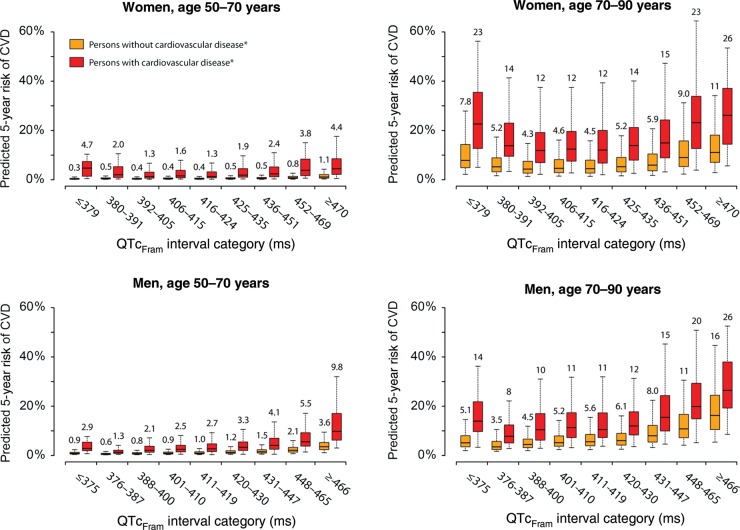
Distributions of 5-year risks of CVD across subgroups are shown in Figure 2 and Supplementary material online, Table S3.
Figure 2.
Predicted 5-year risk of CVD based on subgroups. Both models for CVD and the competing models of non-CVD were independently performed for women and men in age groups 50–70 and 70–90 years, and contained the following covariates: age as a linear parameter, myocardial infarction, heart failure, valvular heart disease, a modified Charlson comorbidity index (0 points, 1 point, or ≥2 points), treatment with ACE-inhibitors or ARBs, beta-blockers or calcium antagonists prior to inclusion, treatment with QTc-prolonging medications or digoxin on the day of ECG recording, and left ventricular hypertrophy on the index ECG. Boxes denote the median risks (horizontal line) and interquartile ranges (lower and upper border) whereas whiskers denote the 5th and 95th percentiles. Numbers above the whiskers denote the median risk for the respective subgroup. Heart rate-correction was based on the Framingham formula (QTcFram).
In women aged 50–70 years with an optimal QTcFram interval (392–405 ms), the median 5-year risk of CVD was 0.4% (IQR 0.2–0.6%) for those without a history of cardiovascular disease and 1.3% (IQR 0.6–3.1%) for those with a history of cardiovascular disease. In the same subgroup of women (age 50–70 years), a QTcFram interval ≥470 ms conferred a 5-year CVD risk of 1.1% (IQR 0.6–2.1%) for those without and 4.4% (2.1–8.5%) for those with a history cardiovascular disease (Figure 2).
In women aged 70–90 with a history of cardiovascular disease, we observed median 5-years risk of 22.6% (IQR 12.7–35.4%), 11.9% (IQR 7.0–19.1%), and 26.1% (IQR 14.4–37.0%) for QTcFram intervals in the ranges of ≤379, 392–405, and ≥470 ms, respectively.
Within age groups, the largest absolute effect of a QTcFram interval in the upper 99th percentile (≥466 ms) was observed in men with a history of cardiovascular disease. In this subgroup, we noted median 5-year CVD risks of 9.8% (IQR 6.2–17.0%) and 26.4% (IQR 19.3–37.7%) for those aged 50–70 and 70–90 years, respectively. The absolute risk of having a very short QTcFram interval was generally lower in men compared with women (Figure 2). For both age groups of men, it is worth noticing that the median 5-year risk was lower for the subgroup with cardiovascular disease and an optimal QTcFram interval (367–387 ms) than for the subgroups of men free of cardiovascular disease but with a QTcFram interval ≥466 ms (Figure 2).
Predicted 5-year risks based on the QTcBaz interval are provided in Supplementary material online, Table S4 and Supplementary material online, Figure S4.
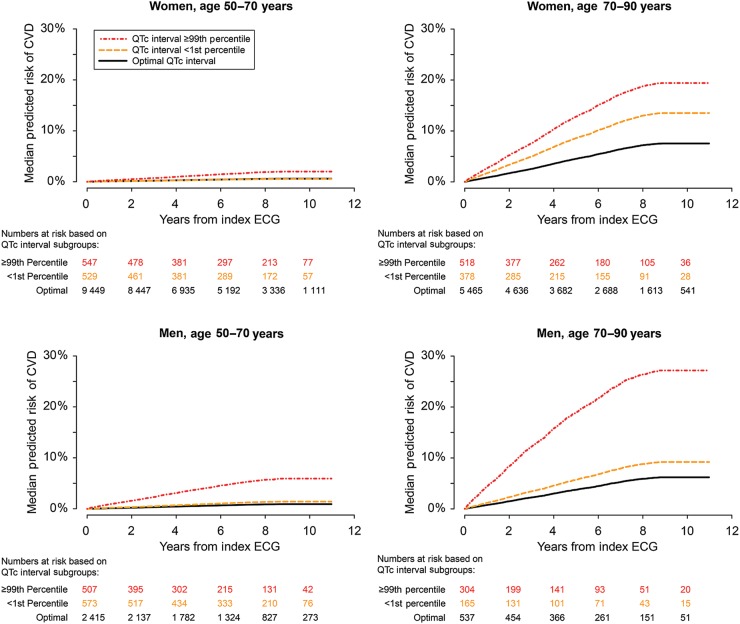
Predicted cumulative incidences of CVD for persons with very short, very long, and optimal QTc intervals for the various age- and gender-determined subgroups are shown in Figure 3.
Figure 3.
Cumulative incidence based on QTcFram interval subgroups. Predictions were based on Cox models fitted within the respective age- and gender-determined subgroups and were adjusted for covariates as described in Figure 2. Optimal QTcFram intervals were the QTcFram intervals that were associated with the lowest relative risk of CVD (Figure 1).
The importance of the QTc interval for individual risk predictions is exemplified by selected risk profiles in Supplementary material online, Table S5.
Measures of discrimination and calibration
C-statistics for both CVD and non-CVD within the age and gender determined subgroups are provided in Table 2. In general, the discrimination ability of the model without the QTcFram interval was higher in persons aged 50–70 years compared with persons aged 70–90 years. Additionally, higher discrimination ability was seen in women compared with men and for predictions of CVD compared with predictions of non-CVD. The ability to discriminate the risk of CVD improved when the QTcFram interval was included in the Cox regression models. The improvement was most pronounced in men aged 70–90. There was generally no improvement with respect to discriminating non-CVD risks (Table 2). Differences in Brier scores before and after adding the QTcFram interval to the Cox regression models did not indicate problems with calibration.
Table 2.
C-statistics (%) with and without QTcFram interval
| Women, age (years) |
Men, age (years) |
||||
|---|---|---|---|---|---|
| Model | 50–70 (n = 63 475) | 70–90 (n = 35 650) | 50–70 (n = 56 589) | 70–90 (n = 17 815) | |
| CVD | Model without QTcFram | 73.0 | 70.4 | 69.9 | 66.8 |
| Model with QTcFram | 73.1 | 70.6 | 70.6 | 67.7 | |
| Difference | +0.1 | +0.2 | +0.7 | +0.9 | |
| Non-CVD | Model without QTcFram | 70.1 | 64.8 | 67.2 | 63.0 |
| Model with QTcFram | 70.0 | 64.8 | 67.6 | 62.9 | |
| Difference | −0.1 | +0.0 | +0.3 | −0.1 | |
C-statistics was based on internal cross validation using 100 bootstrap samples without replacement. Predictions were based on Cox models fitted within the respective age- and gender-determined subgroups and were adjusted for covariates as described in Figure 2.
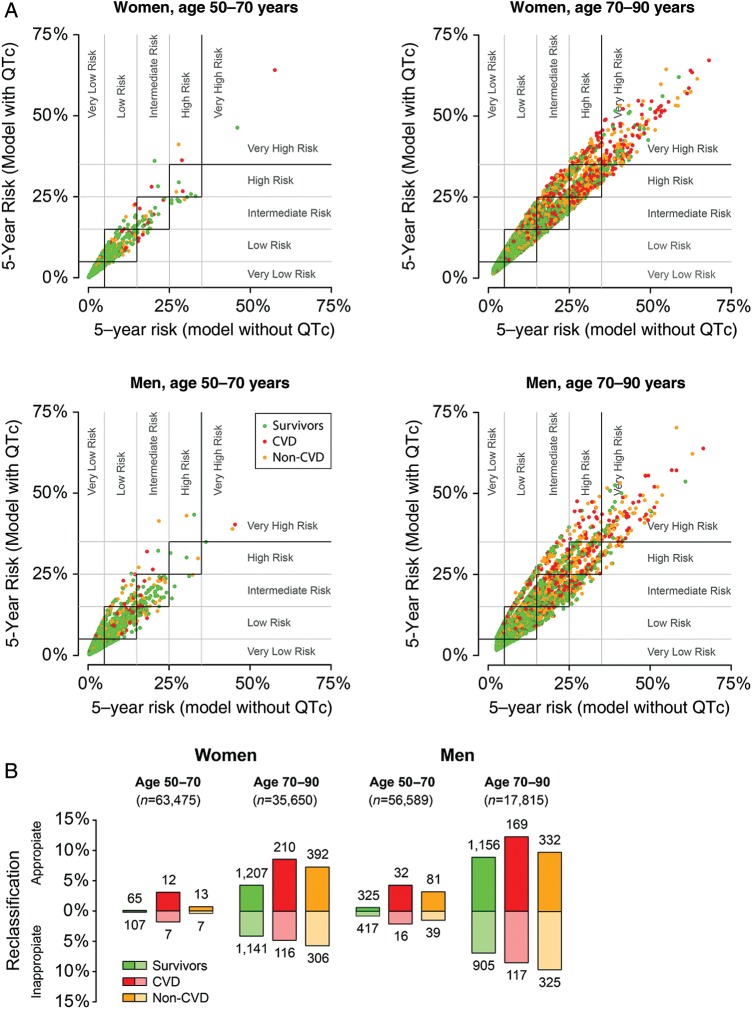
Reclassification
Figure 4 illustrates the effect of QTc interval duration on reclassification within the 5-year risk categories. In general, the largest proportion of appropriate reclassifications was for persons who died from cardiovascular disease (9% appropriate vs. 5% inappropriate across all subgroups), whereas inappropriate reclassifications occurred most frequently for persons who survived the 5-year period (Figure 4B). For women aged 50–70 years, 0.3% were reclassified when the QTc interval was added to the model, and of these, 0.1% of reclassifications were appropriate and 0.2% were inappropriate. A total of 9.5% of women aged 70–90 years were reclassified, comprising 7.2% appropriate and 2.3% inappropriate reclassifications. Of men aged 50–70, 1.6% were reclassified, and of these men, 0.8% were reclassified appropriately and 0.8% inappropriately. Finally, for men aged 70–90 years, a total of 16.9% were reclassified, and of these, 9.3% were reclassified appropriately and 7.6% inappropriately.
Figure 4.
(A) Reclassification within various 5-year risk categories based on predictions with and without QTcFram interval. The predictive models are described in Figure 2. Very low risk was defined as ≤5% risk of dying within 5 years, low risk was >5 to ≤15%, intermediate risk was >15 to ≤25%, high risk was >25% to ≤35%, and very high risk was defined as >35% risk of dying within 5 years (indicated by grey lines). Red and orange dots (CVD and non-CVD, respectively) above and green dots (survivors) below the squares with the black borders denote appropriate reclassifications whereas red and orange dots below and green dots above denote inappropriate reclassifications. Dots within the black-bordered squares denote individuals who were not reclassified. (B) Summary of reclassifications by subgroups. Numbers above or below columns denote the absolute number of appropriate and inappropriate reclassifications, respectively.
Discussion
In a primary care population comprising >170 000 middle-aged and elderly women and men, we estimated long-term prognoses of CVD based on the QTc interval duration across subgroups and calculated measures of discrimination and reclassification.
To enable comparison with previous studies on the QTc interval, we initially investigated the association between QTc interval duration and the risk of death. These analyses revealed an increased risk of all-cause, cardiovascular, and non-CVD with longer QTc intervals, an association that was strongest for CVD in men. This finding is consistent with previous studies that virtually agree on an association between prolonged QTc intervals and the risk of death in the general population.11,12 In contrast to longer QTc intervals, it has been less clear whether very short range QTc intervals also confer an increased risk of death for community-based individuals. We found a dose–response relationship between QTcFram interval shortening and the risk of CVD in women aged 50–90 years. However, in men, this association was only statistically significant for extremely short QTcFram intervals when applying a spline-based approach (Supplementary material online, Figure S2). In a Finnish population comprising ∼11 000 individuals, a very short QTc interval was not associated with an increased risk of death.13 Zhang et al. also performed a study of QTc interval duration and risk of death applying an approach much like ours and by this investigated a wider spectrum of the QTc interval.14 In this study, QTc intervals <5th percentile conferred an increased risk of all-cause mortality but this association was not statistically significant with respect to CVD.
It is noteworthy that relatively large statistical power seems to be required to detect a signal in the shorter QTc interval range compared with the longer range as evidenced by the relatively smaller point estimates and wider confidence limits for shorter compared with longer intervals. This may explain why most previous studies have been unable to identify an increased risk for community-based individuals with QTc intervals in the very short range.2
Although testosterone seems to play an important role in cardiac repolarization,15 we cannot explain the differences in QTc interval risk profiles that we observe for the two genders and future research on this are thus warranted.
Absolute rather than relative risks are preferable for clinical decision making. Therefore, beyond examining associations, we estimated absolute long-term risks of CVD for various subgroups and by this revealed some interesting findings. The absolute effect of QTcFram interval duration on the risk of CVD appears negligible for women aged 50–70 years without a history of cardiovascular disease. However, for women aged 70–90 years and for women aged 50–70 years with a history of cardiovascular disease, the absolute risk related to either a very short or long QTc interval appears to be clinically relevant. Interestingly, we found that middle-aged and elderly women with very short QTcFram intervals and a history of cardiovascular disease had an absolute risk of CVD that was as high or higher as women with a borderline prolonged QTcFram interval. With respect to middle-aged and elderly men, we observed higher average risk of CVD for those with a QTcFram interval that was only borderline prolonged, and even higher risks for those with a very long QTcFram interval, compared with men with a history of myocardial infarction, heart failure, and/or valvular heart disease and a QTcFram interval within the normal range. This finding indicates an on average worse outcome for men with long QTcFram intervals than men with a history of cardiovascular disease. In particular, the one man in a hundred with the longest QTc interval seems to be at an increased risk of CVD to an extent that should not be ignored in clinical practice.
To test the potential implications of using the QTcFram interval in future risk models of cardiovascular mortality, we calculated measures of discrimination and evaluated reclassification across subgroups. Of these two measures, we primarily relied on reclassification because c-statistics is known to be limited when evaluating prediction models for which the task is to assess future risks in a largely healthy population.16 When the QTcFram interval was added to a 5-year risk model that already contained several conventional risk factors for CVD, it did not significantly improve reclassification for the 50–70-year women and men. In fact, only ∼1% of the persons in these two subgroups were reclassified. However, of those who actually died due to cardiovascular causes, reclassification was often appropriate. The impact of the QTcFram interval on personalized risk prediction appears to be of greater value in 70–90-year-old compared with middle-aged. This finding was particularly evident in women where a total of 9.5% of the 70–90-year-old were reclassified and of whom 7.2% were reclassified more accurately. We believe that such an impact on reclassification potentially may have future clinical implications.
All ECGs were analyzed digitally thus avoiding any intra- or inter-observer variability. The 12SL algorithm is widely used, has been approved by the US Food and Drug Administration for use in pharmaceutical studies, and it has been validated extensively with regard to QT interval measurement.17–19 In addition, we undertook our own validation study and found a good overall agreement between the 12SL algorithm and manual measurements of QTcFram intervals <500 ms. The consistency between measurements deteriorated for QTc intervals above 500 ms although there was no evidence of a systematic bias (see Supplementary material online, Appendix and Supplementary material online, Figure S5).
We used the Framingham formula for heart rate-correction of the QT interval because this formula is widely used, because it is based on empirical data from a large population sample rather than hypothetical reasoning,20 and because linear regression functions rather than for instance Bazett's formula has been recommended in recent guidelines.21 However, for comparability with previous studies on the QTc interval, we also provide some results for the widely used Bazett's formula (Supplementary material online, Appendix). Although heart rate-correction with the use of Bazett's formula resulted in a lower risk for short QTc intervals and a higher risk for longer QTc intervals compared with the Framingham formula, using the two different formulas resulted in similar trends.
Limitations
Despite a sample size of >170 000 persons and almost 7000 CVD events, this study has some important limitations.
We restricted our analyses to persons aged 50–90 years old; thus, our data should not be extrapolated to other age groups. We chose to examine only middle-aged and elderly persons because we also aimed to provide absolute risk estimates for the extremes of the QTc interval. Relatively few events of CVD are observed in those younger than 50 years with a QTc interval <1st or ≥99th percentile; thus, risk estimates would be difficult to estimate in this group. Therefore, an alternative approach is necessary for examination of young groups.
Although the average 5-year risk of CVD presented provides some information for the purpose of risk prediction on an individual level, it is obvious that risk prediction should ideally be performed on an individual level rather than for a subgroup, in which individual risks can vary considerably (Figure 2). Although a few examples of some single-subject predictions are presented in Supplementary material online, Table S5, the purpose of this study was not to develop a novel personalized risk model based on ECG parameters but rather to provide the reader with an impression of absolute risks associated with varying degrees of QTc interval duration within relevant subgroups.
Despite the fact that the QTcFram interval might improve risk prediction on an individual level, this does not necessarily mean that lives can be saved. In this regard, future trials have to address whether treatment strategies modified based on the QTcFram interval will improve survival.
Another limitation to our study is that we do not know the indication for the general practitioners referral of an individual to electrocardiography and this has undoubtedly lead to some selection bias. However, we believe that our study population is a clinically relevant representation of individuals in whom an ECG would be considered and hence used as a tool for risk prediction of CVD.
Finally, due to a lack of information on some important cardiovascular risk factors, such as body mass index, blood pressure, and smoking status, we were unable to evaluate the performance of the QTcFram interval with respect to some of the most commonly used cardiovascular risk models.3 However, we were able to adjust our predictive models for several cardiovascular risk factors and diseases that are likely intermediate phenotypes for the possible confounding effect of body mass index, blood pressure, and smoking status on the relationship between QTcFram interval duration and the risk of CVD. Additionally, we were able to adjust for treatment with antihypertensive medication as a proxy for hypertension.
Conclusion
In a contemporary primary care population comprising >170 000 middle-aged and elderly people, we estimated long-term risks of CVD based on QTcFram interval duration and observed important differences between subgroups. We observed an increased risk of CVD for both very short and long QTcFram intervals in both genders. However, QTcFram interval prolongation resulted in the worst prognosis for men whereas in women, a very short QTcFram interval was equivalent in risk to having a borderline prolonged QTcFram interval. In general, the effect of QTcFram interval on the absolute risk of CVD was most pronounced in the elderly and in those with prior cardiovascular disease whereas the effect was negligible for middle-aged women without cardiovascular disease. The QTcFram interval may improve the accuracy of long-term CVD prognosis on an individual level when added to conventional risk models for CVD.
Authors’ contributions
Study concept and design: J.B.N., M.S.O., L.K., T.A.G., and A.G.H. Acquisition of data: C.G., A.P., and B.L. Digital ECG analysis: J.B.N., C.G., and J.J.S. Drafting of the manuscript: J.B.N., C.G., T.A.G., and A.G.H. Critical revision of the manuscript for important intellectual content: C.G., A.P., P.V.R., B.L., M.S.O., J.J.S., S.H., L.K., J.H.S., T.A.G., and A.G.H. Statistical analyses and data interpretation: J.B.N., P.V.R., T.A.G., and A.G.H. Fund raising: J.B.N., C.G., S.H., L.K., J.H.S., and A.G.H.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was supported by the University of Copenhagen, the Danish National Research Foundation, The Danish Council for Independent Research (Grant no. 11-107456), The Villadsen Family Foundation, and The John and Birthe Meyer Foundation. Funding to pay the Open Access publication charges for this article was provided by The John and Birthe Meyer Foundation.
Conflicts of interest: A.G.H. is an employee of Novo Nordisk A/S, Denmark.
Supplementary Material
References
- 1.Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750–763. doi: 10.1016/S0140-6736(08)61307-0. [DOI] [PubMed] [Google Scholar]
- 2.Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–948. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 3.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJM, Vrints C, Wood D, Zamorano JL, Zannad F European Association for Cardiovascular Prevention & Rehabilitation (EACPR), ESC Committee for Practice Guidelines (CPG) European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MS, Haunsø S, Gerds TA, Svendsen JH, Køber L, Holst AG. J-shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013;61:2557–2564. doi: 10.1016/j.jacc.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen JB, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Haunsø S, Gerds TA, Ellinor PT, Køber L, Svendsen JH, Holst AG. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG Study. Heart Rhythm. 2013;10:1249–1256. doi: 10.1016/j.hrthm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Rosthøj S, Andersen PK, Abildstrom SZ. SAS macros for estimation of the cumulative incidence functions based on a Cox regression model for competing risks survival data. Comput Methods Programs Biomed. 2004;74:69–75. doi: 10.1016/S0169-2607(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 7.Wolbers M, Blanche P, Koller MT, Witteman JCM, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014. doi:10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed]
- 8.Steyerberg. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer; 2009. [Google Scholar]
- 9.Gerds TA, Scheike TH, Andersen PK. Absolute risk regression for competing risks: interpretation, link functions, and prediction. Stat Med. 2012;31:3921–3930. doi: 10.1002/sim.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilden J, Gerds TA. A note on the evaluation of novel biomarkers: do not rely on integrated discrimination improvement and net reclassification index. Stat Med. 2013 doi: 10.1002/sim.5804. doi: 10.1002/sim.5804. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22:660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noseworthy PA, Peloso GM, Hwang S-J, Larson MG, Levy D, O'Donnell CJ, Newton-Cheh C. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2012;17:340–348. doi: 10.1111/j.1542-474X.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation. 2007;116:714–720. doi: 10.1161/CIRCULATIONAHA.106.676551. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF, Guallar E. QT-interval duration and mortality rate: results from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1727–1733. doi: 10.1001/archinternmed.2011.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, Bertran G, Arini P, Biagetti MO, Quinteiro RA. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 16.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 17.Rautaharju PM, Prineas RJ, Kadish A, Larson JC, Hsia J, Lund B. Normal standards for QT and QT subintervals derived from a large ethnically diverse population of women aged 50 to 79 years (the Women's Health Initiative [WHI]) Am J Cardiol. 2006;97:730–737. doi: 10.1016/j.amjcard.2005.09.108. [DOI] [PubMed] [Google Scholar]
- 18.Hnatkova K, Gang Y, Batchvarov VN, Malik M. Precision of QT interval measurement by advanced electrocardiographic equipment. Pacing Clin Electrophysiol. 2006;29:1277–1284. doi: 10.1111/j.1540-8159.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 19.Fosser C, Duczynski G, Agin M, Wicker P, Darpo B. Comparison of manual and automated measurements of the QT interval in healthy volunteers: an analysis of five thorough QT studies. Clin Pharmacol Ther. 2009;86:503–506. doi: 10.1038/clpt.2009.34. [DOI] [PubMed] [Google Scholar]
- 20.Al-Khatib SM, LaPointe NMA, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289:2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 21.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.