Abstract
Background
Ovarian cancer is the sixth most common cancer among women and the leading cause of death in women with gynaecological malignancies. Opinions differ regarding the role of ultra-radical (extensive) cytoreductive surgery in ovarian cancer treatment.
Objectives
To evaluate the effectiveness and morbidity associated with ultra-radical/extensive surgery in the management of advanced stage ovarian cancer.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 4), MEDLINE and EMBASE (up to November 2010). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) or non-randomised studies, analysed using multivariate methods, that compared ultra-radical/extensive and standard surgery in adult women with advanced primary epithelial ovarian cancer.
Data collection and analysis
Two review authors independently assessed whether potentially relevant studies met the inclusion criteria, abstracted data and assessed the risk of bias. One non-randomised study was identified so no meta-analyses were performed.
Main results
One non-randomised study met our inclusion criteria. It analysed retrospective data for 194 women with stage IIIC advanced epithelial ovarian cancer who underwent either ultra-radical (extensive) or standard surgery and reported disease specific overall survival and perioperative mortality. Multivariate analysis, adjusted for prognostic factors, identified better disease specific survival among women receiving ultra-radical surgery, although this was not statistically significant (Hazard ratio (HR) = 0.64, 95% confidence interval (CI): 0.40 to 1.04). In a subset of 144 women with carcinomatosis, those who underwent ultra-radical surgery had significantly better disease specific survival than women who underwent standard surgery (adjusted HR = 0.64, 95% CI 0.41 to 0.98). Progression-free survival and quality of life (QoL) were not reported and adverse events were incompletely documented. The study was at high risk of bias.
Authors’ conclusions
We found only low quality evidence comparing ultra-radical and standard surgery in women with advanced ovarian cancer and carcinomatosis. The evidence suggested that ultra-radical surgery may result in better survival. It was unclear whether there were any differences in progression-free survival, QoL and morbidity between the two groups. The cost-effectiveness of this intervention has not been investigated. We are, therefore, unable to reach definite conclusions about the relative benefits and adverse effects of the two types of surgery.
In order to determine the role of ultra-radical surgery in the management of advanced stage ovarian cancer, a sufficiently powered randomised controlled trial comparing ultra-radical and standard surgery or well-designed non-randomised studies would be required.
BACKGROUND
Description of the condition
Ovarian cancer is the sixth most common cancer among women and the leading cause of death in women with gynaecological malignancies. Globally, there are over 200,000 new cases per year, with approximately 6.6 new cases per 100,000 women per year. A woman’s cumulative risk of developing ovarian cancer by the age of 65 years is 0.5%: 0.4% in less developed countries and 0.7% in more developed countries (Ferlay 2004). It is less common in women under the age of 35 years, and its incidence increases with age. In Europe, approximately 37% of women with ovarian cancer are alive five years after diagnosis (EUROCARE 2003), largely because the early stages of the disease often present with very few, if any, specific symptoms so most women present with advanced stage disease (Jemal 2008; Kurman 2008; Lancet 2007; Visintin 2008).
Cancers of the ovary are classified according to their cells of origin. Most ovarian cancers originate from the surface (epithelial) cells of the ovary and are termed epithelial tumours, although some cancers can also arise from the substance of the ovary, called stromal tumours, or from embryological differentiation (sex cord and germ cell tumours). The staging of ovarian cancer is based on the International Federation of Gynaecology and Obstetrics (FIGO) classification system (Shepherd 1989). FIGO staging depends on the findings at the time of surgery. Stages I and II constitute early stage disease, where Stage I is limited to the ovaries and Stage II tumours extend to the pelvis. Stages III and IV constitute advanced disease. In Stage III the tumour extends outside the pelvis, or involves lymph nodes within the pelvis, and Stage IV is where the tumour has spread to distant sites such as the liver, lungs and lymph nodes in the neck.
Description of the intervention
A common and popular treatment for the management of women with epithelial ovarian cancer is surgery followed by combination platinum and taxane based chemotherapy (Ozols 2003). Prognosis depends not only on the stage and histological type of the tumour but also the end result of surgery. Studies have shown that residual disease after initial surgery is a strong independent prognostic factor for survival, with improvements in both overall and progression free survival being greatest in women with no (currently termed complete cytoreduction) or minimal (< 1 cm, currently termed optimal cytoreduction) visible residual disease at the end of surgery (Aletti 2006; Eisenhauer 2006). Women who undergo more extensive surgery are more likely to have tumour deposits of ≤ 2 cm at the end of surgery (Crawford 2005), while each 10% increase in maximal cytoreduction appears to be associated with a 5.5% increase in median survival time (Bristow 2002). The five-year survival for patients having surgery that leaves residual tumour deposits of more than 2 cm and surgery with residual tumour deposits of up to 2 cm at the end of the procedure are similar, further suggesting that optimal cytoreduction is associated with improved survival rates (Bristow 2002). However, the extent of surgical resection required to achieve optimal cytoreduction remains controversial. There appears to be a universally diverse practice with huge variations in the cytoreduction rate of between 22 to 98% (Bristow 2002; Eisenkop 1998).
Although there is a lack of evidence demonstrating a benefit from performing a hysterectomy at the time of debulking surgery, this is accepted practice as it aids the diagnosis of a primary tumour site, for example, serous papillary cancers and mixed mullerian tumours may originate from both the uterus and ovary. It also helps in excluding synchronous primary uterine tumours. While systematic lymphadenectomy may not be standard practice in cytoreductive surgery for ovarian cancer, removing the uterus and cervix, both tubes and ovaries, the omentum and enlarged lymph nodes is part of standard surgery (Aletti 2006; Todo 2003). Patients with widespread disease that is those with upper abdominal disease affecting the diaphragm, liver, spleen and omentum, or widespread disease affecting the bowel, will need much more radical surgery in order to achieve complete or optimal cytoreduction. The complexity of the procedures required to achieve these outcomes undoubtedly increases. Radical surgery including bowel resection, splenectomy, liver resection and diaphragmatic stripping has been described in the literature as treatment for advanced ovarian cancer with low complication rates (Bristow 2003; Eisenkop 2001a; Jaeger 2001; Merideth 2003; Montz 1989). Standard and ultra-radical (extensive) surgery are a continuum, and three types of surgery or procedures have been defined. These are standard surgery, which comprises, as a minimum, hysterectomy, bilateral adnexectomy with excision of the pelvic peritoneum, total omentectomy including the supracolic omentum, appendicectomy, removal of bulky pelvic and lumbo-aortic nodes +/− simple peritonectomies; 2) radical surgery comprising in addition to the above mentioned elements, en bloc removal of the uterus, both ovaries, the pelvic peritoneum and recto-sigmoid with or without simple peritonectomies; and 3) supra-radical surgery, that is, a radical procedure plus at least one of the following: a) extensive peritonectomies including partial resection of the diaphragm, b) resection of subcapsular liver metastases, cholecystectomy, c) splenectomy, resection of that tail of the pancreas and d) other bowel resection, partial gastrectomy, etc. (Pomel 2004).
It has been proposed that multiple factors including tumour biology determine the manner of disease progression, which in turn influences the likelihood of surgical cytoreduction (Eisenkop 2001a; Hoskins 1992; Markman 2007). Supporters of non-radical surgery argue that the initial extent of advanced disease reflects the aggressiveness of the tumour, and ultimately dictates treatment success. Therefore, when radical surgery becomes necessary to achieve optimal cytoreduction, it may not improve survival despite leaving minimal residual disease (Covens 2000). Furthermore, the role of surgery has been questioned because patients who undergo complete cytoreduction and have better survival often represent women who have relatively small pre-operative tumour loads and therefore less biologically aggressive tumours, and that differences in tumour biology account for the survival benefits that are reported to be from surgery (Eisenkop 1998; Hoskins 1992). Perhaps of greater concern is the patient morbidity that is incurred during such radical procedures, both in the perioperative and post-operative periods (Chen 1985; Van Dam 1996; Venesmaa 1992). Furthermore, several authors have reported that patients who underwent extensive debulking procedures such as bowel resection and peritoneal stripping did not have a survival advantage compared with those patients who did not undergo such procedures and had residual disease remaining (Potter 1991).
In showing that survival outcomes of extensive disease managed by ultra-radical (extensive surgery) is equivalent to less extensive disease managed by less radical surgery, whereby complete cytoreduction is being achieved in both cases, would be supportive of the value of cytoreduction in negating the adverse effects of aggressive tumour biology on outcome survival. Also by having equally extensive disease and managing some by ultra-radical (extensive) surgery and some by standard surgery and showing an improvement in survival outcome with ultra-radical surgery would also be supportive of the benefits of cytoreduction.
As would be expected, ultra-radical surgery is associated with a prolonged operating time and exposure to anaesthesia. This increases the risk of hypothermia, respiratory complications such as atelectasis (lung collapse), infection, adult respiratory distress syndrome, blood loss and intraoperative ureteric, bowel and bladder injury. In the postoperative period, these women may require a longer hospital stay and recovery time, with an increased risk of infection (chest, wound, urine), venous thromboembolic disease, poorer mobility and poorer nutritional status. The cost effectiveness of such surgery would also require evaluation.
Why it is important to do this review
To our knowledge, there have been no previous systematic reviews on ultra-radical (extensive) surgery versus standard surgery. In a survey of members of the Society of Gynecologic Oncologists, 45.5% of surgeons cited the lack of evidence for improved survival as a primary rationale against performing aggressive surgical resection (Eisenkop 2001b). Given the differences in opinion regarding the role of extensive debulking surgery in ovarian cancer treatment, the aim of this review was to examine the available evidence for ultra-radical surgery in ovarian cancer management.
OBJECTIVES
To evaluate the effectiveness and morbidity associated with ultra-radical (extensive) surgery in the management of ovarian cancer.
METHODS
Criteria for considering studies for this review
Types of studies
-
Randomised controlled trials (RCTs)
As we expected to find few if any RCTs of surgical interventions (Johnson 2008), the following types of non-randomised studies with concurrent comparison groups were included.
Quasi-randomised trials, non-randomised trials, prospective and retrospective cohort studies, and case series of 100 or more patients.
Case-control studies, uncontrolled observational studies and case series of fewer than 100 patients were excluded.
In order to minimise selection bias, we decided to include only studies that used statistical adjustment for baseline case mix (for example age, performance status, grade, etc) using multivariate analyses.
Types of participants
Adult women diagnosed with Stages III and IV epithelial ovarian cancer. Women having ultra-radical surgery as part of interval debulking surgery (surgery halfway through the course of chemotherapy) were included.
Women with other concurrent malignancies were excluded. Women with recurrent disease were also excluded.
Types of interventions
Intervention: ultraradical surgery defined as total abdominal hysterectomy, bilateral salpingo-öophorectomy, omentectomy, removal of enlarged lymph nodes (para-aortic, pelvic, obturator) and one or more of the following: upper abdominal surgery (splenectomy, diaphragmatic or peritoneal stripping, liver resection), bowel surgery or stoma formation or urinary tract surgery
Comparison: standard surgery defined as total abdominal hysterectomy, bilateral salpingo-öophorectomy, omentectomy either with or without removal of enlarged lymph nodes (paraaortic, pelvic, obturator), and debulking of any other superficial tumour plaques.
The types of interventions defined above have been widely described in the literature and have been adapted from the paper on management of malignant epithelial tumours of the ovary by Pomel 2004.
Types of outcome measures
Primary outcomes
Overall survival: survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Secondary outcomes
Progression-free survival.
Optimal cytoreduction, defined as residual tumour <1 cm, or complete cytoreduction.
Death within 30 days of intervention.
- Adverse events classified according to CTCAE 2006:
- direct surgical morbidity: e.g. vascular injury, injury to bladder, ureter, small bowel or colon, presence and complications of adhesions, febrile morbidity, intestinal obstruction, anastomotic leak, haematoma, collection, local infection.
- surgically related systemic morbidity e.g. chest/wound/urine infection, thrombo-embolic events (deep vein thrombosis and pulmonary embolism), cardiac events (cardiac ischaemia, myocardial infarction and cardiac failure), cerebrovascular accident, transfusion reaction, pulmonary oedema;
- recovery: delayed discharge, unscheduled re-admission
Quality of life (QoL) measured using a scale that has been validated through reporting of norms in a peer-reviewed publication.
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See: Cochrane Gynaecolgical Cancer Group methods used in reviews.
The following electronic databases were searched.
The Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library 2010, Issue 4).
The Cochrane Gynaecological Cancer Collaborative Review Group’s Trial Register
MEDLINE up to November 2010
EMBASE up to November 2010
The MEDLINE, EMBASE and CENTRAL search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3 respectively.
Databases were searched from January 1966 until November 2010. All relevant articles found were identified on PubMed and using the ‘related articles’ feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and grey literature
Metaregister, Physicians Data Query, www.controlled-trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials were searched for ongoing trials.
Reference lists
Reference lists of all included studies were searched for additional studies.
Handsearching
Abstracts of meetings from the International Gynaecological Cancer Society (2000 to 2008), the British Gynaecological Cancer Society (2008), European Society of Gynaecological Oncology (2003, 2005 and 2009) and the Society of Gynecologic Oncologists (2009 and 2010) were handsearched to identify unpublished studies.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were down-loaded to the reference management database Endnote, duplicates were removed and the remaining references were examined by two review authors (CA, KC) independently. Those studies which clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (CA, KC). Disagreements were resolved by discussion between the two review authors and when necessary by a third and fourth review authors (AB, RN). Reasons for exclusion were documented.
Data extraction and management
For included studies, data were abstracted as follows.
Author, year of publication and journal citation (including language).
Country.
Setting.
Inclusion and exclusion criteria.
Study design, methodology.
- Study population, the following will be abstracted by treatment arm if possible:
- total number enrolled.
- patient characteristics.
- age
- ethnicity.
- co-morbidities.
- response to neo-adjuvant chemotherapy.
- Ovarian cancer details at diagnosis:
- FIGO stage (III or IV).
- histological cell type.
- differentiation.
Previous treatment (neoadjuvant chemotherapy subgroup analysis: responders versus non-responders).
- Surgical details:
- type of surgeon (Gynaeoncologist, Gynaecologist, General surgeon).
- type of surgery (ultra-radical (extensive) versus standard).
Risk of bias in study (see below).
Duration of follow-up.
- Outcomes (see above) - overall survival, progression-free survival, QoL and adverse events.
- For each outcome: outcome definition (with diagnostic criteria if relevant).
- unit of measurement (if relevant).
- for scales: upper and lower limits, and whether high or low score is good;
- For results: Number of participants allocated to each intervention group.
- for each outcome of interest: Sample size; Missing participants.
We abstracted the hazard ratio and its 95% confidence interval. For adjusted statistics, we noted the variables used in adjustment. Where possible, all data extracted were those relevant to an intention-to-treat analysis, in which participants were analysed in groups to which they were assigned. The time points at which outcomes were collected and reported was noted.
Data were abstracted independently by two review authors (CA, KC) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author (AB), when necessary.
Assessment of risk of bias in included studies
The risk of bias in included studies was assessed using the Cochrane Collaboration’s tool (Higgins 2008). This included assessment of:
sequence generation;
allocation concealment;
blinding (where assessment of blinding was restricted to blinding of outcome assessors, since it is generally not possible to blind participants and treatment providers to surgical interventions);
- incomplete outcome data; we coded a satisfactory level of loss to follow-up for each outcome as:
- yes, if fewer than 20% of patients were lost to follow up and reasons for loss to follow up were similar in both treatment arms
- no, if more than 20% of patients were lost to follow up or reasons for loss to follow up differed between treatment arms,
- unclear if loss to follow-up was not reported;
selective reporting of outcomes;
other possible sources of bias.
The risk of bias in non-randomised controlled trials was assessed in accordance with the following four additional criteria.
Cohort selection
1. Were relevant details of criteria for assignment of patients to treatments provided?
Yes.
No.
Unclear.
2. Was the group of women who received the experimental intervention (ultra-radical surgery) representative?
Yes, if they were representative of women with advanced stage ovarian cancer.
No, if group of patients was selected.
Unclear, if selection of group was not described.
3. Was the group of women who received the comparison intervention (standard surgery) representative?
Yes, if drawn from the same population as the exposed cohort.
No, if drawn from a different source.
Unclear, if selection of group not described.
Comparability of treatment groups
1. Were there no differences between the two groups or were differences controlled for, in particular with reference to age, FIGO stage, histological cell type, differentiation, previous treatment (neoadjuvant chemotherapy - responders versus non-responders) and type of surgeon (gynae-oncologist, gynaecologist, general surgeon).
Yes, if at least three of these characteristics were reported and any reported differences were controlled for.
No, if the two groups differed and differences were not controlled for.
Unclear, if fewer than three of these characteristics were reported even if there were no other differences between the groups, and other characteristics were controlled for.
The risk of bias tool was applied independently by two reviewers (CA, KC) and differences resolved by discussion or by appeal to a third reviewer (AB). Results were presented in a risk of bias graph.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time to event data, we used the hazard ratio.
Dealing with missing data
We did not impute missing outcome data for any outcomes.
Data synthesis
We were unable to pool results in meta analyses as only one study met our inclusion criteria. Therefore it was not possible to assess heterogeneity between results of studies and we were unable to assess reporting biases using funnel plots or conduct any sub group or sensitivity analyses.
RESULTS
Description of studies
Results of the search
The search strategy identified 2007 references in MEDLINE, 1740 in EMBASE, 320 in CENTRAL and 12 in the Specialised Register. When the search results were merged into EndNote and duplicates were removed, 2965 unique references remained. The title and abstract screening identified 22 references as potentially eligible. The full text screening excluded 21 of these for the reasons described in the table Characteristics of excluded studies. The one remaining reference (Aletti 2006) reported a study that met our inclusion criteria and is described in the table Characteristics of included studies.
Searches of the grey literature did not identify any additional relevant studies.
Included studies
Design
The one included study (Aletti 2006) reported a retrospective analysis of 194 patients from the Mayo clinic in Rochester, Minnesota who had ovarian cancer and received either ultra-radical (n = 83 (43%)) or standard surgery (n = 111 (57%)). Only women with FIGO stage IIIC disease were included in the study, which implies that these women had abdominal tumour deposits of greater than 2 cm and positive retroperitoneal or inguinal lymph nodes. The study also reported the results of 144 of these women with carcinomatosis (n = 68 (47%) and n = 76 (53%) in the ultra-radical and standard surgery groups respectively). As the primary objective of the study was to assess the benefits of tumour cytoreduction, rather than the specific benefits of each type of surgery, baseline demographics were not reported by surgical group.
Participants
The mean and median age at diagnosis of advanced epithelial ovarian cancer for the entire study (n = 194) were 64.4 and 64 years respectively (range 24 to 87). The majority of patients had a serous (126/194; 65%) histological tumour cell type, with endometrioid, mixed and seroanaplastic amounting to most of the other tumour cell types (52/194, 27%). Most patients had a grade III tumour (180/194; 93%) and an ASA score of 2 or 3 (175/194; 90%). The mean and median volume of ascites were 2076 ml and 1,000 ml respectively (range: 0 to 12,000 ml).
Interventions
Initial surgery was performed for diagnosis, staging and surgical cytoreduction. Surgery was classified as ultra-radical if any diaphragmatic surgery, bowel resection, splenectomy, or extensive abdominal peritoneal stripping or resection were performed. Surgery was classified as standard if none of these procedures were performed, but hysterectomy, complete omentectomy, stripping of pelvic peritoneum, or limited resection of peritoneal-based nodules was carried out. Patients were first classified by the extent of peritoneal dissemination. Those with tumour nodules diffusely covering the majority of the bowel serosal surfaces and the parietal peritoneum of the abdomen and pelvis were classified as having carcinomatosis.
The centre’s division of gynaecologic surgery contained a mixed group of surgeons, some being more likely to carry out ultra-radical surgery but all sharing a uniform referral base with similar patient demographics, practicing at a single institution where each surgeon had access to identical services and nursing support. It was reported that: “Radical procedures were performed at the same rate regardless of age (49% for age < 65 years versus 51% for age > 65 years; P= 0.45). Patients with better ASA scores (1 or 2 versus 3 or 4) were more likely to have aggressive procedures performed (59% versus 36%, respectively; P= 0.005), which implies the overall medical condition of the patient at least partially influences the decision to perform aggressive surgery”. However, the numbers of women in each surgical group were not reported. For further details see the table Characteristics of included studies. The mean and median length of follow up were 3.5 years and 2.7 years respectively (range 0.02 to 10.5 years).
Outcomes reported
The study reported disease specific survival and perioperative mortality. Disease specific survival and peri-operative mortality, defined as death within 2 weeks following surgery, are important factors used to assess the risks of a particular surgical intervention and the level of surgical expertise. In this review, we have used ‘death within 30 days’ as a secondary outcome measure because this cut-off has been widely used in the literature and would include patients who die of complications directly related to surgery that may only manifest 1 to 2 weeks after surgery. No other side effects were reported. Progression-free survival, overall survival and QoL were not reported. The paper used the term ‘disease specific overall survival’ to include death from disease or surgical treatment. Although not reported in the original paper, the authors provided estimates of the HR from a multivariable Cox model, comparing death from advanced ovarian cancer in the ultra-radical surgery arm with that in the standard surgery arm for all 194 women and for the 144 women with carcinomatosis. The model for all 194 women was adjusted for: age (continuous), age (> 65 years versus ≤ 65 years), ASA score (3 or 4 versus 1 or 2), carcinomatosis (Yes, No), mesenteric involvement (Yes, No), diaphragmatic involvement (Yes, No), ascites (> 1000 ml versus ≤1000 ml), residual disease (<1 cm versus 0 cm, 1 to 2 cm versus 0 cm, > 2 cm versus 0 cm) and operative time (> 150 min versus ≤150 minutes); the model for 144 women with carcinomatosis was adjusted for: age (continuous), ASA score (3 or 4 versus 1 or 2), histologic grade (3 versus 1 or 2), residual disease (<1 cm versus 0 cm, 1 to 2 cm versus 0 cm, >2 cm versus 0 cm) and operative time (> 150 min versus ≤ 150 min).
Excluded studies
Twenty-one references were excluded after obtaining the full text for the following reasons:
In six studies (Aletti 2009a; Aletti 2009b; Bertelsen 1990; Eisenkop 2001; Eisenkop 2003; Wimberger 2007), a comparison of ultra-radical and standard surgery was not possible.
Patients in the comparison (standard surgery) group also had extensive bowel surgery (which is classified as ultra-radical) in seven studies (Aletti 2006b; Chi 2004; Eisenhauer 2006; Eisenkop 1993; Eisenkop 1998; Kommoss 2010; Kuhn 1998), diaphragmatic stripping in two studies (Tsolakidis 2010a; Tsolakidis 2010b) and extensive upper abdominal surgery in the Chi 2009 study.
In three studies (Aletti 2006a; Cai 2007; Eisenkop 2006), the intervention was a specific form of ultra-radical surgery but it was unclear as to whether those in the comparison group received a different form of ultra-radical surgery or standard surgery.
In the Bristow 1999 study, patients with recurrent disease were included, whereas in the von Hugo 1989 study it was unclear whether women with recurrent disease were included.
For further details of all excluded studies see the table Characteristics of excluded studies.
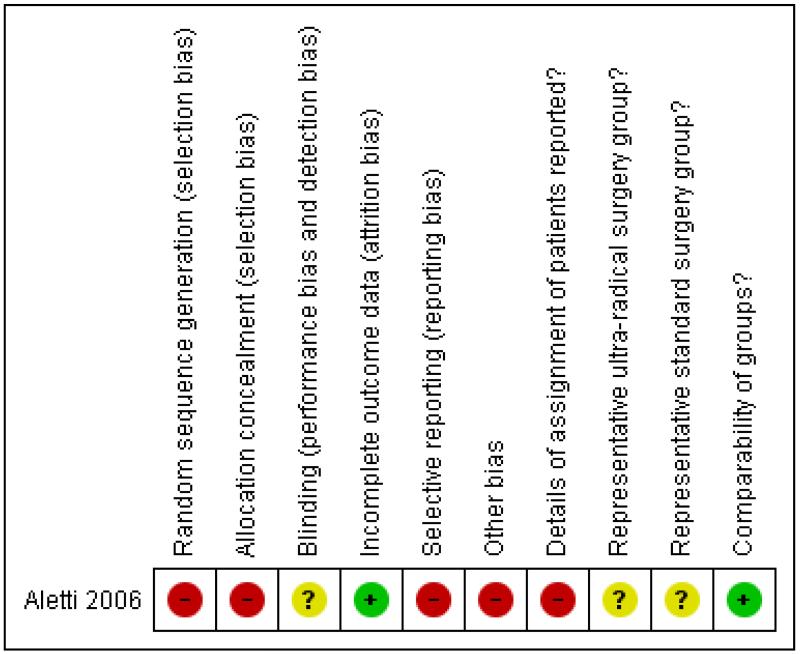
Risk of bias in included studies
The one included study (Aletti 2006) was at high risk of bias. It satisfied only four of the ten criteria that we used to assess risk of bias (see Figure 1). It reported a retrospective analysis so the method of sequence generation and concealment of allocation (which are relevant only to RCTs) were deemed to be unsatisfactory, and ret-rospective group allocation was not based on an intention to treat basis. It did not report details of assignment of patients to groups. It was unclear whether or not the two treatment groups were representative of women with advanced epithelial ovarian cancer as they only included women with stage IIIC disease. We deemed loss to follow up to be satisfactory as all women were analysed. The study did not report whether the outcome assessors were blinded. A multivariate analysis was performed, adjusting for important prognostic factors that were significant predictors of survival in univariate analyses, so the two groups were deemed to be comparable. The authors reported disease specific survival rather than overall survival which is a more appropriate and reliable outcome measure and did not report any QoL data or state if there were any pre-defined outcome measures prior to data analysis, so it is possible that the outcomes may have been selectively reported. There was an additional source of potential bias as the multivariate analysis adjusted for variables that were measured after the time origin in some of the analyses, namely extent of residual disease and operative time (Altman 1995).
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Effects of interventions
Disease specific survival
Overall, optimal cytoreduction (< 1 cm) was achieved in 67% of the 194 women enrolled in the study. Women who had ultra-radical surgery had better disease specific survival than those who underwent standard surgery, although the difference between the groups was not statistically significant. After adjustment for age, ASA score, carcinomatosis, mesenteric involvement, diaphragmatic involvement, ascites, residual disease and operative time, the hazard ratio (HR) comparing the risk of death from advanced epithelial ovarian cancer in women receiving ultra-radical surgery with the risk in women receiving standard surgery was 0.64 95% CI 0.40 to 1.04. The unadjusted estimate was similar in magnitude and also not statistically significant (HR = 0.72, 95% CI 0.51 to 1.02), which suggests that the two groups were well balanced at baseline.
Among the 144 women with carcinomatosis, univariate analysis using the log-rank test indicated that the only significant predictors of disease specific survival were the type of surgery, age (continuous), ASA score, residual disease and operative time. Multivariate analysis using Cox regression found that women who underwent ultra-radical surgery had significantly less chance of death from ovarian cancer than women who underwent standard surgery, after adjustment for grade and for all factors that were significant in univariate analysis (HR = 0.64, 95% CI 0.41 to 0.98). The unadjusted estimate was more extreme and, likewise, statistically significant (HR = 0.43, 95% CI 0.29 to 0.62).
Because of the potential effect of performance status on disease specific overall survival, the authors did a further subgroup analysis on 72 women with carcinomatosis, who had ASA grades of 1 and 2, and found a better outcome in those undergoing ultra-radical surgery: five-year disease specific overall survival was 46% and 13% (P < 0.001) in women undergoing ultra-radical and standard surgery respectively.
Perioperative mortality
In the standard surgery group, three women died within 30 days of surgery and there were no reported cases of perioperative mortality in the ultra-radical surgery group.
DISCUSSION
Summary of main results
We found only one study (Aletti 2006) that met our inclusion criteria. It reported retrospective data for 194 women with stage IIIC disease who underwent either ultra-radical or standard surgery, and also analysed a subset of 144 of these women with carcinomatosis, that is the highest volume of disease. The criteria for as-signment of women to the two surgical procedures were not reported other than stating that some surgeons within the centre were more inclined to perform ultra-radical surgery than other surgeons within the same centre. The assignment of patients into the ultra-radical and standard surgery groups was made depending on the extent of the surgery performed. Three women who underwent standard surgery died within 30 days of surgery, while there were no perioperative deaths in women who underwent ultra-radical surgery. After adjustment for important prognostic factors, multivariate analysis of all 194 patients found that women who underwent ultra-radical surgery had similar disease specific survival to those who underwent standard surgery (HR = 0.64, 95% CI 0.40 to 1.04). As women with stage IIIC ovarian cancer represent a heterogenous group in terms of extent of disease. the authors performed a sub group analysis restricted to women with carcinomatosis. These women had a similar extent of disease, and the study found that the estimated benefit of ultra-radical surgery was similar but the results were statistically significant (HR = 0.64, 95% CI 0.41 to 0.98). This supports the hypothesis that ultra-radical surgery can negate the effect of aggressive tumour biology. A further subgroup analysis on 72 women with carcinomatosis who had ASA grades of 1 and 2 confirmed the better outcome in those undergoing ultra-radical than standard surgery, suggesting that the improved outcomes in the ultra-radical group were unlikely to be related to better overall health of these women.
Overall completeness and applicability of evidence
Overall, the quality of the evidence was low (GRADE Working Group) because the review found only one relevant non-randomised study. This severely limits the conclusions that can be drawn. The one included study did not address many of the objectives of the review. Other adverse events were not documented and QoL was not reported.
The one included study (Aletti 2006) reported disease specific overall survival, which includes deaths from ovarian cancer and deaths from surgical treatment. However, all cause mortality (overall survival) - our primary outcome of interest - was not reported. When trying to assess the effect of ultra-radical against standard surgery, the extent of residual disease is likely to be a consequence of the initial extent of disease (e.g. the pattern rather than the stage and bulk) as well as whether ultra-radical or standard surgery was performed (e.g. the degree of surgical radicality). Adjusting for extent of residual disease in the multivariate model is likely to dilute the estimate of the effect of the type of surgery. This mediating variable is on the causal pathway between type of surgery and outcome. Likewise, operative time is also a mediating variable. Prognostic factors that are known (or could in principle be known) before the operation is performed for example age, ASA score, carcinomatosis, mesenteric involvement, diaphragmatic involvement, ascites, are moderating variables and it is completely valid to adjust for them. Indeed, it is necessary to adjust for them because they are probably confounded with assignment to treatment group.
Another flaw in the study is the assumption that in women with extensive disease in whom complete cytoreduction would not be possible even with ultra-radical surgery (e.g. women with porta hepatis disease or extensive mesenteric involvement) would not have undergone extensive debulking surgery, which also contributes to the risk of bias in this study. Furthermore, surgeons are more likely to perform ultra-radical surgery if the woman is fitter (partially reflected in the ASA grade), and also if the surgeon has a greater level of expertise. For this reason the authors carried out another sub-group analysis on 72 women. The vast majority of women in this study (182/194; 94%) had ASA grades between 1 to 3. Women with advanced ovarian cancer are generally in poor health and have a relatively short life expectancy. A good QoL after treatment is therefore an important issue in this group of women, but unfortunately this review was unable to assess this important outcome as it was not reported.
Quality of the evidence
The one included study analysed a reasonable number of women (n = 194), but was at high risk of bias, largely because it was a retrospective study. Patient characteristics were not reported by surgical arm so it was not possible to assess whether the groups receiving different types of surgery were similar prior to surgery. However, univariate analysis showed which factors were important predictors of disease specific survival on their own and analysis of the type of surgery that adjusted for these prognostic factors gave similar results to the unadjusted results, suggesting that prognostic factors were probably balanced between surgical groups. However, it is possible that factors not significant in univariate analysis could influence the estimates of effect in the multivariate model. Furthermore, the dichotomy of some of the co-variates is also questionable and variables that were not considered in the analysis, such as co-morbidities and ethnicity, could also influence results.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were sifted and data extracted independently by at least two review authors. The review included non-randomised studies and was not restricted to RCTs which provide the strongest level of evidence available. We made every attempt to minimise bias in the review process. We anticipated that selection bias was likely to be a real problem due to the non-randomised assignment of patients to surgery as it was likely that treatment allocation depended on the clinical indication and the level of surgical expertise available. We attempted to minimise this bias by only including RCTs or quasi-RCTs or non-randomised studies of sufficient quality that adjusted for baseline differences between the groups receiving different types of surgery. Unfortunately we were only able to include one study of such quality that met the inclusion criteria.
A further threat to the validity of the review is likely to be the possibility of publication bias. Studies that did not find a statistically significant difference between treatments may not have been published. We were unable to assess this possibility as the analysis was restricted to a single study.
Agreements and disagreements with other studies or reviews
One of the excluded studies (Wimberger 2007) evaluated the impact of different prognostic factors for surgical outcome and evaluated the impact of surgical outcome on survival in women with advanced stage ovarian cancer. In this prospective study, 798 women with FIGO IIB-IV disease from 136 centres within Germany were operated on and then randomised to receive either cisplatin and paclitaxol or carboplatin and paclitaxol chemotherapy. Complete surgical data were obtained from 761 women and were analysed using multivariable logistic regression. Complete cytoreduction with no macroscopic residual tumour was achieved in 29.8% of women, with a significant improved overall survival compared to women with visible, including small remaining disease (P <0.0001). In women with FIGO stages IIIC and IV, complete cytoreduction was less likely in older women, those with a higher pre-operative tumour load, worse performance status, and peritoneal carcinomatosis. FIGO stage was not an independent factor for complete cytoreduction in this group of women. The authors identified a subgroup of 71 centres (type A) which demonstrated the capability of performing ultra-radical surgery having carried out pelvic and/or para-aortic lymphadenectomy and peritoneal stripping in at least one of the enrolled patients in the study. This group included 534 (69.8%) women. The remaining 65 centres were identified as type B centres and treated 227 women. A higher percentage of patients with worse performance status were treated in type A centres (53.9% versus 43.6%, P = 0.009). Type A centres more often achieved complete cytoreduction compared to type B centres (32.8% versus 22.9%, P = 0.007). Treatment in type A centres was associated with greater overall survival compared to treatment in type B centres (45.2 months versus 35 months, p = 0.045). Their results suggest an advantage for aggressive primary surgery and complete cytoreduction in women with more advanced disease when operated on in experienced centres. Although this study was excluded from the review because the comparative groups were by treatment centres that contained a mixed case load of ultra-radical and standard surgery, it does provide some evidence that aggressive primary cytoreductive surgery can negate the effects of aggressive tumour biology in advanced ovarian cancer, with a subsequent improvement in overall survival.
AUTHORS ’ CONCLUSIONS
Implications for practice
We found only low quality evidence comparing ultra-radical and standard surgery in women with advanced ovarian cancer and carcinomatosis. Although the evidence suggested that ultra-radical surgery may result in better survival, it was based on one study at high risk of bias. It was unclear whether there were any differences in progression-free survival, QoL and morbidity between the two groups. the cost-effectiveness of this intervention has not been investigated.
We are, therefore, unable to reach definite conclusions about the relative benefits and adverse effects of the two types of surgery.
Implications for research
To date, most studies of ultra-radical (extensive) surgery for advanced stage ovarian cancer have assessed residual disease as an outcome rather than survival. Other studies which have assessed the role of ultra-radical surgery have not compared it with standard surgery and have included women with recurrent disease, making this a heterogeneous group of women and hence limiting the inferences that can be made about the role of ultra-radical surgery. In order to determine the role of ultra-radical surgery in the management of advanced stage ovarian cancer, it should be compared with standard surgery in women undergoing primary cytoreductive surgery for extensive disease, ideally in a sufficiently powered randomised controlled trial. However, it is acknowledged that there may be some difficulties in designing such trials.
If randomised controlled trials are not feasible, high quality non-randomised studies should be designed. Such studies should include all patients treated in several centres in a specified time period; data collection should use agreed criteria for prognostic factors, including the experience of the treating surgeon; multivariable analysis should allow for baseline prognostic factors but not for variables (such as extent of residual disease or operating time) that were recorded after women were assigned to surgical groups.
PLAIN LANGUAGE SUMMARY.
Ultra-radical surgery for women with advanced ovarian cancer
Ovarian cancer is the commonest cause of death in women with a female cancer. Opinions differ about whether women with advanced ovarian cancer do better if they have ‘ultra-radical’ surgery which is much more extensive than standard surgery.
We systematically searched the scientific literature for reports of studies comparing ultra-radical and standard surgery for women with advanced ovarian cancer. We looked for randomised controlled trials, which are regarded as the best type of study, and for non-randomised studies that were analysed using methods that allow for differences between the groups of women receiving different types of surgery.
We found only one relevant non-randomised study. It analysed data for 194 women recruited at one centre. Analysis that allowed for the differences in the extent of disease of the women who received the two different types of surgery found better disease specific survival among women receiving ultra-radical surgery. The best estimate was that their risk of death from ovarian cancer was about one third lower than for women who had standard surgery, but it might actually have been anywhere between 60% lower and 4% higher. However, the extent of disease in these women varied a lot so the authors also analysed only the 144 women whose cancer had spread throughout their abdomen. Again, the best estimate was that their risk of death was about one third lower than for women who had standard surgery, but it might have been anywhere between 60% lower and only 2% lower. Although this result seems to suggest that ultra-radical surgery might be better than standard surgery, we need to be cautious as the study was not well designed nor analysed, so it may be over-estimating the real benefits of ultra-radical surgery.
The study did not report all deaths, which would have been a more reliable and more important outcome. Neither did it report any differences between the groups in the time before the cancer progressed. It did not report quality of life (QoL) which would be very important to women with this advanced cancer. The cost-effectiveness of this intervention was not investigated.
Therefore, we could not reach any definite conclusions about the relative benefits and adverse effects of the two types of surgery. Better designed, large studies are needed in order to compare ultra-radical and standard surgery for women with advanced ovarian cancer.
ACKNOWLEDGEMENTS
We thank Chris Williams for clinical and editorial advice, Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process. We would also like to thank Professor Cilby and Dr Aletti for providing us with the additional information we required.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG-506
APPENDIX
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Retrospective cohort study of consecutive patients identified from surgical records Surgery carried out at Mayo Clinic. | |
| Participants | Patients with FIGO stage IIIC ovarian cancer, where disease status was extracted from surgical exploration notes The mean and median age at study entry was 64.4 and 64 years respectively (range 24 to 87) All women presented with FIGO stage IIIC: 194/194 (100%) Tumor cell type: Serous 126 (64.9%), Mucinous: 4 (2.1%), Endometrioid: 18 (9.3%), Clear cell: 7 (3.6%), Mixed: 17 (8.8%), Seroanaplastic: 17 (8.8%), Mullerian origin: 2 (1%) Tumor grade: 1: 1 (0.5%), 2: 13 (6.7%), 3: 180 (92.8%) ASA score: 1: 7 (3.6%), 2: 87 (44.8%), 3: 88 (45.4%), 4: 7 (3.6%), Unknown: 5(2. 6%) Ascites: Mean: 2076 ml, Median 1,000 ml, (Range: 0 to 12,000 ml) Extent of disease: carcinomatosis: 144 (74.2%), Diaphragm involvement: 137 (70.6), mesentery: 138 (71.1), cul-de-sac: 163 (84), omentum 168: (86.6), Ascites 160: (82.5) Baseline details for 144 women with carcinomatosis were not reported |
|
| Interventions | Initial surgery was performed for diagnosis, staging, and surgical cytoreduction Intervention: Ultraradical surgery: If any diaphragmatic surgery, bowel resection, splenectomy, or extensive abdominal peritoneal stripping or resection Comparison: Standard surgery: Hysterectomy, complete omentectomy, stripping of pelvic peritoneum, or limited resection of peritoneal-based nodules |
|
| Outcomes | Disease specific overall survival Perioperative mortality |
|
| Notes | Mean and median length of follow up was 3.5 years and 2.7 years respectively (range, 0. 02 to 10.5 years) Patients were first classified by the extent of peritoneal dissemination. Those with tumour nodules diffusely covering the majority of the bowel serosa surfaces and the parietal peritoneum of the abdomen and pelvis were classified as having carcinomatosis In multivariate analysis, only residual disease and radical surgery were independent factors predicting patient survival (Table 4) “When examining the effect of radical surgery on all patients with carcinomatosis (n = 144), we observed an improved disease-specific overall survival rate (38% versus 9%; log-rank test, P= 0.001) favouring patients who underwent radical procedures versus nonradical procedures (Fig. 3)” “Radical procedures were performed at the same rate regardless of age (49% for age 65 years versus 51% for age 65 years; P .45). Patients with better ASA scores (1 or 2 versus 3 or 4) were more likely to have aggressive procedures performed (59% versus 36%, respectively; P .005), which implies the overall medical condition of the patient at least partially influences the decision to perform aggressive surgery” “The 5-year disease-specific overall survival rate was 46% compared with 13% for patients with radical and non-radical surgeries, respectively (log-rank test, P.001; Fig. 4A)” “The rate of optimal resection (residual disease 1 cm) was 84.5% compared with 51% on the basis of surgeon tendency to use radical procedures” “Our division of gynaecologic surgery shares a uniform referral base with similar patient demographics, and we practice at a single institution where each surgeon has access to identical services and nursing support” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective non-randomised study. |
| Allocation concealment (selection bias) | High risk | Retrospective cohort study of patients identified from surgical records |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 194/194 (100%) of all women eligible for analysis, and 144/144 (100%) for women with carcinomatosis |
| Selective reporting (reporting bias) | High risk | Disease specific survival is not a good outcome measure to use for several reasons, namely the coding of death certificates is notoriously error-prone. If someone dies because of the treatment they receive, this may not be counted as a death from OC. But it is just as important to the patient as a death from OC and the evaluation of the relative benefits of the treatments should include these deaths. The study authors would have had access to data for death from all causes |
| Other bias | High risk | There is a serious problem in the multivariate analysis. It adjusted for variables that were measured after the time origin, namely extent of residual disease and operative time (Altman 1995). |
| Details of assignment of patients reported? | High risk | Not reported. |
| Representative ultra-radical surgery group? | Unclear risk | All patients had stage IIIC disease so may not have been representative of all women with advanced stage disease (III and IV) |
| Representative standard surgery group? | Unclear risk | All patients had stage IIIC disease so may not have been representative of all women with advanced stage disease (III and IV) |
| Comparability of groups? | Low risk | “Significant variables from the univariate analysis were included in the multivariable model” Multivariate Cox model included type of surgery, residual disease, age, ASA score, carcinomatosis, bowel mesentery, diaphragm, ascites and operative time The unadjusted HR for overall survival was similar to the multivariate one, so it is likely that the two groups were well balanced at baseline, as most prognostic factors were statistically significant in univariate analyses Multivariate analysis of subset of 144 patients with carcinomatosis also used adjustment for significant variables in univariate analyses |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Aletti 2006a | Intervention was ultra-radical (removal of tumour from diaphragm), but unclear as to whether those in comparison group received different form of ultra-radical surgery |
| Aletti 2006b | Patients in comparison standard surgery group also had extensive bowel surgery which is ultra-radical. It was also unclear whether women with recurrent disease were included |
| Aletti 2009a | Comparison of ultra-radical versus standard surgery groups not possible - low complexity scores also included possible small bowel resection |
| Aletti 2009b | Comparison of ultra-radical versus standard surgery groups not possible - low complexity scores also included possible small bowel resection |
| Bertelsen 1990 | Comparison of ultra-radical versus standard surgery groups not possible. It was also unclear whether women with recurrent disease were included |
| Bristow 1999 | Patients with recurrent disease also included. |
| Cai 2007 | Comparisons were made between bowel resection versus no bowel resection regardless of the nature of surgery, so those in the no bowel resection group may have still received a form of ultra-radical surgery. It was also unclear whether women with recurrent disease were included |
| Chi 2004 | Patients in comparison standard surgery group also had extensive bowel surgery which is ultra-radical. It was also unclear whether women with recurrent disease were included |
| Chi 2009 | Comparison between standard surgery and ultra-radical surgery groups not possible as all women underwent extensive upper abdominal surgery |
| Eisenhauer 2006 | Patients in comparison standard surgery group also had extensive bowel surgery which is ultra-radical. Also unclear if women with recurrent disease included |
| Eisenkop 1993 | Patients in comparison standard surgery group also had extensive bowel surgery and/or diaphragmatic stripping which is ultra-radical. It was also unclear whether women with recurrent disease were included |
| Eisenkop 1998 | Patients in comparison standard surgery group also had extensive bowel surgery and/or diaphragmatic stripping which is ultra-radical. Patients with recurrent disease were also included |
| Eisenkop 2001 | Comparison of ultra-radical versus standard surgery groups not possible. |
| Eisenkop 2003 | Comparison of ultra-radical versus standard surgery groups not possible. Patients with recurrent disease were also included |
| Eisenkop 2006 | Comparisons were made between splenectomy versus no splenectomy regardless of the nature of surgery, so those in the no splenectomy group may have still received a form of ultra-radical surgery. Patients with recurrent disease were also included |
| Kommoss 2010 | Comparison between groups not possible as both standard surgery group also included patients undergoing bowel resection |
| Kuhn 1998 | Patients in comparison standard surgery group also had extensive bowel surgery and/or diaphragmatic stripping which is ultra-radical. Patients with recurrent disease were also included |
| Tsolakidis 2010a | Comparison between 'standard surgery' and 'ultra-radical surgery' groups not possible as all women underwent diaphragmatic stripping |
| Tsolakidis 2010b | Comparison between standard surgery and ultra-radical surgery groups not possible as all women underwent diaphragmatic stripping |
| von Hugo 1989 | Unclear if women with recurrent disease were included. |
| Wimberger 2007 | Comparison of ultra-radical versus standard surgery groups not possible, as the comparative groups include patients who had both types of surgery |
DATA AND ANALYSES
This review has no analyses.
Appendix 1. MEDLINE search strategy
Medline Ovid 1950 to November week 3, 2010
exp Ovarian Neoplasms/
(ovar* adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
1 or 2
exp Surgical Procedures, Operative/
surg*.mp.
surgery.fs.
4 or 5 or 6
debulk*.mp.
cytoreduc*.mp.
(ultraradical or ultra-radical or ultra radical).mp.
exp Omentum/
omentum.mp.
bowel.mp.
abdom*.mp.
exp Spleen/
spleen.mp.
exp Liver/
liver.mp.
exp Diaphragm/
diaphragm*.mp.
exp Lymph Nodes/
(lymph adj node*).mp.
exp Peritoneum/
peritone*.mp.
exp Urinary Tract/
(urinary adj tract).mp.
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
7 and 27
exp Splenectomy/
splenectomy.mp.
exp Hysterectomy/
(abdom* adj5 hysterectomy).mp.
abdominohysterectomy.mp.
exp Lymph Node Excision/
(lymph adj node adj excision).mp.
(bilateral adj salpingo adj oophorectomy).mp.
omentectomy.mp.
exp Surgical Stomas/
stoma.mp.
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39
28 or 40
3 and 41
“randomized controlled trial”.pt.
“controlled clinical trial”.pt.
randomized.ab.
randomly.ab.
trial.ab.
groups.ab.
exp Cohort Studies/
cohort*.mp.
(case adj series).mp.
43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51
42 and 52
Animals/
Humans/
54 not (54 and 55)
53 not 56
key: mp = title, original title, abstract, name of substance word, subject heading word
Appendix 2. EMBASE search strategy
Ovid Embase 1980 to 2010 week 47
exp Ovary Tumor/
(ovar* adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
1 or 2
exp Surgery/
surg*.mp.
su.fs.
4 or 5 or 6
debulk*.mp.
cytoreduc*.mp.
(ultraradical or ultra-radical or ultra radical).mp.
exp Omentum/
omentum.mp.
bowel.mp.
abdom*.mp.
exp Spleen/
spleen.mp.
exp Liver/
liver.mp.
exp Diaphragm/
diaphragm*.mp.
exp Lymph Node/
(lymph adj node).mp.
exp Peritoneum/
peritone*.mp.
exp Urinary Tract/
(urinary adj tract).mp.
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
7 and 27
exp Splenectomy/
splenectomy.mp.
exp Hysterectomy/
(abdom* adj5 hysterectomy).mp.
abdominohysterectomy.mp.
exp Lymphadenectomy/
(lymph adj node adj excision).mp.
(bilateral adj salpingo adj oophorectomy).mp.
omentectomy.mp.
exp Stoma/
stoma.mp.
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39
28 or 40
3 and 41
exp Controlled Clinical Trial/
randomized.ab.
randomly.ab.
trial.ab.
groups.ab.
exp Cohort Analysis/
cohort*.mp.
(case adj series).mp.
50 or 49 or 46 or 45 or 43 or 44 or 48 or 47
42 and 51
exp Animal/
Human/
53 not (53 and 54)
52 not 55
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
ab=abstract
fs=floating subheading
Appendix 3. CENTRAL search strategy
CENTRAL Issue 4, 2010
MeSH descriptor Ovarian Neoplasms explode all trees
ovar* near/5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)
(#1 OR #2)
MeSH descriptor Surgical Procedures, Operative explode all trees
surg*
Any MeSH descriptor with qualifier: SU
(#4 OR #5 OR #6)
debulk*
cytoreduc*
ultradical or ultra-radical or ultra radical
MeSH descriptor Omentum explode all trees
omentum
bowel
abdom*
MeSH descriptor Spleen explode all trees
spleen
MeSH descriptor Liver explode all trees
liver
MeSH descriptor Diaphragm explode all trees
diaphragm*
MeSH descriptor Lymph Nodes explode all trees
lymph next node*
MeSH descriptor Peritoneum explode all trese
peritone*
MeSH descriptor Urinary Tract explode all trees
urinary next tract
(#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR 23 OR #24 OR #25 OR #26)
(#7 AND #27)
MeSH descriptor Splenectomy explode all trees
splenectomy
MeSH descriptor Hysterectomy explode all trees
abdom* near/5 hysterectomy
abdominohysterectomy
MeSH descriptor Lymph Node Excision explode all trees
lymph next node next excision
bilateral next salpingo next oophorectomy
omentectomy
MeSH descriptor Surgical Stomas explode all trees
stoma
(#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39)
(#28 OR #40)
(#3 AND #41)
WHAT’S NEW
Last assessed as up-to-date: 3 March 2011.
| Date | Event | Description |
| 26 February 2014 | Amended | Contact details updated. |
HISTORY
Protocol first published: Issue 2, 2009
Review first published: Issue 4, 2011
| Date | Event | Description |
| 28 July 2011 | Amended | Author contact details updated |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We added the following study constraint in the Types of studies section, as it was apparent that selection bias would have been problematic.
In order to minimise selection bias, we decided to include only studies that used statistical adjustment for baseline case mix (for example age, performance status, grade, etc) using multivariate analyses.
We removed discussion of unadjusted results from the data synthesis, subgroup analysis and investigation of heterogeneity and sensitivity analysis sections as we do not plan to use unadjusted results in future updates due to the risk of selection bias.
Only one study met the inclusion criteria for the review and this did not report dichotomous or continuous outcomes and we were unable to use the methods specified for a meta analysis. Should more studies be identified for updates of the review, the following methods will be employed.
Data extraction and management
Data on outcomes will be extracted as below.
For time to event data (overall survival), we extracted the log of the hazard ratio (HR) and its standard error from trial reports; if these were not reported, we attempted to estimate the log (HR) and its standard error using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events or deaths, if it was not possible to use a hazard ratio), we will extract the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio.
For continuous outcomes (e.g. QoL measures), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow up, in order to estimate the mean difference between treatment arms and its standard error.
Measures of treatment effect
We will use the following measures of the effect of treatment.
For dichotomous outcomes, we will use the risk ratio.
For continuous outcomes, we will use the mean difference between treatment arms.
Dealing with missing data
We will not impute missing outcome data. For the primary outcome, if data are missing or only imputed outcome data are reported, we will contact trial authors to request data on the outcomes among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies will be assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, if possible, by sub group analysis (see below). If there is evidence of substantial heterogeneity, the possible reasons for this will be investigated and reported.
Assessment of reporting biases
Funnel plots corresponding to meta-analysis of the primary outcome will be examined to assess the potential for small study effects. When there is evidence of small-study effects, publication bias will be considered as only one of a number of possible explanations. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random-effects model, sensitivity analysis will be performed using fixed-effects models.
Data synthesis
If sufficient clinically similar studies are available, their results will be pooled in a meta-analysis. Adjusted summary statistics will be used.
For time-to-event data, hazard ratios will be pooled using the generic inverse variance facility of RevMan 5.
For any dichotomous outcomes, the relative risk will be calculated for each study and these will then be pooled.
For continuous outcomes, the mean differences between the treatment arms at the end of follow up will be pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences will be pooled.
Random effects models with inverse variance weighting will be used for all meta-analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Sub-group analysis will be performed, grouping the trials by:
women who received neoadjuvant chemotherapy (responders versus non-responders);
stage of disease (Stage III versus Stage IV, as progression-free survival and overall survival will differ).
Factors such as age, type of surgeon, length of follow up and adjusted/unadjusted analysis will be considered in interpretation of any heterogeneity.
Sensitivity analysis
Sensitivity analysis will be performed excluding studies at high risk of bias.
Footnotes
DECLARATIONS OF INTEREST
None.
INDEX TERMS
Medical Subject Headings (MeSH)
*Neoplasms, Glandular and Epithelial [pathology; surgery]; *Ovarian Neoplasms [pathology; surgery]; Neoplasm Invasiveness [pathology]; Neoplasm Staging
MeSH check words
Adult; Female; Humans
REFERENCES
* Indicates the major publication for the study
References to studies included in this review
- Aletti 2006.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstetrics and Gynecology. 2006;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [published data only] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aletti 2006a.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecologic Oncology. 2006;100(2):283–7. doi: 10.1016/j.ygyno.2005.08.027. [published data only] [DOI] [PubMed] [Google Scholar]
- Aletti 2006b.Aletti GD, Podratz KC, Jones MB, Cliby WA. Role of rectosigmoidectomy and stripping of pelvic peritoneum in outcomes of patients with advanced ovarian cancer. Journal of the American College of Surgeons. 2006;203(4):521–6. doi: 10.1016/j.jamcollsurg.2006.06.027. [published data only] [DOI] [PubMed] [Google Scholar]
- Aletti 2009a.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope RC, Wilson TO, et al. Quality Improvement in the Surgical Approach to Advanced Ovarian Cancer: The Mayo Clinic Experience. Journal of the American College of Surgeons. 2009;208:614–20. doi: 10.1016/j.jamcollsurg.2009.01.006. [published data only] [DOI] [PubMed] [Google Scholar]
- Aletti 2009b.Aletti GD, Podratz KC, Moriarity JP, Cliby WA, Long KH. Aggressive and complex surgery for advanced ovarian cancer: An economic analysis. Gynecologic Oncology. 2009;112:16–21. doi: 10.1016/j.ygyno.2008.10.008. [published data only] [DOI] [PubMed] [Google Scholar]
- Bertelsen 1990.Bertelsen K. Tumor reduction surgery and long-term survival in advanced ovarian cancer: a DACOVA study. Gynecologic Oncology. 1990;38(2):203–9. doi: 10.1016/0090-8258(90)90042-j. [published data only] [DOI] [PubMed] [Google Scholar]
- Bristow 1999.Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecologic Oncology. 1999;72(3):278–87. doi: 10.1006/gyno.1998.5145. [published data only] [DOI] [PubMed] [Google Scholar]
- Cai 2007.Cai HB, Zhou YF, Chen HZ, Hou HY. The role of bowel surgery with cytoreduction for epithelial ovarian cancer. Clinical Oncology (Royal College of Radiologists) 2007;19(10):757–62. doi: 10.1016/j.clon.2007.06.015. [published data only] [DOI] [PubMed] [Google Scholar]
- Chi 2004.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecologic Oncology. 2004;94(3):650–4. doi: 10.1016/j.ygyno.2004.01.029. [published data only] [DOI] [PubMed] [Google Scholar]
- Chi 2009.Chi DS, Zivanovic O, Kolev V, Huh J, Joseph D, Leitao MM, et al. Incidence of major surgical complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal and peritoneal carcinomas. Gynecologic Oncology. 2009;112(2):S2–3. doi: 10.1016/j.ygyno.2010.05.031. Suppl 1. [published data only] [DOI] [PubMed] [Google Scholar]
- Eisenhauer 2006.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecologic Oncology. 2006;103(3):1083–90. doi: 10.1016/j.ygyno.2006.06.028. [published data only] [DOI] [PubMed] [Google Scholar]
- Eisenkop 1993.Eisenkop SM, Nalick RH, Wang HJ, Teng NN. Peritoneal implant elimination during cytoreductive surgery for ovarian cancer: impact on survival. Gynecologic Oncology. 1993;51(2):224–9. doi: 10.1006/gyno.1993.1277. [published data only] [DOI] [PubMed] [Google Scholar]
- Eisenkop 1998.Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecologic Oncology. 1998;69(2):103–8. doi: 10.1006/gyno.1998.4955. [published data only] [DOI] [PubMed] [Google Scholar]
- Eisenkop 2001.Eisenkop SM, Spirtos NM. Procedures required to accomplish complete cytoreduction of ovarian cancer: is there a correlation with “biological aggressiveness” and survival? Gynecologic Oncology. 2001;82(3):435–41. doi: 10.1006/gyno.2001.6313. [published data only] [DOI] [PubMed] [Google Scholar]
- Eisenkop 2003.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecologic Oncology. 2003;90(2):390–6. doi: 10.1016/s0090-8258(03)00278-6. [published data only] [DOI] [PubMed] [Google Scholar]
- Eisenkop 2006.Eisenkop SM, Spirtos NM, Lin WC. Splenectomy in the context of primary cytoreductive operations for advanced epithelial ovarian cancer. Gynecologic Oncology. 2006;100(2):344–8. doi: 10.1016/j.ygyno.2005.08.036. [published data only] [DOI] [PubMed] [Google Scholar]
- Kommoss 2010.Kommoss S, Rochon J, Harter P, Heitz F, Grabowski JP, Ewald-Riegler N. Prognostic impact of additional extended surgical procedures in advanced-stage primary ovarian cancer. Annals of Surgical Oncology. 2010;17:279–86. doi: 10.1245/s10434-009-0787-8. [published data only] [DOI] [PubMed] [Google Scholar]
- Kuhn 1998.Kuhn W, Florack G, Roder J, Schmalfeldt B, Pache L, Rust M, et al. The influence of upper abdominal surgery on perioperative morbidity and mortality in patients with advanced ovarian cancer FIGO III and FIGO IV. International Journal of Gynecological Cancer. 1998;8(1):56–63. [published data only] [Google Scholar]
- Tsolakidis 2010a.Tsolakidis D, Amant F, Van Gorp T, Leunen K, Neven P, Vergote I. The role of diaphragmatic surgery during interval debulking after neoadjuvant chemotherapy. International Journal of Gynecological Cancer. 2010;20:542–51. doi: 10.1111/IGC.0b013e3181d4de23. [published data only] [DOI] [PubMed] [Google Scholar]
- Tsolakidis 2010b.Tsolakidis D, Amant F, Van Gorp T, Leunen K, Neven P, Vergote I. Diaphragmatic surgery during primary debulking in 89 patients with stage IIIB-IV epithelial ovarian cancer. Gynecologic Oncology. 2010;116:489–96. doi: 10.1016/j.ygyno.2009.07.014. [published data only] [DOI] [PubMed] [Google Scholar]
- von Hugo 1989.von Hugo R, Holscher M, Janicke F. [Morbidity, mortality and quality of life following radical surgical interventions in advanced ovarian cancer]. [German] Archives of Gynecology & Obstetrics. 1989;245(1-4):625–7. doi: 10.1007/BF02417463. [published data only] [DOI] [PubMed] [Google Scholar]
- Wimberger 2007.Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR) Gynecologic Oncology. 2007;106(1):69–74. doi: 10.1016/j.ygyno.2007.02.026. [published data only] [DOI] [PubMed] [Google Scholar]
Additional references
- Altman 1995.Altman DG, de Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. British Journal of Cancer. 1995;72:511–8. doi: 10.1038/bjc.1995.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow 2003.Bristow RE, del Carmen MG, Kaufman HS, Montz FJ. Radical oophrectomy with primary stapled colorectal anastomosis for resection of locally advanced epithelial ovarian cancer. Journal of the American College of Surgeons. 2003;197:565–74. doi: 10.1016/S1072-7515(03)00478-2. [DOI] [PubMed] [Google Scholar]
- Bristow 2002.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. Journal of Clinical Oncology. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- Chen 1985.Chen SS, Bochner R. Assessment of morbidity and mortality in primary cytoreductive surgery for advanced ovarian carcinoma. Gynecologic Oncology. 1985;20:190–95. doi: 10.1016/0090-8258(85)90141-6. [DOI] [PubMed] [Google Scholar]
- Covens 2000.Covens AL. A critique of surgical cytoreduction in advanced ovarian cancer. Gynecologic Oncology. 2000;78:269–74. doi: 10.1006/gyno.2000.5926. [DOI] [PubMed] [Google Scholar]
- Crawford 2005.Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. Journal of Clinical Oncology. 2005;23:8802–11. doi: 10.1200/JCO.2005.02.1287. [DOI] [PubMed] [Google Scholar]
- CTCAE 2006.CTCAE. Common Terminology Criteria for Adverse Events 2006 Aug 9th; ( http://ctep.cancer.gov/forms/CTCAEv3.pdf) Vol. v3.0 (CTCAE)
- Deeks 2001.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition BMJ Publication Group; London: 2001. [Google Scholar]
- DerSimonian 1986.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Eisenkop 2001a.Eisenkop SM, Spirtos NM. Procedures required to accomplish complete cytoreduction in ovarian cancer: is there a correlation with “biological aggressiveness” and survival? Gynecologic Oncology. 2001;82:435–41. doi: 10.1006/gyno.2001.6313. [DOI] [PubMed] [Google Scholar]
- Eisenkop 2001b.Eisenkop SM, Spirtos NM. What are the current surgical objectives, strategies, and technical capabilities of gynecologic oncologists treating advanced epithelial ovarian cancer? Gynecologic Oncology. 2001;82:489–97. doi: 10.1006/gyno.2001.6312. [DOI] [PubMed] [Google Scholar]
- EUROCARE 2003.Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, et al. EUROCARE Working Group. EUROCARE-3: survival of cancer patients diagnosed 1990-94 - results and commentary. Annals of Oncology. 2003;14(Suppl 5):v61–v118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- Ferlay 2004.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARCPress; Lyon: 2004. Vol. IARC CancerBase No. 5, version 2.0. [Google Scholar]
- GRADE Working Group.GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2003.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008.The Cochrane Collaboration Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] 2008 Available from www.cochrane-handbook.org.
- Hoskins 1992.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free survival in small-volume stage III epithelial ovarian cancer: A Gynecologic Oncology Group study. Gynecological Oncology. 1992;47:159–66. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- Jaeger 2001.Jaeger W, Ackermann, Kessler H, Katalinic A, Lang N. The effect of bowel resection on survival in advanced epithelial ovarian cancer. Gynecologic Oncology. 2001;83:286–91. doi: 10.1006/gyno.2001.6375. [DOI] [PubMed] [Google Scholar]
- Jemal 2008.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics. CA: A Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Johnson 2008.Johnson NP, Selman T, Zamora J, Khan KS. Gynaecologic surgery from uncertainty to science: evidence-based surgery is no passing fad. Human Reproduction. 2008;23(4):832–9. doi: 10.1093/humrep/dem423. [DOI] [PubMed] [Google Scholar]
- Kurman 2008.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. American Journal of Obstetrics and Gynecology. 2008;198(4):351–6. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet 2007.An experiment in earlier detection of ovarian cancer. Lancet. 2007;369(9579):2051. doi: 10.1016/S0140-6736(07)60952-0. Editorial. [DOI] [PubMed] [Google Scholar]
- Markman 2007.Markman M. Concept of optimal cytoreduction in advanced ovarian cancer: A brief critique and a call for action. Journal of Clinical Oncology. 2007;25(27):4168–70. doi: 10.1200/JCO.2007.11.8992. [DOI] [PubMed] [Google Scholar]
- Merideth 2003.Merideth MA, Cliby WA, Keeney GL, Lesnick TG, Nagorney DM, Podratz KC. Hepatic resection for metachronous metastases from ovarian cancer. Gynecologic Oncology. 2003;89:16–21. doi: 10.1016/s0090-8258(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Montz 1989.Montz FJ, Schlaerth JB, Berek JS. Resection of diaphragmatic peritoneum and muscle: role in cytoreductive surgery for ovarian cancer. Gynecologic Oncology. 1989;35:338–40. doi: 10.1016/0090-8258(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Ozols 2003.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- Parmar 1998.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pomel 2004.Pomel C, Dauplat J. Management of malignant epithelial tumours of the ovary. Journal de Chirurgie NLM. 2004;141:277–84. doi: 10.1016/s0021-7697(04)95334-3. [DOI] [PubMed] [Google Scholar]
- Potter 1991.Potter ME, Partridge EE, Hatch KD, Soong SJ, Austin JM, Shingleton HM. Primary surgical therapy of ovarian cancer: how much and when. Gynecological Oncology. 1991;40:195–200. doi: 10.1016/0090-8258(90)90277-r. [DOI] [PubMed] [Google Scholar]
- Shepherd 1989.Shepherd JH. Revised FIGO staging for gynaecological cancer. BJOG. 1989;96(8):889–92. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- Todo 2003.Todo Y, Sakuragi N, Oikawa M, Negishi H, Yamamoto R, Yoshiaki K, et al. Cytoreductive surgery combined with organ resection for advanced ovarian cancer. International Journal of Clinical Oncology. 2003;8:90–6. doi: 10.1007/s101470300016. [DOI] [PubMed] [Google Scholar]
- Van Dam 1996.Van Dam PA, Tjalma W, Weyler J. Ultraradical debulking of epithelial ovarian cancer with the ultrasonic surgical aspirator: a prospective randomised trial. Obstetrics and Gynecology. 1996;174:943–50. doi: 10.1016/s0002-9378(96)70331-9. [DOI] [PubMed] [Google Scholar]
- Venesmaa 1992.Venesmaa P, Ylikorkala O. Morbidity and mortality associated with primary and repeat operations for ovarian cancer. Obstetrics and Gynecology. 1992;79:168–72. [PubMed] [Google Scholar]
- Visintin 2008.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, Tenthorey J, et al. Diagnostic markers for early detection of ovarian cancer. Clinical Cancer Research. 2008;14:1065–72. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- *.Indicates the major publication for the study