Abstract
Although often overshadowed by the motor dysfunction associated with Parkinson’s disease (PD), autonomic dysfunction including urinary bladder and bowel dysfunctions are often associated with PD and may precede motoric changes; such autonomic dysfunction may permit early detection and intervention. Lower urinary tract symptoms are common in PD patients and result in significant morbidity. The current studies focus on non-motor symptoms in PD using a transgenic mouse model with overexpression of human α-synuclein, the peptide found in high concentrations in Lewy body neuronal inclusions, the histopathologic hallmark of PD. We examined changes in the physiological, molecular, chemical, and electrical properties of neuronal pathways controlling urinary bladder function in transgenic mice. The results of these studies reveal that autonomic dysfunction (i.e., urinary bladder) can precede motor dysfunction. In addition, mice with human α-synuclein overexpression in relevant neuronal populations is associated with alterations in expression of neurotransmitter/ neuromodulatory molecules (PACAP, VIP, substance P, neuronal NOS) within neuronal pathways regulating bladder function as well as with increased NGF expression in the urinary bladder. Changes in the electrical and synaptic properties of neurons in the major pelvic ganglia that provide postganglionic innervation to urogenital tissues were not changed as determined with intracellular recording. The urinary bladder dysfunction observed in transgenic mice likely reflects changes in peripheral (i.e., afferent) and/or central micturition pathways or changes in the urinary bladder. SYN-OE mice provide an opportunity to examine early events underlying the molecular and cellular plasticity of autonomic nervous system pathways underlying synucleinopathies.
Keywords: quantitative PCR, conscious cystometry, intracellular recording, neurochemistry, hyperreflexia
INTRODUCTION
~We are pleased to contribute an article as part of this volume honoring Dr. Ira Black. Dr. Black was an inspiration to many young investigators as well as established neuroscientists with common interests in autonomic neurobiology, neural injury and plasticity. Of particular importance, Dr. Black was a mentor and longstanding colleague and friend of Dr. Robert Hamill. As mentor, Dr. Black was instrumental in guiding Bob during his development as a neuroscientist.~
“Synucleinopathies” are neurodegenerative disorders characterized by changes in α-synuclein expression that are correlated with clinical dysfunction. For example, studies of dominantly inherited Parkinson disease (PD) led to the discovery that missense (A53T, A30P, E46k) mutations in the synuclein gene caused the disorder (Polymeropoulos et al., 1997; Kruger et al., 1998; Zarranz et al., 2004). Subsequent neuropathological studies confirmed that α-synuclein is the major constituent of the ‘Lewy body’ (LB) aggregates that characterize the neuropathology of sporadic PD, the second most common neurodegenerative disease in humans (Spillantini et al., 1997). Further investigations revealed that cytoplasmic and nuclear accumulations of α-synuclein occurred in neurons and glia in a range of disorders: dementia with Lewy bodies, diffuse Lewy body disease, multiple system atrophy, neurodegeneration with brain iron accumulation type 1 (NBIA 1) [formally known as Hallevorden Spatz Disease] and amyotrophic lateral sclerosis (Kruger et al., 2000). Thus, evolving data suggest that abnormalities in α-synuclein may play a broad pathogenic role in a number of neurodegenerative disorders and the term ‘Synucleinopathies’ was coined (Spillantini and Goedert, 2000; Galvin et al., 2001).
More recent studies of the clinical and neuropathological features of PD suggest that LB pathology including Lewy neurites (LN), appear in the peripheral autonomic nervous system (ANS) and may occur early in the disease process (Braak et al., 2003; Braak et al., 2004; Braak and Del Tredici, 2008). Importantly, Lewy himself observed LB pathology in the sympathetic nervous system in his original studies of PD (Lewy, 1913) and the more recent extensive studies by Braak and colleagues have revealed involvement of brainstem, spinal, and peripheral autonomic neuronal populations (Braak et al., 2003; Braak et al., 2004; Braak and Del Tredici, 2008). Furthermore, in many patients the ANS may be involved quite early in the disease process. A proposed staging system indicated that olfactory and autonomic neuronal populations are involved at the earliest stage (Stage I) whereas substantia nigra involvement emerges along with motor signs and symptoms at Stage III. These carefully performed human studies suggest that trans-synaptic mechanisms may underlie the systemic degenerations that occur in these disorders (i.e., the disease may start in ‘peripheral’ neuronal systems and progress centripetally and then caudal to rostral within the central nervous system (Braak et al., 2003; Braak et al., 2004; Braak and Del Tredici, 2008). Complementing these pathological studies are clinical observations indicating that symptoms related to disordered ANS function may precede the appearance of motor symptoms by many years (Simuni and Sethi, 2008; Tolosa and Poewe, 2009).
The development of synuclein transgenic (Tg) mice has permitted major advances in understanding the molecular genetics and pathology of the synucleinopathies (Masliah et al., 2000; Giasson et al., 2002; Richfield et al., 2002). Although the neuropathology of these animal models does not fully mimic the human condition, Tg mice have been instrumental in revealing potential mechanisms of disease pathogenesis (Moore and Dawson, 2008). Tg animals also offer the opportunity to examine the emergence of clinical dysfunction in a longitudinal fashion. Previous studies have focused on the motor system (Masliah et al., 2000; Giasson et al., 2002; Richfield et al., 2002) but as noted above, non-motor symptoms in human synucleinopathies may precede the evolution of motor dysfunction.
The A53T human α-synuclein mouse was chosen for the present study because it is a well-characterized, murine synucleinopathy model that exhibits major motor dysfunctions (Giasson et al., 2002). With this model, we investigated whether dysfunction within the peripheral ANS occurred before the onset of central motor abnormalities. The current multidisciplinary study examined the changes in the physiological, molecular, chemical, and electrical properties of neuronal pathways controlling urinary bladder function in the A53T mouse. The results of these studies reveal that autonomic dysfunction can precede motor dysfunction. In addition, mice with human α-synuclein overexpression (SYN-OE) in relevant neuronal populations is associated with alterations in expression of neurotransmitter/neuromodulatory molecules within neuronal pathways regulating bladder function as well as with increased neurotrophic factor (e.g., nerve growth factor, NGF) expression in the urinary bladder. Thus, SYN-OE mice provide an opportunity to examine the response of autonomic neurons as they change from functional to dysfunctional and provide new insights into the earliest events underlying the molecular and cellular plasticity underlying synucleinopathies.
METHODS
Animals
Mice hemizygous for human α-synuclein (α-SYN) were obtained from The Jackson Laboratory (Bar Harbor, ME). The Tg mice were generated on a C57BL/C3H background and expressed endogenous α-SYN and human A53T mutant α-SYN driven by a mouse prion protein promoter (Giasson et al., 2002). According to the manufacturer, a human α–synuclein cDNA sequence encoding a mutated protein with an Ala53Thr mutation and the mouse prion protein gene promoter, together with its 5′ and 3′ untranslated regions were used as the transgene to create α-SYN Tg mice on a C57BL/C3H background. The influence of different promoters on the expression pattern of mutated human α–synuclein in transgenic mice has been evaluated for Tg mice under the control of three different promoters: the chicken β-actin, the mouse tyrosine hydroxylase 9.6 kb and the mouse prion protein (Maskri et al., 2004). Although, all three promoters were found to successfully drive the expression of the transgene, the mouse prion protein promoter resulted in the highest level of transgene expression in the brain and specific neuron types (Maskri et al., 2004). Differences were noted in the transgene expression pattern in peripheral organs as well as the number and distribution of expressing cells in the brain (Maskri et al., 2004). Mice used in this study were bred locally at The University of Vermont College of Medicine using a hemizygous backcross breeding scheme. PCR genotyping of tail snips identified human α-SYN overexpressing (SYN-OE) and littermate wildtype (WT) mice. Mice were housed (12-hr light/dark cycle) in groups (5) in the UVM animal vivarium with water and food provided ad libitum. The litters were of normal size and weight and feeding and drinking behaviors appeared normal. All experimental protocols involving animal use were approved by the UVM Institutional Animal Care and Use Committee (IACUC #08-085). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress.
Open Voiding Cystometry in Conscious, Unrestrained Mice
Open voiding cystometry in conscious, unrestrained mice was conducted as previously described (Studeny et al., 2008; Schnegelsberg et al., 2009; May and Vizzard, 2010). Bladder function was characterized monthly in littermate WT and SYN-OE Tg mice of both genders beginning at 1 month of age through 16 months of age (n = 5-8 at each age for both WT and SYN-OE mice) using conscious cystometry with open outlet, continuous intravesical instillation of saline. The bladder was exposed though a lower midline abdominal incision under general anesthesia (isoflurane 2.5-3.5%). A saline filled PE-10 cannula with the end flared by heat was inserted into the dome of the bladder and secured with a 6-0 nylon purse string suture. The distal end of the cannula was sealed, tunneled subcutaneously to the back and exteriorized. Muscle and skin layers were closed separately using absorbable and nonabsorbable sutures, respectively. The exteriorized part of the cannula was placed in the subcutaneous space and the mice were returned to normal caging for 72 hr to ensure complete recovery. Postoperative analgesics were given for a period of 48 hr. Mice were placed conscious and unrestrained in recording cages with a balance and pan for urine collection and measurement placed below. Intravesical pressure changes were recorded using a Small Animal Cystometry System (Med Associates, Inc., St Albans, VT). Room temperature saline was infused at a rate of 25 μl/min to elicit repetitive bladder contractions. At least four reproducible micturition cycles were recorded after an initial stabilization period of 25 to 30 min. Voided saline was collected to determine void volume. After each void volume was collected the infusion was stopped and residual volume was determined by withdrawing the residual saline through the intravesical catheter. Inter-contraction interval, maximal voiding pressure, pressure threshold for voiding and baseline resting pressure were measured (Maggi et al., 1986). The number of non-voiding bladder contractions (NVC) per voiding cycle, maximal NVC pressure and frequency of NVC were assessed. For these studies, NVC were defined as rhythmic intravesical pressure rises (greater than 5 cm H2O from baseline pressure) without a release of fluid from the urethra. Mice were excluded from the study when adverse events occurred such as ≥ 20% reduction in body weight post-surgery, a significant post-operative event, lethargy, pain or distress not relieved by our IACUC-approved regimen of postoperative analgesics. In the present study, no mice were excluded from the study or from analysis due to post-surgical complications. In addition, behavioral movements such as grooming, standing, walking and defecation rendered bladder pressure recordings during these events unusable (Streng et al., 2006). Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements (Dorr, 1992). Mice were euthanized at the conclusion of study by isoflurane (4%) and thoracotomy.
Urination Patterns
In addition to characterizing voiding function in SYN-OE mice using open voiding cystometry in conscious, unrestrained mice (Schnegelsberg et al., 2009), we have also determined urination patterns on filter paper in SYN-OE mice, which does not necessitate the need for an intravesical catheter (May and Vizzard, 2010). Male and female WT and SYNOE littermate (n = 10 for each; 5-6 months of age) mice were placed individually in standard cages for 1 hour (h) (6, 56) in the morning (9-11 am) with the bedding replaced with Whatman Grade 3 filter paper. Food and water were provided ad libitum. Mice were habituated to cages for 1 h in the morning (9-11 am) on each of two consecutive days prior to data accumulation. Urine spots were photographed under UV light (Studeny et al., 2008), area (cm2) of spots determined and large (0.2 – 10 cm2) (Birder et al., 2002) and small (< 0.2 cm2) (Birder et al., 2002) spots counted. A standard curve of urine volume versus urine spot area was created to estimate void volumes (20 points, r2= 0.997). Mice were placed into cages and data analyzed by an individual blinded to mouse strain; groups were decoded after data analysis.
Motor Behavioral Tests
RotaRod
The effect of SYN overexpression on motor function was tested with a RotaRod (Dunham and Miya, 1957) using two fixed speed rotations (16, 32 rotations per minute (rpm). WT or SYN-OE mice of both genders (n = 12-15; evaluated monthly from 1 month of age through 17 months of age) were placed on the RotaRod rotating at 16 rpm for a maximum of 200 sec or until they fell off (latency to fall). Regardless of completion or fall, each animal was allowed to rest for 2 min between each trial. Each testing session consisted of 10 trials. Animals were tested at 16 rpm and 32 rpm on consecutive days.
Hindlimb extension
WT or SYN-OE mice of both genders (n = 8-10; evaluated every two months from 2 months of age through 12 months of age) were suspended by the tail and the extent of hindlimb extension was observed and scored as previously described (Jaworski et al., 2006). A score of 0 corresponds to the absence of hindlimb extension. A score of 1 corresponds to an extension reflex of only one hindlimb or extension of both hindlimbs without splayed toes. A score of 2 corresponds to a normal extension reflex in both hindlimbs, including splaying of toes. Only hindlimbs extended > 90 ° were scored.
Gait analysis
An analysis of gait was assessed based on measurements made from walking tracks (de Medinaceli et al., 1982; Jaworski et al., 2006). WT or SYN-OE mice of both genders (n = 10-12; evaluated at 4, 6 and 12 months of age) were manually restrained and their hindpaws dipped in non-toxic ink. Animals were then allowed to walk on white paper within a restricted area (70 × 400 mm). Stride length (mean distance between consecutive left or right paw prints), gait base of support (mean distance perpendicular to parallel left and right paw prints), and intrastep distance (mean distance between alternate left and right paw prints) were measured.
Measurement of Urinary Bladder NGF Content by ELISAs
Elevated levels of neurotrophins (i.e., nerve growth factor, NGF) have been detected in the urine of women with interstitial cystitis (IC)/bladder pain syndrome (BPS) (Okragly et al., 1999) and in the urothelium of individuals with IC/BPS or other painful bladder conditions (Lowe et al., 1997). It has also been recently demonstrated that urinary NGF levels are increased in patients with overactive bladder symptoms associated with detrusor overactivity, stress urinary incontinence, or bladder outlet obstruction (Okragly et al., 1999; Liu and Kuo, 2007; Liu et al., 2008a; Liu et al., 2008b; Liu and Kuo, 2008a; Liu and Kuo, 2008b; Yokoyama et al., 2008). Bladder function in patients with synucleinopathies exhibit hyperactive bladder function with detrusor-sphincter-dyssnergia and urgency and frequent urination, including increasing nocturia, and urinary retention; however, patients also experience urinary incontinence from failed internal urethral sphincter control (Fowler et al., 2010; Ragab and Mohammed, 2011).
We aimed to determine if NGF bladder content was altered in littermate WT or SYN-OE mice using enzyme-linked immunoassays (ELISAs) as previously described (Vizzard, 2000b; Cheppudira et al., 2008; Schnegelsberg et al., 2009). Whole urinary bladders (n = 5-7 each for WT and SYN-OE mice; 5-7 months of age) were homogenized separately in tissue protein extraction agent (T-PER; Roche, Indianapolis, IN), a commercially available, mild zwitterionic dialyzable detergent in 25 mM bicine, 150 mM sodium chloride (pH 7.6) containing a protease inhibitor mix (Sigma-Aldrich, St. Louis, MO; 16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor and 2 μg/ml pepstatin A) and aliquots were removed for protein assay as previously described (Klinger and Vizzard, 2008). The supernatants were used for quantification as previously described (Vizzard, 2000b). Total protein was determined by the Coomassie Plus (Bradford) Protein Assay Kit (Fisher Scientific, Pittsburgh, PA). According to the manufacturer, the NGF E-max immunoassay system (Promega Corporation, Madison, WI) demonstrates very low cross-reactivity with structurally related growth factors at concentrations up to 10 - 100 ng/ml. The standards provided with these systems generated linear standard curves (r2 = 0.996 – 0.998, P ≤ 0.001). Absorbance values of standards and samples were corrected by subtraction of the background value (absorbance due to nonspecific binding). No samples fell below the detection limits of the assays and samples were not diluted prior to assay. Curve fitting of standards and evaluation of NGF content of samples was performed using a least squares fit (Vizzard, 2000b; Schnegelsberg et al., 2009).
Euthanasia and Tissue Harvest
Wildtype (WT) and SYN-OE littermate (n = 3 - 7 for each; 4-6 months of age) mice were deeply anesthetized with isoflurane (3–4%) and then euthanized via thoracotomy. The urinary bladder and lumbosacral DRG were quickly dissected under RNase-free conditions. The bladder was cut open along the midline and pinned to a sylgard-coated dish and the urothelium was removed with the aid of fine forceps and a dissecting microscope and all tissues were snap-frozen on dry ice prior to processing as previously described (Arms et al., 2010). The urothelium has suburothelial structures associated with it; the term urothelium in this paper refers to both urothelial and suburothelial structures. DRG were identified and isolated as previously described (Vizzard, 1997; Vizzard, 2000a; Vizzard, 2000c). Lumbosacral DRG (L6, S1) and spinal cord were specifically chosen for real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR) analysis based upon the previously determined segmental representation of urinary bladder circuitry (Donovan et al., 1983; Keast and de Groat, 1992; Nadelhaft and Vera, 1995). Bladder afferents are not distributed within the L4–L5 DRG (Donovan et al., 1983; Keast and de Groat, 1992) that contain only somatic afferents nor are neurons that are involved in urinary bladder function observed in the L4–L5 spinal segments (Nadelhaft and Vera, 1995). The urinary bladder and reproductive organs receive direct innervation from neurons that reside within the pelvis. In rodents, these neurons are clustered into major pelvic ganglia (MPG) that are located on the pelvic floor, near the base of the bladder. The MPG and superior cervical ganglia (SCG) were identified and isolated. In order to determine whether changes in expression of the neuropeptide and nNOS transcripts were restricted to pelvic ganglia, we also chose to also examine the SCG. Additionally, cardiac noradrenergic and pupillary deafferentation in PD suggests involvement of SCG and stellate ganglion (Tipre and Goldstein, 2005; Goldstein et al., 2011). We chose to examine the SCG as part of these initial studies.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (Q-PCR)
We sought to determine if components of the micturition reflex pathways in SYNOE mice exhibited changes in transcript expression of neuroactive compounds including neuropeptides (e.g., pituitary adenylate cyclase activating polypeptide (PACAP), vasoactive intestinal polypeptide (VIP), substance P (sub P) and neuronal nitric oxide synthase (nNOS)) previously shown to be altered with urinary bladder dysfunction (Lecci et al., 1994; Zvara et al., 2004; Braas et al., 2006; Girard et al., 2008; Studeny et al., 2008; May and Vizzard, 2010; Tompkins et al., 2010). In addition, we determined expression of human α-SYN and endogenous mouse α-SYN in tissues from micturition pathways, superior cervical ganglia (SCG) and brain from WT and SYN-OE mice. Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test‘B’, Friendswood, TX, USA) as previously described (Girard et al., 2002; Klinger et al., 2008). One microgram (1 μg) of RNA per sample was used to synthesize complementary DNA using SuperScript II reverse transcriptase and a mix of random hexamer and oligo dT primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA, USA) in a 25-μl final reaction volume.
The quantitative PCR standards for all transcripts were prepared with the amplified human α-SYN, mouse α-SYN, PACAP, VIP, Sub P, nNOS, L32 and 18S cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Real-time quantitative PCR was performed using SYBR Green I detection (Girard et al., 2002; Klinger et al., 2008; Arms et al., 2010). Complementary DNA templates, diluted 5-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using SYBR Green I JumpStartTM. Taq ReadyMixTM (Sigma, St. Louis, MO, USA) containing 5 mM MgCl2, 200 mM dATP, dGTP, dCTP and dTTP, 0.64 U Taq DNA polymerase and 300 nM of each primer in a final 25-μl reaction volume. The real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) (Girard et al., 2002; Klinger et al., 2008; Arms et al., 2010) using the following standard conditions: (i) 94 °C for 2 min followed by serial heating at 94 °C for 15 s and 60 to 64 °C depending on the primer sets for 40 s; (ii) amplification over 40 cycles.
The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences for PACAP, VIP, sub P (Girard et al., 2002), L32 and 18S (Girard et al., 2002; Klinger et al., 2008) used in these studies have been previously described. We also used the following primer sequences: Human α-SYN (hSNCA) upper 5′ TGTGGCTGCTGCTGAGAAAA3′; hSNCA lower 5′ TCCTTCTTCATTCTTGCCCAACT 3′; nNOS upper 5′TACCACGAGGACATCTTTG 3′; nNOS lower 5′ CTGAAAACCTCATCTGTGTCT 3′. To identify mouse α-SYN (mSNCA) mRNA we used the hSNCA upper primer in combination with mSNCA lower 5′CCCCTCCTCACCCTTGCCCCACT 3′. For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using the Sequence Detection Software version 1.3.1 (Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the reference gene. WT samples were set equal to 100%; SYN-OE samples were expressed relative to WT samples.
Electrophysiological recording
The urinary bladder receives direct innervation from neurons that reside within the pelvis (Keast et al., 1989). The MPG are comprised of heterogeneous populations of neurons, not only with regard to their target tissues, but also because MPG contain both parasympathetic and sympathetic postganglionic neurons (Keast et al., 1989). As action potentials originating in the cell body and traveling down the axon are the principal activators of neurotransmitter release from nerve terminals in the target tissue, the electrical properties of MPG neurons have a critical influence on the function of these reflex pathways. The purpose of this investigation was to undertake an initial examination of the active and passive electrical properties of MPG neurons to establish if differences exist between littermate WT and SYN-OE mice in light of urinary bladder dysfunction. Current research indicates that α-synuclein expression affects neurotransmitter release, synaptic function and membrane conductance in various cell lines and tissues (Feng et al., 2010; Nemani et al., 2010; Lim et al., 2011).
MPG, isolated from male littermate (WT) and SYN-OE mice (n = 6-7 for both WT and SYN-OE mice; 5-7 months of age), were pinned out on Sylgard coated (Dow Corning, Midland MI) petri dishes and superfused (2-3 ml/min) with a HEPES-buffered solution containing in mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 HNaHCO3, 1.2 NaH2PO4, 8 glucose and 10 HEPES, pH = 7.4 and temperature 30-32 °C.
Neurons were visualized with an inverted microscope equipped with Hoffman optics and impaled using high impedance borosilicate microelectrodes (2 M KCl-filled; 80-120 MΩ). Membrane potential, input resistance and action potentials were recorded from the impaled neurons using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 (Molecular Devises; Sunnyvale, CA). Input resistance was determined from the change in membrane potential produced by long hyperpolarizing current pulses. The amplitude and duration of the afterhyperpolarization (AHP) following an action potential was determined by eliciting single action potentials with 5 msec suprathreshold depolarizing current pulses. AHP duration was measured at 2/3rds the peak AHP amplitude.
Neuronal excitability was determined as the number of action potentials generated during 1 second depolarizing current steps of increasing magnitude (0.1-0.4 nA). If the number of action potentials generated during all depolarizing steps was 4 or less, then the cell was classified as “phasic”. However, if the number of action potentials elicited during a step was 5 or greater, then the action potential firing pattern was considered “multiple firing”. Multiple firing neurons included those exhibiting a burst of action potentials at the beginning of the depolarization or those exhibiting tonic firing throughout the depolarization. If required, hyperpolarizing current was injected through the recording electrode to electrotonically maintain the resting membrane potential between −55 and −65 mV. This ensured that action potential generation was tested at the same potential in all cells.
Nerve evoked potentials were elicited by brief (2 ms) focal stimulation of a pelvic nerve branch using concentric bipolar electrodes (FHC, Inc., Bowdoin, ME). Constant current stimuli (10-300 μA) were generated with a Grass S88X (Grass Technologies of Astro-Med, Inc., West Warwick, RI) stimulator coupled with a PSIU6X photoelectric stimulus isolation unit (Grass Technologies of Astro-Med, Inc.).
Statistical Analyses
Data are presented as mean ± S.E.M. Variance between subjects within a litter was determined prior to pooling data from multiple litters. One-way analysis of variance was used to evaluate differences among groups for Q-PCR, motor tests, ELISAs and bladder function parameters. Gender and age were tested as blocks in the ANOVA. When F ratios exceeded the critical value (p ≤ 0.05), the Newman-Keul’s post-hoc test was used to compare the experimental means. Membrane properties were compared within strains (phasic vs. tonic) and between strains (phasic vs. phasic, tonic vs. tonic) using an unpaired t-test. Differences were considered statistically significant if p ≤ 0.05.
RESULTS
General Properties of WT and SYN-OE Mice and Urination Patterns
In experimental approaches where mice of both genders were evaluated, statistical analyses showed no gender by genotype difference. Thus data from male and female mice were analyzed together and presented as pooled data for all results described except for electrophysiological studies where only male WT and male SNY-OE mice were evaluated.
Male WT and SYN-OE mice and female WT and SYN-OE mice were of similar body mass; however, the urinary bladder of SYN-OE mice (71.3 ± 4.9 mg) exhibited significantly (p ≤ 0.001) increased mass compared to littermate WT mice (54.6 ± 3.7 mg). Urine spots from SYN-OE mice, as quantified on filter paper over a 1 h period, were significantly (p ≤ 0.01) greater in number (38.2 ± 3.5 voids/h vs. 12.8 ± 2.5 voids/h) but smaller in area compared to those made by littermate WT mice (3.1 ± 1.4 cm2 vs. 7.4 ± 1.2 cm2, respectively). A significant (p ≤ 0.01) increase in the number of both large (0.2 -10 cm2) and small (< 0.2 cm2) diameter urine spots was observed for SYN-OE mice. Using a standard curve to estimate urine volumes from urine spot diameter, no difference in total void volume/h was observed between WT (59.7 ± 4.5 μl) and SYN-OE (61.3 μl ± 4.5 μl) mice. Fluid intake measured over a 24 h period was similar among littermates (7.5 ± 1.0 ml/24 h vs. 7.8 ± 0.8 ml/24 h).
Human α-SYN Transcript Expression in Micturition Reflex Pathways, Superior Cervical Ganglia (SCG) and Brain of SYN-OE mice
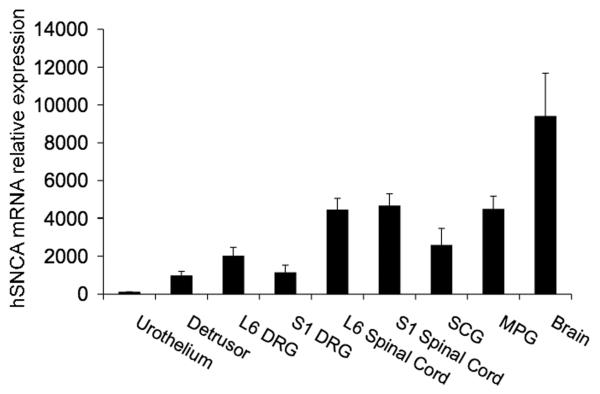
Human α-SYN transcript was expressed in all tissues examined (urothelium, detrusor smooth muscle, L6-S1 dorsal root ganglia (DRG) and spinal cord segments, major pelvic ganglia (MPG) and brain) from SYN-OE mice (Fig. 1). No human α-SYN transcript was detected in the same tissues from littermate WT mice (data not shown). Both WT and SYN-OE mice had similar expression of endogenous mouse α-SYN transcript in tissues examined (data not shown).
Fig. 1.
Human α-synuclein (hSNCA) mRNA overexpression in micturition reflex pathways and superior cervical ganglia (SCG) and brain from human α-synuclein overexpressing (SYNOE) mice. In all tissues examined (urothelium, detrusor smooth muscle, L6-S1 dorsal root ganglia (DRG), L6-S1 spinal cord, SCG, major pelvic ganglia (MPG) and brain), hSNCA mRNA is overexpressed. The same tissues harvested from littermate wildtype mice only expressed endogenous mouse α-synuclein mRNA similar to that demonstrated in SYN-OE mice (data not shown). SYN-OE mice samples were normalized to the relative expression of the reference gene, 18S.
Motor Behavior is Altered in SYN-OE Mice with Advancing Age
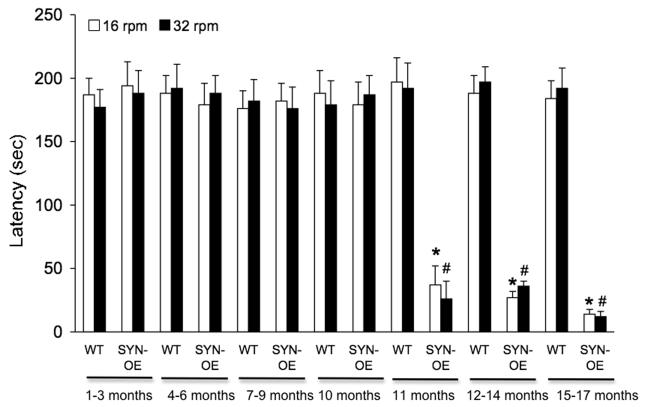
Motor function was assessed on a RotaRod (Dunham and Miya, 1957) using a fixed speed testing strategy. Both male and female animals were tested. Thirty-seven mice began the assessments of motor function but 24.3% of this group (n = 9) exhibited hemiparesis, partial limb paralysis, trembling or inability to stand beginning at 11 months of age through the endpoint of testing (17 months of age). These signs and symptoms required euthanasia of affected mice; therefore, these animals did not complete the entire temporal sequence of motor function testing. Since ANOVA showed no gender by genotype difference, data from male and female animals were analyzed together. SYN-OE are able to maintain motor activity on the rod comparable to littermate WT mice when the rod rotated at 16 or 32 rpm from 1 month of age through 10 months of age. Beginning at 11 months of age, SYN-OE mice fall significantly (p ≤ 0.01) faster (decreased latency to fall) at 16 and 32 rpm compared to littermate WT mice (Fig. 2). This decreased latency to fall exhibited by SYN-OE mice persisted through the end of the testing period (17 months of age) (Fig. 2). The RotaRod is a complex motor task that involves coordination, balance and motor learning; therefore, additional tests were performed to assess individual aspects of motor function.
Fig. 2.
SYN-OE mice exhibit altered locomotor function with advancing age. Wildtype (WT) and synuclein overexpressing (SYN-OE) mice littermates were tested at 16 and 32 rpm on consecutive days on a motorized rotating rod. SYN-OE maintain motor activity on the rod comparable to littermate WT mice when the rod rotated at 16 or 32 rpm from 1 month of age through 10 months of age. Beginning at 11 months of age, SYN-OE mice fall significantly (p ≤ 0.01) faster (decreased latency to fall) at 16 and 32 rpm compared to littermate WT mice. This decreased latency to fall exhibited by SYN-OE mice persisted through the end of the testing period (17 months of age). *, p ≤ 0.01 relative to WT at 16 rpm; #, p ≤ 0.01 relative to WT at 32 rpm.
Motor neuron disease was assessed by testing hindlimb extension. Normally, an extension reflex of the hindlimb is observed when a mouse is suspended in the air by its tail (Jaworski et al., 2006). However, in mice with motor neuron disease, hindlimb retraction is more commonly observed (Jaworski et al., 2006). Both WT (1.8 ± 0.1) and SYN-OE mice (1.7 ± 0.2) exhibited similar hindlimb extension from the earliest age evaluated (2 months of age) through 10 months of age. At the 12 month testing point, SYN-OE mice (0.8 ± 0.4) exhibited significantly (p ≤ 0.01) reduced hindlimb extension compared to WT mice (1.8 ± 0.2).
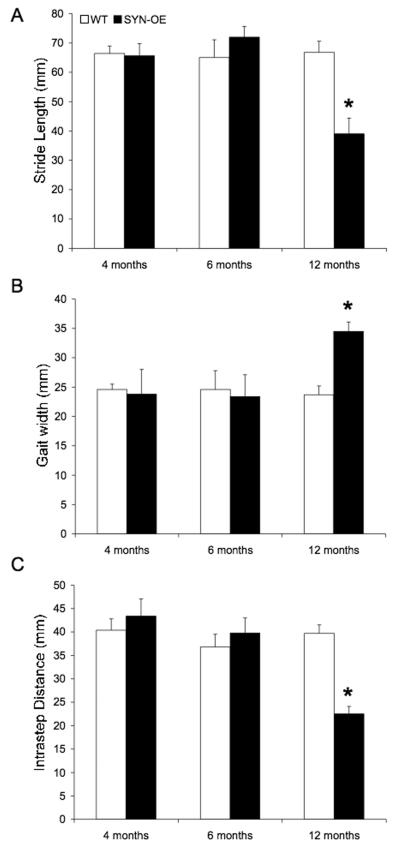
To quantify potential differences in walking patterns of littermate SYN-OE and WT mice, the stride length, gait width, and intrastep distance between right and left paw prints were measured (Fig. 3). Stride length, gait and intrastep distance are similar in WT and SYN-OE mice evaluated at 4 and 6 months of age (Fig. 3A-C). At 12 months of age, SYNOE mice display a significant (p ≤0.01) increase in gait width (Fig. 3B) with a significant decrease in stride length and intrastep distance compared to littermate WT mice (Fig. 3A, C).
Fig. 3.
Stride length, gait width and intrastep distance is altered in SYN-OE mice with advancing age. A) Stride length is decreased in SYN-OE mice evaluated at 12 months of age, but is unaltered in wildtype (WT) mice. No difference in stride length in SYN-OE mice is observed at 4 or 6 months of age compared to WT. B) Gait width is increased in SYN-OE mice at 12 months of age. No difference in gait in SYN-OE mice is observed at 4 or 6 months of age compared to WT. C) Intrastep distance is decreased in SYN-OE mice evaluated at 12 months of age, but is unaltered in WT mice. No difference in gait width in SYN-OE mice is observed at 4 or 6 months of age compared to WT. *, p ≤ 0.01 relative to WT at 12 months.
Urinary Bladder NGF Content is Increased in SYN-OE Mice
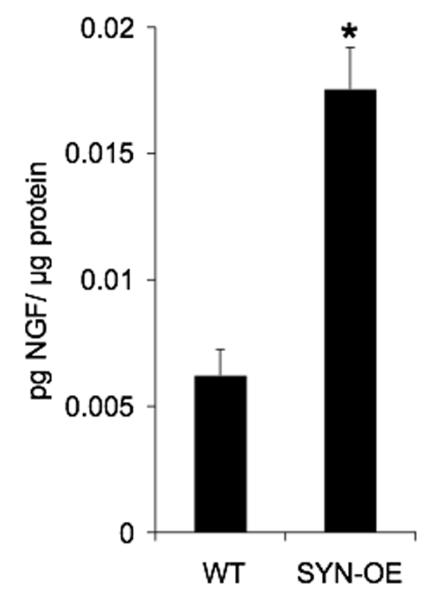
NGF bladder content is significantly (p ≤ 0.01) increased (2.8-fold) in SYN-OE mice (Fig. 4).
Fig. 4.
Nerve growth factor (NGF) content in the urinary bladders of WT and SYN-OE transgenic (Tg) mice. NGF content in whole urinary bladder was determined in littermate WT and Tg mice. Urinary bladder NGF was significantly (p ≤ 0.01) greater in Tg mouse bladders compared to WT.
Early Onset of Altered Urinary Bladder Function in SYN-OE Mice
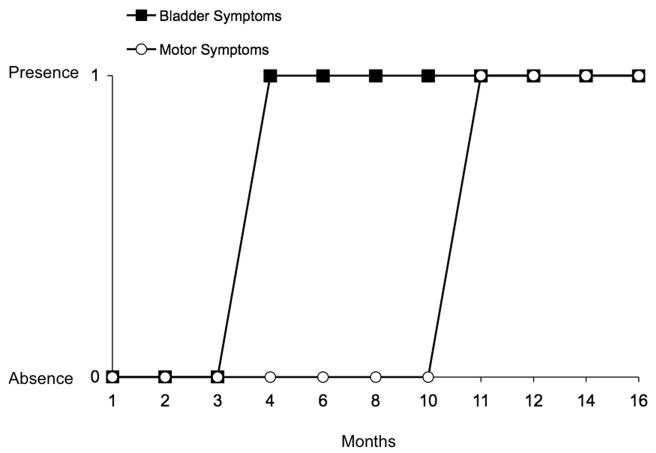
Bladder function was characterized in littermate WT and SYN-OE mice beginning at one month of age and continuing through sixteen months of age (n = 4-5 at each age for WT and SYN-OE). A variety of cystometric observations suggestive of lower urinary tract dysfunction were revealed with conscious cystometry and included: urinary bladder hyperreflexia with increased voiding frequency and non-voiding bladder contractions (72% of SYN-OE mice examined), urinary incontinence suggestive of an incompetent urethral closure mechanism (evidence of leakage of saline from the urethra without evidence of increases in bladder pressure; 10% of SYN-OE mice examined) and urinary bladder hyporeflexia associated with decreased voiding frequency and increased bladder capacity (18% of SYN-OE mice examined). There was no correlation of any particular type of bladder dysfunction with SYN-OE mouse age and each described lower urinary tract dysfunction was observed at each age examined in SYN-OE mice. Urinary bladder dysfunction was observed in SYN-OE mice at 4 months of age and persisted to the last age studied (16 months of age). The presence of urinary bladder dysfunction preceded motor dysfunction by 7 months in SYN-OE mice (Fig. 5). Urinary bladder hyperreflexia was the most commonly observed (72% of SYN-OE mice examined) cystometric finding in SYN-OE mice; therefore, this dysfunction is the main focus of this report.
Fig. 5.
Urinary bladder dysfunction onset precedes motor dysfunction in SYN-OE mice. Urinary bladder dysfunction was observed in SYN-OE mice at 4 months of age and persisted to the last age studied (16 months of age). The presence (score of 1) of urinary bladder dysfunction preceded motor dysfunction by 7 months in SYN-OE mice. Motor dysfunction was not observed (absence; score of 0) in SYN-OE evaluated using rotarod testing (Fig. 2) and gait analyses (Fig. 3) from 1-10 months of age. Urinary bladder dysfunction was not observed (absence; score of 0) using conscious cystometry in SYN-OE mice evaluated from 1-3 months of age.
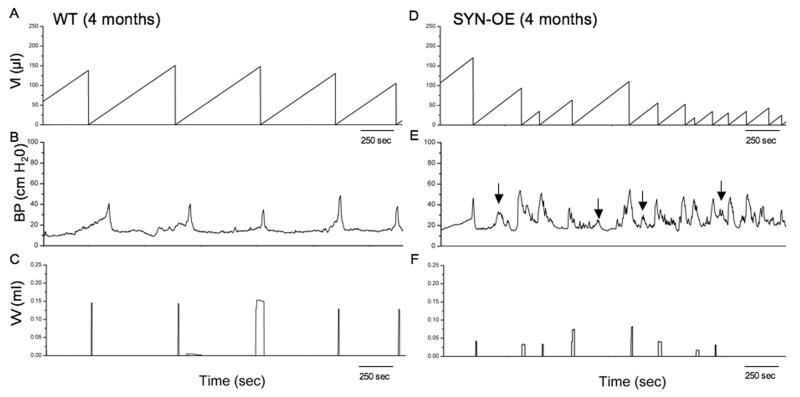
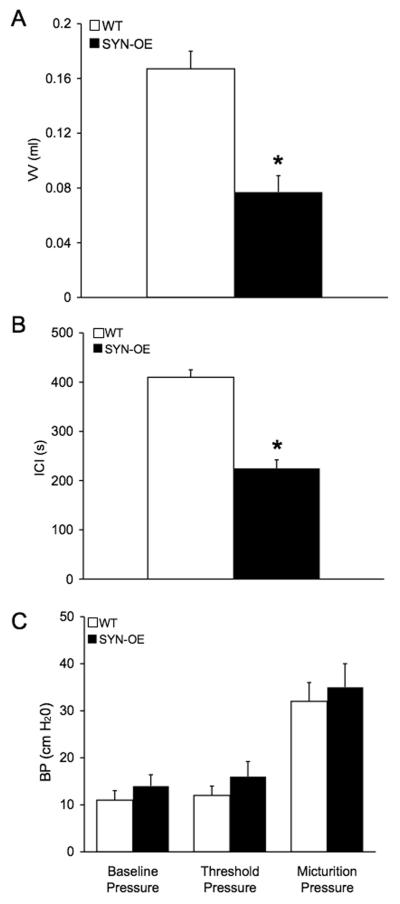
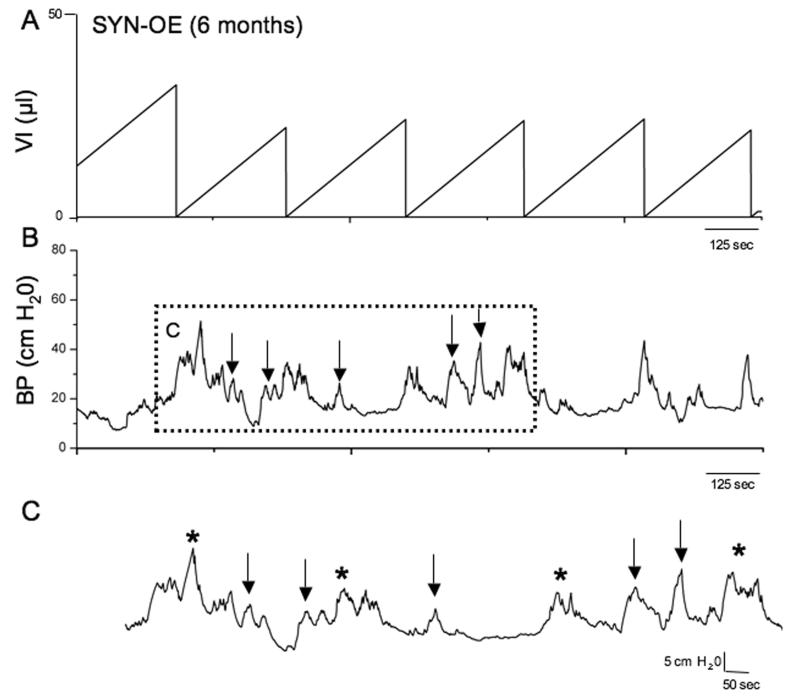
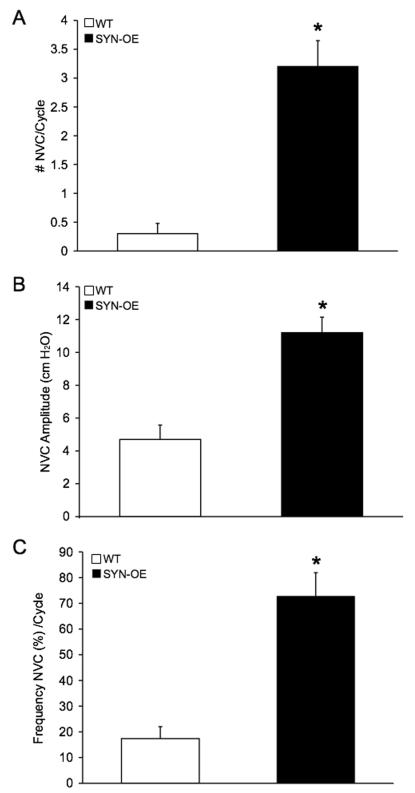
To determine whether increased NGF bladder content resulted in changes in urinary bladder function, we carried out a series of cystometry studies on littermate WT and SYNOE mice. Figure 6 shows representative open voiding cystometrograms in conscious, unrestrained mice in which voiding reflexes were measured in response to a continuous infusion of saline. In contrast to WT littermate controls where normal micturition contractions were observed (Fig. 6A-C), 72% of SYN-OE mice examined (n = 35) exhibited urinary bladder hyperreflexia (Fig. 6D-F) with significantly reduced (p ≤ 0.01) void volumes and intercontraction intervals compared to WT mice (Fig. 7A,B). The reductions in void volume and intercontraction interval were observed in both male and female transgenic mice with a similar magnitude of change; thus, data from both genders were analyzed together. No changes in baseline resting pressure, micturition threshold pressure or maximum voiding pressure were observed between WT and SYN-OE mice (Fig. 7C). Non-voiding bladder contractions (NVC) were observed in both WT and SYN-OE mice under conscious cystometry conditions but NVCs were not observed with each voiding cycle for either group (Fig. 8 and data not shown). However, the number of NVCs/voiding cycle, the amplitude of NVCs and the frequency of occurrence of NVCs/voiding cycle were significantly (p ≤ 0.01) increased in SYN-OE mice (Fig. 9A-C) compared to WT littermate controls. Of the SYNOE mice with urinary bladder hyperreflexia, the majority (78%) exhibited significantly (p ≤ 0.01) increased residual volume compared to littermate WT mice (47 ± 12 μl vs. 10 ± 8 μl).
Fig. 6.
Open cystometry in 4 month old, conscious WT and SYN-OE transgenic mice. Representative cystometrogram trace from a conscious, unrestrained WT (A-C) and SYN-OE (D-F) mouse with continuous intravesical infusion (25 μl/min) of room temperature saline. Bladder pressure (BP, cm H20), volume infused (VI, μl) and voided volume (VV, ml) are shown. Arrows in E indicate examples of non-voiding contractions. Seconds, sec.
Fig. 7.
Summary bar graphs from open cystometry. Bar graphs of VV (ml, A), ICI (seconds, s; B) and BP (cm H20, C) from conscious cystometry in unrestrained WT and SYN-OE transgenic mice with continuous infusion of saline. A) Voided volume (VV) in transgenic mice were significantly (p ≤ 0.01) smaller compared to WT VV. B) Intercontraction intervals (ICI) were significantly (p ≤ 0.01) reduced in transgenic mice compared to WT mice. C) No changes in baseline, threshold or maximum micturition pressure were observed between WT and transgenic mice using conscious cystometry. Data represent the mean ± S.E.M.
Fig. 8.
Non-voiding contractions (NVCs) in SYN-OE transgenic mice from open cystometry. Representative cystometrogram trace from a conscious, unrestrained transgenic mouse with continuous intravesical infusion (25 μl/min) of room temperature saline. VI (μl, A) and BP (cm H20, B) are shown. Dashed box in B is focusing on micturition events associated with the occurrence of NVCs. C) The dashed box in B is expanded with arrows indicating NVCs and asterisks indicating void events.
Fig. 9.
Summary bar graphs of non-voiding contractions (NVCs) properties in WT and SYNOE transgenic mice. Evidence of NVCs was observed in both WT and transgenic mice under conscious cystometry conditions but NVCs were not observed with each voiding cycle in either WT or transgenic mice. The number of NVCs/voiding cycle (A), amplitude of NVCs (cm H20, B) and frequency of occurrence (%, C) of NVCs/voiding cycle were significantly (p ≤ 0.01) increased in transgenic mice. Data represent the mean ± S.E.M.
Neuropeptide and nNOS Transcript Expression in Micturition Reflex Pathways, Superior Cervical Ganglia (SCG) and Brain of WT and SYN-OE mice
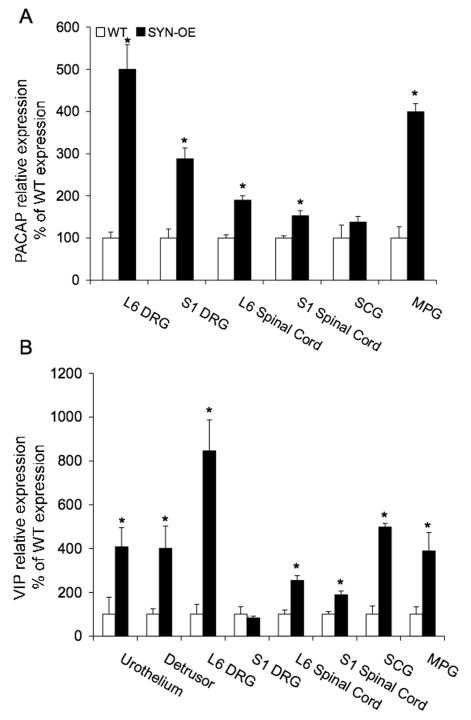
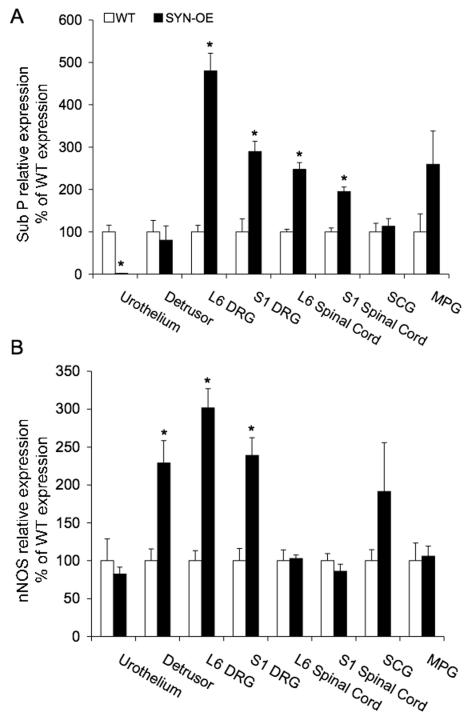
PACAP transcript expression significantly (p ≤ 0.01) increased all tissues examined in SYN-OE mice that demonstrated amplification of PACAP transcript except SCG (Fig. 10A). The most robust increases in PACAP transcript expression were observed in L6 DRG (4.9-fold increase) and MPG (3.9-fold increase)(Fig. 10A) in SYN-OE mice. No amplification of PACAP transcript was observed in the urothelium or detrusor smooth muscle of the urinary bladder. VIP transcript expression significantly (p ≤ 0.01) increased in all tissues examined in SYN-OE mice except S1 DRG (Fig. 10B). The most robust changes in VIP transcript expression were observed in the urothelium (4.1-fold increase) and detrusor smooth muscle (4-fold increase), L6 DRG (8.5-fold increase), SCG (4.9-fold increase) and MPG (3.9-fold increase) in SYN-OE mice (Fig. 10B). Sub P transcript expression significantly (p ≤ 0.01) increased in L6-S1 DRG and spinal cord in SYN-OE mice (Fig. 11A). In contrast, Sub P transcript expression significantly (p ≤ 0.01) decreased in the urothelium of the urinary bladder in SYN-OE mice (Fig. 11A). No changes were observed in detrusor smooth muscle, SCG or MPG. The most robust increase (4.8-fold) in Sub P transcript expression was observed in the L6 DRG of SYN-OE mice whereas the urothelium exhibited the most robust decrease (62.5-fold) in Sub P transcript expression in SYN-OE mice (Fig. 11A). nNOS transcript expression significantly (p ≤ 0.01) increased in detrusor smooth muscle and L6-S1 DRG in SYN-OE mice (Fig. 11B). No changes in nNOS transcript expression were observed in the urothelium, L6-S1 spinal cord, SCG or MPG in SYN-OE mice (Fig. 11B). Detrusor smooth muscle, L6 and S1 DRG exhibited similar increases (2.3-3-fold) in nNOS transcript expression in SYN-OE mice (Fig. 11B).
Fig. 10.
Modulation of pituitary adenylate cyclase activating polypeptide (PACAP; A) and vasoactive intestinal polypeptide (VIP; B) transcript expression in littermate wildtype (WT) and synuclein overexpressing (SYN-OE) mice in micturition reflex pathways, superior cervical ganglia (SCG) and brain. WT samples were set equal to 100% and normalized to the relative expression of the reference gene, L32. SYN-OE samples were expressed relative to WT samples and normalized to the relative expression of the reference gene, L32. A) PACAP mRNA expression in tissues sampled (L6-S1 dorsal root ganglia (DRG), L6-S1 spinal cord, SCG, major pelvic ganglia (MPG) and brain). PACAP transcript expression significantly (p ≤ 0.01) increased all tissues examined in SYN-OE mice that demonstrated amplification of PACAP transcript except SCG. No amplification of PACAP transcript was observed in the urothelium or detrusor smooth muscle of the urinary bladder. B) VIP mRNA expression in tissues sampled (urothelium, detrusor smooth muscle, L6-S1 DRG, L6-S1 spinal cord, SCG, MPG and brain). VIP transcript expression significantly (p ≤ 0.01) increased in all tissues examined in SYN-OE mice except S1 DRG. Values are mean ± SEM. *, p ≤ 0.01 versus WT.
Fig. 11.
Modulation of substance P (Sub P; A) and neuronal nitric oxide synthase (nNOS; B) transcript expression in littermate wildtype (WT) and synuclein overexpressing (SYN-OE) mice in micturition reflex pathways, superior cervical ganglia (SCG) and brain. WT samples were set equal to 100% and normalized to the relative expression of the housekeeping gene, L32. SYN-OE samples were expressed relative to WT samples and normalized to the relative expression of the reference gene, L32. A) Sub P mRNA expression in tissues sampled (urothelium, detrusor smooth muscle, L6-S1 dorsal root ganglia (DRG), L6-S1 spinal cord, SCG, major pelvic ganglia (MPG) and brain). Sub P transcript expression significantly (p ≤ 0.01) increased in L6-S1 DRG and spinal cord in SYN-OE mice. Sub P transcript expression significantly (p ≤ 0.01) decreased in the urothelium of the urinary bladder in SYN-OE mice. B) nNOS transcript expression significantly (p ≤ 0.01) increased in detrusor smooth muscle and L6-S1 DRG in SYN-OE mice. Values are mean ± SEM. *, p ≤ 0.01 versus WT.
Basic Electrical Properties of Pelvic Ganglia Neurons are not Altered in SYN-OE mice
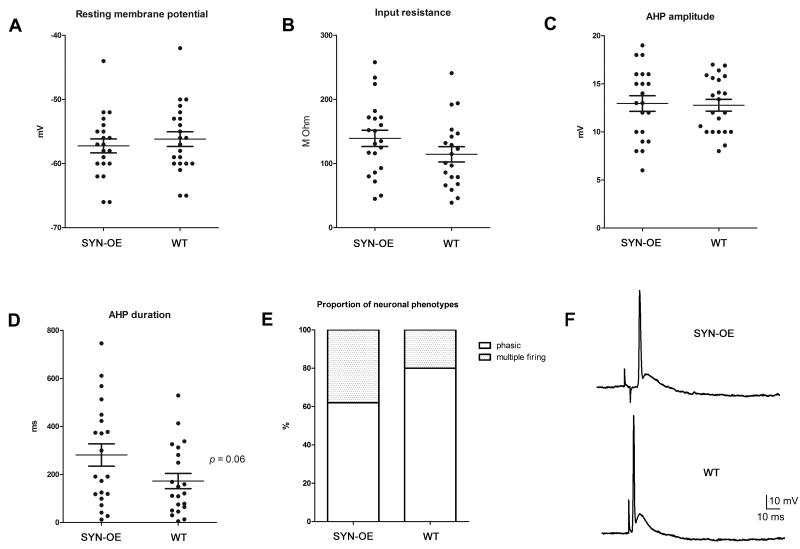
To test whether the observed alteration in bladder function might be due in part to an alteration in ganglionic transmission or change in the basic electrical properties of MPG neurons, intracellular recordings were obtained from neurons in pelvic ganglia whole mount preparations taken from male WT and SYN-OE mice. The input resistance was determined from the amplitude of the voltage change evoked by injection of long hyperpolarizing current steps and properties of the afterhyperpolarization (AHP) were determined by eliciting action potentials with brief depolarizing current steps applied to the cell. The results of recordings from 21 cells in 6 SYN-OE ganglia and 22 cells in 7 WT ganglia, which are summarized in Figure 12A-D, indicate that averaged values of the resting membrane potential, input resistance, and amplitude and duration of the AHP were not different in cells from the two genotypes.
Fig. 12.
Membrane properties of major pelvic ganglia (MPG) neurons from synuclein overexpressing (SYN-OE) and wildtype (WT) mice. Resting membrane potential (A), input resistance (B), and afterhyperpolarization amplitude (C) and duration (D) were not different between strains (21 cells from 6 SYN-OE MPG; 22 cells from 7 WT MPG). E) the proportion of phasic neurons (cells firing fewer than 4 action potentials in response to long-duration depolarization) also was not different between the two genotypes (16/20 WT vs. 13/21 SYNOE, p = 0.6). F) Ganglionic transmission elicited by pelvic nerve stimulation was comparable in the MPG from WT and SYN-OE mice. Responses recorded from cells in the MPG from a WT and an SYN-OE mouse evoked by suprathreshold stimulation of the pelvic nerve.
Subsequent experiments tested whether there was a change in the excitability pattern of the MPG neurons from SYN-OE mice. Excitability was characterized from the response to 1 second depolarizing current pulses of increasing magnitude. The proportion of neurons exhibiting a phasic firing pattern (4 or less APs) or multiple firing (>4 APs) was not different in the MPG from WT or SYN-OE mice (Fig. 12E).
Additional experiments tested whether ganglionic transmission was altered in the MPG from the SYN-OE mice. Pelvic nerve stimulation was used to evoke responses in MPG neurons from WT and SYN-OE mice. The EPSPs elicited with low frequency, suprathreshold stimulation (0.2 Hz) of the pelvic nerve were sufficient to evoke an action potential in all MPG neurons tested from both strains (Fig. 12F). Also, the amplitude and duration of MPG neuron action potentials evoked by nerve stimulation were comparable in both strains (6 cells from WT MPG and 5 cells from SYN-OE MPG). The response to repetitive stimulation (10 Hz) of the pelvic nerve was determined in 6 cells in MPG from WT mice and in 2 cells in MPG from the SYN-OE mice. In all cases, the EPSP remained suprathreshold so that action potentials were evoked during a 5 sec period of 10Hz pelvic nerve stimulation (data not shown). Although limited in scope, these experiments suggest ganglionic transmission remains intact in the MPG from SYN-OE mice.
DISCUSSION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, after Alzheimer’s disease, and affects 1% of the population at 65 years of age (Venda et al., 2010). The prevalence increases with age: currently approximately 1.0 - 1.5 million Americans are living with PD. The scope of PD is broadening: classic motor features may be preceded by mood or sleep disorders, olfactory, cardiovascular, gastrointestinal or lower urinary tract dysfunction (i.e., ‘nonmotor/premotor’ manifestations of PD (Fowler et al.; Simuni and Sethi, 2008; Tolosa and Poewe, 2009). The disease has also evolved from being considered a sporadic disease to a complex genetic disorder requiring mutational analyses to characterize familial forms of PD (Moore and Dawson, 2008). The recognition of specific monogenic forms has provided opportunities to develop transgenic mouse models of PD and further explore mechanisms of neurodegeneration, and sites of potential intervention with therapeutic agents (Moore and Dawson, 2008). Using a human α-synuclein overexpressing (SYN-OE) transgenic mouse model we have identified changes in peripheral autonomic nervous system control of the lower urinary tract (i.e., bladder) months prior to the onset of motor dysfunction. Transgenic mice also exhibited increased nerve growth factor (NGF) content in the urinary bladder and increased neuropeptide/neuromodulator (PACAP, VIP, Sub P and nNOS) (Arms and Vizzard, 2011) transcript expression in lower urinary tract tissues. In contrast, when transgenic mice were evaluated at ages that demonstrated bladder dysfunction, no changes in ganglionic transmission or neuronal electrophysiology were demonstrated in the major pelvic ganglion (MPG) that provides postganglionic innervation to urogenital tissues including the urinary bladder. Thus, these transgenic mice offer good construct (genetic etiology) and face (symptoms and pathology) validity for evaluating α-synuclein’s role in the cascade of clinical events that might begin in the peripheral control of ANS function and progress centripetally (from disordered autonomic function to altered central systems controlling motor function). The current study demonstrates human α-synuclein (hSNCA) mRNA overexpression in micturition reflex pathways and SCG and brain from SYN-OE mice. In all tissues examined (urothelium, detrusor smooth muscle, L6-S1 DRG, L6-S1 spinal cord, SCG, MPG and brain), hSNCA mRNA is overexpressed. The same tissues from littermate wildtype mice only expressed endogenous mouse α-synuclein mRNA similar to that demonstrated in SYN-OE mice. Future studies are necessary to demonstrate synuclein protein expression in the relevant tissues and cell types (e.g., urothelium, detrusor smooth muscle, sensory neurons) in target tissues (i.e., urinary bladder) and to determine correlation between hSNCA mRNA and synuclein protein expression in SYN-OE mice.
The bladder dysfunction in the transgenic model parallels the altered bladder function in patients with synucleinopathies (i.e., hyperactive bladder dysfunction predominates with detrusor-sphincter-dyssnergia and urgency and frequent urination, including increasing nocturia, and urinary retention) (Fowler et al., 2010; Ragab and Mohammed, 2011). In addition, PD patients also will experience urinary incontinence from failed sphincter control primarily reflecting an internal sphincter malfunction (Fowler et al., 2010). In the SYN-OE mouse, urinary bladder hyperreflexia with increased voiding frequency, decreased voided volumes and the presence of non-voiding contractions was the predominant bladder dysfunction observed in the present study. However, urinary bladder hyporeflexia as well as incontinence were also observed, although less frequently, in the transgenic mouse model. Human studies demonstrate a higher presence of LB pathology in vesicoprostate plexi compared to other autonomic ganglia suggesting that this transgenic model system may provide insights into neuronal vulnerability to the pathogenic process of alterations in the processing of the synuclein protein (Minguez-Castellanos et al., 2007). Consistent with this is the demonstration in the present study that changes in transcript expression of neuroactive compounds in the SCG were only demonstrated in 1 of 4 neurochemicals (i.e., VIP) examined in contrast to robust changes in each neuroactive compound examined in micturition reflex components. Further, in the autonomic nervous system, the involvement of the genitourinary system is higher than the enteric nervous system occurring in a fairly high percentage of ‘healthy appearing elderly’ (26% vs. 4%)(Minguez-Castellanos et al., 2007). In addition to LB pathology in peripheral ganglia, studies demonstrate LB pathology in autonomic nuclei in lumbar and sacral spinal cord in human PD (Wakabayashi et al., 1990). Thus, human studies support the unique vulnerability of peripheral autonomic neural structures in PD.
Increased NGF bladder content was demonstrated in SYN-OE mice with urinary bladder dysfunction. The urinary bladder hyperreflexia observed was similar to that recently described in a transgenic mouse with chronic overexpression of NGF (NGF-OE) in the urothelium of the urinary bladder (Schnegelsberg et al., 2009). Altered NGF content is associated with stretch of the urinary bladder (Clemow et al., 2000) as well as urinary bladder inflammation and dysfunction in both rodents and humans where it may underlie neurochemical (Vizzard, 2001; Braas et al., 2006; Zvarova and Vizzard, 2006; Klinger et al., 2008), organizational (Vizzard and Boyle, 1999; Vizzard, 2000a) and electrophysiological (Yoshimura and de Groat, 1999) changes of micturition reflex pathways. In rodents, urinary bladder inflammation induced by noxious chemical or mechanical stimuli increased NGF mRNA and/or protein within the urothelium or detrusor smooth muscle (Vizzard, 2000b; Bjorling et al., 2001; Dupont et al., 2001) and caused morphological changes in bladder sensory and motor neurons (Dupont et al., 2001). Urinary bladder inflammation (Vizzard, 2000a; Murray et al., 2004; Guerios et al., 2008; Klinger and Vizzard, 2008) also produced changes in NGF or NGF receptor (TrkA and p75NTR) expression in micturition reflex pathways. NGF also increased in hypertrophied bladders following spinal cord injury or bladder outlet obstruction (BOO) (Kim et al., 2005; Kim et al., 2006; Liu and Kuo, 2007; Liu et al., 2008a; Liu et al., 2008b; Liu and Kuo, 2008a; Liu and Kuo, 2008b) and in bladders of spontaneously hypertensive rats (Clemow et al., 1998) where it appears to correlate with neuronal hypertrophy (Steers et al., 1991) and persistent detrusor overactivity (DO) (Clemow et al., 1998; Kim et al., 2004). Future studies involving the SYN-OE mice will address morphological changes in bladder sensory and motor neurons, expression of NGF receptor (TrkA and p75NTR) expression in micturition reflex pathways and cellular/tissue (e.g., urothelium, detrusor smooth muscle, suburothelial nerve plexus) sources of increased NGF expression in the urinary bladder of SYN-OE mice.
A number of addition or subtraction studies have demonstrated role(s) for NGF in urinary bladder hyperreflexia. Intrathecal NGF administration increased NGF expression in bladder afferents, increased bladder afferent hyperexcitability and caused urinary bladder hyperreflexia, indicating that increased NGF levels in the afferent limb of the micturition reflex contributes to changes in bladder sensory function (Yoshimura et al., 2006). Increased bladder NGF expression via adenoviral delivery (Lamb et al., 2004) also increased voiding frequency, while chronic administration of NGF into the bladder wall (Zvara and Vizzard, 2007) increased voiding frequency, augmented central responses to bladder distension (increased Fos protein) and increased neuropeptide (substance P, calcitonin gene-related peptide) expression in the bladder and lumbosacral spinal cord, suggesting an NGF-mediated re-organization of micturition reflex pathways. In contrast, sequestration of NGF using a TrkA-IgG fusion protein (Dmitrieva et al., 1997) or the NGF scavenging agent REN1820 (Hu et al., 2005) reduced urinary bladder hyperreflexia (Dmitrieva et al., 1997; Hu et al., 2005). Future studies involving NGF sequestration approaches in the SNY-OE transgenic mouse will be pursued to reduced urinary bladder hyperreflexia.
Numerous neuropeptide and neuromodulatory systems including vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), substance P (sub P) and neuronal nitric oxide synthase (nNOS) are expressed in the lower urinary tract (LUT) in both neural and non-neural (e.g., urothelium) components (Vizzard, 2001; Arms and Vizzard, 2011). LUT neuropeptide and nNOS immunoreactivity is present in afferent and autonomic efferent neurons innervating the bladder and urethra and in the urothelium of the urinary bladder. Neuropeptides and nNOS have tissue-specific distributions and functions in the LUT and exhibit neuroplastic changes in expression and function with LUT dysfunction following neural injury, inflammation and disease (Arms and Vizzard, 2011). Neuropeptides in the afferent pathways to the LUT exhibit either excitatory or inhibitory actions. Non-neural sources of peptides in the LUT include plasma, sites of tissue inflammation or injury, detrusor smooth muscle cells, bladder fibroblasts and the urothelium (Arms and Vizzard, 2011). Pathology, neural injury and target organ pathology (e.g., bladder inflammation) can alter the known balance of neuropeptides either in the periphery and/or central pathways conceivably shifting the balance to a hyper- or hypo-active reflex state (Arms and Vizzard, 2011).
The demonstration of increased expression of neuropeptides and nNOS transcripts in micturition reflex pathways in SYN-OE mice is consistent with demonstrated facilitatory effects of these neuroactive compounds in micturition reflex pathways and this neurochemical plasticity may contribute to urinary bladder dysfunction (Arms and Vizzard, 2011). Additional studies are needed to determine if protein expression of the examined neuroactive compounds correlates with demonstrated increased transcript expression in micturition reflex pathways. We have previously demonstrated direct facilitatory effects of PACAP on bladder smooth muscle contractility (Braas et al., 2006). In a urinary bladder inflammation model, intrathecal or intravesical administration of a PAC1 receptor antagonist, PACAP6-38, reduced cystitis-induced bladder hyperreflexia (Braas et al., 2006). In the MPG, local application of PACAP or maxadilan, a PAC1-selective agonist or VIP, decreased the afterhyperpolarization and increased neuronal excitability in a subpopulation of neurons (Tompkins et al., 2010). Consistent with facilitatory effects of PACAP and VIP in micturition reflexes, PACAP null mice (May and Vizzard, 2010) and VIP null mice (Studeny et al., 2008) exhibit hyporeflexia (i.e., increased bladder capacity, voided volumes and longer intercontraction intervals). Current research also supports the suggestion that PACAP and VIP are regulators of bladder physiology at the level of the urothelium through ATP release (Girard et al., 2008). Furthermore, it has been demonstrated (Lecci et al., 1994) that intrathecal injection of sub P antagonists reduced bladder inflammation-induced bladder hyperreflexia. Much less is known regarding the contribution of nNOS to urinary bladder dysfunction; however, BOO is associated with increased nNOS expression in bladder afferent neurons in DRG (Zvara et al., 2004). Therefore, PACAP, VIP, sub P and nNOS signaling in the urinary bladder, sensory neurons and/or MPG may represent diverse sites for neuromodulation of bladder function in SYN-OE mice.
There is growing evidence that expression of α-synuclein is associated with decreased release of neurotransmitter and synaptic dysfunction in neuronal populations (Larsen et al., 2006; Garcia-Reitbock et al., 2010; Nemani et al., 2010; Scott et al., 2010). Specifically, SYN-OE mice demonstrate impaired synaptic vesicle exocytosis in hippocampal neurons (Yavich et al., 2004) and ventral midbrain dopaminergic neurons transfected with α-synuclein similarly demonstrate impaired synaptic vesicle exocytosis as well as reductions in the readily releasable and recycling synaptic vesicle pools (Nemani et al., 2010). In the current studies, intracellular recording and nerve stimulation did not reveal changes in the electrical or synaptic properties of postganglionic neurons in the MPG of SYN-OE mice at postnatal ages with demonstrated urinary bladder dysfunction. The absence of changes in electrical and synaptic properties in MPG neurons suggests that urinary bladder dysfunction in SYN-OE mice does not reflect a change in efferent limb of the micturition reflex despite some changes in the neurochemistry in the MPG of SYN-OE mice. Rather, urinary bladder dysfunction in SYN-OE mice may reflect changes in the properties of the afferent limb of the micturition reflex, target organ (i.e., urinary bladder) and/or changes in central micturition reflex circuitry. This suggestion is consistent with the likely substrates underlying urinary bladder hyperreflexia in NGF-OE mice (Schnegelsberg et al., 2009) (Tompkins et al., unpublished observations). Similar to SYN-OE mice, postganglionic neurons in the MPG do not exhibit changes in electrical or synaptic properties but do demonstrate robust changes in neurochemistry in bladder afferent and central micturition circuitry (Schnegelsberg et al., 2009). Future studies evaluating the electrical and synaptic properties of bladder afferent neurons as well as recording afferent nerve activity in response to urinary bladder distention may provide additional insight into changes in the afferent limb of the micturition reflex in SYN-OE mice that may underlie urinary bladder dysfunction.
Perspectives and Significance.
The human α-synuclein overexpressing mouse model permits longitudinal examination of autonomic function and motoric function/dysfunction and provides an opportunity to test interventions in autonomic function long before motor dysfunction appears. Also, pharmacological management of bladder dysfunction may be examined. The transgenic mouse model permits the opportunity for early intervention in pre-clinical phase of ANS dysfunction and examine whether onset of genitourinary dysfunction might be altered by disease modifying interventions. Future studies are necessary to determine the presence of additional autonomic dysfunction (e.g., gastrointestinal, cardiac) in SYN-OE mice as well as to confirm early onset of urinary bladder dysfunction in other pre-clinical animal models of PD.
Acknowledgements
The authors thank Abbey Peterson and Susan Malley for valuable technical assistance.
Sources of Financial Support This work was supported in part by NIH grants DK051369, DK060481, DK065989 and UVM College of Medicine New Research Initiative Funding to MAV, Department of Surgery research support provided to RTK and NIH Grant P20 RR16435 to RLP.
REFERENCES
- Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011:395–423. doi: 10.1007/978-3-642-16499-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms LA, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298:F589–600. doi: 10.1152/ajprenal.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, deGroat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Jacobsen HE, Blum JR, Shih A, Beckman M, Wang ZY, Uehling DT. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int. 2001;87:697–702. doi: 10.1046/j.1464-410x.2001.02138.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R951–962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F826–836. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Reg Integr Comp Physiol. 1998;44:R1279–1286. doi: 10.1152/ajpregu.1998.275.4.R1279. [DOI] [PubMed] [Google Scholar]
- Clemow DB, Steers WD, Tuttle JB. Stretch-activated signaling of nerve growth factor secretion in bladder and vascular smooth muscle cells from hypertensive and hyperactive rats. J Cell Physiol. 2000;183:289–300. doi: 10.1002/(SICI)1097-4652(200006)183:3<289::AID-JCP1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed W, Wyatt R. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, Shelton D, Rice ASC, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997;78:449–459. doi: 10.1016/s0306-4522(96)00575-1. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Winternitz SR, Wyss JM. An analysis of the sensory innervation of the urinary system of the rat. Brain Res Bull. 1983;11:321–324. doi: 10.1016/0361-9230(83)90168-5. [DOI] [PubMed] [Google Scholar]
- Dorr W. Cystometry in mice--influence of bladder filling rate and circadian variations in bladder compliance. J Urol. 1992;148:183–187. doi: 10.1016/s0022-5347(17)36549-7. [DOI] [PubMed] [Google Scholar]
- Dunham N, Miya T. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharmacol Assoc. 1957:46. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166:1111–1118. [PubMed] [Google Scholar]
- Feng LR, Federoff HJ, Vicini S, Maguire-Zeiss KA. Alpha-synuclein mediates alterations in membrane conductance: a potential role for alpha-synuclein oligomers in cell vulnerability. Eur J Neurosci. 2010;32:10–17. doi: 10.1111/j.1460-9568.2010.07266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Dalton C, Panicker JN. Review of neurologic diseases for the urologist. Urol Clin North Am. 2010;37:517–526. doi: 10.1016/j.ucl.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- Garcia-Reitbock P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Ghetti B, Della Corte L, Spano P, Tofaris GK, Goedert M, Spillantini MG. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept. 2002;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36:310–320. doi: 10.1007/s12031-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D, Sewell L, Sharabi Y. Autonomic dysfunction in PD: A window to early detection. J Neurol Sci. 2011 doi: 10.1016/j.jns.2011.04.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173:1016–1021. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- Jaworski D, Soloway P, Caterina J, Falls W. Tissue inhibitor of metalloproteinase-2 (TIMP-2) deficient mice display motor deficits. J Neurobiol. 2006;66:82–94. doi: 10.1002/neu.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR, booth AM, de Groat WC. Distribution of neurons in the major pelvic ganglion of the rat which supply the bladder, colon or penis. Cell Tissue Res. 1989;256:105–112. doi: 10.1007/BF00224723. [DOI] [PubMed] [Google Scholar]
- Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Kim JC, Kim DB, Seo SI, Park YH, Hwang TK. Nerve growth factor and vanilloid receptor expression, and detrusor instability, after relieving bladder outlet obstruction in rats. BJU Int. 2004;94:915–918. doi: 10.1111/j.1464-4096.2003.05059.x. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol. 2005;12:875–880. doi: 10.1111/j.1442-2042.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol. 2006;175:1773–1776. doi: 10.1016/S0022-5347(05)00992-4. discussion 1776. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Girard B, Vizzard MA. p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol. 2008;507:1379–1392. doi: 10.1002/cne.21627. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F1778–1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kruger R, Muller T, Riess O. Involvement of alpha-synuclein in Parkinson’s disease and other neurodegenerative disorders. J Neural Transm. 2000;107:31–40. doi: 10.1007/s007020050002. [DOI] [PubMed] [Google Scholar]
- Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Giulani S, Santiciolo P, Maggi CA. Involvement of spinal tachykinin NK1 and NK2 receptors in detrusor hyperreflexia during chemical cystitis in anaesthetized rats. Eur J Pharmacol. 1994;259:129–135. doi: 10.1016/0014-2999(94)90501-0. [DOI] [PubMed] [Google Scholar]
- Lewy F. Zur Pathologischen Anatomie der Paralysis Agitans. Deutsche Zeitschrift fur Nervenheilkunde. 1913;50:50–55. [Google Scholar]
- Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, Lee VM. {alpha}-Syn Suppression Reverses Synaptic and Memory Defects in a Mouse Model of Dementia with Lewy Bodies. J Neurosci. 2011;31:10076–10087. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int. 2008a;102:1440–1444. doi: 10.1111/j.1464-410X.2008.07757.x. [DOI] [PubMed] [Google Scholar]
- Liu HT, Chancellor MB, Kuo HC. Urinary Nerve Growth Factor Levels are Elevated in Patients with Detrusor Overactivity and Decreased in Responders to Detrusor Botulinum Toxin-A Injection. Eur Urol. 2008b;56:700–706. doi: 10.1016/j.eururo.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007;70:463–468. doi: 10.1016/j.urology.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol. 2008a;179:2270–2274. doi: 10.1016/j.juro.2008.01.146. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008b;72:104–108. doi: 10.1016/j.urology.2008.01.069. [DOI] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- Maskri L, Zhu X-R, Fritzen S, Kühn K, Ullmer C, Engels P, Andriske M, Stichel CC, Lübbert H. Influence of different promoters on the expression pattern of mutated human α–synuclein in transgenic mice. Neurodegenerative Dis. 2004;1:255–265. doi: 10.1159/000085064. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP-/- mice. J Urol. 2010;183:772–779. doi: 10.1016/j.juro.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders? a cohort study. Neurology. 2007;68:2012–2018. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Dawson TM. Value of genetic models in understanding the cause and mechanisms of Parkinson’s disease. Curr Neurol Neurosci Rep. 2008;8:288–296. doi: 10.1007/s11910-008-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172:2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359:443–456. doi: 10.1002/cne.903590307. [DOI] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–442. [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Ragab MM, Mohammed ES. Idiopathic Parkinson’s disease patients at the urologic clinic. Neurourol Urodyn. 2011 doi: 10.1002/nau.20983. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2009;298:R534–547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simuni T, Sethi K. Nonmotor manifestations of Parkinson’s disease. Ann Neurol. 2008;64(Suppl 2):S65–80. doi: 10.1002/ana.21472. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. Journal of Clinical Investigations. 1991;88:1709–1715. doi: 10.1172/JCI115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI. Phasic non-micturition contractions in the bladder of the anaesthetized and awake rat. BJU Int. 2006;97:1094–1101. doi: 10.1111/j.1464-410X.2006.06137.x. [DOI] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)-/- mice. J Mol Neurosci. 2008;36:175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipre D, Goldstein D. Cardiac and extracardiac sympathetic denervation in Parkinson’s disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med. 2005;46:1775–1781. [PubMed] [Google Scholar]
- Tolosa E, Poewe W. Premotor Parkinson disease. Neurology. 2009;72:S1. doi: 10.1212/wnl.0b013e318198dace. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Girard BM, Vizzard MA, Parsons RL. VIP and PACAP effects on mouse major pelvic ganglia neurons. J Mol Neurosci. 2010;42:390–396. doi: 10.1007/s12031-010-9367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. alpha-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010;33:559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Dev Neurosci. 1997;19:232–246. doi: 10.1159/000111212. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R1027–1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000b;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000c;420:335–348. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res. 1999;844:174–187. doi: 10.1016/s0006-8993(99)01936-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Kumon H, Nagai A. Correlation of urinary nerve growth factor level with pathogenesis of overactive bladder. Neurourol Urodyn. 2008;27:417–420. doi: 10.1002/nau.20519. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zvara P, Folsom JB, Kliment J, Jr., Dattilio AL, Moravcikova A, Plante MK, Vizzard MA. Increased expression of neuronal nitric oxide synthase in bladder afferent cells in the lumbosacral dorsal root ganglia after chronic bladder outflow obstruction. Brain Res. 2004;1002:35–42. doi: 10.1016/j.brainres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvarova K, Vizzard MA. Changes in galanin immunoreactivity in rat micturition reflex pathways after cyclophosphamide-induced cystitis. Cell Tissue Res. 2006;324:213–224. doi: 10.1007/s00441-005-0114-z. [DOI] [PubMed] [Google Scholar]