Introduction
Youth aged 15–24 years in sub-Saharan Africa are at high risk for HIV acquisition [1]. In South Africa, the country with the world’s largest burden of HIV infection, an estimated 21% of girls and 5% of boys have acquired HIV by the time they are 24 years old [2,3]. Thus, youth in this age range urgently need effective HIV prevention interventions to slow HIV incidence.
Although HIV counseling and testing (HCT) is designed to target HIV risk behaviors and has been widely implemented throughout the region, the impact of HCT on HIV acquisition has never been studied among youth in sub-Saharan Africa. Among HIV-uninfected adults living in sub-Saharan Africa, research with HIV endpoints suggests HCT leads to stable or modestly elevated HIV acquisition rates [4–7], a finding that may be due to behavioral disinhibition following HCT [8], confounding or other biases, or spurious associations. The implications of these findings are unclear for youth, who are behaviorally and socially different from adults [9] and more likely to be HIV-uninfected, especially at younger ages. Many youth have not yet experienced sexual debut, and early behavioral interventions, including HCT, may be more effective at establishing or reinforcing safer sexual behavior during this formative period [10]. Additionally, sexually active youth are not typically in marital or cohabitating partnerships [11], and condom use may be easier to negotiate in these less established partnerships. A cluster randomized trial, Project Accept, assessed the impact of intensive community-based HCT versus standard clinic-based HCT on HIV incidence among persons 18–24 years [12] at the community level and found no significant reduction in HIV incidence [13]. However, the impact of experiencing HCT versus not at the individual level has not been assessed among youth.
We assessed HIV acquisition among youth 15–24 years comparing those who had not been exposed to HCT to those who had been exposed to HCT using data from a household and HIV surveillance conducted by the Africa Centre for Health and Population Studies in South Africa [14].
Methods
Setting
The study was conducted in Hlabisa, a rural sub-district in KwaZulu-Natal. Overall HIV prevalence has been estimated at 23%, and HIV incidence at 3.4 per 100 person-years [15, 16]. By 2005, HCT was available in all primary health centers in the sub-district to support a decentralized HIV care and treatment program [17]. From 2005–2010, HCT was provided to persons presenting voluntarily and offered to patients presenting for antenatal care, sexually transmitted infections, and tuberculosis with high uptake [18]. Starting in mid-2010, HCT was expanded and offered to all patients. Since 2008, home- and mobile-HCT have also been available in some parts of the sub-district [19]. All clinic-, home-, and mobile-HCT services are counselor-delivered and incorporate education about how HIV is transmitted and counseling on sexual behavior risk reduction. All clients are provided with same-day results. The impact of these HCT services on HIV acquisition among youth was assessed using data sources described below.
Data Sources and Study Population
Within an enumerated part of the Hlabisa sub-district, two data sources collected within the same catchment area can be linked together: a household surveillance and an HIV and health survey. The household surveillance contains demographic and household characteristics of residents and non-residents of any age. The household surveillance is collected biannually by trained interviewers conducting in-person interviews with a household proxy. The HIV and health survey includes self-reported sexual behavior and HCT information, as well as anonymised laboratory-based HIV results. This survey is offered annually to all residents 15 years and older and 12.5% of non-residents. Data are collected by trained field-workers of the same gender. All questions are administered in isiZulu.
We conducted a cohort study using data collected from these two sources. For this analysis, in each year from 2006 to 2011, one household surveillance record and one HIV survey record were merged together using a unique identifier. These annual records were then combined over time so an individual’s HCT history, covariates, and HIV status could be assessed in a time-varying manner.
Participants were eligible for inclusion in the analysis if they were 15–24 years at any point from 2006–2010 with at least two records in the HIV survey. Participants had to have sufficient self-reported information to determine whether and when they received HCT, have been HIV-uninfected at the origin, and have at least one year of follow-up.
Exposure, Outcome, and Covariates
The primary factor of interest was if and when a participant became aware of his or her HIV status from HCT. This was assessed based on a participant’s response to a question from the HIV and health survey: “Do you know your HIV status from previous HIV testing?” The time origin for this analysis was the midpoint between the first two responses to this question. Using the midpoint allowed for an estimate of when HCT had occurred, the natural time origin for this research question. Participants who responded “no” at their first time point and “yes” at their second time point initially were considered “HCT-exposed.” Participants who responded “no” at both their first and second time points initially were considered “HCT-unexposed.” Participants who did not have at least two responses and those who responded “yes” at their first time point were excluded. Persons with two initial “yes” responses were excluded because there was missing person-time between HCT occurrence and the initiation of follow-up. Because of this missing person-time, we could not calculate a meaningful origin, determine what covariate values would have been at that origin, assess how long after the origin an event had occurred, or compare these observations to others experiencing the same amount of person-time [20].
After the origin, exposure to HCT was treated as time-varying. Participants who were HCT-exposed at the origin had all of their person-time characterized as HCT-exposed. Participants who were HCT-unexposed at the origin and all subsequent time points had all of their person-time characterized as HCT-unexposed. Participants who were initially HCT-unexposed, but later HCT-exposed had their person time split: the first portion time was characterized as HCT-unexposed and the second portion, after HCT occurred, as HCT-exposed.
The primary outcome of interest was HIV seroconversion, obtained from the HIV and health survey. For consenting participants, blood was obtained via finger prick and prepared into dried blood spots. A serial HIV testing algorithm with two ELISAs was performed in a laboratory: Vironostika HIV-1/HIV-2 Microelisa System (Biomérieux, Durham, NC, USA), followed by Wellcozyme HIV 1+2 GACELISA (Murex Diagnostics, Benelux B.V., Breukelen, The Netherlands) [21]. In the initial rounds of the HIV and health survey, participants could retrieve their HIV test results. However, due to extremely low uptake this practice was ceased, a decision that received ethical approval. Instead, all participants were encouraged to seek HCT at local health centers described above.
The laboratory-based HIV test results were used to determine the end of follow-up. The timing of seroconversion was the midpoint between the last HIV-negative result and the first HIV-positive result minus thirty days. Thus, if a change in exposure and change in outcome occurred in the same survey round, we presume the outcome preceded the exposure. Non-seroconverters were censored at the time of their last HIV-negative or indeterminate HIV test minus thirty days. Thirty days were subtracted to allow for a window period between HIV acquisition and detection through antibodies [21]. Persons who refused to provide blood for the HIV survey after their last HIV-negative test were characterized as “refusals” and censored.
Household-level characteristics, such as distance to the nearest primary health center, and individual demographic traits came from the household surveillance. Self-reported sexual behavior came from the HIV and health survey. Covariates were treated as time-varying, when appropriate.
Statistical Analyses
A directed acyclic graph was used to identify the covariates used in this analysis [22]. The covariates were gender, age, year of first HCT report, distance to the nearest primary health center, education, ever pregnant, fatherhood status, whether sexual debut had occurred, number of sex partners in the last year, and condom use at last sex. For some participants (21%), information was missing for at least one of the following covariates: education, pregnancy, fatherhood, sexual debut, number of sex partners, and condom use. Multiple imputation using Markov Chain Monte Carlo simulation was used to impute missing values of these covariates separately for the HCT-exposed and HCT-unexposed participants [23]. Five complete imputed data sets were generated using PROC MI and results were combined using PROC MIANALYZE in SAS 9.3 [24].
Descriptive statistics were calculated based on HCT status at the origin (Table 1). HIV incidence rates were calculated within strata of time-varying variables (Table 2). Cox proportional hazard models were used to estimate the association of HCT with time to HIV acquisition. We conducted two main hazard analyses: unweighted (i.e. crude) and inverse probability of exposure and censoring weighted (i.e. a marginal structural model) (Table 3) [25]; the latter of these can appropriately address time-varying confounding. In the marginal structural model, inverse probability of exposure and censoring weights were created separately based on time-fixed and time-varying covariates and then multiplied to produce final weights. Final weights were stabilized with time-fixed variables, and censored at the 1st and 99th percentiles to mitigate the influence of extreme weights [26]. Weights were distributed appropriately with a mean of 1.02 (standard deviation=0.34) and a median of 0.98 (range 0.10 – 7.78). The hazard of HIV acquisition by HCT status was estimated in a dataset with one observation per person quarter (i.e. three-month increment) using discrete time hazard models. These hazard models were estimated with pooled logistic regression using robust variance estimators and exchangeable correlation matrices. Time of HCT was used as the time origin [27].
Table 1. Characteristics of the Study Population.
Table 1 displays the distribution of factors between in the HCT-exposed and HCT-unexposed groups observed at the origin. Covariate totals may not add up to the column total due to missing data.
| HCT-unexposed N=2792 |
HCT-exposed N=1167 |
Total N=3959 |
||||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 1247 | 45% | 690 | 59% | 1937 | 49% |
| Male | 1545 | 55% | 477 | 41% | 2022 | 51% |
| Age | ||||||
| 15–19 | 2268 | 81% | 856 | 73% | 3124 | 79% |
| 20–24 | 524 | 19% | 311 | 27% | 835 | 21% |
| Year of study entry | ||||||
| 2006 | 1887 | 68% | 609 | 52% | 2496 | 63% |
| 2007 | 553 | 20% | 220 | 19% | 773 | 20% |
| 2008 | 309 | 11% | 273 | 23% | 582 | 15% |
| 2009 | 43 | 2% | 65 | 6% | 108 | 3% |
| Distance to Nearest Clinic | ||||||
| <2 km | 838 | 30% | 410 | 35% | 1248 | 32% |
| 2–5 km | 1398 | 50% | 559 | 48% | 1957 | 49% |
| >5km | 556 | 20% | 198 | 17% | 754 | 19% |
| Education | ||||||
| ≤primary | 657 | 25% | 206 | 19% | 863 | 23% |
| some secondary | 1801 | 68% | 719 | 66% | 2520 | 67% |
| ≥secondary | 188 | 7% | 170 | 16% | 358 | 10% |
| Reported sexual debut | ||||||
| Yes | 728 | 29% | 465 | 47% | 1193 | 34% |
| No | 1749 | 71% | 532 | 53% | 2281 | 66% |
| Condom Use at Last Sex (among sexually active) | ||||||
| Yes | 305 | 43% | 192 | 42% | 497 | 43% |
| No | 407 | 57% | 261 | 58% | 668 | 57% |
| Sex Partners in Last 12 months* | ||||||
| 0 | 1799 | 73% | 555 | 56% | 2354 | 68% |
| 1 | 595 | 24% | 384 | 39% | 979 | 28% |
| ≥2 | 82 | 3% | 46 | 5% | 128 | 4% |
| Ever Pregnant (females) | ||||||
| Yes | 159 | 14% | 146 | 24% | 305 | 17% |
| No | 984 | 86% | 462 | 76% | 1446 | 83% |
| Ever Father (males) | ||||||
| Yes | 51 | 4% | 34 | 8% | 85 | 5% |
| No | 1341 | 96% | 381 | 92% | 1722 | 95% |
Table 2. Incidence of HIV Acquisition by HCT Exposure and Covariates.
Table 2 presents number of events, person years, incidence rates, and confidence intervals by time-fixed and time-varying variables.
| Variable | Events | PYs | IR | (95% CI) |
|---|---|---|---|---|
| HCT | ||||
| Unexposed | 131 | 4702 | 2.79 | (2.35, 3.31) |
| Exposed | 117 | 3834 | 3.05 | (2.55, 3.66) |
| Gender | ||||
| Female | 190 | 4344 | 4.37 | (3.79, 5.04) |
| Male | 58 | 4193 | 1.38 | (1.07, 1.79) |
| Age | ||||
| 15–19 | 188 | 6570 | 2.86 | (2.48, 3.30) |
| 20–24 | 60 | 1966 | 3.05 | (2.37, 3.93) |
| Distance to Nearest Clinic | ||||
| <2 km | 81 | 2804 | 2.89 | (2.32, 3.59) |
| 2–5 km | 129 | 4166 | 3.10 | (2.61, 3.68) |
| >5km | 38 | 1567 | 2.43 | (1.76, 3.33) |
| Education | ||||
| ≤ primary | 41 | 1724 | 2.38 | (1.75, 3.23) |
| some secondary | 177 | 5733 | 3.09 | (2.66, 3.58) |
| ≥secondary | 30 | 1080 | 2.78 | (1.94, 3.97) |
| Reported sexual debut | ||||
| Yes | 171 | 3731 | 4.58 | (3.95, 5.32) |
| No | 77 | 4806 | 1.60 | (1.28, 2.00) |
| Condom use at last sex | ||||
| Yes | 56 | 1397 | 4.01 | (3.08, 5.21) |
| No | 115 | 2333 | 4.93 | (4.11, 5.92) |
| Sex Partners in Last 12 months | ||||
| 0 | 84 | 4859 | 1.73 | (1.40, 2.14) |
| 1 | 152 | 3291 | 4.62 | (3.94, 5.42) |
| ≥2 | 12 | 389 | 3.10 | (1.76, 5.46) |
| Ever Pregnant (females) | ||||
| Yes | 78 | 1153 | 6.77 | (5.42, 8.45) |
| No | 112 | 3191 | 3.51 | (2.92, 4.22) |
| Ever Father (males) | ||||
| Yes | 6 | 219 | 2.72 | (1.22, 6.04) |
| No | 52 | 3972 | 1.31 | (1.00, 1.72) |
PY=person-year; IR=incidence rate; CI=confidence interval
Table 3. Hazard Ratios Comparing HCT-Exposed to HCT-Unexposed Participants.
All hazard ratios compare the hazard of HIV acquisition among HCT-exposed to HCTunexposed participants. The main unweighted analysis displays the results of a model with no weights and the main weighted analysis displays the results of a model with inverse probability of exposure and censoring weights. The three stratified analyses display the results of the main weighted model with interaction terms (for gender, age, and time since HCT). Sensitivity analyses display results of main effect models in a non-imputed dataset (i.e. a complete case analysis), in a model with only exposure weights, and in model with standard covariate adjustment, rather than weights. Except in the specified sensitivity analysis, all models are implemented in a dataset with imputed values. All models are implemented in a discrete time dataset.
| HR | (95% CI) | |
|---|---|---|
| Main Analyses | ||
| Unweighted | 1.02 | (0.79, 1.31) |
| Weighted | 0.59 | (0.45, 0.78) |
| Stratified Analyses (weighted and imputed) | ||
| Gender Stratified | ||
| Women | 0.57 | (0.41, 0.78) |
| Men | 0.66 | (0.37, 1.18) |
| Age Stratified | ||
| 15–19 | 0.59 | (0.43, 0.82) |
| ≥20 | 0.54 | (0.31, 0.97) |
| Time Stratified | ||
| ≤1.5 years | 0.65 | (0.40, 1.05) |
| >1.5 years | 0.57 | (0.40, 0.79) |
| Sensitivity Analyses | ||
| Weighted, complete case | 0.64 | (0.46, 0.89) |
| Weighted with exposure weights only | 0.59 | (0.44, 0.78) |
| Covariate Adjusted | 0.65 | (0.50, 0.86) |
HCT=HIV counseling and testing, HR=hazard ratio, CI=confidence interval
Sensitivity Analyses
We conducted additional analyses to explore whether results were sensitive to model structure and assumptions. We implemented a marginal structural model with only inverse probability of exposure weights, but no censoring weights, to assess whether exposure or censoring weights were driving the difference between the unweighted and weighted effects. Next, we implemented a complete case analysis (i.e. an analysis among individuals with no missing data) to assess the extent to which the imputation assumptions could be driving the results. Finally, we implemented a model using covariate adjustment, rather than weights because such methods are more familiar. However, in the presence of time-varying confounding affected by prior exposure, covariate-adjusted Cox models can result in biased estimates of effect [25, 26].
Statistical analyses were implemented using SAS 9.3.
Ethics Approval
Permission for collecting the household surveillance and HIV and health survey data was obtained from the University of KwaZulu-Natal research ethics committee, and renewed annually. The current analysis was approved by the Public Health-Nursing Institutional Review Board at the University of North Carolina, Chapel Hill.
Results
Study Population
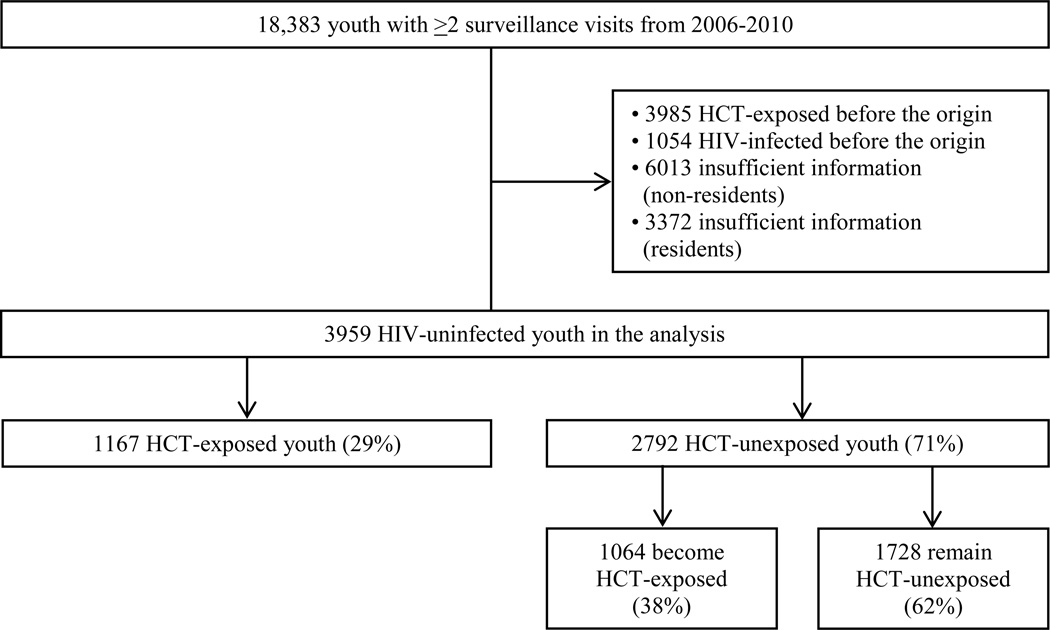
Overall, 18,383 persons 15–24 years were enumerated in the catchment area in at least two surveillance rounds from 2006–2010 (Figure 1). Youth who were HIV-infected before the origin (N=1054, 6%) were excluded as were youth who reported knowing their HIV status at their first HCT report (N=3985, 22%), since we could not estimate the origin. Persons with missing HCT status, HIV status, or both were also excluded. Most of these excluded youth were migrants enumerated as household members but not residing in the catchment area (N=6013, 33%). Others resided in the catchment area but had insufficient information to classify their exposure (HCT), outcome (HIV status), or both (N=3372, 18%). The study sample included the remaining youth (N=3959, 22%). Of these 1167 (29%) were initially HCT-exposed and 2792 (71%) initially HCT-unexposed. Of the persons who were initially HCT-unexposed, 38% became HCT-exposed during follow-up (N=1064) and the rest remained HCT-unexposed (N=1728). Among persons excluded for having missing information, some had sufficient HCT information for initial classification (N=1611, 48%). Of these excluded persons, the initial prevalence of HCT exposure was comparable (25%) to the initial prevalence of HCT exposure among included youth (29%) and covariate distributions were similar.
Figure 1. Study Population.
Figure 1 illustrates the number of youth 15–24 in the catchment area with at least two surveillance time points from 2006–2010. It shows the proportion included and excluded from the analysis, and the proportion who experienced HCT initially and over time.
Population Characteristics
The study population was 51% male and 49% female with a median age of 17 (interquartile range: 16–20) (Table 1). The minority of women (17%) reported ever having been pregnant and few men (5%) reported ever having fathered a child. Initially, one third of participants reported sexual debut (34%) and ≥1 sex partners in the last year (32%), and 4% reported ≥2 partners in the last year. Of those who were sexually active, 43% reported condom use at last sex. Persons initially HCT-exposed were more likely to be female (59% versus 45%), sexually experienced (47% versus 29%), and previously pregnant (24% versus 14%) than those who were HCT-unexposed.
Main Analyses
Youth experienced 248 seroconversions over 8536 person-years of follow-up, an incidence rate of 2.91 per 100 person-years [95% confidence interval (CI): 2.56, 3.28]. In periods after HCT exposure, participants experienced 117 HIV events over 3834 person-years (HIV incidence rate: 3.05 per 100 person-years). In periods of HCT non-exposure, participants experienced 131 HIV events over 4702 person-years (HIV incidence rate: 2.79 per 100 person-years) (Table 2).
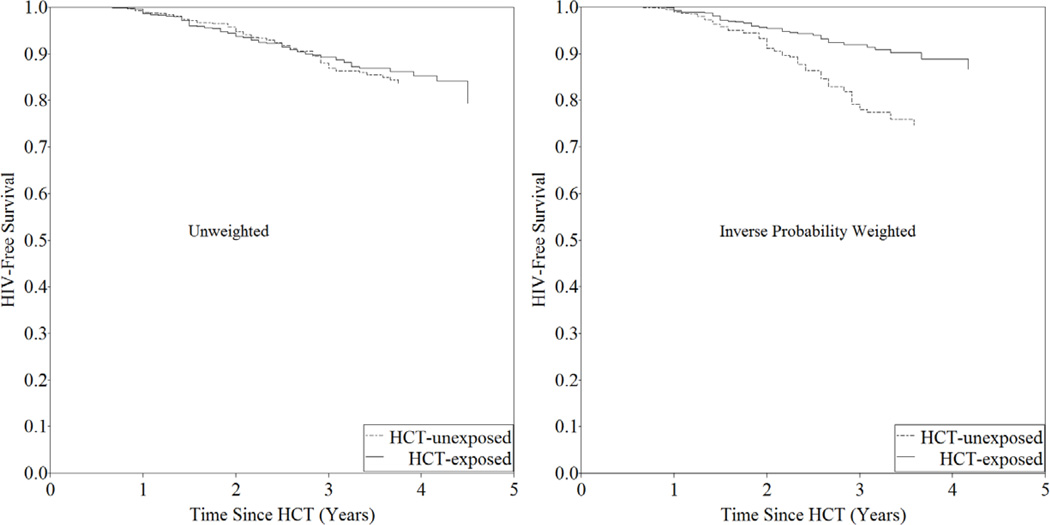
In unweighted Cox analysis, comparing HCT-exposed persons to HCT-unexposed persons, the hazard of HIV acquisition was 1.02 (95% CI: 0.79, 1.31) (Table 3, Figure 2). However, in the marginal structural model with inverse probability of exposure and censoring weights, the hazard of HIV acquisition was 0.59 (95% CI: 0.45, 0.78) (Table 3). Most of the change in estimate was attributed to exposure weighting for gender, sexual debut, and pregnancy. Hazard ratios were similar in the first 1.5 years after HCT (HR: 0.65 (95% CI: 0.40, 1.05) as in the next three years after HCT (HR: 0.57, 95% CI: 0.40, 0.79), suggesting durability. The effect was similar among men (HR: 0.66, 95% CI: 0.37, 1.38) and women (HR: 0.57, 95% CI: 0.41, 0.78), as well as between those <20 years (HR: 0.59, 95% CI: 0.43, 0.82) and those who were ≥20 years (HR: 0.56, 95% CI: 0.31, 0.97).
Figure 2. HIV-free Survival by HIV Counseling and Testing Status: Unweighted and Inverse Probability Weighted Curves.
Figure 2 depicts the unweighted and weighted Kaplan Meier survival curves comparing those who were HCT-unexposed to those who were HCT-exposed. The inverse probability weighted graph is constructed in a population with time divided into person-months.
Sensitivity Analyses
In a marginal structural model with inverse probability of exposure weights, but no censoring weights, the hazard ratio was essentially identical to the main effect: 0.59 (95% CI: 0.44, 0.78), suggesting confounding, not refusal, was driving the difference between the unweighted and weighted effects (Table 3). In a marginal structural model without imputed covariates (i.e. a complete case analysis), the hazard ratio was similar, but estimated less precisely (HR: 0.64, 95% CI: 0.46, 0.89), suggesting the imputation model was not driving the results. In the covariate-adjusted model the hazard ratio was 0.65 (95% CI: 0.50, 0.86). The similarity between time-varying adjusted model and the marginal structural model suggests most of the confounding was simple time-varying confounding, not time-varying confounding from covariates being affected by prior exposure.
Discussion
We observed that youth in this high prevalence setting experienced a 41% reduction in the hazard of HIV acquisition following HCT, an effect sustained for 4.5 years. Youth who had experienced HCT were more likely to be female, pregnant, and to have experienced sexual debut. In short, the HCT-exposed youth were at greater risk for HIV acquisition. After accounting for these risk factors in longitudinal multivariable analyses, HCT was associated with lower HIV incidence.
In contrast, Project Accept, a study assessing community HCT compared to clinic HCT had no effect on HIV acquisition among persons 18–24 [13]. The difference between our results and Project Accept results may be explained by the differences in study design. Our study compared any HCT versus no HCT, whereas Project Accept compared community HCT to clinic HCT. Additionally, we measured HIV acquisition at the individual level, whereas Project Accept measured HIV incidence at the community level.
HCT may affect youth differently than adults. Among adults, HCT appears to have no effect or a small harmful effect on HIV acquisition [4–7]. The differential effects between youth and adults may be related to differences in life stages. Youth are still in a formative period with recent or no sexual debut, and may be better able to adopt counseling messages than adults, who have formed behavioral habits [10]. Additionally, in this setting, youth are primarily in non marital, non-cohabiting partnerships [11]. HCT may more readily facilitate protective sexual behaviors in these less stable partnerships [28].
Understanding the mechanism underlying the effect is a key question for future investigation. Changes in sexual behavior following HCT are one possible explanation as those in the catchment area who are aware of their HIV status are more likely to report condom use than those who are unaware [29]. Another potential explanation is that youth who sought HCT and knew they were HIV-uninfected were more likely to select other HIV-uninfected partners, though the prevalence and effect of sero-sorting in the African context is poorly understood. HCT referral to medical male circumcision, a prevention intervention for HIV-uninfected males, is unlikely to explain the effect, as it was not available until the end of the study period with low coverage.
Expanding age-appropriate access to HCT among youth could have important public health benefits. South Africa’s recent decision to provide HCT in secondary schools is an important step towards greater access. However, questions about optimal timing and delivery modalities remain. South African youth often report stigmatizing care-seeking environments, and assessing whether youth-oriented HCT models improve service uptake and effectiveness in community-, clinic-, and school-based settings is an area for future investigation [9].
The benefits of HCT will likely be enhanced by additional prevention interventions. In this analysis, HIV-uninfected youth who received HCT still experienced a high HIV incidence rate (3 per 100 person-years), underscoring the importance of combination prevention. For HIV-uninfected youth, HCT can be an entry-point for medical male circumcision among young men and pre-exposure prophylaxis for high risk young men and women [30]. For HIV-infected youth, early HCT is necessary for early antiretroviral therapy for “treatment as prevention [31].” Developing appropriate linkages from HCT to these biomedical services for both HIV-infected and HIV-uninfected youth is a priority.
Large groups of youth were excluded or lost from this analysis, raising concerns about selection bias and non-generalizability. First, many persons who were HCT-exposed at the time of first report were excluded because it was not possible to estimate the time of HCT exposure or covariate values at that time. Exclusion of this high risk group could have made HCT appear more protective than it truly was. Additionally, we excluded persons with insufficient HCT information, HIV information, or both. About two thirds (64%) of these persons were nonresidents, typically persons residing outside of the catchment area for work. In contrast, only 4% of youth in the analysis were non-residents, suggesting findings generalize best to residents. Of the remaining excluded youth, those with sufficient HCT information for initial classification had a comparable exposure and covariate distribution to the included youth, suggesting the excluded youth may not have been different from the included youth.
We did not have a perfect measure of HCT. We wanted to explore whether the frequency of HCT had an impact on HIV incidence, but were only able to assess first HCT, due to the phrasing of the question. Additionally, assessment of HCT was based on self-report, with the potential for misreporting. One possible sign of misreporting was that some youth reported HCT, even though they had not reported sexual debut. However, HCT in non-sexually active persons is expected when HCT is routinely offered, such as clinical settings with opt-out HCT. In spite of possible misreporting, trends in the HCT data are consistent with observed trends in the catchment area: females were more likely to report HCT than males, pregnant females were more likely to report HCT than non-pregnant females, and increases in age and calendar year were associated with increases in HCT. These associations are consistent with expectation and lend credibility to the exposure assessment.
This analysis may not have fully accounted for all factors influencing the effect of HCT on HIV acquisition. Youth who sought HCT may have had unmeasured traits that made them less risky than those who did not seek HTC. Additionally, some sexual behaviors, such as sexual debut, may have been under-reported due to social desirability, a likely phenomenon as many HIV acquisition events (77/248) occurred among persons who reported no sexual behavior. An alternative explanation for acquisition among youth who did not report debut is sexual abuse. In South Africa, boys 15–26 frequently report perpetrating rape [31] and school-age boys and girls report being victims of forced or coerced sex [33], events that may not have been reported in questions about debut and partnerships.
Given these potential sources of bias, replication of these results is warranted. In light of the rapid scale-up of HCT in the region and the unethical nature of withholding HCT, this question must be studied in non-randomized settings.
HCT is a necessary first step for reducing HIV transmission through linkage of HIV-infected persons to treatment. Based on our observations, HCT appears effective at reducing HIV acquisition among HIV-uninfected youth, even in the absence of biomedical prevention. These findings provide support to South Africa’s recent decision to implement HCT in secondary schools [34], and suggest HCT may contribute to slowing HIV acquisition among this at-risk population.
Supplementary Material
Acknowledgments
NER conceptualized the study under the guidance of all co-authors. NER conducted analyses with guidance from DW, AEP, WCM, TB, and M-LN. M-LN and TB are investigators on the demographic and HIV surveillance and oversee data collection. NER drafted the initial manuscript and revisions were made by all co-authors. All co-authors approved the final draft.
Funding
The Africa Centre for Health and Population Studies receives core funding for its household surveillance and HIV and health surveillance from the Wellcome Trust, UK (grant # 082384/Z/07/Z). NER was supported by the DHHS/NIH/NIAID (T32 AI 007001-34), the UNC Hopkins Morehouse Tulane Fogarty Global Health Fellows Program (R25TW009340), and University of North Carolina Center for AIDS Research (NIH P30 AI50410). DW was supported by NIH/NIAID 2P30-AI064518-06, the Duke Center for AIDS Research. TB was supported by R01 HD058482-01 from the National Institute of Child Health and Development and R01 MH083539-01 from the National Institute of Mental Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Duplicate publication
Results have not been published previously, but will be presented at the Conference on Retroviruses and Opportunistic Infections in March 2013 as a research poster.
References
- 1.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010 [Google Scholar]
- 2.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people's sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19:1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 3.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Pillay-van Wyk V, et al. South African national HIV prevalence, incidence, behavior and communication survey 2008: a turning tide among teenagers? Cape Town. 2009 [Google Scholar]
- 4.Matovu JK, Gray RH, Makumbi F, Wawer MJ, Serwadda D, Kigozi G, et al. Voluntary HIV counseling and testing acceptance, sexual risk behavior and HIV incidence in Rakai, Uganda. AIDS. 2005;19:503–511. doi: 10.1097/01.aids.0000162339.43310.33. [DOI] [PubMed] [Google Scholar]
- 5.Corbett EL, Makamure B, Cheung YB, Dauya E, Matambo R, Bandason T, et al. HIV incidence during a cluster-randomized trial of two strategies providing voluntary counselling and testing at the workplace, Zimbabwe. AIDS. 2007;21:483–489. doi: 10.1097/QAD.0b013e3280115402. [DOI] [PubMed] [Google Scholar]
- 6.Sherr L, Lopman B, Kakowa M, Dube S, Chawira G, Nyamukapa C, et al. Voluntary counselling and testing: uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. AIDS. 2007;21:851–860. doi: 10.1097/QAD.0b013e32805e8711. [DOI] [PubMed] [Google Scholar]
- 7.Machekano R, McFarland W, Mbizvo MT, Bassett MT, Katzenstein D, Latif AS. Impact of HIV counselling and testing on HIV seroconversion and reported STD incidence among male factory workers in Harare, Zimbabwe. Cent Afr J Med. 1998;44:98–102. [PubMed] [Google Scholar]
- 8.Cremin I, Nyamukapa C, Sherr L, Hallett TB, Chawira G, Cauchemez S, et al. Patterns of self-reported behaviour change associated with receiving voluntary counselling and testing in a longitudinal study from Manicaland, Zimbabwe. AIDS Behav. 2010;14:708–715. doi: 10.1007/s10461-009-9592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacPhail C, Pettifor A, Moyo W, Rees H. Factors associated with HIV testing among sexually active South African youth aged 15–24 years. AIDS Care. 2009;21:456–467. doi: 10.1080/09540120802282586. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel HM, Futterman DC. Adolescents and HIV: prevention and clinical care. Curr HIV/AIDS Rep. 2009;6:100–107. doi: 10.1007/s11904-009-0015-y. [DOI] [PubMed] [Google Scholar]
- 11.Hosegood V, McGrath N, Moultrie T. Dispensing with marriage: Marital and partnership trends in rural KwaZulu-Natal, South Africa, 2000–2006. Demogr Res. 2009;20:279–312. doi: 10.4054/DemRes.2009.20.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khumalo-Sakutukwa G, Morin SF, Fritz K, Charlebois ED, van Rooyen H, Chingono A, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008;49:422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates T, Eshleman S, Chariyalertsak S, Chingono A, Gray G, Mbwambo J, et al. Community-level Reductions in Estimated HIV Incidence: HIV Prevention Trials Network 043, Project Accept. Conference on Retroviruses and Opportunistic Infections. 2013 20th annual: session 8, abstract 30. [Google Scholar]
- 14.Tanser F, Hosegood V, Barnighausen T, Herbst K, Nyirenda M, Muhwava W, et al. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37:956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welz T, Hosegood V, Jaffar S, Batzing-Feigenbaum J, Herbst K, Newell ML. Continued very high prevalence of HIV infection in rural KwaZulu-Natal, South Africa: a population-based longitudinal study. AIDS. 2007;21:1467–1472. doi: 10.1097/QAD.0b013e3280ef6af2. [DOI] [PubMed] [Google Scholar]
- 16.Barnighausen T, Tanser F, Newell ML. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Res Hum Retroviruses. 2009;25:405–409. doi: 10.1089/aid.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, Newell ML. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010;88:593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimbindi N, Barnighausen T, Newell ML. Almost universal coverage: HIV testing among TB patients in a rural public programme. Int J Tuberc Lung Dis. 2012;16:708. doi: 10.5588/ijtld.11.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maheswaran H, Thulare H, Stanistreet D, Tanser F, Newell ML. Starting a home and mobile HIV testing service in a rural area of South Africa. J Acquir Immune Defic Syndr. 2012;59:e43–e46. doi: 10.1097/QAI.0b013e3182414ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole SR, Hudgens MG. Survival analysis in infectious disease research: describing events in time. AIDS. 2010;24:2423–2431. doi: 10.1097/QAD.0b013e32833dd0ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BioMerieux. Vironostika (R) HIV-1 Plus O Microelisa System. Durham, NC: 2003. [Google Scholar]
- 22.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 23.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 24.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development. Rockville, MD: SAS Institute; [cited 2012 May 24]. Available from: http://support.sas.com/rnd/app/papers/multipleimputation.pdf. [Google Scholar]
- 25.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westreich D, Cole SR, Tien PC, Chmiel JS, Kingsley L, Funk MJ, et al. Time scale and adjusted survival curves for marginal structural cox models. Am J Epidemiol. 2010;171:691–700. doi: 10.1093/aje/kwp418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehle TM, Hallett TB, Shisana O, Pillay-van Wyk V, Zuma K, Carrara H, et al. A decline in new HIV infections in south africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS One. 2010;5:e11094. doi: 10.1371/journal.pone.0011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath N, Eaton JW, Tanser F, Bärnighausen T, Newell ML. Sexual behaviour trends by gender in a rural South African population-based cohort during the era of scaled-up access to VCT and ART, 2005–2010. (Oral Poster) XIX International AIDS Conference; 22–27 Jul 2012; Washington DC, USA. [Google Scholar]
- 30.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jewkes R, Nduna M, Jama Shai N, Dunkle K. Prospective study of rape perpetration by young South african men: incidence & risk factors. PLoS One. 2012;7:e38210. doi: 10.1371/journal.pone.0038210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson N, Paredes-Solis S, Milne D, Omer K, Marokoane N, Laetsang D, et al. Prevalence and risk factors for forced or coerced sex among school-going youth: national cross-sectional studies in 10 southern African countries in 2003 and 2007. BMJ Open. 2012;2:e000754. doi: 10.1136/bmjopen-2011-000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naughton J, Hughes H, Wilkinson L, Boyles T. HIV counselling and testing in South African schools. Lancet. 2011;377:1748. doi: 10.1016/S0140-6736(11)60735-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.