Abstract
BACKGROUND:
The birth prevalence of cerebral palsy varies over time among very preterm infants, and the reasons are poorly understood.
OBJECTIVE:
To describe the variation in the prevalence of cerebral palsy among very preterm infants over time, and to relate these differences to other maternal or neonatal factors.
METHODS:
A population-based cohort of very preterm infants was evaluated over a 20-year period (1988 to 2007) divided into four equal epochs.
RESULTS:
The prevalence of cerebral palsy peaked in the third epoch (1998 to 2002) while mortality rate peaked in the second epoch (1993 to 1997). Maternal anemia, tocolytic use and neonatal need for home oxygen were highest in the third epoch.
CONCLUSIONS:
Lower mortality rates did not correlate well with the prevalence of cerebral palsy. Maternal risk factors, anemia and tocolytic use, and the newborn need for home oxygen were highest during the same epoch as the peak prevalence of cerebral palsy.
Keywords: Cerebral palsy, Infant, Population-based, Premature
Abstract
HISTORIQUE :
La prévalence de paralysie cérébrale à la naissance varie au fil du temps chez les nourrissons très prématurés, et on en comprend mal les raisons.
OBJECTIF :
Décrire la variation de la prévalence de paralysie cérébrale chez les nourrissons très prématurés au fil du temps et les relier à d’autres facteurs relatifs à la mère ou à la période néonatale.
MÉTHODOLOGIE :
Les chercheurs ont évalué une cohorte de nourrissons très prématurés sur 20 ans (1988 à 2007), divisée en quatre périodes d’égale longueur.
RÉSULTATS :
La prévalence de paralysie cérébrale a atteint un pic pendant la troisième période (1998 à 2002), tandis que le pic du taux de mortalité est survenu pendant la deuxième période (1993 à 1997). L’anémie et l’utilisation de tocolytiques chez la mère, ainsi que l’assistance ventilatoire néonatale à domicile, étaient plus élevées pendant la troisième période.
CONCLUSIONS :
Les taux de mortalité plus faibles n’étaient pas bien corrélés avec la prévalence de paralysie cérébrale. Les facteurs de risque de la mère, c’est-à-dire l’anémie et l’ utilisation de tocolytiques, de même que l’assistance ventilatoire du nouveau-né à domicile, étaient tous plus élevés pendant la période qui s’associait à la plus forte prévalence de paralysie cérébrale.
The prevalence of cerebral palsy in the general population is 1.5 to 2.5 per 1000 live births. In the preterm infant, this figure is approximately 10 times higher (1,2). Because cerebral palsy is associated with potentially life-altering impairments and early death (3), especially if it is severe or associated with gastrostomy feeding (4), it is important to understand trends in the prevalence of this condition.
In preterm infants, an increasing prevalence of cerebral palsy has been documented and is believed to be due to improving survival rates (5), although this has not been universally supported (6). As noted in a Swedish cohort, when examined over time, the prevalence of cerebral palsy appears to follow an undulating pattern (7). To provide a population perspective of cerebral palsy prevalence in the preterm infant, the denominator should be the entire birth population, as in the ‘fetus at risk’ model (8). Previous work has shown that the prevalence of cerebral palsy among very preterm infants increased in Nova Scotia between 1993 and 2002 (5). Increasing survival at the borderline of viability may partially explain the increase in the prevalence of cerebral palsy; however, the explanation is likely to be more complex.
The purpose of the present study was to extend the previous observations to examine the prevalence of cerebral palsy in the five-year epochs preceding and following this initial work, and to identify trends over time.
METHODS
Study population
All very preterm liveborn infants (<31 weeks’ gestational age) born to mothers who resided in Nova Scotia were enrolled in the Perinatal Follow-Up Program, with the exception of the first five years (1988 to 1992). During this time period, infants in the four counties in Cape Breton and Cumberland County were not enrolled in the Perinatal Follow-Up Program; therefore, the population includes the remaining 13 counties in Nova Scotia. In 1993, the Perinatal Follow-Up Program was expanded to include all 18 counties in Nova Scotia.
Setting
Three tertiary-level hospitals cared for very preterm infants born to Nova Scotia residents: the IWK Health Centre (Halifax, Nova Scotia); the Cape Breton Regional Hospital (Sydney, Nova Scotia); and the Moncton City Hospital (Moncton, New Brunswick). All three hospitals were routinely surveyed for any very preterm infants born to Nova Scotia residents cared for at their institutions and included in the Perinatal Follow-Up Program. To ensure completeness of the Perinatal Follow-Up Program database, the medical director of the program (MJV) travelled to the two hospitals outside Halifax and obtained maternal and newborn information for inclusion in the database. The maternal and infant charts at the IWK Health Centre in Halifax were abstracted by the program database manager and routinely audited by the medical director to ensure accuracy of the data. Deaths in the labour and delivery ward at any hospital in Nova Scotia (tertiary or regional or the Moncton City Hospital) were also noted and their data included in the database. These strategies were used to ensure that the present study was truly a population-based study.
Follow-up
Following discharge from the neonatal intensive care unit (NICU), the infant was scheduled for visits in the Perinatal Follow-Up Program at four, eight, 12, 18 and 24 months’ corrected gestational age, which included a complete history, physical and neurological examination, and a developmental screening test. Although these were the scheduled times for assessments, assessments occasionally occurred outside these target dates (eg, on travel clinics). A final assessment occurred at 36 months’ corrected age and included a Bayley Scales of Infant Development (later the Bayley Scales of Infant and Toddler Development, third edition). For the purposes of the present study, a neurological examination between 12 and 42 months’ corrected gestational age was used to determine the presence or absence of cerebral palsy and to define the gross motor functional classification (9). Cerebral palsy was defined as a disorder of control of movement or posture secondary to a nonprogressive brain lesion (10). Travel clinics were conducted twice per year in five other centres in Nova Scotia to improve the attendance rate of families enrolled in the program and to improve cooperation of the child for assessments.
Data sources
The Nova Scotia Atlee Perinatal Database (11,12) was used to determine the entire live birth population of Nova Scotia in four five-year epochs (1988 to 1992, 1993 to 1997, 1998 to 2002 and 2003 to 2007, with the exception of the five counties previously identified from 1988 to 1992). This database contains information about all live born infants, deaths in live born infants and information regarding their mothers, who were residents of Nova Scotia. All live-born infants <31 weeks’ gestation born to residents in Nova Scotia during the four epochs were enrolled in the Perinatal Follow-Up Program. The Perinatal Follow-Up Program Database is modelled on the Nova Scotia Atlee Perinatal Database (11,12). It includes illnesses and treatments for mothers during the pregnancy, and for newborns during the NICU hospital admission. It also includes follow-up of the infants after discharge including the presence or absence of cerebral palsy. The database uses the Scientific Information Retrieval (SIR version XS-01-12) platform.
Definitions
Infant death was defined as death before one year of age, and the mortality rate in the very preterm infant was defined as the deaths in this group as a proportion of the entire live birth population in Nova Scotia for each five-year epoch. Similarly, cerebral palsy prevalence was defined as the number of very preterm infants with cerebral palsy as a proportion of the entire live birth population in Nova Scotia for each epoch. Gestational age was determined shortly after birth in the following hierarchical order:
conception dating if mother was receiving fertility treatments;
last menstrual period if it corresponded to ultrasound dating (13) within 10 days (14);
ultrasound if it was >10 days difference from the last menstrual period or no dates were known;
physical examination of the infant at birth if none of the three preceding estimates were available.
RESULTS
In 2011, Nova Scotia had a population of approximately 950,000 (15). All 1430 very preterm liveborn infants (<31 weeks’ gestational age) born between January 1, 1988 and December 31, 2007 to mothers in Nova Scotia were included in the present study (Figure 1). Basic demographic data are presented in Table 1 and indicate that maternal age increased throughout the 20 years of the study (P<0.001) but the proportion of single-parent families was not different (P not significant). Birth weight increased during the 20 years (P<0.03) but gestational age did not (P not significant).
Figure 1).
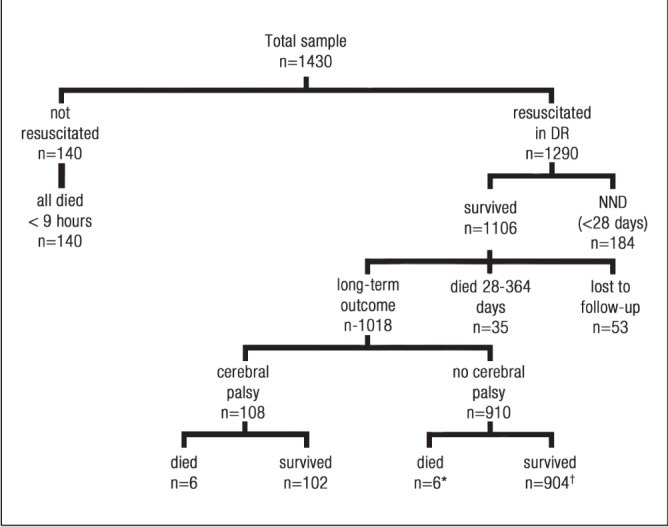
Flow of patients in the study. *Two children died during the 12- to 42-month window but were believed to be free from cerebral palsy before the window; †One child was not examined during the 12- to 42-month window but was subsequently examined at 51 months of age and was free from cerebral palsy
TABLE 1.
Demographic data regarding very preterm infants (<31 weeks’ gestational age) in Nova Scotia according to five-year epochs, 1988 to 2007
| Characteristic | Epoch | P | |||
|---|---|---|---|---|---|
|
| |||||
| 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | ||
| Maternal age, years, mean ± SD | 27.0±5.8 | 28.1±5.9 | 28.6±6.2 | 28.8±5.6 | 0.001 |
| Single-parent family, n/n (%) | 57/267 (21.3) | 53/336 (15.8) | 77/331 (23.3) | 62/339 (18.3) | NS |
| Birth weight, g, mean ± SD | 998±333 | 994±346 | 1048±399 | 1062±379 | 0.03 |
| Gestational age, weeks, mean ± SD | 27.0±2.4 | 27.2±2.6 | 27.0±2.7 | 27.1±2.6 | NS |
NS Not statistically significant
A total of 140 (9.8%) infants were believed to be nonviable and, thus, were not resuscitated in the delivery room (all died within 9 h of birth, most within the first hour). Of the remaining 1290, 1106 (85.7%) survived at least 28 days. Of the 1106 survivors, 1018 survived beyond one year, 35 died between 28 and 364 days and 53 survived but were lost to follow-up. Of the 1018 infants surviving for at least one year, 108 (10.6%) were known to have cerebral palsy and 910 (89.4%) were known to not have cerebral palsy. Of those with cerebral palsy, six (5.6%) died at >1 year of age and, of those without cerebral palsy, six (<1%) died at >1 year of age (P<0.001). One of the infants with cerebral palsy was initially believed to be nonviable and, therefore, was not resuscitated in the delivery room. He was found to be alive >1 h later and was subsequently resuscitated. He developed severe quadriplegic cerebral palsy and eventually died of a respiratory illness at 21 months of age.
The birth population of Nova Scotia (except for five counties in the first five-year epoch; see above) for the entire 20 years was 191,477. The births were divided into four five-year epochs (1988 to 1992 [epoch 1], 1993 to 1997 [epoch 2], 1998 to 2002 [epoch 3] and 2003 to 2007 epoch 4] [Table 2]). The very preterm birth rate, as a proportion of the total birth population, significantly increased over the 20-year time period (P<0.05); however, the mortality rate, which varied from a high of 21.21 to a low of 15.37 per 10,000 live births, did not show a statistically significant trend. The proportion of very preterm infants not resuscitated in the delivery room was also not significantly different. Because of the increasing birth rate of very preterm infants, the proportion of surviving very preterm infants was significantly higher with time (P<0.002). The prevalence of cerebral palsy peaked in epoch 3 (the peak year was 2001) at 9.18 per 10,000 live births (P<0.002). Of note, this increase was exclusively in level 1 (9), the mild end of the spectrum of cerebral palsy severity (Table 3). While there have been changes in personnel during this 20-year time period, the Medical Director (MJV) has been the same over this time and confirmed the diagnosis and severity of cerebral palsy by a direct chart audit in all infants with cerebral palsy. Several intrapartum factors showed significant differences with time, but only two showed peak prevalences during the third epoch (maternal anemia [hemoglobin level <100 g/L] and use of tocolytics) (Table 4). For newborn risk factors, only one peaked during epoch 3 (the need for home oxygen) (Table 5). Neither intraventricular hemorrhage nor periventricular leukomalacia were significantly different according to time.
TABLE 2.
Infants not resuscitated, infant mortality and cerebral palsy prevalence as a proportion of the entire live birth population of Nova Scotia in very preterm infants (<31 weeks’ gestational age) according to five-year epoch, 1988 to 2007
| Epoch | P | ||||
|---|---|---|---|---|---|
|
| |||||
| 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | ||
| Total number of live births in Nova Scotia | 48,841 | 53,860 | 45,770 | 43,006 | |
| Total number of very preterm infants born, n (rate per 10,000 live births) | 319 (65.31) | 418 (77.61) | 348 (76.03) | 345 (80.22) | 0.05 |
| Number of very preterm infants not resuscitated at birth, n (rate per 10,000 live births) | 27 (5.5) | 49 (9.1) | 36 (7.9) | 28 (6.5) | NS |
| Number of very preterm infant deaths, n (rate per 10,000 live births) | 97 (19.9) | 114 (21.2) | 82 (18.0) | 66 (15.4) | 0.19 |
| Total number of very preterm infants surviving at least one year, n (rate per 10,000 live births) | 222 (45.45) | 304 (56.44) | 266 (58.12) | 279 (64.88) | 0.002 |
| Number of very preterm infants with cerebral palsy, n (rate per 10,000 live births) | 27 (5.5) | 23 (4.3) | 42 (9.2) | 16 (3.7) | 0.002 |
Excludes four Cape Breton counties and Cumberland county in the first epoch. NS Not statistically significant
TABLE 3.
Severity of cerebral palsy in very preterm infant (<31 weeks’ gestational age) survivors using Palisano gross motor functional classification in Nova Scotia according to five-year epoch, 1988 to 2007
| Epoch | Total | ||||
|---|---|---|---|---|---|
|
| |||||
| 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | ||
| No cerebral palsy | 185 (87.3) | 265 (92.0) | 209 (83.3) | 246 (93.9) | 906 (89.3) |
| Level 1 (mild cerebral palsy) | 17 (8.0) | 12 (4.2) | 31 (12.4) | 11 (4.2) | 71 (7.0) |
| Level 2 to 5 (moderate to severe cerebral palsy) | 10 (4.7) | 11 (3.8) | 11 (4.4) | 5 (1.9) | 37 (1.2) |
Data presented as n (%)
TABLE 4.
Changes in intrapartum risk factors in very preterm infants (<31 weeks’ gestational age) as a proportion of the entire live birth population of Nova Scotia according to five-year epoch, 1988 to 2007
| Epoch | P | ||||
|---|---|---|---|---|---|
|
| |||||
| 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | ||
| Total number of live births in Nova Scotia | 48,841 | 53,860 | 45,770 | 43,006 | |
| Antenatal corticosteroids, n (rate per 10,000 live births) | 140 (28.8) | 308 (57.5) | 258 (56.7) | 262 (61.3) | 0.001 |
| Multiple births, n (rate per 10,000 live births) | 72 (14.8) | 107 (19.9) | 91 (19.9) | 105 (24.5) | 0.012 |
| Chorioamnionitis, n (rate per 10,000 live births) | 73 (15.0) | 65 (12.1) | 40 (8.8) | 31 (7.2) | 0.002 |
| Maternal MgSO4 use, n (rate per 10,000 live births) | 51 (8.3) | 97 (18.0) | 69 (15.1) | 29 (6.7) | 0.001 |
| Maternal anemia (hemoglobin level <100 g/L), n (rate per 10,000 live births) | 26 (5.3) | 45 (8.4) | 42 (9.2) | 12 (2.8) | 0.001 |
| Tocolytic use, n (rate per 10,000 live births) | 7 (1.4) | 75 (3.9) | 113 (24.8) | 80 (18.6) | 0.001 |
Excludes four Cape Breton counties and Cumberland county in the first epoch
TABLE 5.
Changes in neonatal risk factors in very preterm infants (<31 weeks’ gestational age) as a proportion of the entire live birth population of Nova Scotia according to five-year epoch, 1988 to 2007
| Epoch | P | ||||
|---|---|---|---|---|---|
|
| |||||
| 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | ||
| Total number of live births in Nova Scotia | 48,841 | 53,860 | 45,770 | 43,006 | |
| Grade 3 or 4 IVH, n (rate per 10,000 live births) | 41 (8.4) | 33 (6.1) | 35 (7.6) | 33 (7.7) | NS |
| Cystic PVL, n (rate per 10,000 live births) | 17 (3.5) | 28 (5.2) | 18 (3.9) | 17 (4.0) | NS |
| Steroids for BPD, n (rate per 10,000 live births) | 49 (10.0) | 101 (18.8) | 76 (16.6) | 54 (12.6) | 0.001 |
| Home oxygen use, n (rate per 10,000 live births) | <5 (<0.9)* | 5 (0.9) | 21 (4.6) | <5 (<0.9)* | 0.001 |
| Cystic bronchopulmonary dysplasia, n (rate per 10,000 live births) | 27 (5.5) | 38 (7.1) | 58 (12.7) | 62 (14.4) | 0.001 |
| Caffeine use, n (rate per 10,000 live births) | 138 (28.3) | 226 (42.1) | 198 (43.4) | 233 (54.5) | 0.001 |
Excludes four Cape Breton counties and Cumberland county in the first epoch;
Cell sizes <5 suppressed as required by the Perinatal Follow-Up Data Management Committee. BPD Bronchopulmonary dysplasia; IVH Intraventricular hemmorhage; NS Not statistically significant; PVL Periventricular leukomalacia
DISCUSSION
The infants in the present study comprised a cohort of live-born, very preterm infants born over a 20-year period (1988 to 2007) to mothers residing in Nova Scotia. The main strength of the present study was that it involved a population-based cohort of very pre-term infants with a very low rate of loss to follow-up (4%).
The current study was performed for two reasons. The first was to describe the variation in cerebral palsy among very preterm infants over time; the second was to determine whether changes in maternal or neonatal treatments or conditions were related to this variation in cerebral palsy over time. There was a noticeable increase in the prevalence of cerebral palsy in the third epoch (1998 to 2002) compared with the preceding two epochs and the subsequent epoch (Table 2). Why this occurred is unclear, although it was believed to be due to lower mortality rates (5). If the lower mortality is the explanation for a higher prevalence of cerebral palsy, epoch 4 should have shown the highest prevalence of cerebral palsy; however, it showed the lowest prevalence of cerebral palsy (Table 2). Thus, this is an unlikely explanation for the higher prevalence of cerebral palsy in epoch 3. A comparable population-based dataset from the United Kingdom also failed to show a relationship between declining mortality rates and rising cerebral palsy rates (6).
Because lower mortality appears to be unlikely to explain the changing prevalence of cerebral palsy, other possible explanations need to be considered, including:
Tocolytics: Tocolytic use was the highest in the same epoch as the peak of cerebral palsy prevalence (Tables 2 and 4); however, ritrodrine, which was associated with a higher prevalence of cerebral palsy (16), was withdrawn from the market around 1998, before the peak of cerebral palsy. Since then, nifedipine has been the major tocolytic used and it appears to be safe (17); thus, tocolytic use appears to be an unlikely explanation for the peak of cerebral palsy in epoch 3.
Reproductive technologies: There have been concerns about reproductive technologies leading to a greater prevalence of cerebral palsy due to the results of a population-based cohort study of >500,000 individuals from Denmark (18); however, this appears to be mediated through increased multiple births because neither in vitro fertilization nor induction of ovulation remained predictive of cerebral palsy after adjusting for multiple births and prematurity. When multiple births are considered in very preterm or very low birth weight infants, there is controversy regarding whether multiple births predict cerebral palsy (19,20). The current data has shown a steady increase in multiple births in the four epochs (Table 4); thus, this appears to be unlikely to explain the higher rate of cerebral palsy in epoch 3.
Antenatal corticosteroids: Antenatal corticosteroid use has increased over the past 20 years, which should be associated with a steady downward trend in cerebral palsy prevalence; however, this was not observed in the current data. In a systematic review, no correlation between antenatal corticosteroid use and the prevalence of cerebral palsy has been noted (21).
Magnesium sulfate: Magnesium sulfate has recently been used for fetal neuroprotection in the intrapartum period (22). The current study was conducted before neuroprotective magnesium sulfate use; regardless, there was no pattern of lower cerebral palsy rates in eras with higher use.
Chorioamnionitis: Although chorioamnionitis has been shown to be associated with cerebral palsy (23,24), intrapartum antibiotics have failed to show that they prevent cerebral palsy (24–26) and the composite outcome of cerebral palsy or death was actually linked to the use of erythromycin (26). Our data showed a general downward trend of chorioamnionitis over time; however, this may have been an artifact because fewer placentas were being sent for pathological examination. Thus, it would not be possible to evaluate the role of chorioamnionitis in cerebral palsy from the current data.
Maternal anemia: This was defined as a hemoglobin level <100 g/L and was noted to be the highest in epoch 3. Maternal anemia has not been known to be associated with cerebral palsy in the medical literature, but will need further consideration as a potential explanation for the increased risk of cerebral palsy in epoch 3.
Bronchopulmonary dysplasia: Cystic bronchopulmonary dysplasia has been steadily rising in each epoch, while the use of postnatal steroids for bronchopulmonary dysplasia peaked in the second epoch, suggesting that it was not linked to cerebral palsy, although a systematic review suggests that dexamethasone may be causally linked to cerebral palsy (27). The use of home oxygen increased until the third epoch and subsequently declined; thus, oxygen-dependant bronchopulmonary dysplasia may help to explain changes in the prevalence of cerebral palsy.
CONCLUSION
The prevalence of cerebral palsy peaked between 1998 and 2002 compared with the previous decade and subsequent five-year period. Lower mortality was not clearly related to this peak because it continued to decline in the fourth epoch, when cerebral palsy prevalence was the lowest. This appears to contradict previous work from Nova Scotia (5); therefore, alternative explanations need to be sought. An examination of intrapartum and newborn risk factors revealed that maternal anemia, use of tocolytics and newborns being sent home on oxygen were also highest in the third epoch.
Acknowledgments
The assistance of the staff in the Perinatal Follow-Up Program was essential in evaluating children and collecting data used in this study. The authors extend their appreciation to all the children and their families who contributed time and patience so that this study could be completed. The assistance of the Reproductive Care Program of Nova Scotia was essential in providing data from the Nova Scotia Atlee Database regarding Nova Scotia births.
REFERENCES
- 1.Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol. 2006;33:251–67. doi: 10.1016/j.clp.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Blair E. Epidemiology of the cerebral palsies. Orthop Clin North Am. 2010;41:441–55. doi: 10.1016/j.ocl.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Crichton JU, Mackinnon M, White CP. The life-expectancy of persons with cerebral palsy. Dev Med Child Neurol. 1995;37:567–76. doi: 10.1111/j.1469-8749.1995.tb12045.x. [DOI] [PubMed] [Google Scholar]
- 4.Westbom L, Bergstrand L, Wagner P, Nordmark E. Survival at 19 years of age in a total population of children and young people with cerebral palsy. Dev Med Child Neurol. 2011;53:808–14. doi: 10.1111/j.1469-8749.2011.04027.x. [DOI] [PubMed] [Google Scholar]
- 5.Vincer MJ, Allen AC, Joseph KS, Stinson DA, Scott H, Wood E. Increasing prevalence of cerebral palsy among very preterm infants: A population-based study. Pediatrics. 2006;118:e1621–6. doi: 10.1542/peds.2006-1522. [DOI] [PubMed] [Google Scholar]
- 6.D’Amore A, Broster S, Le Fort W, Curley A, East Anglian Very Low Birthweight Project Two-year outcomes from very low birthweight infants in a geographically defined population across 10 years, 1993–2002: Comparing 1993–1997 with 1998–2002. Arch Dis Child Fetal Neonatal Ed. 2011;96:F178–85. doi: 10.1136/adc.2009.171876. [DOI] [PubMed] [Google Scholar]
- 7.Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287–94. doi: 10.1111/j.1651-2227.2005.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 8.Joseph KS, Allen AC, Lutfi S, Murphy-Kaulbeck L, Vincer MJ, Wood E. Does the risk of cerebral palsy increase or decrease with increasing gestational age? BMC Pregnancy Childbirth. 2003;3:8. doi: 10.1186/1471-2393-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 10.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 11.Joseph KS, Fahey J, Canadian Perinatal Surveillance System Validation of perinatal data in the discharge abstract database of the Canadian Institute for Health Information. Chronic Dis Can. 2009;29:96–100. [PubMed] [Google Scholar]
- 12.Fair M, Cyr M, Allen AC, Wen SW, Guyon G, MacDonald RC. An assessment of the validity of a computer system for probabilistic record linkage of birth and infant death records in Canada. The Fetal and Infant Health Study Group. Chronic Dis Can. 2000;21:8–13. [PubMed] [Google Scholar]
- 13.Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown-rump length: Reevaluation of relation to menstrual age (5–18 weeks) with high-resolution real-time US. Radiology. 1992;182:501–5. doi: 10.1148/radiology.182.2.1732970. [DOI] [PubMed] [Google Scholar]
- 14.Gray PH, Jones P, O’Callaghan MJ. Maternal antecedents for cerebral palsy in extremely preterm babies: A case-control study. Dev Med Child Neurol. 2001;43:580–5. doi: 10.1017/s0012162201001074. [DOI] [PubMed] [Google Scholar]
- 15.Canada’s population estimates < www.statcan.gc.ca/daily-quotidien/100628/t100628a2-eng.htm> (Accessed November 19, 2012).
- 16.Takahashi R, Yamada M, Takahashi T, et al. Risk factors for cerebral palsy in preterm infants. Early Hum Dev. 2005;81:545–53. doi: 10.1016/j.earlhumdev.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Pryde PG, Janeczek S, Mittendorf R. Risk-benefit effects of tocolytic therapy. Expert Opin Drug Saf. 2004;3:639–54. doi: 10.1517/14740338.3.6.639. [DOI] [PubMed] [Google Scholar]
- 18.Hvidtjorn D, Grove J, Schendel D, et al. Multiplicity and early gestational age contribute to an increased risk of cerebral palsy from assisted conception: A population-based cohort study. Hum Reprod. 2010;25:2115–23. doi: 10.1093/humrep/deq070. [DOI] [PubMed] [Google Scholar]
- 19.Bodeau-Livinec F, Zeitlin J, Blondel B, et al. Do very preterm twins and singletons differ in their neurodevelopment at 5 years of age? Arch Dis Child Fetal Neonatal Ed. 2013;98:F480–7. doi: 10.1136/archdischild-2013-303737. [DOI] [PubMed] [Google Scholar]
- 20.Wadhawan R, Oh W, Vohr BR, et al. Neurodevelopmental outcomes of triplets or higher-order extremely low birth weight infants. Pediatrics. 2011;127:e654–60. doi: 10.1542/peds.2010-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009;(1):CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–9. doi: 10.1002/mrdd.10003. [DOI] [PubMed] [Google Scholar]
- 24.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: A meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):387–92. doi: 10.1097/AOG.0b013e3181e90046. [DOI] [PubMed] [Google Scholar]
- 25.Leviton A, Kuban K, O’Shea TM, et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: The ELGAN study. J Pediatr. 2011;158:897, 903.e1–5. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 26.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet. 2008;372:1310–8. doi: 10.1016/S0140-6736(08)61202-7. [DOI] [PubMed] [Google Scholar]
- 27.Barrington KJ. The adverse neurodevelopmental effects of postnatal steroids in the preterm infant: A systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


