Abstract
The recent introduction of the seven-valent pneumococcal conjugate vaccine has led to changes in the proportion of disease caused by different serotypes. The serotypes targeted by the vaccine have been reduced, and Streptococcus pneumonia serotype 19A is now the most commonly isolated serotype causing invasive pneumococcal disease. This serotype has been associated with antibiotic resistance. The authors of this article conducted a review of cases of invasive pneumococcal disease diagnosed between 2000 and 2010 in Calgary, Alberta, to examine the disease course of serotype 19A invasive pneumococcal disease compared with other serotypes.
BACKGROUND:
Streptoccocus pneumoniae serotype 19A (ST19A) became an important cause of invasive pneumococcal disease (IPD) after the introduction of the conjugate vaccine.
OBJECTIVE:
To examine the severity and outcome of ST19A IPD compared with non-ST19A IPD.
METHODS:
The Calgary Area Streptococcus pneumoniae Epidemiology Research (CASPER) study collects clinical and laboratory data on all IPD cases in Calgary, Alberta. Analysis was performed on data from 2000 to 2010 comparing ST19A and non-ST19A IPD cases. Adjusted linear and logistic regression models were used to examine outcomes of duration of appropriate intravenous antibiotic therapy and intensive care unit admission, respectively.
RESULTS:
ST19A tended to cause disease in younger patients. ST19A isolates were more often multidrug resistant (19% versus 0.3%; P<0.001). Adjusted logistic regression showed no difference in intensive care unit admission between ST19A and non-ST19A IPD cases (OR 1.4 [95% CI 0.8 to 2.7]). An adjusted linear regression model showed patients <18 years of age with a diagnosis of bacteremia and no risk factors infected with ST19A were, on average, treated with antibiotics 1.4 times (95% CI 1.1 to 1.9) as long as patients with non-19A IPD and the same baseline characteristics.
DISCUSSION:
ST19A IPD was associated with an increase in average time on antibiotics. Although many of the infecting strains of ST19A were within the threshold for susceptibility, they may be sufficiently resilient to require a longer duration of antibiotic therapy or higher dose to clear the infection.
CONCLUSIONS:
ST19A is more common in younger individuals, is more antibiotic resistant and may require longer average treatment duration.
Keywords: Antibiotic resistance, Meningitis, Mortality, Serotype 19A, Streptococcus pneumoniae
Abstract
HISTORIQUE :
Le Streptoccocus pneumoniae du sérotype 19A (ST19A) est devenu une cause importante de pneumococcie invasive (PI) depuis l’introduction du vaccin conjugué.
OBJECTIF :
Examiner la gravité et les issues de la PI ST19A par rapport aux PI non ST19A.
MÉTHODOLOGIE :
L’étude CASPER de recherche épidémiologique sur le Streptococcus pneumoniae dans la région de Calgary s’intéresse à la collecte de données cliniques et de données de laboratoire sur tous les cas de PI à Calgary, en Alberta. Les chercheurs ont analysé les don-nées de 2000 à 2010 pour comparer les cas de PI ST19A aux cas de PI non ST19A. Ils ont utilisé des modèles de régression linéaire et logistique ajustés pour examiner les résultats de la durée d’une antibiothérapie intraveineuse pertinente et de l’hospitalisation à l’unité de soins intensifs, respectivement.
RÉSULTATS :
Le ST19A avait tendance à susciter la maladie chez des patients plus jeunes. Les isolats de ST19A étaient plus souvent multi-résistants (19 % par rapport à 0,3 %; P<0,001). La régression logistique ajustée ne démontrait aucune différence dans les hospitalisations aux soins intensifs des cas de PI ST19A et des cas de PI non ST19A (RC 1,4 [95 % IC 0,8 à 2,7]). Un modèle de régression linéaire ajusté a révélé que les patients de moins de 18 ans chez qui on avait diagnostiqué une bactériémie, mais qui n’avaient pas de facteurs de risque et qui étaient infectés par le ST19A, étaient traités en moyenne 1,4 fois plus longtemps (95 % IC 1,1 à 1,9) que ceux qui étaient atteints d’une PI non ST19A et qui présentaient les mêmes caractéristiques de départ.
EXPOSÉ :
La PI ST19A s’associait à une période moyenne d’antibiothérapie plus longue. Même si bon nombre de souches infectieuses du ST19A se situaient dans le seuil de susceptibilité, elles sont peut-être assez résilientes pour qu’une antibiothérapie plus longue ou à plus forte dose puisse éliminer l’infection.
CONCLUSIONS :
Le ST19A est plus courant chez les plus jeunes, résiste davantage aux antibiotiques et a peut-être besoin d’être traité pendant une période moyenne plus longue.
Following introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) for children, the total incidence of invasive pneumococcal disease (IPD) decreased through reduction of vaccine serotypes in the community (1,2). While vaccine serotypes became less prevalent, Streptococcus pneumoniae serotype 19A became the most frequently isolated serotype from cases of IPD and mucosal pneumococcal disease (3,4). The increasing presence of serotype 19A as well as the concern over antibiotic resistance of the prevalent 19A strains has raised questions and concerns regarding outcomes in patients infected with serotype 19A (5–8).
Several countries saw an increase in serotype 19A before the vaccine introduction, suggesting a temporal trend (9). Choi et al (9) showed that multidrug-resistant 19A had increased substantially before vaccine introduction, suggesting that resistance was allowing serotype 19A an advantage over other serotypes through selective pressure. Serotype 19A was the ninth most prevalent serotype causing IPD in children <2 years of age in the United States in 1998 (10). Only PCV7 serotypes and serotype 6A, a vaccine-related serotype, were more common. Serotype 19A was poised to fill the niche left by a reduction in the prevalence of PCV7 serotypes through vaccination (11). The purpose of the present study was to examine the severity and outcome of serotype 19A IPD compared with IPD caused by other serotypes in Calgary, Alberta from 2000 to 2010.
METHODS
Surveillance
The Calgary Area Streptococcus pneumoniae Epidemiology Research (CASPER) study group collects clinical and laboratory data on all IPD cases that present to a hospital in the Calgary zone of Alberta Health Services. The present study included all CASPER data collected between January 2000 and December 2010. Surveillance is performed through the Calgary Laboratory Services (CLS), a centralized laboratory system serving the entire Calgary zone. When a clinical isolate is positive for S pneumoniae, CLS notifies the CASPER staff and the patient is recruited into the study. All patients in the Calgary area with a culture positive for S pneumoniae are included. Once a patient is enrolled, a chart review and interview are conducted. If the patient is unable to provide an interview, then their next of kin are approached. Written informed consent was obtained from all patients or their next of kin unless the patient could not be contacted; a chart review was then conducted. For patients who refused participation, only data from the laboratory reports and notifiable disease report forms were collected. S pneumoniae is a notifiable disease, and the Province of Alberta Department of Health mandates that all invasive illness be reported through a notifiable disease report form.
Microbiology
CLS identifies S pneumoniae using standard laboratory procedures including colony morphology, hemolysis, optochin susceptibility and bile solubility tests. Serotyping is performed using the Quellung reaction with serotype-specific antisera from Statens Serum Institute (Denmark) and is conducted at the Provincial Laboratory for Public Health (Edmonton, Alberta). Minimum inhibitory concentrations (MICs) are calculated using broth microdilution with a MicroScan MICroSTREP plus (Siemens Healthcare Diagnostics, USA) panel. Interpretation of the MIC results for susceptibility and resistance are performed following the Clinical Laboratory Standards Institute guidelines (12). For analysis, samples were considered to be either nonsusceptible (resistance or intermediate resistance) or susceptible. For clinical relevance, the MIC cutpoints for meningitis were applied to patients with meningitis, and the nonmeningitis MIC cutpoints were applied to patients with a nonmeningitis disease manifestation when determining susceptibility/nonsusceptibility. Nonsusceptibility to amoxicillin is only reported for nonmeningitis isolates because amoxicillin is an oral drug that is not appropriate for the treatment of meningitis. If the patient’s primary diagnosis was unavailable, the isolate was excluded from all analyses.
Antibiotic durations
The primary purpose of the present study was to examine whether serotype 19A IPD required longer duration of antibiotic therapy because this may indicate more severe disease. In the analysis, only antibiotics that were appropriate for the infecting strain were considered and included in the variable for duration of antibiotic treatment. The antibiotic had to be appropriate for treatment of S pneumoniae and the strain of S pneumoniae causing the infection had to be susceptible to the antibiotic, taking into consideration the disease manifestation (ie, for meningitis cases, meningitis-appropriate antimicrobials that were susceptible according to Clinical Laboratory Standards Institute guidelines were used). Time spent on appropriate antibiotics was generated by first selecting for antibiotics that met the definition of appropriate. The start date and time from the first appropriate antibiotic the patient received was then subtracted from the end date and time of the last appropriate antibiotic the patient received. The half-life of the last antibiotic the patient received was added to the final number to generate a more accurate representation of total time on antibiotics.
Due to variations in antibiotic times and possible missing information, several assumptions were made with calculating the antibiotic durations. Approximately 10% of patients with >360 h of antibiotic time had gaps of varying lengths (ranging from 6 h to 30 days) during which it appeared that all antibiotics were stopped, and then the patient was reinitiated on antibiotics at a later time. The records of all IPD patients who received >360 h of time on antibiotics were carefully checked under the assumption that they were most likely to have gaps in treatment. For patients for whom the gap was >7 days, it was assumed that the patient received antibiotics for two different illnesses and antibiotics that were not given near the culture date were excluded from the total time. If the gap was ≤7 days, the duration of antibiotics was left as calculated. The presence of gaps among patients treated for <360 h is unknown and the times generated were used as calculated. It was assumed that any gaps present would fall into the category of ≤7 days for these shorter treatment times. This was performed to maintain consistency because it was not feasible to check the entire dataset for gaps in treatment time and it is difficult to retrospectively assess the reasons for such gaps to occur.
Statistical analysis
Descriptive analysis of antibiotic resistance was conducted using Pearson’s χ2 test. A significance level of alpha = 0.05 was used, and 95% CIs are reported where appropriate. Logistic regression was used to examine whether serotype 19A IPD increased risk of admission to the intensive care unit (ICU). Linear regression with log transformation was used to compare the duration of appropriate antibiotic therapy between serotype 19A and non-serotype 19A IPD. Both multivariable models were adjusted for age, sex, primary diagnosis and for the presence of a risk factor that is an indication for receipt of a provincially funded vaccine according to the Alberta Immunization Manual (13). It was assumed that risk factors that were an indication for vaccination could be assumed to increase a patient’s risk of severe disease and a poor outcome. Primary diagnosis was grouped into meningitis, pneumonia/empyema or bacteremia without focus/other invasive. Other invasive samples included peritonitis, septic arthritis and eye infections. Age was included as <18 or ≥18 years of age for the linear regression model, and according to the age groups <5 years of age, five to 17 years of age and ≥18 years of age in the logistic regression model. All analysis was performed using STATA/IC version 11 (STATA Corporation, USA).
Ethics approval
CASPER has ethics approval for data collection and analysis through the Conjoint Health Research Ethics Board of the University of Calgary and Calgary Zone of Alberta Health Services.
RESULTS
From 2000 to 2010, 1271 patients with IPD presented to a health care centre in Calgary. One patient was excluded from the analysis due to an unusual IPD presentation of endophthalmitis. Thirty-four patients did not have a complete chart review (3%) due to patient refusal to participate or a missing/inaccessible chart. These missing patients did not differ from the rest of the sample with regard to age or sex. Only patients with complete information (n=1229 [97%]) were included in the logistic regression, and only patients who were admitted and had complete antibiotic information could be included in the linear regression investigating time on antibiotics (n=1040 [82%]).
Serotype 19A caused 59 (4.6%) of 1270 IPD cases. Serotypes were available for 1259 of the samples. Serotyping could not be performed on samples that were not frozen/saved (n=6), and samples that were not viable when thawed (n=5). Six samples were not typeable, and were included in the non-serotype 19A group. Complete antibiotic information, including susceptibility testing and treatment information, was available for 1043 IPD cases, of which 51 (4.9%) were serotype 19A.
Susceptibility testing was available for 1268 isolates. Among serotype 19A isolates, 19.6% were penicillin nonsusceptible, compared with 0.2% of non-serotype 19A isolates (P<0.001) (Table 1). Similarly, multidrug resistance was observed in 19.6% of serotype 19A isolates and 0.2% of non-serotype 19A isolates (P<0.001). All isolates that were nonsusceptible to ceftriaxone and amoxicillin were serotype 19A.
TABLE 1.
Antibiotic nonsusceptibility in Streptococcus pneumoniae serotype 19A (ST19A) compared with non-ST19A isolates (n=1229)
| Antibiotic | ST19A (n=56) | Non-ST19A (n=1173) | P |
|---|---|---|---|
| Penicillin nonsusceptible | 11 (19.6) | 2 (0.2) | <0.001 |
| Amoxicillin nonsusceptible* | 4 (8.7) | 0 (0.0) | <0.001 |
| Ceftriaxone nonsusceptible | 2 (3.6) | 0 (0.0) | <0.001 |
| Trimethoprim-sulfamethoxazole nonsusceptible | 24 (42.9) | 294 (25.1) | 0.003 |
| Erythromycin nonsusceptible | 27 (48.2) | 86 (7.3) | <0.001 |
| Clindamycin nonsusceptible | 20 (35.7) | 22 (1.9) | <0.001 |
| Multidrug resistance† | 11 (19.6) | 2 (0.2) | <0.001 |
Data presented as n (%) unless otherwise indicated.
Resistance to amoxicillin is inferred from penicillin for 2000 to 2008. All penicillin minimum inhibitory concentrations were ≤2 μg/mL for 2000 to 2008 (12). Amoxicillin is appropriate only for nonmeningitis treatment; therefore, resistance was only calculated for nonmeningitis cases (total n=1155; ST19A n=56; non-ST19A n=1109).
Multidrug resistant defined as nonsusceptible to three or more classes of antibiotics
Figure 1 shows the age distribution among patients infected with serotype 19A and patients infected with a non-19A serotype. Serotype 19A was more common in young children, the age group at which meningitis is more common as a proportion of all IPD. Among patients with serotype 19A IPD, 30.5% were <5 years old, compared with 11.8% of patients with IPD due to a non-19A serotype (P<0.001). Additionally, meningitis was the primary diagnosis in 14.2% of IPD cases occurring in children <5 years old, and 4.6% of IPD in children and adults ≥5 years old. Thus, in univariable analysis there was an association between meningitis and serotype 19A infection, with 15.3% of IPD caused by serotype 19A presenting as meningitis compared with only 5.4% of infection caused by non-19A serotypes (P=0.0018). Empyema was a complication in 8% of non-19A infections and 12% of serotype 19A infections, but this difference was not statistically significant (P=0.2589).
Figure 1).
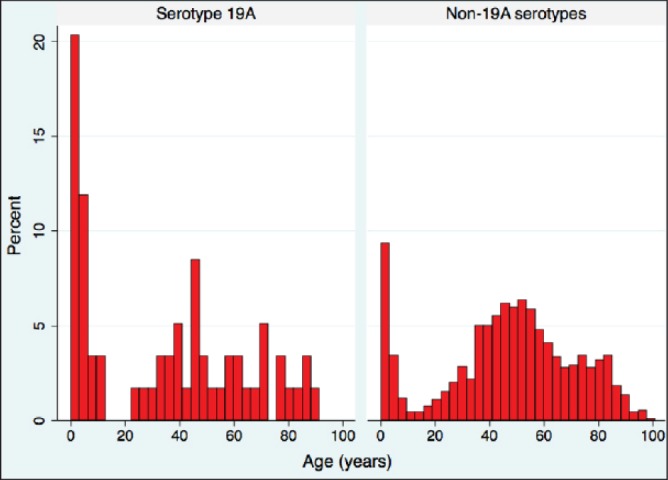
Distribution of age for Streptococcus pneumoniae serotype 19A and non-serotype 19A invasive pneumococcal disease
In a test of proportions there was no difference in 30-day case fatality between patients infected with serotype 19A or non-19A (8.8% versus 10.5%; P=0.6798). An adjusted logistic regression model revealed no difference in ICU admission between serotype 19A and non-serotype 19A IPD, while meningitis was an independent risk factor for ICU admission (Table 2). An adjusted linear regression model showed a significantly longer duration of antibiotics for patients infected by serotype 19A compared with patients with IPD due to non-19A serotypes. The linear regression results are reported in Table 3. The mean time on antibiotics for patients with all baseline factors (<18 years of age, no risk factor, primary diagnosis of bacteremia/other invasive and non-19A serotype) was 66.6 h (55.8 h to 79.4 h). Patients presenting with serotype 19A spent, on average, 1.4 (1.1 to 1.9) times as long on antibiotics, which translates to a mean of 93.3 h. Patients <18 years of age with no risk factor, presenting with meningitis due to a non-19A serotype spent, on average, 2.4 (1.8 to 3.1) times as long on antibiotics as the same demographic with bacteremia.
TABLE 2.
Logistic regression of risk factors for intensive care unit admission (n=1229)
| Risk factor | OR | 95% CI |
|---|---|---|
| Serotype | ||
| Non-ST19A infection | Reference group | |
| ST19A infection | 1.4 | 0.8–2.7 |
| Primary diagnosis | ||
| Bacteremia | Reference group | |
| Pneumonia | 2.4 | 1.5–3.8 |
| Meningitis | 21.4 | 10.8–42.3 |
| Presence of risk factor* | ||
| No risk factor | Reference group | |
| Risk factor | 1.9 | 1.4–2.6 |
Risk factor is defined as presence of an indication to receive a provincially funded vaccination (13). ST19A Streptococcus pneumoniae serotype 19A
TABLE 3.
Adjusted linear regression examining the influence of Streptococcus pneumoniae serotype and clinical factors on duration of appropriate in-hospital antibiotic therapy (n=1040)
| Risk factor | Average time on appropriate antibiotic therapy, h | 95% CI |
|
| ||
| Reference group: Bacteremia due to non-19A serotype, age <18 years and no risk factor | 66.6 | 55.8–79.4 |
| Risk factor | Ratio of increase in time spent on antibiotics due to risk factor* | 95% CI |
|
| ||
| Serotype 19A | 1.4 | 1.1–1.9† |
| Pneumonia diagnosis | 1.1 | 0.9–1.3 |
| Meningitis diagnosis | 2.4 | 1.8–3.1† |
| Risk factor‡ | 1.1 | 1.0–1.3† |
| Age ≥18 years | 1.3 | 1.0–1.5† |
See results text for sample interpretation of values;
P<0.05;
Risk factor is defined as presence of an indication to receive pneumococcal vaccination (13)
DISCUSSION
The present study revealed that serotype 19A was a prevalent cause of disease in children <5 years of age, which is consistent with previous studies (11,14). In univariable analysis, an apparent association between serotype 19A and meningitis was observed. Meningitis and infection with serotype 19A are both more common in children, resulting in a strong association between serotype 19A and meningitis in a test of proportions. Therefore, although serotype 19A may have an aptitude for causing meningitis, age is still a more important determining factor and largely accounts for the association between serotype 19A and meningitis.
Similar to previous findings, a high proportion of antibiotic-nonsusceptible serotype 19A isolates was observed (3,11,14,15). In England and Wales, a significant association of serotype 19A with antibiotic resistance was noted before introduction of the PCV7 vaccine (1). Following PCV7 introduction in 2007/2008, Kaplan et al (8) found 30% of serotype 19A isolates to be multidrug resistant. Cohen et al (14) showed daycare attendance, young age (<12 months), recent antibiotic use and acute otitis media to be predictive of nasopharyngeal carriage of serotype 19A in a population with a high proportion of vaccine uptake.
Serotype 19A became a prevalent cause of IPD following the PCV7 vaccine introduction (3,7,11). Hanage et al (16) found that serotype 19A has a high invasive potential. Serotype 19A is also causing disease in healthy children, which further supports a higher invasive potential compared with more opportunistic serotypes (8). Harboe et al (17) suggested that there is a greater risk of mortality when infected with serotype 19A compared with serotype 1, although this could only be demonstrated in individuals >5 years of age due to a low number of child deaths. Despite serotype 19A having an apparent ability to cause disease, the results of the present study suggest that the disease caused by serotype 19A does not result in a worse outcome when compared with all other serotypes.
While there does not appear to be a difference in severity of 19A IPD with regard to ICU admissions and death, serotype 19A IPD was associated with an increase in average time on antibiotics compared with non-19A serotype IPD in an adjusted linear regression model. This is a clinically relevant finding because it may suggest more severe disease due to serotype 19A, or that treatment is less effective. Disease presentation of meningitis increases risk for ICU admission and increases time on antibiotics due to the nature of the infection, which makes it more difficult to treat. Patients who are older (≥18 years of age) with IPD also tend to require an increased average duration of antibiotic treatment. However, both of these factors were considered in the linear regression model indicating meningitis and age are independent risk factors for increased duration of antibiotic treatment, but cannot account for the association between serotype 19A and antibiotic duration.
It is possible that the increased antibiotic resistance in serotype 19A may play a role in the longer duration of antibiotics. This variable accounted for MIC when indicating whether an antibiotic was appropriate; therefore, actual resistance cannot account for the possible association. It is notable that no difference was observed in ICU admission, which may also be considered a proxy for severity. The discrepancy in the results of these two separate proxies for severity of disease suggest that it may not be that serotype 19A causes more severe disease. It is possible that while actual antibiotic nonsusceptibility is accounted for in the time on antibiotics variable, it does not account for the fact that the prevalent serotype 19A strain may require a longer duration of antibiotic therapy due to its higher tendency for resistance. Although many of the infecting strains of serotype 19A did not reach the threshold to be considered ‘nonsusceptible’, they may be sufficiently resilient to require a longer duration of antibiotic therapy or higher dose for the infection to be cleared.
Although infection with serotype 19A resulted in increased mean time on antibiotic therapy, disease presentation remains a more important factor than serotype in determining severity and length of treatment. Presentation with meningitis increased risk of ICU admission and death, as well as causing an increase in mean time on antibiotics compared with presentation of bacteremia, which was the baseline group for this analysis.
Outbreaks of serotype 5 and 8 occurred in Calgary adults 18 to 64 years of age during the study period (18). However, the results in the linear and logistic regressions did not change when the analysis was performed with serotype 5 excluded or with both serotypes 5 and 8 excluded. In the analysis for which both serotypes 5 and 8 were excluded, the risk factor variable was no longer significant as an independent risk factor for increased duration of time on antibiotics; this is likely because there was a considerable number of homeless individuals in the serotype 5 outbreak, and homelessness is a risk factor that is an indication for receipt of the pneumococcal vaccine for adults (13).
The present study examined durations of time on appropriate antibiotic therapy, taking into account antibiotic resistance. These data are complex and, therefore, prone to error; however, these data are also underutilized in the current literature. Due to considerable variations in antibiotic times and possible missing information, we had to make several assumptions in the antibiotic durations. As a result, there may be some durations that are overestimated due to gaps in treatment. However, we have no reason to expect that the gaps would vary according to serotype. Therefore, not removing the gaps should not alter our results in terms of the relative difference in time on antibiotics between serotype 19A and non-serotype 19A IPD. Although the absolute value of the mean may change, our conclusions would not change. Furthermore, several patients had true antibiotic treatment times that exceeded 1000 h; therefore, these real outliers will affect the results more than some smaller outliers that are a result of gaps.
We assume more severe disease is indicated by longer treatment times; however, it is possible that longer treatment is used by the physician due to antibiotic resistance, which is more common in serotype 19A. However, we only considered treatment times of antibiotics to which the strain was susceptible and, presumably, the physician would have had this susceptibility information within a few days of treatment choice and would have switched the patient to an antibiotic to which the strain was susceptible early in the treatment. Therefore, it should not be an indication of antibiotic resistance; however, it is not feasible to determine what dictates physician antibiotic and therapy duration choices. It is possible that, while physicians would not know the serotype, they may favour a longer treatment regime in serotype 19A patients because the antibiogram indicates a less susceptible strain.
SUMMARY
Streptococcus pneumoniae serotype 19A is more common in younger individuals, and cases are more likely to be antibiotic resistant. Serotype 19A is not independently associated with worse clinical course such as death or ICU admissions. However, there is a statistically significant and clinically relevant increase in duration of antibiotics for treatment of serotype 19A IPD.
Acknowledgments
The authors acknowledge the contributions of the other CASPER investigators (Drs Deirdre Church, Judy MacDonald and David Scheifele) and staff (Joslyn Fernandes, Linda Hastie, Sarah Merrill and Shannon Pyra), and Tracie Lloyd from CLS. The authors also acknowledge the statistical expertise and support from Alberto Nettel-Aguirre with the Alberta Children’s Hospital Research Foundation.
REFERENCES
- 1.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: An observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 2.Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: Update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis. 2009;49:205–12. doi: 10.1086/599827. [DOI] [PubMed] [Google Scholar]
- 3.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 4.Lepoutre A, Varon E, Georges S, Gutmann L, Levy-Bruhl D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001–2006. Euro Surveill. 2008;13:1–6. doi: 10.2807/ese.13.35.18962-en. [DOI] [PubMed] [Google Scholar]
- 5.Mahjoub-Messai F, Doit C, Koeck JL, et al. Population snapshot of Streptococcus pneumoniae serotype 19A isolates before and after introduction of seven-valent pneumococcal vaccination for French children. J Clin Microbiol. 2009;47:837–40. doi: 10.1128/JCM.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shouval DS, Greenberg D, Givon-Lavi N, Porat N, Dagan R. Site-specific disease potential of individual Streptococcus pneumoniae serotypes in pediatric invasive disease, acute otitis media and acute conjunctivitis. Pediatr Infect Dis J. 2006;25:602–7. doi: 10.1097/01.inf.0000220231.79968.f6. [DOI] [PubMed] [Google Scholar]
- 7.Demczuk WH, Martin I, Griffith A, et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada during the introduction of the 13-valent pneumococcal conjugate vaccine, 2010. Can J Microbiol. 2012;58:1008–17. doi: 10.1139/w2012-073. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125:429–36. doi: 10.1542/peds.2008-1702. [DOI] [PubMed] [Google Scholar]
- 9.Choi EH, Kim SH, Eun BW, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14:275–81. doi: 10.3201/eid1402.070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–35. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 11.Moore MR, Gertz RE, Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Laboratory and Standards Institute . Zone Diameter and MIC Interpretive Standards for Streptococcus pneumoniae. 1ed. Wayne: Clinical and Laboratory Standards Institute; 2011. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-first Informational Supplement; pp. 96–9. [Google Scholar]
- 13.Alberta Health and Wellness . Alberta Immunization Manual. Edmonton, Alberta: Public Health Division, Alberta Health and Wellness; 2007. [Google Scholar]
- 14.Cohen R, Levy C, Bonnet E, et al. Risk factors for serotype 19A carriage after introduction of 7-valent pneumococcal vaccination. BMC Infect Dis. 2011;11:95. doi: 10.1186/1471-2334-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dortet L, Ploy M-C, Poyart C, Raymond J. Emergence of Streptococcus pneumoniae of serotype 19A in France: Molecular capsular serotyping, antimicrobial susceptibilities, and epidemiology. Diagn Microbiol Infect Dis. 2009;65:49–57. doi: 10.1016/j.diagmicrobio.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Hanage WP, Kaijalainen TH, Syrjanen RK, et al. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect Immun. 2005;73:431–5. doi: 10.1128/IAI.73.1.431-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: A population-based cohort study. PLoS Med. 2009;6:e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderkooi OG, Church DL, Macdonald J, Zucol F, Kellner JD. Community-based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pneumoniae serotypes 5 and 8 in Calgary, Canada. PloS One. 2011;6:e28547. doi: 10.1371/journal.pone.0028547. [DOI] [PMC free article] [PubMed] [Google Scholar]


