Abstract
The dorsal column pathway consists of direct projections from primary afferents and of ascending fibers of the post-synaptic dorsal column (PSDC) cells. This pathway mediates touch but may also mediate allodynia after nerve injury. The role of PSDC neurons in nerve injury-induced mechanical allodynia is unknown. Repetitive gentle, tactile stimulus or noxious pinch was applied to the ipsilateral hindpaw of rats with spinal nerve ligation (SNL) or sham surgery that had previously received tetramethylrhodamine dextran in the ipsilateral n. gracilis. Both touch and noxious stimuli produced marked increases in FOS expression in other cells throughout all laminae of the ipsilateral dorsal horn after nerve injury. However, virtually none of the identified PSDC cells expressed FOS immunofluorescence in response to repetitive touch or pinch in either the nerve-injured or sham groups. In contrast, labeled PSDC cells expressed FOS in response to ureter ligation and labeled spinothalamic tract (STT) cells expressed FOS in response to noxious pinch. Identified PSDC neurons from either sham-operated or SNL rats did not express immunoreactivity to substance P, CGRP, NPY, PKCg, MOR, the NK1 and the NPY-Y1 receptor. Retrogradely labeled DRG cells of nerve injured rats were large diameter neurons, which expressed NPY, but no detectable CGRP or substance P. Spinal nerve injury sensitizes neurons in the spinal dorsal horn to repetitive light touch but PSDC neurons apparently do not participate in touch-evoked allodynia. Sensitization of these non-PSDC neurons may result in activation of projections integral to the spinal/supraspinal processing of enhanced pain states and of descending facilitation, thus priming the central nervous system to interpret tactile stimuli as being aversive.
Keywords: PSDC cells, spinal cord, neuropathic pain, FOS expression
Introduction
Neuropathic pain is characterized by spontaneous burning pain and, in some patients, as touch-evoked allodynia. Tactile allodynia is likely mechanistically distinct from normal nociceptive processing. Differential nerve blocks applied to patients demonstrated that allodynia is mediated through non-nociceptive, large diameter Aβ fibers whereas thermal hyperalgesia is mediated through the unmyelinated C-fiber nociceptors (Campbell et al. 1988; Koltzenburg et al. 1992; Koltzenburg et al. 1994). Selective desensitization of C-fibers or presynaptic inhibition of C-fiber output with spinal morphine abolished thermal, but not tactile, hyperesthesias in rats with spinal nerve ligation (SNL) (Bian et al. 1995; Lee et al. 1995; Ossipov et al. 1999). Likewise, inhibition of C-fiber activity did not alter behavioral responses to light brush in nerve-injured rats (Field et al. 1999). The dorsal column pathway consists of direct afferent projections from large-diameter Aβ fibers and projections from post-synaptic dorsal column (PSDC) neurons (Bennett et al. 1983; Hendry et al. 1999; Palecek, 2004; Willis and Coggeshall, 2004). It is well established that the PSDC neurons mediate visceral pain (Al-Chaer et al. 1996a,b; Hirshberg et al. 1996; Houghton et al. 1997; Nauta et al. 1997; Houghton et al. 2001). For example, the physiologic responses of PSDC neurons to colorectal distension are opioid-sensitive and behavioral responses to noxious visceral stimuli are blocked by lesions of the dorsal columns (Al-Chaer et al. 1996a; Houghton et al. 2001; Palecek et al. 2002). The distribution of PSDC neuronal cell bodies coincides with the terminations of Aβ fibers, but not C-fibers, to include laminae III to lamina VI of the dorsal horn and the central canal, and they could receive tactile sensory inputs from the periphery (Bennett et al. 1983; Bennett et al. 1984; Giesler et al. 1984; Wang et al. 1999; Todd, 2002).
The dorsal column pathway has also been implicated in experimental neuropathic pain. Lesions of the dorsal column or microinjection of lidocaine, anti-NPY antiserum or Y1 antagonists into n. gracilis abolished nerve injury-induced hindpaw tactile allodynia, but not thermal hyperalgesia (Sun et al. 2001; Ossipov et al. 2002). The enhanced activity of thalamic wide-dynamic range (WDR) neurons after nerve injury was also blocked by lesions of the dorsal columns or the n. gracilis (Miki et al. 2000). While these observations support a role for the dorsal column pathway in the mediation of nerve injury-induced tactile allodynia, the role of the PSDC neurons in this response is unknown.
The evoked expression of the proto-oncogene product FOS in the spinal cord is related to neuronal excitation and correlates with nociceptive stimulation (Menetrey et al. 1989; Bullitt, 1990; Presley et al. 1990; Traub et al. 1992). FOS expression is normally not elicited by light touch, but can be evoked by repetitive light touch in nerve-injured rats, indicating a state of spinal sensitization (Catheline et al. 1999). In the present study, we explored whether SNL elicited a sensitization of PSDC neurons by evaluating FOS expression of these neuronal profiles in response to light touch.
Materials and Methods
Animals
Male, Sprague Dawley rats (Harlan, Indianapolis, IN), 250–300 gm at time of testing, were maintained in a climate-controlled room on a 12-hr light/dark cycle (lights on at 6 A.M.), and food and water were available ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for the Study of Pain and National Institutes of Health guidelines for the handling and use of laboratory animals and received approval from the Institutional Animal Care and Use Committee of the University of Arizona.
Spinal Nerve Ligation (SNL)
Peripheral nerve injury was produced by tight ligation of the L5 and L6 spinal nerves as described by (Kim and Chung, 1992). Anesthesia was induced with 2% halothane in O2 at 2 l/min and maintained with 0.5% halothane in O2. The dorsal vertebral column from L4 to S2 was exposed, and the L5 and L6 spinal nerves of the left hindpaw were identified and carefully isolated. The L5 and L6 spinal nerves were tightly ligated distal to the dorsal root ganglion with a 4-0 silk suture and the incision was closed. Sham control rats underwent the same surgery and handling as the experimental animals but without SNL.
Ligation of the Ureter
Rats were anesthetized with a veterinary preparation of 80 mg/kg of ketamine and 20 mg/kg of xylazine. Ligation of the ureter was performed as described by Avelino and colleagues (1997). A midline abdominal incision was made, the left ureter was exposed and a loose ligature of 3-0 silk suture were placed around the ureter between 2 and 3 cm below the pyeloureteral junction. The free ends of the silk suture were exteriorized through the dorsal wall of the abdominal cavity and secured to the skin on the animal’s back, and the wounds were closed. The animals were allowed to recover for 8 days in order to allow healing and also to minimize the possibility of artefactual FOS expression resulting from the surgical trauma itself. The animals were then briefly anesthetized under halothane anesthesia and the free exteriorized threads on the rat back were pulled tightly, knotted and secured to the skin. The animals were allowed to recover from the anesthesia and 4 hours later they were killed by anesthetic overdose and perfused for immunolabeling as described below.
Tracer injections
Tracer microinjections into either the n. gracilis or the thalamus were made in rats anesthetized with of 80 mg/kg of ketamine and 20 mg/kg of xylazine by stereotaxic microinjection. The rats were mounted in a stereotaxic instrument (Stoelting, Wood Dale, IL) the skull was exposed and a small hole drilled above the n. gracilis. An injection cannula was lowered to the level of the left (i.e.; ipsilateral to SNL or sham surgery) n. gracilis or the right (contralateral) thalamus and 0.2 µl of 10% tetramethylrhodamine dextran (TMR Dextran; 3000 MW, Molecular Probes, Eugene, OR) was microinjected over 10 sec. Sterotactic coordinates for the n. gracilis were 6.5 mm caudal to the intra-aural line (AP -6.5), 0.6 mm left of midline (L 0.6) and 7.5 mm below the cranium (H -7.5) (Paxinos and Watson, 1986). In order to maximize retrograde labeling of neurons terminating in the thalamic nuclei, 4 injections of 0.5 µl of 10% tetramethylrhodamine dextran were made. The injections were applied either one week after SNL, when behavioral evidence of tactile hypersensitivity was confirmed, or at the same time as ligation of the ureter. The animals were subjected to stimulation and perfusion one week after microinjection of the tracer.
Non-noxious and noxious mechanical stimulation
Two weeks after SNL or sham surgery, gentle tactile stimuli were applied to the left hindpaw of unanesthetized rats. Consistent with our previous investigations (Ossipov et al. 2002), only SNL rats with established tactile hyperesthesia, defined by a paw withdrawal threshold of less than 4.7 grams, were used. The rats were loosely restrained under a towel and the flat portion of the thumb was placed on the center of the plantar hindpaw and stroked gently towards the distal footpad over a period of 2 sec. The stimulus was applied at a frequency of every 4 sec over a period of 10 minutes, as described in previous reports (Catheline et al. 1998; Catheline et al. 1999). Separate groups of rats received noxious pinch, which was applied by briefly squeezing the hindpaw with forceps every 12 sec for 10 min (Ma and Woolf, 1996).
Immunofluorescent labeling
The rats were deeply anesthetized with 100 mg/kg of a 10:1 mixture of ketamine and xylazine and perfused transcardially with 250 ml of phosphate-buffered saline (PBS; 0.1 µM; pH 7.4), containing 15,000 IU/L of heparin, followed by 4% paraformaldehyde in PBS for 20 min. The brain, spinal cord, and L4–L6 DRGs were removed, postfixed in 4% paraformaldehyde for 4 hr, and cryoprotected in 30% sucrose in PBS overnight at 4°C. Sections 40 µm thick were cut through the DRG, the coronal plane of the L2-S1 spinal cord and the caudal medulla at the level of the n. gracilis and rostrally at the level of the thalamus. The sections were preincubated in a solution of 4% normal goat serum in PBS with 1% bovine serum albumin and 0.2% Triton X-100 for 30 min. The spinal cord and DRG sections were then incubated in primary antiserum for 1 to 3 days. The antibodies employed (with dilution factors) were polyclonal rabbit anti-SP (Peninsula, San Carlos, CA, 1:10,000); NPY (Peninsula 1:10,000); CGRP, (Peninsula 1:10,000); CCK39 (Peninsula 1:10,000); galanin (Chemicon, Temecula, CA, 1:10.,000); NK1 (Chemicon, 1:1000); NPY-Y1 receptor (ImmunoStar, Hudson, WI, 1:1000); MOR (Neuromics, Minneapolis, MN, 1:10,000); P2X3 (Neuromics, 1:1000); PKCγ (Santa Cruz Biotechology, Santa Cruz, CA, 1:10,000); monoclonal mouse anti-calbindin D (Sigma, St. Louis, MO, 1:10,000); and parvalbumin (Sigma 1:10,000). Visualization of FOS expression in the spinal cord and the n. gracilis sections was accomplished by incubation with a polyclonal rabbit antiserum to FOS (Ab-5, Oncogene, San Diego, CA, 1:3,000) for 1–3 nights at 4°C. The sections incubated with the primary antibodies were then washed in PBS (three times for 10 min) and incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:1,000; Molecular Probes, Eugene, OR) for 60 min at room temperature. After two 5 min washes in PBS, the sections were mounted on gelatin-coated glass slides and air-dried overnight and coverslipped with DPX. The sections were examined with a Nikon E800 fluorescence microscope equipped with a standard filter for FITC and a Nikon Y-2E/C filter to detect fluorescence for tretramethylrhodamine. Images were acquired with a Hamamatsu C5810 color CCD camera and its proprietary Image Processor software (Hamamatsu Photonic Systems, Bridgewater, NJ). The acquired images were analyzed and measurement of cross-sectional diameters of neuronal profiles was accomplished with the aid of the imaging and analysis software Metamorph (Universal Imaging Corp., West Chester, PA). Neuronal profiles were defined with the aid of the Integrated Morphometry Analysis function of Metamorph and profiles with a nucleus were counted. Because this imaging analysis software does not perform unbiased stereology, references to “cell counts” is avoided. In all studies where the retrograde tracer was used, only sections from animals that showed correct placement of the tetramethylrhodamine dextran injection (i.e.; n. gracilis or thalamus) were used.
Counting of FOS labeled profiles
The expression of FOS in dorsal horn sections obtained from either the nerve-injured, sham-operated or the animals subjected to ligation of the ureter were analyzed by examining 40 µm thick transverse spinal sections taken from L3 through S1 with the image acquisition system described above. A minimum of 10 sections was taken from each rat, and a minimum of 6 rats were culled from each treatment group. The number of profiles within each of the dorsal horn section that showed immunoreactivity for FOS was counted and the results are expressed as the mean ± SEM per section. The profiles were also expressed according to laminar distribution designated as superficial (laminae I and II), intermediate (laminae III and IV) and deep (laminae V and VI). Significant (p ≤ 0.05) differences in profile counts were determined by ANOVA followed by Fisher's Least Significant Difference test. Similarly, a minimum of 10 sections from the n. gracilis from each of 6 rats per treatment group were examined for immunoreactivity to FOS. In addition, at least 10 spinal cord sections per animal were taken from separate groups of rats with retrograde tracer microinjected into the n. gracilis or the thalamus and subjected to sham surgery, SNL or ureter distension. The numbers of retrogradely labeled profiles within the spinal dorsal horn that also expressed labeling for FOS as well as those that did not show FOS expression were counted.
Results
Retrogradelly labeled PSDC and STT neurons in rats not subjected to SNL or sham-surgery
Fluorescent microscopy revealed that the labeling with tetramethylrhodamine dextran was restricted to the n. gracilis, providing confirmation of the injection site (Figure 1A). The fact that the entire nucleus showed red fluorescence provides for a high degree of confidence that retrograde labeling of neurons with projections into the n. gracilis would occur. Similarly, microinjection of tetramethylrhodamine dextran into the right thalamus resulted in marked fluorescence for the marker, confirming successful microinjection into the thalamus. This outcome provides a high level of confidence that STT neurons would be retrogradely labeled (Figure 1B).
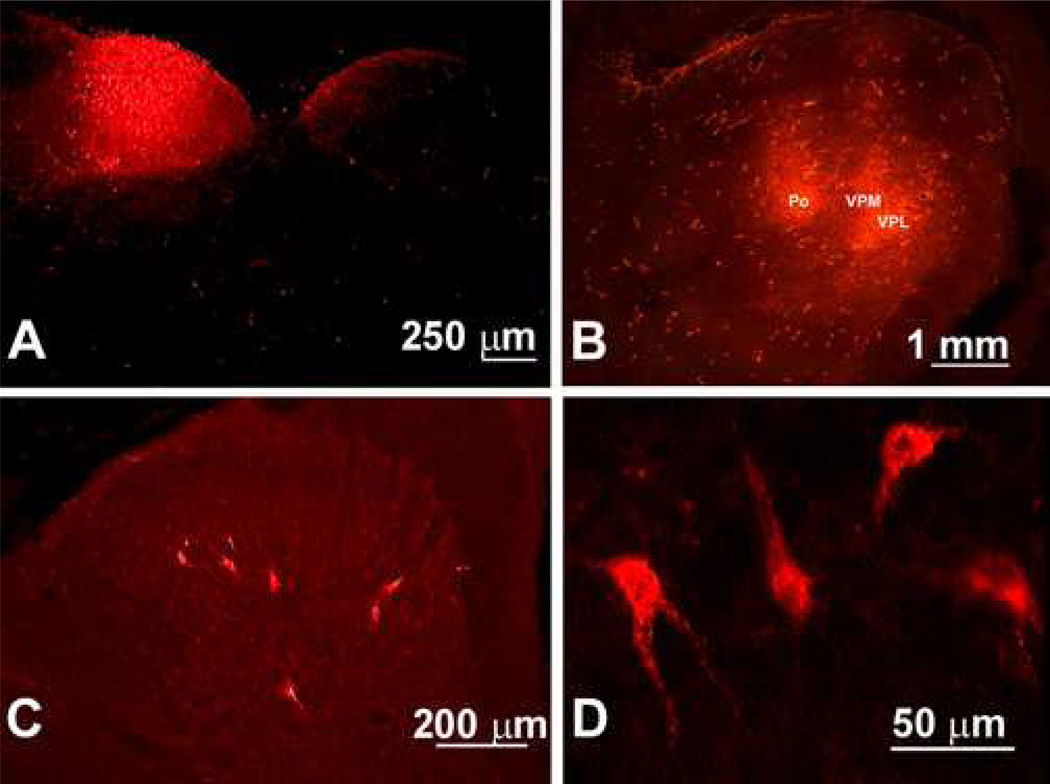
Figure 1.
Fluorescent images indicating labeling of the n. gracilis and the thalamus with tetramethylrhodamine dextran are shown. (A). The transverse section of the caudal medulla illustrates the site of injection of the retrograde tracer, identified by the red fluorescence within the n. gracilis. (B). Two pairs of injections of the rhodamine-dextran retrograde tracer were made 1 mm apart from each other into the right side of the thalamus. (C) Retrograde labeling from microinjection of the retrograde tracer into the n. gracilis is found in the labeled PSDC profiles in laminae III – IV of the dorsal horn ipsilateral to the injection of tracer in rats. (D). High power magnification of the retrogradely labeled PSDC profiles is shown.
Spinal dorsal horn sections from 6 sham-operated rats and 6 SNL rats were taken from L2 to S1 one week after tetramethylrhodamine dextran microinjection into the n. gracilis. The red fluorescence of tetramethylrhodamine dextran is clearly visible in the perikarya and proximal dendrites of the labeled PSDC neurons (Figure 1C and 1D). The PSDC neurons were found throughout the dorsal horn ipsilateral to the microinjection and around the central canal of the spinal cord. There was little or no labeling in the spinal dorsal horn contralateral to the n. gracilis that received the tracer. An average 3 retrogradely-labeled PSDC profiles per section were identified. The highest concentration of labeled neuronal profiles, ranging as high as 13 per section, was found between L4 and L6. The retrogradely-labeled profiles were found principally in laminae III to V, although a few were found in the superficial laminae of the spinal dorsal horn (Figure 1C and 1D). In addition, DRG ipsilateral to the injected n. gracilis displayed retrograde label, and the preponderance of tetramethylrhodamine dextran was found in large-diameter DRG neurons, which is consistent with the known projection of large diameter, myelinated Aβ fibers to the n. gracilis (Figure 2).
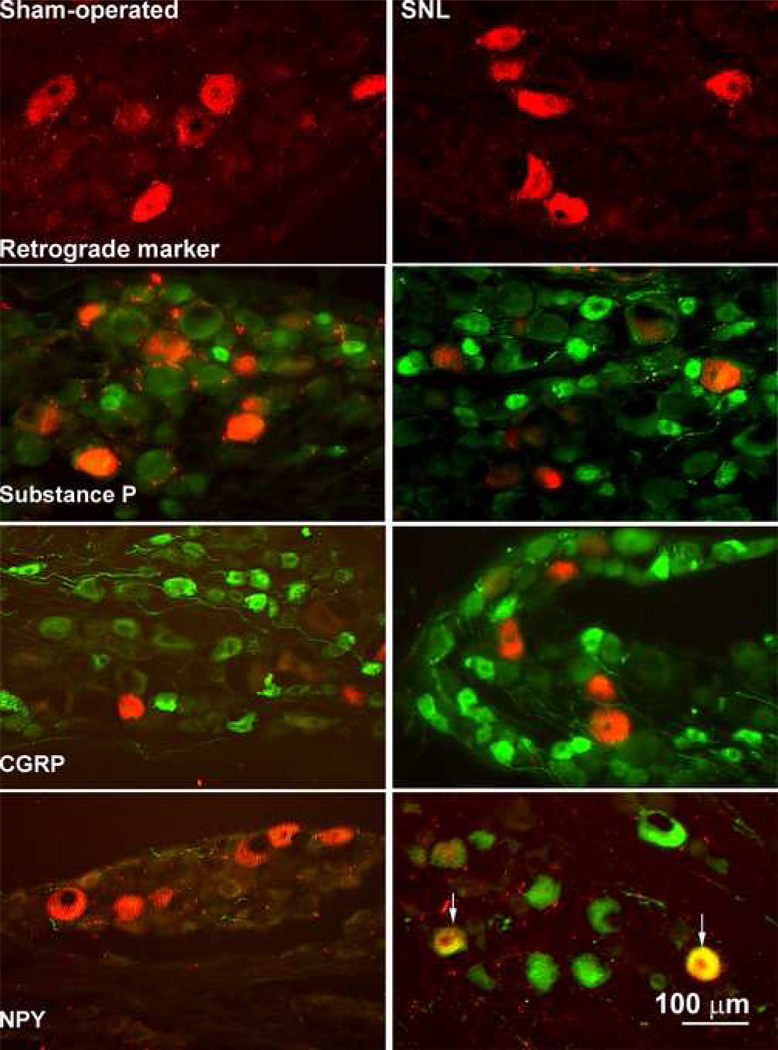
Figure 2.
Photomicrographs of the L5 DRG obtained from sham-operated (left panels) and nerve-injured (right panels) rats are shown. The red immunofluorescence of the retrograde tracer is found in medium to large diameter cellular profiles of the DRG. Green immunofluorescence indicates labeling for substance P, CGRP and NPY in the DRG profiles. There is no evidence of colocalization of either substance P or of CGRP with tetramethylrhodamine dextran obtained from either the sham-operated or nerve-injured rats. Furthermore, the DRG obtained from sham-operated animals do not show labeling for NPY whereas it is present in the DRG of nerve-injured rats. Labeling for NPY is found in the medium to large diameter profiles of the DRG that also demonstrate the presence of the retrograde tracer.
Immunofluorescent Markers and PSDC Profiles
Spinal cord sections were obtained from sham-operated and nerve-injured rats 1 week after the microinjection of tetramethylrhodamine dextran into the n. gracilis ipsilateral to the surgery. Behavioral assessment for tactile hyperesthesia was performed prior to microinjection of tetramethylrhodamine dextran in order to confirm the presence of experimental neuropathic pain in rats with SNL, which was indicated by significant reductions in paw withdrawal thresholds to probing the hindpaw with von Frey filaments compared with the sham-operated group (data not shown). Because the greatest concentration of retrogradely-labeled profiles was found in the region of L5, 10 sections from this region were obtained from 6 sham-operated and 6 SNL rats for the examination of colabeling of the immunostaining markers with the retrograde tracer. An average of 5 retrogradely-labeled PSDC profiles were observed per section, and approximately 50 PSDC profiles were available for examination of colabeling with one of the immunofluorescent markers. The spinal sections obtained from the more rostral sites exhibited few (<3) to no retrogradley-labeled PSDC profiles. Fluorescent immunoreactivity to substance P, CGRP, NPY, PKCγ, MOR, the NK1 receptor and the NPY-Y1 receptor was observed in spinal cord sections obtained from both sham-operated and nerve-injured rats. As expected, the distribution of these immunofluorescent labels was predominant in the superficial laminae of the dorsal horn. For both the sham-operated and the nerve-injured groups, there was no evidence that any of the retrogradely-labeled PSDC neurons also showed immunofluorescence for any these markers (data not shown). Moreover, there was no evidence of co-labeling of PSDC profiles with P2X3, CCK, galanin or calbindin D in sham-operated or nerve-injured rats.
Immunofluorescent Markers and DRG Neurons
At least 10 DRG sections were taken from 3 to 6 sham-operated and nerve-injured rats with confirmed microinjections of tetramethylrhodamine dextran into the n. gracilis ipsilateral to the surgery. The retrogradely-labeled DRG neurons were identified by red fluorescence, and immunofluorescence to substance P, CGRP or NPY was identified by green immunofluorescence (Figure 2). Immunofluorescence for substance P and CGRP was evident in small to medium diameter neurons of the DRG of sham-operated rats, and there was no co-labeling of these profiles with those that were labeled with the retrograde tracer (Figure 2). There was no detectable expression of NPY in the DRG of sham-operated rats. In contrast, most of the DRG profiles with the retrograde tracer obtained from rats with SNL also demonstrated immunofluorescence for NPY (Figure 2). This observation is consistent with the reported upregulation of NPY after SNL in large-diameter Aβ fibers that project to the dorsal column nuclei (Li et al. 1999b; Ma and Bisby, 2000; Ossipov et al. 2002).
Evoked Expression of FOS in the Spinal Cord
In order to evaluate if light touch can evoke FOS expression in PSDC neurons of sham-operated or nerve-injured rats, the number of profiles expressing FOS after light stroking of the hindpaw was counted in each section (Figure 3). FOS was expressed principally in the dorsal horn of the spinal cord, and in some profiles around the central canal, with very little FOS expression being present in the ventral horns (Table 1). The expression of FOS in response to light tactile stimulation in the unanesthetized sham-operated rats was relatively low. The mean number of neuronal profiles that expressed FOS per section ipsilateral to the tactile stimuli were 17.8 ± 1.4 (L4), 21.3 ± 2.5 (L5) and 17.3 ± 1.1 (L6) (Table 1). Of all the profile bodies that expressed FOS immunofluorescence within L5, 62% of these were found in the middle laminae (III to IV) of the spinal dorsal horn; 22% were found in the superficial laminae and 16% were found in the deeper laminae (V to VI) (Table 1). A similarly small number of profiles, between 15.3 ± 1.5 and 19.3 ± 2.0, were found to express FOS contralateral to the applied stimulus.
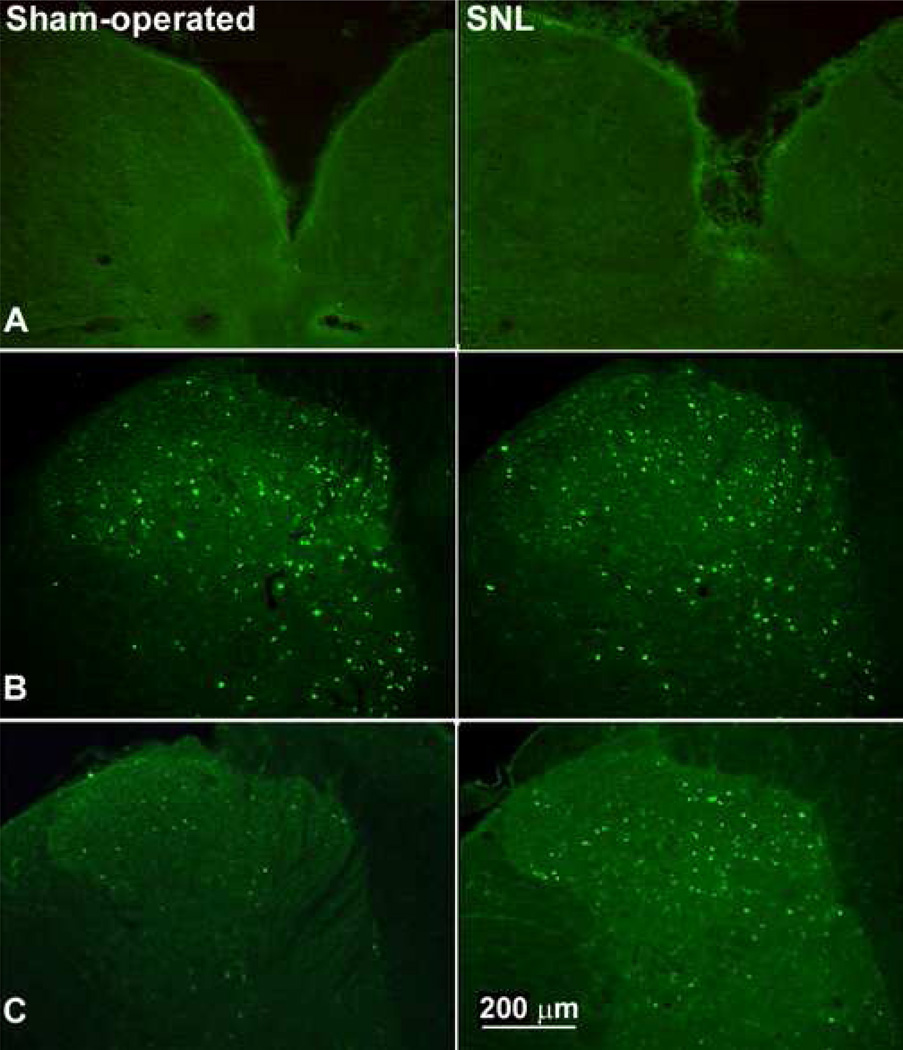
Figure 3.
Immunofluorescence for FOS expression in the n. gracilis and the L5 spinal dorsal horn of sham-operated rats and of rats with SNL are shown in response to mechanical stimuli. (A). FOS expression was completely absent from the n. gracilis of sham-operated or nerve-injured rats after noxious pinch. This result is similar to observations made after light brush (not shown). (B). Both sham-operated and nerve-ligated rats demonstrate similar levels of evoked FOS expression throughout the dorsal horn of the spinal cord in response to noxious pinch. (C). FOS expression evoked by lightly stroking the hindpaw is increased throughout the dorsal horn of the nerve-injured rats relative to the sham-operated rats.
Table 1.
Mean Number of Profiles Expressing FOS in the Spinal Dorsal Horn
| SNL- Ipsilateral |
SNL - Contralateral |
Sham - Ipsilateral | Sham - Contralateral |
||
|---|---|---|---|---|---|
| L4 | I–II | 12.4 ± 1.4 | 4.7 ± 1.0 | 4.1 ± 1.1 | 2.1 ± 0.5 |
| III–IV | 30.8 ± 2.4* | 17.1 ± 1.2 | 11.4 ± 1.1 | 10.2 ± 0.8 | |
| V–VI | 13.2 ± 2.7 | 6.9 ± 1.2 | 3.8 ± 0.6 | 3.1 ± 0.9 | |
| Total | 55.6 ± 5.1* | 28.7 ± 2.7 | 17.8 ± 1.4 | 15.3 ± 1.5 | |
| L5 | I–II | 12.4 ± 1.4 | 4.7 ± 1.0 | 4.5 ± 0.6 | 3.0 ± 0.7 |
| III–IV | 34.0 ± 2.4* | 17.9 ± 1.2 | 14.2 ± 2.1 | 13.4 ± 1.6 | |
| V–VI | 16.8 ± 2.4 | 10.0 ± 1.5 | 3.1 ± 0.8 | 2.4 ± 0.7 | |
| Total | 64.3 ± 4.9* | 35.2 ± 3.1 | 21.3 ± 2.5 | 19.3 ± 2.0 | |
| L6 | I–II | 8.6 ± 1.3 | 3.4 ± 0.7 | 4.4 ± 0.6 | 4.1 ± 0.7 |
| III–IV | 31.9 ± 3.0* | 16.4 ± 1.2 | 10.2 ± 0.5 | 9.8 ± 1.4 | |
| V–VI | 16.4 ± 1.9 | 6.6 ± 1.2 | 3.4 ± 1.1 | 3.0 ± 1.0 | |
| Total | 57.6 ± 5.0* | 26.4 ± 2.3 | 17.3 ± 1.1 | 16.9 ± 2.3 | |
Gentle mechanical stimulation of the hindpaw evokes FOS expression in the lumbar dorsal horn. FOS expression in laminae III–IV ipsilateral to SNL is significantly (* p < 0.05) increased when compared to the contralateral side.
Spinal nerve ligation was associated with a significant (p ≤ 0.05) increase in FOS expression in response to repeated light tactile stimulation (Figure 3). The mean number of neurons expressing immunofluorescence for FOS per section was markedly elevated, compared to the sham-operated group, to 55.6 ± 5.1 (L4), 64.3 ± 4.9 (L5) and 57.6 ± 5.0 (L6) (Table 1). These values represent a highly significant (p < 0.001), approximately 3-fold increase in the numbers of FOS-expressing neuronal profiles. Of all the profiles that expressed FOS immunofluorescence within L5, 54% of these were found in the middle laminae (III to IV) of the spinal dorsal horn; 22% were found in the superficial laminae and 24% were found in the deeper laminae (V to VI) (Table 1). Likewise, the expression of FOS contralateral to tactile stimuli was significantly (p ≤ 0.05) elevated when compared to the sham-operated group. Between 26.4 ± 2.3 and 35.2 ± 3.1 profiles were found to express FOS in the contralateral sections from L4 to L6, representing a doubling of responsive neurons. No FOS expression was found in the n. gracilis after light touch in either sham-operated or nerve-injured rats (not shown).
Similarly, noxious pinch also evoked FOS expression throughout the dorsal horn of the spinal cord, to include the medial laminae (Figure 3). In contrast to light touch, noxious pinch produced similar levels of expression of FOS in sham-operated or nerve-injured rats. No FOS expression was observed in the n. gracilis of nerve-injured or sham-operated rats after noxious pinch (Figure 3).
Absence of Colocalization of Tetramethylrhodamine dextran and FOS in PSDC of Nerve-injured Rats
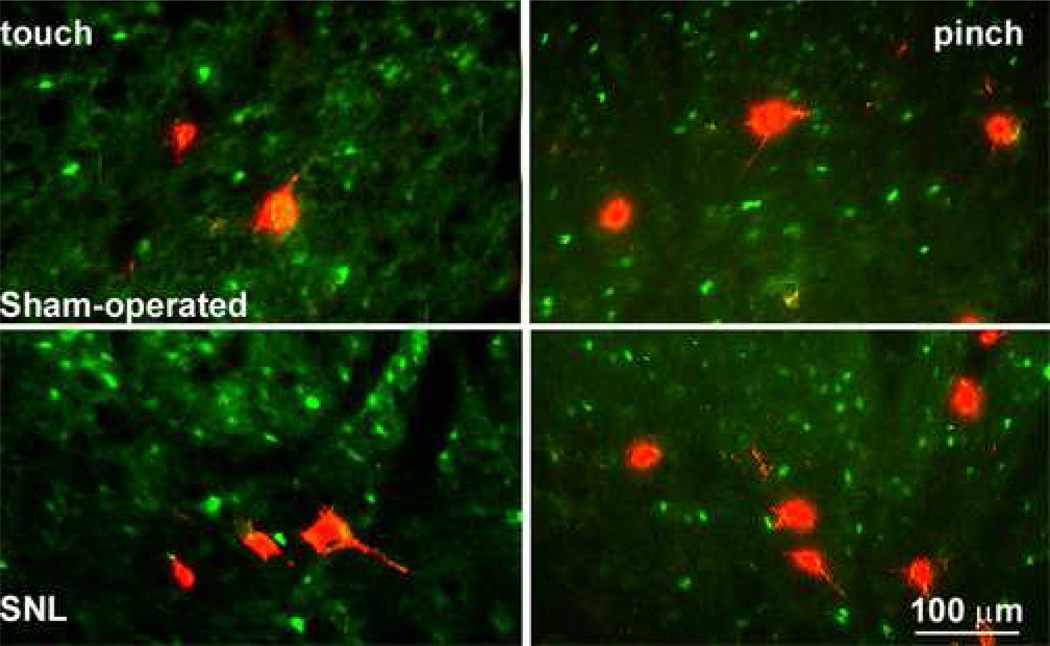
Retrogradely-labeled PSDC neurons were readily visualized in the ipsilateral dorsal horns of the spinal cord, and were predominantly found within laminae III to IV (Figure 4). Similarly, the neuronal profiles expressing FOS demonstrated strong immunofluorescence for the marker, and were also found predominantly in the medial laminae of the ipsilateral dorsal horn (Figure 4). There was no colocalization between labeling with tetramethylrhodamine dextran and for FOS in spinal sections obtained from sham-operated rats, and virtually none for the spinal sections from rats with SNL after gentle tactile stimulation (Figure 4). Only 3 of the 507 PSDC profiles counted that were labeled with tetramethylrhodamine dextran also expressed immunofluorescence for touch-evoked FOS in SNL rats. Similarly, none of 150 identified PSDC neurons showed immunofluorescence for FOS after noxious mechanical stimulation in the SNL rats (Figure 4).
Figure 4.
Double-labeling studies indicating retrograde tracer (red) and immunofluorescence for FOS (green) are shown for sham-operated and nerve-injured rats. FOS expression was evoked by light touch or by noxious pinch. There was very little to no labeling for FOS in any of the identified PSDC profiles in either the sham-operated or nerve-injured groups after either light or noxious stimuli.
In order to verify that these results did not represent either an inability of PSDC neurons to express FOS, and also to demonstrate that the methods employed in the present study were indeed capable of detecting immunofluorescence for FOS expression in profiles that have been retrogradely labeled with tetramethylrhodamine dextran, additional experiments were performed as positive control studies. It is well established that noxious pelvic visceral stimuli may activate PSDC neurons (al-Chaer et al, 1996a,1996b; Palecek et al, 2002), and that noxious visceral stimuli evokes enhanced FOS expression in PSDC cells (Palecek et al, 2003). In the current investigation, ligation of the ureter caused marked increases in spinal FOS expression. The FOS-positive profiles were found extending from T8 to S2 and showed a peak increase in the region of T13, L1 and L2. Most profiles expressing FOS were located around the central canal (lamina X), with fewer being found in the ipsilateral laminae V–VI and in lamina I, and were virtually absent from other laminae of the spinal cord. Importantly, immunofluorescence for FOS was found in profiles that also were labeled by tetramethylrhodamine dextran. These double labeled profiles showed the red fluorescence for tetramethylrhodamine dextran in the perikarya and proximal dendrites with no labeling in the nuclei, and green immunofluorescence for FOS in the nuclei (Figure 5). Among the 480 labeled PSDC profiles, 55 of these also expressed immunofluorescence for FOS, representing 11.5% of the retrogradely labeled PSDC profiles. These double labeled profiles were found principally within the medial portion of laminae IV and V of the lumbar dorsal horn.
Figure 5.
Double-labeling studies indicating retrograde tracer (red) and immunofluorescence for FOS (green) are shown after ureter ligation (top panels) or noxious pinch (bottom panels). The upper panels also show PSDC profiles retrogradely labeled from the n. Gracilis and the lower panels show STT profiles retrogradely labeled from the thalamus. Low magnification images showing the dorsal horn are shown on the left and higher magnification images from the same section are shown on the right. Ligation of the ureter caused a prominent expression of FOS in the spinal dorsal horn, predominantly in the intermediate and deep laminae. Also visible are PSDC profiles that were retrogradely labeled by injection of tetramethylrhodamine dextran into the n. gracilis. The arrow indicates a retrogradely-labeled PSDC profiles that also expresses FOS in response to ureter ligation. Noxious mechanical pinch evoked FOS expression throughout the dorsal horn of the spinal cord. Retrograde labeling of STT neurons was accomplished by microinjection of tetramethylrhodamine dextran into the contralateral thalamus. The arrow indicates a retrogradely-labeled STT neuron that also expresses immunoreactivity for FOS.
In addition, lumbar spinal cord sections were examined about 1 week after microinjection of tetramethylrhodamine dextran into the thalamus. The red fluorescence indicative of retrogradely-labeled spinothalamic tract (STT) neurons was found in each of the sections examined between L2 and S1 (Figure 5). Most of the labeled STT profiles located in the dorsal horn contralateral (left) to the thalamic injection, although a few labeled profiles were identified on the ipsilateral side. A few labeled profiles were also found around the central canal. These results indicate successful retrograde labeling of neurons projecting to the thalamus from the spinal cord. Although most of the retrogradely labeled STT neurons did not express FOS in response to noxious pinch, sufficient numbers of STT profiles were responsive and evidence of double-labeling was clearly evident (Figure 5). As was observed with the PSDC profiles, the red fluorescence for tetramethylrhodamine dextran was found in the perikarya and proximal dendrites with no labeling in the nuclei, and green immunofluorescence for FOS was found in the nuclei (Figure 5). Among the 690 labeled STT profiles, 83 of these also expressed immunofluorescence for FOS, representing 12% of the retrogradely labeled STT profiles. These double labeled profiles were found principally within the medial portion of the deep dorsal horn, and some double-labeled profiles were found in the superficial laminae and around the central canal (Figure 5).
Discussion
Central sensitization results from repeated, prolonged stimulation of C, but not Aβ, fibers (Li et al. 1999a). With nerve-injury, however, repetitive gentle touch can elicit FOS expression, which is indicative of a “sensitized” spinal dorsal horn (Molander et al. 1998; Shortland and Molander, 1998; Catheline et al. 1999). The present results showed that enhanced FOS expression throughout the spinal dorsal horn was evoked by gentle touch or noxious pinch in nerve-injured rats. However, we suggest that the “sensitized” neurons exclude the PSDC cells or those of the n. gracilis, since neither touch nor pinch evoked FOS expression even after nerve injury. These observations indicate that although a sensitized spinal cord may be required for expression of nerve injury-induced tactile allodynia, direct sensitization of elements of the dorsal column pathway is not essential.
Although the function of the PSDC neurons is unclear, it is not likely that they encode nociception. Electrophysiologic studies demonstrated that most of the PSDC neurons respond to light mechanical stimuli, 36% also respond to pinch and might represent WDR neurons (Giesler and Cliffer, 1985). However, 93% of the PSDC neurons studied did not respond to repeated thermal stimuli, suggesting little input from C or Aδ thermal or C polymodal nociceptors, although they may receive inputs from the small population of Aδ nociceptors that do not respond to noxious heat (Giesler and Cliffer, 1985; Kamogawa and Bennett, 1986). Pinch-sensitive PSDC neurons became sensitive to noxious heat only after prolonged and repetitive exposure, suggesting that they are responsive to high-threshold myelinated mechanoreceptors, but do not receive inputs from unmyelinated nociceptors (Lu et al. 1983; Kamogawa and Bennett, 1986). Furthermore, PSDC cells around the central canal are responsive to noxious visceral and to cutaneous mechanical, but not thermal, inputs (Al-Chaer et al. 1996a, b). Critically, it was demonstrated in the present investigation that the absence of FOS expression evoked by touch or pinch in PSDC cells was not a result of an inability of these cells to express FOS, nor was it an artefact induced by the double-labeling procedures employed. Noxious visceral stimuli evoked FOS expression in retrogradely labeled PSDC neurons, consistent with previous studies (Al-Chaer et al. 1999; Palecek et al, 2003). Likewise, our observation that retrogradely labeled STT cells also demonstrated immunofluorescence for FOS after noxious cutaneous stimuli (Al-Chaer et al. 1999; Palecek et al, 2003).
The laminar distribution of retrogradely labeled PSDC profiles of sham-operated and nerve-injured groups were similar, indicating no change due to peripheral nerve injury. Moreover, the immunofluorescent profile of these cells also remained unchanged, indicating that injury did not elicit apparent phenotypic changes. The identified PSDC profiles did not co-express fluorescent labeling for parvalbumin, calbindin D, CGRP, substance P, PKCg, NPY and galanin or for the MOR, Y1, NK1 or P2X3 receptors in either group of animals. The profile found in the present study is consistent with previous observations (Zhang et al. 1999; Ossipov et al. 2002; Todd, 2002; Gardell et al. 2003). Earlier electrophysiologic studies indicated that transmission of visceral nociception to the n. gracilis is mediated through the PSDC neurons (Palecek et al, 2003; Al-Chaer et al. 1996a). Furthermore, morphine abolished the responses of PSDC neurons to visceral or somatic nociceptive stimuli, but not to innocuous tactile stimuli, suggesting the possibility that modulation of the PSDC pathway by spinal morphine is mediated through opioid receptors at presynaptic sites or on interneurons (al-Chaer et al, 1996a; Willis et al. 1999). Our observation that retrogradely-labeled PSDC profiles did not show immunofluorescence for MOR is consistent with this interpretation. Moreover, immunofluorescence for MOR has not been identified outside of lamina II of the spinal dorsal horn (Spike et al. 2002), and it is possible that MOR may be present at densities below the limits of detection. The presence of tetramethylrhodamine dextran in large diameter DRG neurons is consistent with direct projections of Aβ fibers to the n. gracilis. These cell bodies do not express substance P or CGRP in either the basal or injured state (Ma and Bisby, 1998; Macdonald et al. 2001; Hammond et al. 2004). Retrogradely labeled large-diameter DRG neurons expressed NPY after nerve injury, but not after sham surgery, which is consistent with previous observations showing novel expression of NPY in projections of large-diameter myelinated Aβ neurons to the n. gracilis, and that this neuroplastic change may mediate allodynia (Ma and Bisby, 2000; Ossipov et al. 2002).
The evoked expression of FOS in the spinal cord is a reliable indicator for activation of neurons and increased expression correlates with spinal sensitization (Hunt et al. 1987; Harris, 1998). Noxious-evoked FOS is found in laminae I, II and V (Hunt et al. 1987; Willis and Coggeshall, 1991, 2004). Touch-evoked FOS expression was found in laminae III and IV, and electrical stimulation of Aβ fibers evoked FOS expression in PSDC neurons and the n. gracilis, but only in rats with injury or inflammation (Shortland and Molander, 1998; Catheline et al. 1999; Tokunaga et al. 1999; Catheline et al. 2001). Expression of FOS has been evoked in PSDC neurons by ureter ligation or repetitive pinch in anesthetized rats (Day et al. 2001; Palecek et al. 2003). We found that FOS expression in response to gentle stroking or noxious pinch was enhanced in laminae I through V of conscious rats after nerve injury, but FOS was not expressed in retrogradely-labeled PSDC neurons. Similarly, no FOS expression was evoked in the n. gracilis of these unanesthetized rats. These results are consistent with the conclusion that a sensitized dorsal column pathway is not required to mediate allodynia.
We previously demonstrated that injury-induced tactile allodynia may be mediated through direct projections of Aβ fibers to the n. gracilis (Ossipov et al. 2002). However, spinal sensitization appears to be a key element in the expression of nerve injury-induced allodynia since ablation of lamina I neurons that express the NK-1 receptor abolished behavioral allodynia (Nichols et al. 1999; Porreca et al. 2001; Suzuki et al. 2002; 2004). This treatment also abolished sensitization of laminae III/IV WDR neurons after nerve injury or inflammation and attenuated formalin-evoked FOS expression in lamina V (Nichols et al. 1999; Suzuki et al. 2002; 2004). Spinal sensitization after nerve injury is also maintained by activation of descending pain facilitatory systems from the rostral ventromedial medulla (RVM)(Porreca et al. 2001; Mantyh and Hunt, 2004; Suzuki et al. 2004). Non-PSDC neurons in laminae III and IV that express the NK1 receptor and that contribute to ascending nociceptive pathways have been identified (Todd et al. 2000; Todd, 2002). The NK1-expressing neurons of laminae III and IV are in close contact with descending serotonergic terminals from the RVM and receive inputs from primary afferent nociceptors (Stewart and Maxwell, 2000; Todd, 2002). Accordingly, they may be sensitized by afferent inputs and/or by activation of descending facilitation (Hunt et al. 1987; Suzuki et al. 2002; Suzuki et al. 2004), and may correspond to the FOS-expressing cells of the intermediate laminae in the present study. Selective destruction of descending pronociceptive facilitatory neurons of the RVM abolished evoked touch-evoked FOS expression in the intermediate laminae of the spinal dorsal horn of nerve-injured rats, suggesting that descending facilitation maintains the injury-induced enhanced pain state (Ossipov et al. 2001; Burgess et al., 2002; Porreca et al. 2002; Vera-Portocarrero et al, 2006). Microinjection of lidocaine into the n. gracilis 14 days after SNL (maintenance phase) abolished behavioral signs of tactile, but not thermal, hyperesthesia (Ossipov et al., 2002). A sensitized spinal/supraspinal projection system may be more receptive to tactile inputs from the dorsal column pathway, allowing the interpretation of innocuous tactile sensory input as nociceptive, or aversive. Evidence indicates that tactile inputs mediated through the dorsal column/medial lemniscal pathways converge with inputs from the spinothalamic tract in the ventrobasal complex of the thalamus and may serve as a site of interaction between noxious and innocuous sensory inputs (Ma et al. 1987). Moreover, peripheral nerve injury enhanced the responses of low-threshold mechanoreceptive and WDR neurons of the ventroposterolateral nucleus of the thalamus (Miki et al. 2000) and increased the basal levels of FOS expression in that region (Narita et al. 2003).
Substantial evidence supports the crucial role of the dorsal column pathway in experimental nerve injury. While it is not clear if PSDC neurons are transiently sensitized during the initial phase immediately following nerve injury, it appears that neither the PSDC neurons nor those in n. gracilis remain sensitized after injury, suggesting that sensitization-mediated maintenance of allodynia likely occurs through an alternate mechanism. Sensitization of non-PSDC neurons of the intermediate laminae may activate projections consistent with spinal/supraspinal processing of enhanced pain states and of descending facilitation. The results of this investigation suggest that spinal sensitization may prime the other sites within the central nervous system to interpret tactile stimuli as aversive, but does not require that the tactile pathway itself become sensitized. Clarification of the ascending mechanisms which mediate nerve injury-induced tactile allodynia will be essential in ultimately identifying strategies which can lead to the development of therapeutics for treatment of abnormal tactile hypersensitivity in patients with neuropathic pain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Chaer ED, Feng Y, Willis WD. Comparative study of viscerosomatic input onto postsynaptic dorsal column and spinothalamic tract neurons in the primate. J Neurophysiol. 1999;82:1876–1882. doi: 10.1152/jn.1999.82.4.1876. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996a;76:2675–2690. doi: 10.1152/jn.1996.76.4.2675. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol. 1996b;76:2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- Avelino A, Cruz F, Coimbra A. Sites of renal pain processing in the rat spinal cord. A c-fos study using a percutaneous method to perform ureteral obstruction. J Auton Nerv Syst. 1997;67:60–66. doi: 10.1016/s0165-1838(97)00105-7. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Nishikawa N, Lu GW, Hoffert MJ, Dubner R. The morphology of dorsal column postsynaptic spinomedullary neurons in the cat. J Comp Neurol. 1984;224:568–578. doi: 10.1002/cne.902240406. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Seltzer Z, Lu GW, Nishikawa N, Dubner R. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosens Res. 1983;1:131–149. doi: 10.3109/07367228309144545. [DOI] [PubMed] [Google Scholar]
- Bian D, Nichols ML, Ossipov MH, Lai J, Porreca F. Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats. Neuroreport. 1995;6:1981–1984. doi: 10.1097/00001756-199510010-00007. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Catheline G, Guilbaud G, Kayser V. Peripheral component in the enhanced antinociceptive effect of systemic U-69,593, a kappa-opioid receptor agonist in mononeuropathic rats. Eur J Pharmacol. 1998;357:171–178. doi: 10.1016/s0014-2999(98)00597-4. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Besson JM. Intravenous morphine does not modify dorsal horn touch-evoked allodynia in the mononeuropathic rat: a Fos study. Pain. 2001;92:389–398. doi: 10.1016/S0304-3959(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Honore P, Besson JM. Are there long-term changes in the basal or evoked Fos expression in the dorsal horn of the spinal cord of the mononeuropathic rat? Pain. 1999;80:347–357. doi: 10.1016/s0304-3959(98)00234-6. [DOI] [PubMed] [Google Scholar]
- Day AS, Wen CY, Shieh JY, Sun WZ, Lue JH. Somatic noxious mechanical stimulation induces Fos expression in the postsynaptic dorsal column neurons in laminae III and IV of the rat spinal dorsal horn. Neurosci Res. 2001;40:343–350. doi: 10.1016/s0168-0102(01)00245-0. [DOI] [PubMed] [Google Scholar]
- Field MJ, Bramwell S, Hughes J, Singh L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: are they signalled by distinct primary sensory neurones? Pain. 1999;83:303–311. doi: 10.1016/s0304-3959(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DW, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Cliffer KD. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res. 1985;326:347–356. doi: 10.1016/0006-8993(85)90044-7. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. Journal of Neurophysiology. 1984;51:260–275. doi: 10.1152/jn.1984.51.2.260. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Hsaio SS, Bushnell MC. Somatic Sensation. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego: Academic Press; 1999. pp. 761–789. [Google Scholar]
- Hirshberg RM, Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Is there a pathway in the posterior funiculus that signals visceral pain? Pain. 1996;67:291–305. doi: 10.1016/0304-3959(96)03127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AK, Kadura S, Westlund KN. Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport. 1997;8:3795–3800. doi: 10.1097/00001756-199712010-00028. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Wang CC, Westlund KN. Do nociceptive signals from the pancreas travel in the dorsal column? Pain. 2001;89:207–220. doi: 10.1016/s0304-3959(00)00364-x. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Kamogawa H, Bennett GJ. Dorsal column postsynaptic neurons in the cat are excited by myelinated nociceptors. Brain Res. 1986;364:386–390. doi: 10.1016/0006-8993(86)90853-x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Lundberg LE, Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117(Pt 3):579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- Lee YW, Chaplan SR, Yaksh TL. Systemic and supraspinal, but not spinal, opiates suppress allodynia in a rat neuropathic pain model. Neurosci Lett. 1995;199:111–114. doi: 10.1016/0304-3940(95)12034-2. [DOI] [PubMed] [Google Scholar]
- Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999a;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Li WP, Xian C, Rush RA, Zhou XF. Upregulation of brain-derived neurotrophic factor and neuropeptide Y in the dorsal ascending sensory pathway following sciatic nerve injury in rat. Neurosci Lett. 1999b;260:49–52. doi: 10.1016/s0304-3940(98)00958-6. [DOI] [PubMed] [Google Scholar]
- Lu GW, Bennett GJ, Nishikawa N, Hoffert MJ, Dubner R. Extra- and intracellular recordings from dorsal column postsynaptic spinomedullary neurons in the cat. Exp Neurol. 1983;82:456–477. doi: 10.1016/0014-4886(83)90417-x. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996;67:307–316. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Increase of preprotachykinin mRNA and substance P immunoreactivity in spared dorsal root ganglion neurons following partial sciatic nerve injury. Eur J Neurosci. 1998;10:2388–2399. doi: 10.1046/j.1460-9568.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Partial sciatic nerve ligation induced more dramatic increase of neuropeptide Y immunoreactive axonal fibers in the gracile nucleus of middle-aged rats than in young adult rats. J Neurosci Res. 2000;60:520–530. doi: 10.1002/(SICI)1097-4547(20000515)60:4<520::AID-JNR11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ma W, Peschanski M, Ralston HJ., 3rd The differential synaptic organization of the spinal and lemniscal projections to the ventrobasal complex of the rat thalamus. Evidence for convergence of the two systems upon single thalamic neurons. Neuroscience. 1987;22:925–934. doi: 10.1016/0306-4522(87)92970-8. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Bingham S, Bond BC, Parsons AA, Philpott KL. Determination of changes in mRNA expression in a rat model of neuropathic pain by Taqman quantitative RT-PCR. Brain Res Mol Brain Res. 2001;90:48–56. doi: 10.1016/s0169-328x(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP. Setting the tone: superficial dorsal horn projection neurons regulate pain sensitivity. Trends Neurosci. 2004;27:582–584. doi: 10.1016/j.tins.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Gannon A, Levine JD, Basbaum AI. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989;285:177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Miki K, Iwata K, Tsuboi Y, Morimoto T, Kondo E, Dai Y, Ren K, Noguchi K. Dorsal column-thalamic pathway is involved in thalamic hyperexcitability following peripheral nerve injury: a lesion study in rats with experimental mononeuropathy. Pain. 2000;85:263–271. doi: 10.1016/s0304-3959(99)00279-1. [DOI] [PubMed] [Google Scholar]
- Molander C, Hongpaisan J, Shortland P. Somatotopic redistribution of c-fos expressing neurons in the superficial dorsal horn after peripheral nerve injury. Neuroscience. 1998;84:241–253. doi: 10.1016/s0306-4522(97)00375-8. [DOI] [PubMed] [Google Scholar]
- Narita M, Ozaki S, Ise Y, Yajima Y, Suzuki T. Change in the expression of c-fos in the rat brain following sciatic nerve ligation. Neurosci Lett. 2003;352:231–233. doi: 10.1016/j.neulet.2003.08.052. [DOI] [PubMed] [Google Scholar]
- Nauta HJ, Hewitt E, Westlund KN, Willis WD., Jr Surgical interruption of a midline dorsal column visceral pain pathway. Case report and review of the literature. J Neurosurg. 1997;86:538–542. doi: 10.3171/jns.1997.86.3.0538. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Bian D, Malan TP, Jr, Lai J, Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr, Vanderah TW, Porreca F. Tonic descending facilitation as a mechanism of neuropathic pain. In: Hansson PT, Fields HL, Hill RG, Marchettini P, editors. Neuropatic Pain: Pathophysiology and Treatment. Seattle: IASP Press; 2001. pp. 107–124. [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek J. The role of dorsal columns pathway in visceral pain. Physiol Res. 2004;53(Suppl 1):S125–S130. [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. The roles of pathways in the spinal cord lateral and dorsal funiculi in signaling nociceptive somatic and visceral stimuli in rats. Pain. 2002;96:297–307. doi: 10.1016/S0304-3959(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. Fos expression in spinothalamic and postsynaptic dorsal column neurons following noxious visceral and cutaneous stimuli. Pain. 2003;104:249–257. doi: 10.1016/s0304-3959(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland P, Molander C. The time-course of abeta-evoked c-fos expression in neurons of the dorsal horn and gracile nucleus after peripheral nerve injury. Brain Res. 1998;810:288–293. doi: 10.1016/s0006-8993(98)00940-8. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Sakamoto H, Stewart W, Watt C, Todd AJ. MOR-1-immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for nonsynaptic innervation by substance P-containing primary afferents and for selective activation by noxious thermal stimuli. Eur J Neurosci. 2002;15:1306–1316. doi: 10.1046/j.1460-9568.2002.01969.x. [DOI] [PubMed] [Google Scholar]
- Stewart W, Maxwell DJ. Morphological evidence for selective modulation by serotonin of a subpopulation of dorsal horn cells which possess the neurokinin-1 receptor. Eur J Neurosci. 2000;12:4583–4588. [PubMed] [Google Scholar]
- Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Kondo E, Fukuoka T, Miki K, Dai Y, Tsujino H, Noguchi K. Excitability of spinal cord and gracile nucleus neurons in rats with chronically injured sciatic nerve examined by c-fos expression. Brain Res. 1999;847:321–331. doi: 10.1016/s0006-8993(99)02074-0. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49:393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wang CC, Willis WD, Westlund KN. Ascending projections from the area around the spinal cord central canal: A Phaseolus vulgaris leucoagglutinin study in rats. J Comp Neurol. 1999;415:341–367. doi: 10.1002/(sici)1096-9861(19991220)415:3<341::aid-cne3>3.0.co;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord, Vol. 1, Primary Afferent Neurons and the Spinal Dorsal Horn. New York: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- Zhang X, Tong YG, Bao L, Hokfelt T. The neuropeptide Y Y1 receptor is a somatic receptor on dorsal root ganglion neurons and a postsynaptic receptor on somatostatin dorsal horn neurons. Eur J Neurosci. 1999;11:2211–2225. doi: 10.1046/j.1460-9568.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]