Abstract
Opioids can induce hyperalgesia in humans and in animals. Mechanisms of opiate-induced hyperalgesia and possibly of spinal antinociceptive tolerance may be linked to pronociceptive adaptations occurring at multiple levels of the nervous system including activation of descending facilitatory influences from the brainstem, spinal neuroplasticity, and changes in primary afferent fibers. Here, the role of NK-1 receptor-expressing cells in the spinal dorsal horn in morphine-induced hyperalgesia and spinal antinociceptive tolerance was assessed by ablating these cells with intrathecal injection of SP-saporin (SP-SAP). Ablation of NK-1 receptor expressing cells prevented (a) morphine-induced thermal and mechanical hypersensitivity, (b) increased touch-evoked spinal FOS expression, (c) upregulation of spinal dynorphin content and (d) the rightward displacement of the spinal morphine antinociceptive dose-response curve (i.e., tolerance). Morphine-induced hyperalgesia and antinociceptive tolerance were also blocked by spinal administration of ondansetron, a serotonergic receptor antagonist. Thus, NK-1 receptor expressing neurons play a critical role in sustained morphine-induced neuroplastic changes which underlie spinal excitability reflected as thermal and tactile hypersensitivity to peripheral stimuli, and to reduced antinociceptive actions of spinal morphine (i.e., antinociceptive tolerance). Ablation of these cells likely eliminates the ascending limb of a spinal-bulbospinal loop that engages descending facilitation and elicits subsequent spinal neuroplasticity. The data may provide a basis for understanding mechanisms of prolonged pain which can occur in the absence of tissue injury.
Keywords: Opioid-induced hyperalgesia, descending facilitation, spinal plasticity, spinal tolerance, NK-1 receptors, projection cells
1. Introduction
Opioid-induced “paradoxical pain” has been repeatedly described in both clinical and preclinical settings (Ali, 1986; Stillman et al., 1987; Arner and Meyerson, 1988; De Conno et al., 1991; Devulder, 1997; Yaksh et al., 1986; Yaksh and Harty, 1988; Mao et al., 1994, 1995, 1998; Larcher et al., 1998; Celerier et al., 2000, 2001; Vanderah et al., 2000, 2001a,b; Angst and Clark, 2006). Preclinical studies have shown opioid-induced neuroplastic changes including upregulation of dynorphin (Gardell et al., 2002) and NK-1 receptors (King et al., 2005) in the spinal cord, and upregulation of spinal substance P and CGRP content and release in primary afferents (King et al., 2005; Gardell etl al., 2002), findings that support increased excitability in nociceptive pathways. Additionally, sustained morphine administration produces plasticity in the rostral ventromedial medulla (RVM) which results in activation of descending facilitation, in part, through enhanced activity of cholecystokinin (CCK) in this region (Vanderah et al., 2001b; Xie et al., 2005). Blockade of descending facilitatory pathways has been shown to block sustained opiate-induced thermal and tactile hypersensitivity, the upregulation of spinal dynorphin (Vanderah et al, 2001b; Xie et al., 2005) and the expression of antinociceptive tolerance to spinal morphine (Vanderah et al, 2001b).
Lamina I cells expressing the NK1 receptor project to supraspinal areas that mediate nociceptive processing (Marshall et al., 1996; Todd et al., 2000; 2002). This same population of cells plays a critical role in the maintenance of injury-induced hyperalgesia as their ablation blocks capsaicin-induced mechanical and thermal hyperalgesia (Mantyh et al., 1997), as well as persistent inflammatory and neuropathic pain (Nichols et al., 1999; Suzuki et al., 2002). Ablation of spinal NK-1 receptor expressing cells has also been demonstrated to block sensitization of spinal dorsal horn cells following intraplantar injection of capsaicin (Khasabov et al., 2002). Elimination of NK1-receptor expressing cells inhibited CFA-induced hyperalgesia and diminished formalin-induced hyperexcitability of wide dynamic range neurons (WDR) (Suzuki et al., 2002). In conditions of tissue injury, it has been proposed that spinal NK1 receptor expressing cells in lamina I may act as a critical component in an ascending pathway which results in activation of descending facilitation from the brainstem through serotonergic projections to the spinal dorsal horn (Suzuki et al., 2002). Supporting this concept, descending facilitation was blocked by spinal ondansetron, a 5HT3 receptor antagonist (Suzuki et al., 2002).
The present study explored the possible role of spinal NK-1 receptor expressing cells in a hyperalgesic condition which is independent of tissue injury. Our data suggest that spinal NK-1 receptor expressing cells play a critical role in the development of sustained opiate-induced spinal excitability, thermal and mechanical hypersensitivity, and antinociceptive tolerance. These data support the importance of spinal NK-1 receptor expressing cells as a gateway to engagement of descending facilitation by opioids in the absence of tissue injury. Such mechanisms may be relevant to clinical conditions in which pain occurs in the absence of prominent tissue injury.
2. Methods
2.1 Animals and experimental design
Male Sprague-Dawley rats (Harlan; Indianapolis, IN), 200–300 g at time of testing were maintained in a climate-controlled room on a 12-h light/dark cycle (lights on at 06:00 h) with food and water available ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals and received approval from the Institutional Animal care and Use Committee of the University of Arizona. Groups of 6 to 8 rats were used in all experiments.
All animals received intrathecal injections of SP-SAP, or respective controls. Twenty-eight days later, these animals were implanted s.c. with minipumps delivering either morphine or saline. Separate groups of animals were tested for (a) mechanical and thermal thresholds, (b) spinal morphine antinociceptive tolerance, (c) spinal dynorphin content and (d) touch-evoked expression of spinal FOS. Additionally, separate groups of animals were implanted s.c. with minipumps delivering either morphine or saline and the effects of spinal ondansetron on morphine-induced hypersensitivity and antinociceptive tolerance were examined.
2.2 Spinal pretreatments
Rats underwent surgery for implantation of an intrathecal catheter under halothane anesthesia (polyethylene-10 tubing; 7.5 cm) as described previously (Yaksh and Rudy, 1976) for drug administration at the level of the lumbar cord. Seven days later rats received a single i.th. injection of either SP-SAP (10 µM), SAP alone (10 µM) or saline in a volume of 5 µl followed by a saline flush in a volume of 9 µl according to the protocol established by Mantyh and colleagues (Mantyh et al., 1997). Rats were behaviorally monitored and any animals presenting motor deficits were excluded from the study; fewer than 5% of rats showed signs of motor deficits resulting from the spinal surgery. SAP and SP-SAP were purchased from Advanced Targeting Systems (San Diego, CA). In pilot studies, animals were injected with i.th. SP as an additional control. Animals injected with SP presented typical transient pain behaviors immediately after the injection which dissipated within minutes. No effects of the i.th. SP pretreatment on baseline sensory thresholds were observed at 28 day after the injection. Therefore, SP-treated animals were not studied in order to minimize the number of animals used and to adhere to the protocol used in previous studies (Mantyh et al., 1997).
2.3 Sustained morphine administration
Morphine (64 mg/kg) was administered by s.c. osmotic minipumps ( Alza, Mountain View, CA) which were implanted 28 days after the i.th. pretreatments with SP-SAP, SAP or saline. Control groups received saline minipumps. The osmotic minipumps delivered saline at 1 µl/hr or morphine at 45 µg ˙ µl−1 ˙ hr−1 for 7 days. Minipumps were filled according to manufacturers specifications. This dose of morphine was chosen since it is the maximum dose that could be dissolved and allow for proper function of the minipump. The use of the osmotic pump insures a continuous delivery of morphine avoiding intermittent periods of withdrawal.
2.4 Behavioral testing
Behavioral responses to thermal and tactile stimuli were determined before the i.th. pretreatments and every 7 days afterwards until implantation of the osmotic minipumps. Behavioral thresholds were then recorded each day after minipump implantation until day 6. Response thresholds to innocuous mechanical stimuli were assessed with calibrated von Frey filaments. Each filament was applied perpendicularly to the plantar surface of the paw of rats kept in suspended wire-mesh cages. The withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (“up and down” method, (Chaplan et al., 1994) and analyzed using a Dixon nonparametric test (Dixon, 1980). Data are expressed as the mean withdrawal threshold ± standard error of the mean (SEM) in grams. A significant reduction in paw withdrawal threshold from before implantation of the minipumps indicated tactile hypersensitivity. Pairwise comparisons were performed with Student’s t test. Significance was set at the level of p < 0.05. Response thresholds to noxious thermal stimuli were evaluated using the method of Hargreaves and colleagues (Hargreaves et al., 1988). In brief, rats were allowed to acclimate within a plexiglass enclosure on a clear glass plate maintained at 30°C. A radiant heat source (i.e., high intensity projector lamp) was activated with a timer and focused onto the plantar surface of the hindpaw. Paw-withdrawal latency was determined by a motion detector that halted both lamp and timer when the paw was withdrawn. A maximal cut-off of 40 sec was employed to prevent tissue damage. A significant reduction in paw withdrawal latency from before implantation of the osmotic minipump was characterized as thermal hyperalgesia. Pairwise comparisons were performed with Student’s t-test. Significance was set at the level of p < 0.05.
2.5 Antinociceptive tolerance
Assessment of antinociception was accomplished by placing the distal third of the tail of the rat in a water bath maintained at 52°C. The latency to withdrawal was measured to 0.1 sec and a cutoff latency of 10 sec was used to prevent tissue injury. The tail-flick test was used to determine the antinociceptive A50 dose (the dose produce 50% antinociception from the dose-response curve) of morphine injected though the i.th. catheter. A straight line was fitted to the linear portion of the log dose-effect curve using linear regression as previously described (Tallarida et al., 1987). The possible expression of antinociceptive tolerance following s.c. delivery of morphine by minipump was assessed by constructing the i.th. morphine dose-response curve at the 30 min post-injection time point, a time previously demonstrated to produce maximal antinociception. Dose response curves were compared before and after implantation of the osmotic minipumps. Data were converted to %MPE by the following formula (response latency - baseline latency)/(cutoff - baseline latency) × 100. Tolerance to the antinociceptive effect of morphine was indicated by a significant rightward shift in the dose-response curve.
2.6 Dynorphin immunoassay
Rats were deeply anesthetized with ether and sacrificed on day 6 after osmotic minipump implantation. The spinal cord was ejected with ice-cold saline and placed on an iced glass Petri dish, and the dorsal lumbar cord rapidly dissected. The tissue samples were immediately frozen on dry ice and stored at −70°C. Thawed tissue was placed in 1N acetic acid, disrupted with a Polytron homogenizer, and incubated for 20 min at 95°C. After centrifugation at 10,000 × g for 20 min (4°C), the supernatant was lyophilized and stored at −70°C. Protein concentrations were determined by the use of the bicinchoninic acid method with bovine serum albumin as a standard. Immunoassay was performed by the use of a commercial enzyme immunoassay kit with an antibody specific for dynorphin A(1–17) (Peninsula Laboratories, Belmont, CA). Standard curves were constructed and dynorphin content was determined with Graph Pad Prism (San Diego, CA).
2.7 Immunohistochemistry
Rats from all groups were deeply anesthetized with a mixture of ketamine/xylazine and perfused transcardially with 100 ml of saline containing heparin (1500 IU/l), followed by 500 ml of cold 4% paraformaldahyde. After perfusion the spinal cords and DRGs were isolated and post-fixed for 4 hr in 4% paraformaldehyde and then cryoprotected with 30% sucrose in PBS overnight at 4°C. Coronal frozen sections (30 µm) were cut from the lumbar enlargement of the spinal cord and processed as floating sections. Sections from lumbar DRGs were cut at a thickness of 20 µm and mounted on gel slides for staining procedures. Spinal cord sections were immunolabeled either with a rabbit antiserum against the NK1 receptor (a generous gift from Dr. Patrick Mantyh, 1:5000), antiserum against substance P (Chemicon, Temecula, CA; 1:5000), antiserum against CGRP (Peninsula, San Diego, CA, 1:5000) or antiserum against FOS protein (Oncogene, San Diego, CA 1:10000). In brief, the spinal cord sections were washed six times for 5 min each in PBS and then preincubated with PBS containing 5 % normal goat serum (NGS), 0.3% Triton X-100 for 30 min at room temperature. The sections were then incubated in the primary antiserum diluted in 2% NGS overnight. The following day the sections were washed six times in PBS for 5 min each, followed by incubation with secondary antibody CY3-conjugated goat anti-rabbit IgG (1:500; Jackson ImmunoResearch, West Grove, PA) for 2 hr. The sections were rinsed and mounted in Vectashield. Fluorescent digital images were captured using a Nikon (Tokyo, Japan) E800 fluorescence microscope outfitted with a Hamamatsu (Bridgewater, NJ) C5810 color CCD camera. Photographs were taken and cells counted manually by an experimenter blinded to the experimental conditions using Metamorph (Universal Imaging Corporation, PA). The data are expressed as the percentage of cells showing positive staining from the total number of cells in the DRG.
2.8 Assessment of touch-evoked FOS
On day 6 after osmotic minipump implantation, rats were gently restrained using a towel and received repetitive non-noxious tactile stimulation of the hindpaw as previously described (Ma and Woolf, 1996). Animals were gently restrained under a towel and the flat portion of the experimenter’s thumb was placed in the center of the plantar hindpaw and stroked gently towards the distal foot pad over a period of 2 sec ever 4 sec over a period of 10 min. Two other groups of rats received either tactile stimulation without any previous surgical manipulations or no tactile stimulation at all. Two hours following stimulation, animals were perfused transcardially with PBS followed by 4% paraformaldehyde. The lumbar spinal cord was harvested and processed for FOS expression though immunohistochemical analysis. FOS-positive cells were counted using Metamorph with a minimum of 10 sections per animal. There were 5 animals per group.
2.9 Spinal Ondansetron and morphine-induced hypersensitivity
Rats were implanted with intrathecal catheters as described above. Seven days after surgery, rats were implanted with osmotic minipumps for sustained morphine administration or the respective saline minipumps. On day 6 after osmotic minipump implantation, rats were tested for mechanical and thermal sensitivity before i.th. injections of either saline vehicle or the 5HT3 receptor antagonist ondansetron (30 µg/ 9 µl, followed by a 5 µl saline flush). Rats were then tested at 40 min after i.th. injection since previous studies have shown this time point to be the time peak of ondansetron effects (Suzuki et al., 2002; 2005).
2.10 Spinal Ondansetron and Tolerance
Rats were implanted with i.th. catheters and osmotic minipumps as described before. On day 6 after minipump implantation, rats were injected with saline or ondansetron (30 µg) i.th. and then 10 min later with different doses of morphine (1, 3, 10 and 30 µg, n=6 per dose). At 30 min after spinal morphine administration, rats underwent antinociceptive testing using the tail-flick test as described above. The A50 antinociceptive dose was calculated from the generated dose-response curve for all groups. Data were converted to %MPE. Tolerance was indicated by a significant rightward shift in the dose-response curve. Pairwise comparisons between treatments were determined by Student’s t-test. Significance was established at the p< 0.05 level.
3. Results
3.1 Spinal NK-1 receptors
Twenty-eight days after the single i.th. treatment with SP-SAP, saporin or saline, animals that had been treated with sustained systemic (s.c.) morphine or saline exposure across an additional 6 days were sacrificed and the lumbar cord was processed for NK-1 receptor immunohistochemistry. There was an increase in staining of the NK-1 receptor (data not shown) in sections from animals treated with morphine when compared to controls treated with saline in agreement with our previous studies (King et al., 2005). Sections from animals treated with SP-SAP demonstrated a decrease in NK-1 staining regardless of the treatment suggesting that the sustained morphine-induced increased expression of NK-1 receptors within the spinal dorsal horn is not likely due to novel NK-1 receptor expression.
3.2 Sensory thresholds following sustained morphine administration
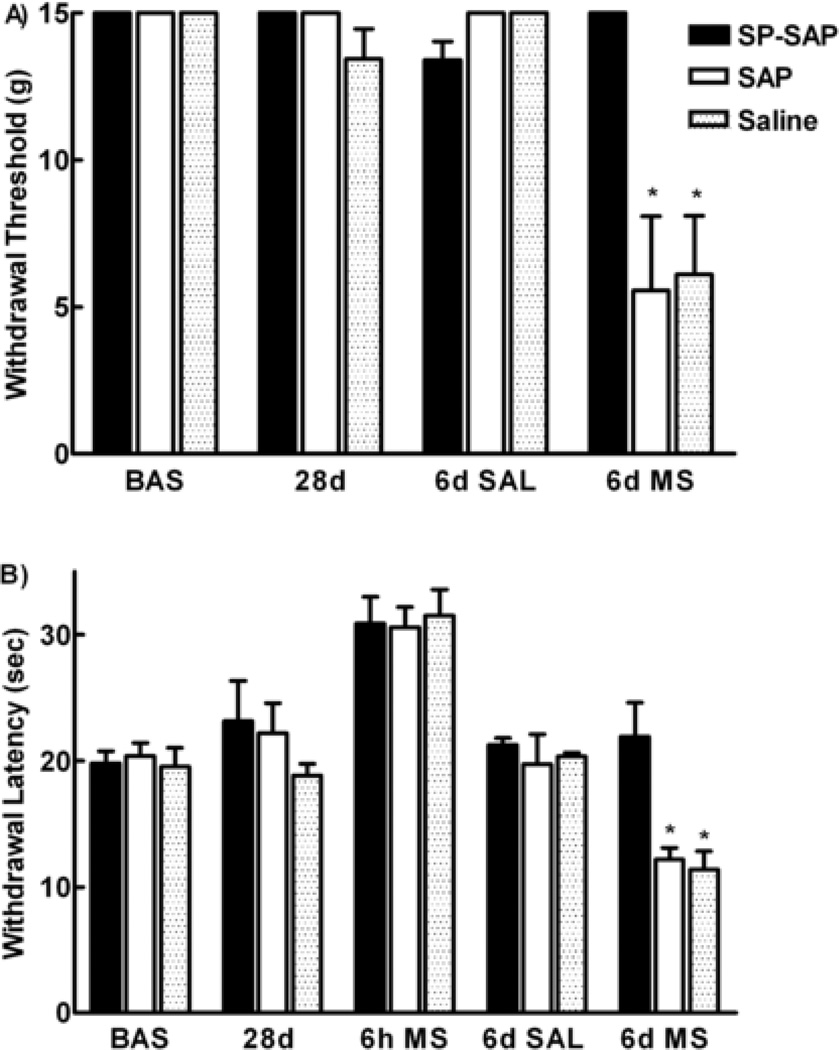
The mean pretreatment (i.e., baseline) paw-withdrawal threshold to probing of the hindpaw with von Frey filaments was 15 ± 0 gm, and the paw-withdrawal latency to noxious radiant heat applied to the plantar aspect of the hindpaw was 20.4 ± 1.1 sec. No significant changes in baseline sensory thresholds were observed after a single i.th. pretreatment with SP-SAP, SAP or saline across the 28 day post-injection period in agreement with previous studies (data not shown) (Mantyh et al., 1997). On day 28 after i.th. injection, animals were evaluated for baseline sensory thresholds and were then implanted with morphine or saline minipumps. No significant differences in sensory thresholds were observed following s.c. saline minipumps and spinal pretreatments with saline, SAP or SP-SAP. Animals previously injected with SP did not show any changes in sensory thresholds when tested 28 days later (data not shown). Animals receiving morphine-filled minipumps and previous i.th. injection of saporin or saline demonstrated mechanical and thermal hypersensitivity on day 6 after minipump implantation. In contrast, the sensory thresholds of the group receiving morphine minipumps following i.th. pretreatment with SP-SAP were not different from baseline (Figure 1A).
Figure 1.
Mechanical and thermal hypersensitivity induced by sustained morphine exposure is prevented by SP-SAP. Male Sprague Dawley rats received a single i.th. injection of either SP-SAP, SAP or saline. Twenty–eight days later animals were implanted with morphine or saline minipumps. A) Mechanical threshold to stimulation with von Frey filaments was no different from baseline before implantation of the minipumps (28d). Six days after saline minipump implantation, the mechanical threshold did not change. Animals with morphine minipumps demonstrated a decrease in mechanical threshold (SAP and saline) (* denotes p< 0.05 compared to baseline and 28d values). In contrast animals with a single i.th. injection of SP-SAP had mechanical threshold similar to baseline. B) Paw withdraw latencies were no different from baseline before implantation of the minipumps (28d). Animals receiving morphine minipumps demonstrated analgesia represented by an increase in thermal latencies above baseline (6h ms). Six days after saline minipump implantation, the paw withdrawal latencies did not change. Animals with morphine minipumps demonstrated decreased paw withdrawal latencies in the SAP and saline groups (* denotes p < 0.05 compared to baseline and 28d values). In contrast, animals with a single i.th. SP-SAP injection had paw withdrawal latencies similar to baseline. There were 8 animals per group.
Similar results were obtained when measuring thermal thresholds. Animals implanted with morphine minipumps demonstrated antinociception to thermal nociceptive tests when tested at 6 hours after minipump implantation as previously described (King et al., 2005; Figure 1B). Six days after morphine minipump implantation, thermal withdrawal latencies were decreased indicating the development of thermal hypersensitivity (Figure 1B). In contrast, animals which had received i.th. SP-SAP pretreatment showed similar thermal withdrawal latencies prior to minipump implantation and 6 days following morphine minipump implantation, indicating that these animals did not develop thermal hypersensitivity. There were no statistical differences between the control groups of saline and SAP in any of the behavioral paradigms.
3.3 Spinal antinociceptive tolerance
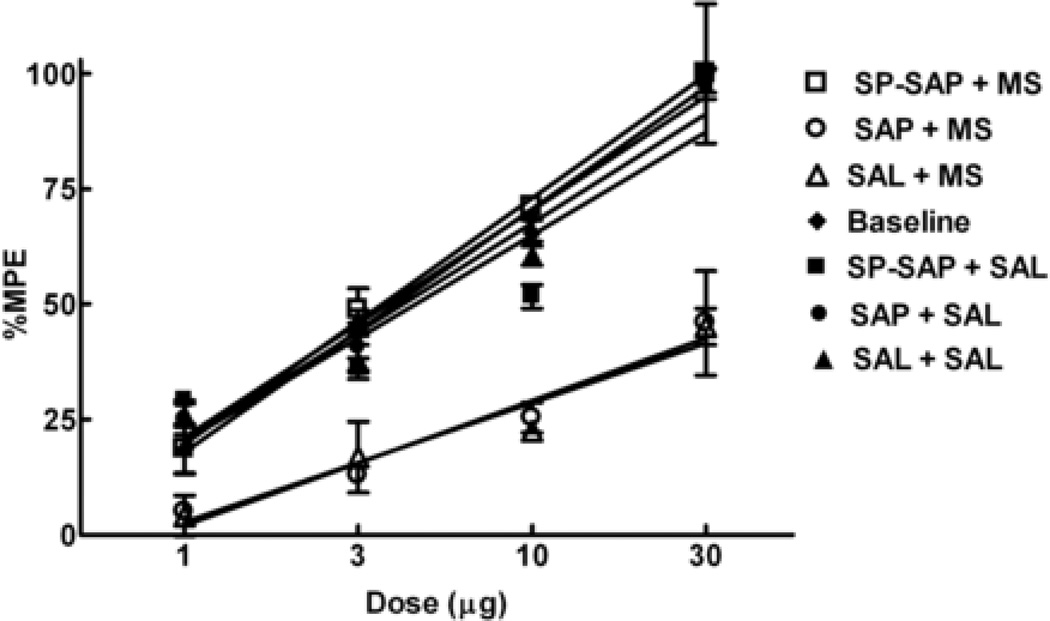
Twenty-eight days after i.th. treatment, dose-response curves for spinal morphine induced antinociception were generated for saline, SAP and SP-SAP groups before osmotic minipump implantation. There were no significant differences between the three dose-response curves so they were collapsed into one (Figure 2, baseline). Following implantation of saline minipumps, the A50 values for i.th. morphine were 3.7 µg (2.3–5.9 µg), 4.19 µg (1.7–10.2 µg) and 4.5 (0.9–21.9 µg) for SAP, saline and SP-SAP pretreated animals respectively. Following implantation of morphine minipumps, the A50 values for i.th. morphine showed a rightward shift to 57.2 µg (24.0–136.1 µg) and 67.1 µg (22.2–202.0 µg) for SAP and saline treated animals respectively, indicating development of spinal antinociceptive tolerance. In contrast, there was no shift in the dose-response curve of the SP-SAP pre-treated group, which had an A50 value of 3.5 µg (2.6–4.8 µg), indicating no development of antinociceptive tolerance.
Figure 2.
Antinociceptive tolerance to spinal morphine induced by sustained morphine exposure is prevented by SP-SAP. Male Sprague Dawley rats received i.th. injections of SP-SAP, SAP or saline and 28 days later either morphine (open symbols) or saline minipumps (closed symbols). Antinociceptive dose-response functions for i.th. morphine were generated before minipump implantation (Baseline) and 6 days after minipump implantation in the 52°C hot water tail-flick test. Each group of rats was tested with only one dose, 30 min after i.th. morphine injection. The dose-effect curve for i.th. morphine in groups with morphine minipumps and previous i.th. injections of SAP or saline was shifted significantly to the right of that for animals with saline minipumps (P<0.05). This dose-effect curve of animals with morphine minipumps and previous i.th. injection of SP SAP was not different from that of the animals with saline minipumps or baseline. There were 6 animals per dose.
3.4 Morphine-induced upregulation of spinal dynorphin
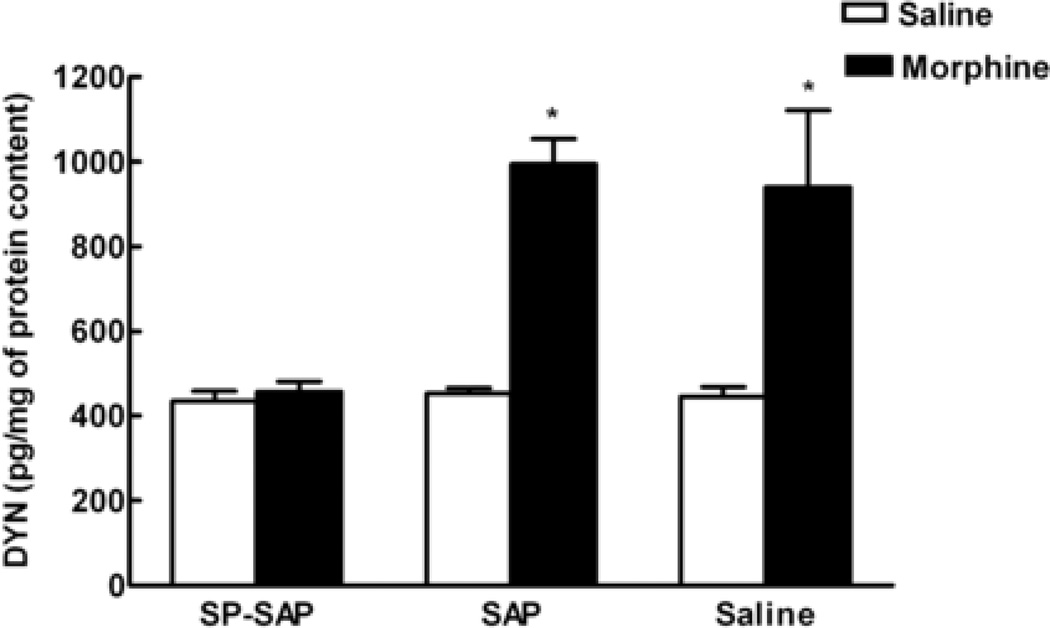
Dynorphin content was measured in the spinal cord dorsal horn on day 6 after morphine minipump implantation in animals that had received i.th. pre-treatment of SP-SAP, SAP or saline and treatment with s.c. osmotic minipumps containing saline or morphine (Figure 3). No differences in dynorphin content were observed in saline treated rats pretreated with i.th. SP-SAP, SAP or saline, with spinal dynorphin content of 134.25 ± 23.36, 153.69 ± 12.07 and 145.36 ± 23.90 pg/mg, respectively. In contrast, animals pretreated with i.th. SAP or saline that had morphine minipumps showed a significant increase in spinal dynorphin levels 6 days after minipump implantation, (394.94 ± 59.24 and 540.07 ± 181.98 pg/mg, respectively). SP-SAP pretreated rats that had sustained morphine exposure did not show increased spinal dynorphin levels (157.69 ± 24.57 pg/mg). Thus, sustained s.c. morphine induced upregulation of spinal dynorphin levels in rats pretreated with i.th. SAP or saline but not in those animals pretreated with i.th. SP-SAP.
Figure 3.
The increase in dynorphin content in the spinal cord induced by sustained morphine exposure is prevented by SP-SAP. Male Sprague Dawley rats received single it.h injections of SP-SAP, SAP or saline and 28 days later implanted subcutaneously with morphine or saline (placebo) osmotic minipumps. At day 6 after minipump implantation, the spinal cords were removed, and the dorsal half of the lumbar cords were assayed for dynorphin content. The dorsal lumbar cords of rats with morphine minipumps and previous i.th. injections of SAP or saline showed significantly greater levels of dynorphin (* denotes P< 0.05 vs. saline groups) than tissues from rats with saline minipumps. The levels of spinal dynorphin from rats with saline minipumps were not different between the 3 groups (SP-SAP, SAP, saline). Morphine minipumps failed to significantly increase the levels of dynorphin in spinal tissues taken from animals with a previous single i.th. injection of SP-SAP. These levels were not different from those seen with saline minipumps. Each treatment group consisted of 8 animals.
3.5 Touch-evoked FOS in the spinal cord dorsal horn
Animals without repetitive touch stimulation (non-stimulated controls) showed a mean of 4.9 ± 0.7 cells expressing FOS overall in the spinal dorsal horn. Repetitive, non-noxious stimulation of the hindpaw in awake, non-anesthetized animals implanted with morphine minipumps resulted in increased FOS expression in the ipsilateral spinal cord dorsal horn when compared with animals implanted with saline minipumps (Figure 4, Table 1). Animals pretreated with saline or SAP, and implanted twenty-eight days later with saline minipumps had mean FOS counts of 28.5 ± 2.4 and 30.1 ± 2.0, respectively. These FOS counts are consistent with previous studies examining FOS expression after tactile stimulation in normal animals (Abbadie et al, 1993) or sham operated animals (Hao et al, 2003a,b; Vera-Portocarrero et al., 2006) but higher than some studies reporting lower levels of FOS expression in control animals (Catheline et al., 1998; 2001) perhaps reflecting methodological differences which enhance variability (see Coggeshall et al, 2005 for review). Sustained morphine increased mean FOS counts to 50.2 ± 3.2 and 58.4 ± 4.1 in the spinal dorsal horn of SAP and saline pretreated rats, respectively. The sustained morphine-induced increase in the number of touch-evoked FOS cells was prevented in animals pretreated spinally with SP-SAP (33.5 ± 2.8 cells in tissues from saline minipumps animals vs. 32.7 ± 3.4 cells in tissues from morphine minipump animals, Figure 4). There was no difference in the number of FOS-expressing cells in the spinal dorsal horn contralateral to the side of tactile stimulation in any of the treatment groups (Table 1).
Figure 4.
Treatment with SP-SAP prevents the increase in touch-evoked FOS induced by sustained morphine exposure. Bar graph representing the number of FOS-positive cells in the spinal cord dorsal horn at the L5 levels counted in the side ipsilateral to tactile stimulation. Sprague Dawley rats received i.th. injections of SP-SAP, SAP or saline and 28 days later implanted with morphine or saline minipumps. The number of touch-evoked FOS positive cells in animals with morphine minipumps was increased compared with the number of FOS cells in animals with saline minipumps (* denotes P<0.05 vs. saline groups). This increase was only seen in animals with previous i.th. injection of SAP or saline. Animals with single i.th. injections of SP-SAP demonstrated similar numbers of touch-evoked FOS positive cells after morphine minipumps and saline minipumps. There were no differences in the number of touch-evoked FOS positive cells in the contralateral side to stimulation in any of the experimental groups.
TABLE 1.
| Saline+Saline | SAP+Saline | SP- SAP+Saline |
Saline+Morphine | SAP+Morphine | SP- SAP+Morphine |
|
|---|---|---|---|---|---|---|
| Ipsilateral | 28.5±2.4 | 30.1±2.0 | 33.5±2.8 | 58.4±4.1* | 50.2±3.2* | 32.7±3.4 |
| Contralateral | 19.3±1.6 | 16.2±1.5 | 19.2±1.5 | 17.9±1.7 | 17.2±1.3 | 17.3±1.7 |
Mean number of FOS positive cells per section in L5 spinal cord dorsal horn after non-noxious stimulation (*P<0.05 vs. saline pumps).
3.6 Spinal ondansetron and morphine-induced mechanical and thermal hypersensitivity
Animals implanted with morphine minipumps had enhanced sensitivity to mechanical and thermal stimulation on day 6 after minipump implantation (Figure 5). Prior to morphine administration, baseline tactile thresholds were 15 ± 0 gm. On day 6, mechanical withdrawal threshold was 5.29 ± 0.7 gm for animals with sustained morphine indicating development of tactile hypersensitivity. Spinal application of ondansetron (30 µg) reversed the morphine-induced tactile hypersensitivity with a maximal response at 40 min after injection. After ondansetron injection, mechanical withdrawal threshold was 9.75 ± 1.4 gm, which was significantly different from the threshold value before injection (P< 0.05; Figure 5A). Similar results were obtained when measuring thermal thresholds. Prior to morphine administration, the baseline thermal threshold was 19.4 ± 0.2 sec. On day 6, thermal withdrawal latency for animals with morphine minipumps was 12.8 ± 0.6 sec, indicating the development of thermal hypersensitivity. Spinal application of ondansetron (30 µg) reversed morphine-induced thermal hypersensitivity at with a maximal response at 40 min after injection. After ondansetron injection, thermal withdrawal latency was 17.3 ± 0.5 sec, which was significantly different from the latency before injection (P< 0.05; Figure 5B). Spinal application of ondansetron did not have any effects on tactile and thermal thresholds in animals implanted with saline minipumps. As an additional control, animals with morphine and saline minipumps were given saline spinally. Spinal application of saline did not affect tactile and thermal thresholds of animals implanted with morphine or saline minipumps (Figure 5).
Figure 5.
Spinal administration of ondansetron reduces mechanical and thermal hypersensitivity induced by sustained morphine exposure. Male Sprague Dawley rats were implanted saline or morphine minipumps and 6 days later the effects of ondansetron on sustained morphine-induced hypersensitivity were studied. Rats with morphine minipumps demonstrated a decrease in mechanical (A) and thermal thresholds (B) at 6 days after minipump implantation. Injection of ondansetron (30 µg) it.h. attenuated the mechanical and thermal hypersensitivity 40 min after injection. It.h. injection of saline did not have any effects. (* denotes P<0.05 vs before it.h. injection). Animals with saline minipumps did not demonstrate changes in mechanical and thermal thresholds 6 days after implantation and after ondasentron injection.
3.7 Spinal ondansetron and antinociceptive tolerance
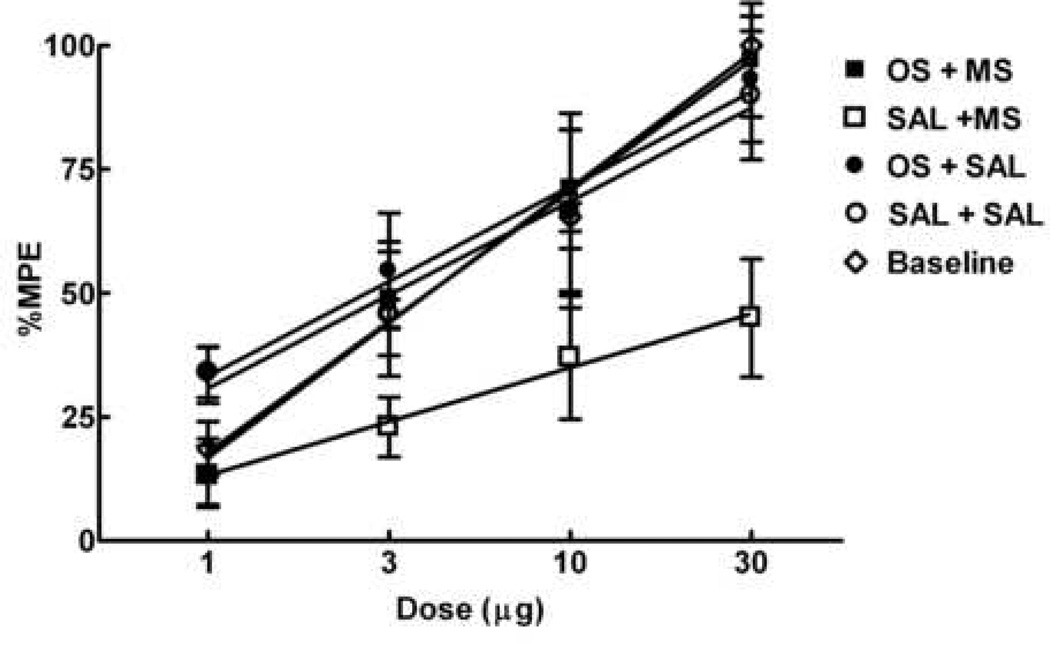
The spinal dose-response curve for morphine was generated prior to implantation of morphine or saline minipumps. The A50 value for spinal morphine antinociception was 3.9 µg (2.3–6.5 µg). Following saline minipump implantation, the antinociceptive A50 value for i.th. morphine in animals pretreated with saline or ondansetron were 3.1 µg (1.8–5.2 µg) and 2.6 µg (1.4–4.7 µg), respectively; these values were not significantly different from the antinociceptive A50 value observed prior to minipump implantation. Following morphine minipump implantation, the A50 value for animals pretreated with saline was 45.7 µg (33.1–63.1 µg), an 11.7-fold rightward shift from baseline, indicating antinociceptive tolerance. Pretreatment with ondansetron (30 µg) prevented the right shift observed in animals receiving morphine minipumps; the antinociceptive A50 value observed in these animals was 2.7 µg (1.4–5.1 µg), which was not significantly different from baseline, or from the antinociceptive A50 values of animals pretreated with saline (Figure 6).
Figure 6.
Spinal administration of ondansetron reduces antinociceptive tolerance to spinal morphine induced by sustained morphine exposure. Male Sprague Dawley rats received either morphine (squares) or saline minipumps (circles). Antinociceptive dose-response functions for i.th. morphine were generated before minipump implantation (Baseline) and 6 days after minipump implantation in the 52°C water tail-flick test. Each group of rats was tested with only one dose, 30 min after i.th. morphine injection. The dose-effect curve for i.th. morphine in groups with morphine minipumps and previous i.th. injections of saline 10 min before morphine injection was shifted significantly to the right of that for animals with saline minipumps (P<0.05). The dose-effect curve of animals with morphine minipumps and previous i.th. injection of ondansetron (30 µg) was not different from that of the animals with saline minipumps or baseline. There were 6 animals per dose.
3.8 Neurochemical markers in the spinal cord dorsal horn and dorsal root ganglion (DRG)
Spinal and DRG tissues from animals pre-treated with i.th. saline, SAP or SP-SAP were evaluated for expression of SP and CGRP 6 days post s.c. morphine or saline minipump implantation. Spinal pretreatments with controls (saline or SAP) or SP -SAP did not affect SP or CGRP staining. In the L5 DRG, animals with morphine minipumps demonstrated an increased number of cells positively stained for SP and CGRP (Table 2). Treatment with SP-SAP did not modify the sustained morphine-induced enhanced staining of SP or CGRP (Table 2). Quantification of SP-ir and CGRP-ir positive cells in the L5 DRG confirmed that there was a higher percentage of cells with positive staining for SP and CGRP in animals with morphine minipumps when compared to animals receiving saline minipumps (Table 2).
TABLE 2.
| Saline+Saline | SP+Saline | SP- SAP+Saline |
Saline+Morphine | SAP+Morphine | SP- SAP+Morphine |
|
|---|---|---|---|---|---|---|
| SP | 19.2 | 17.3 | 15.3 | 28.0* | 30.3* | 34.3* |
| CGRP | 31.4 | 28.7 | 32.5 | 52.2* | 57.0* | 54.8* |
Percentage of SP and CGRP positive cells per L5 DRG (*P<0.05 vs. saline pumps).
4. Discussion
The present study demonstrates that ablation of spinal dorsal horn cells expressing the NK-1 receptor with SP-SAP prevents (a) enhanced expression of spinal FOS, a measure of neuronal excitability, following repetitive, light touch in morphine-treated rats; (b) morphine-induced upregulation of spinal dynorphin, (c) expression of tactile and thermal hypersensitivity in morphine-treated rats; and (d) the rightward displacement of the spinal morphine antinociceptive dose-response curve (i.e., tolerance). These effects occurred without altering sustained morphine-induced changes in excitatory transmitter expression in primary afferent fibers. Additionally, the effects of SP-SAP on morphine-induced behavioral hypersensitivity and spinal morphine antinociceptive tolerance were replicated by spinal administration of ondansetron, a 5HT3 receptor antagonist. Three main conclusions can be drawn from these data: first, spinal NK-1 receptor expressing cells are critical for the maintenance of opiate-induced spinal plasticity which likely underlies behavioral hypersensitivity and central sensitization; second, these cells are critical for the behavioral expression of opiate-induced antinociceptive tolerance; and third, these cells appear to be an initial relay which leads to the activation of descending facilitatory pathways. It is noteworthy that all of these changes are induced by opiate administration in the absence of tissue injury.
Spinal NK-1 receptor-expressing cells play a crucial role in the transmission of ascending nociceptive information (e.g., parabrachial region, Marshall et al., 1996; Todd et al., 2000). Ablation of these cells abolishes responses to prolonged noxious stimuli (Mantyh et al., 1997; Nichols et al., 1999; Yezierski et al., 2004) without altering baseline thresholds. NK-1 receptor-expressing cells are also essential in capsaicin-induced central sensitization (Khasabov et al., 2002). Deep dorsal horn cells in mice lacking the NK-1 gene have deficits encoding mechanical and thermal stimuli (De Felipe et al., 1998; Suzuki et al., 2003). Our findings with sustained morphine parallel the role of the NK-1 receptor cells in injury-induced pain. After sustained morphine, NK-1 receptor expression is upregulated in the dorsal horn (King et al., 2005 and present results) and there is an increase in SP content and evoked release (King et al. 2005). Blockade of the NK-1 receptor reversed morphine-induced hypersensitivity and mice lacking the NK-1 receptor did not develop morphine-induced hyperalgesia (King et al., 2005).
In addition to blockade of opiate-induced hypersensitivity, ablation of NK-1 receptor expressing cells also inhibited opiate-induced changes indicative of central sensitization. FOS expression has been used to identify increased neuronal excitability after noxious stimulation (Hunt et al. 1987; Harris, 1998). While innocuous tactile stimuli do not normally induce FOS expression, they do so following tissue injury, suggesting sensitization of the spinal cord (Ma and Woolf, 1996; Wei et al., 1999; Abbadie et al., 1993). Similarly, sustained morphine resulted in enhancement of touch-evoked FOS in animals previously receiving saline or SAP, but this was prevented following ablation of NK-1 cells with SP-SAP. NK-1 receptor-expressing cells are thus critical in expression of spinal sensitization and of morphine-induced hypersensitivity. Injuries, or prolonged opioid exposure, increase expression of spinal dynorphin (Rattan and Tejwani, 1997; Vanderah et al., 2000) which can act in a pronociceptive fashion (Bian et al., 1999; Malan et al., 2000; Gardell et al., 2004). Such upregulation of spinal dynorphin depends on descending facilitation from the RVM (Gardell et al., 2002). We now report that upregulation of spinal dynorphin following morphine exposure is prevented by ablation of the spinal NK-1 receptor expressing cells.
Antagonism of spinal 5HT-3 receptors reversed morphine-induced hypersensitivity, findings consistent with suggestions of Dickenson and colleagues (Suzuki et al., 2002; 2004; 2005; Dickenson and Suzuki, 2005) that a spinal-supraspinal-spinal loop may maintain injury-induced central sensitization and pain. Activation of ascending inputs from spinal lamina I cells are thought to lead to enhanced activation of descending serotonergic projections from the RVM that facilitate further nociceptive inputs through 5-HT3 receptors, thus maintaining central sensitization and enhanced pain (Suzuki, et al., 2002; 2004; Polgar et al., 2002). Ablation of NK1-expressing projection neurons by SP-SAP abolished injury-induced sensitization (Suzuki et al., 2002; 2004; 2005; Nichols et al.; 1999; Mantyh and Hunt, 2004; Khasabov et al., 2002) as well as opiate-induced sensitization and hyperalgesia. Collectively, these findings suggest that elimination of spinal NK-1 receptor expressing cells prevents the engagement of descending facilitation from the RVM in both injury-induced and opiate-induced hyperalgesia.
Opioids are increasingly used for the treatment of chronic non-malignant pain conditions and the need for dose-escalation to attain satisfactory pain relief is often observed (Way et al., 1969; Cox, 1990; Foley, 1993, 1995). In preclinical models, opioids have been repeatedly demonstrated to produce antinociceptive tolerance. The mechanisms by which antinociceptive tolerance occur are unknown. Numerous cellular mechanisms such as receptor downregulation, desensitization, internalization and recycling, effects on second messengers and others have been proposed (for review, see Childers, 1991). In addition to these cellular mechanisms, other studies have proposed that the behavioral expression of antinociceptive tolerance may be linked to opiate-induced pronociceptive actions (Vanderah et al., 2000, 2001a,b; Ossipov et al., 2004; Mao et al., 1995). Such suggestions have been based on the fact that the blockade of antinociceptive tolerance can be achieved by multiple classes of anti-hyperalgesic substances. Opiate antinociceptive tolerance is blocked, for example, by NMDA antagonists (Mao et al., 1998), COX inhibitors (Powell et al., 1999), NOS inhibitors (Powell et al, 1999), CGRP antagonists (Menard et al., 1996; Powell et al., 1999), calcium channel blockers (Aley and Levine, 1997; Dogrul et al., 2005) and numerous other types of anti-hyperalgesic agents (Ossipov et al., 2005, for review). Consistent with these previous studies, a role for SP and the NK-1 receptor in antinociceptive tolerance has also been suggested (Powell et al., 2000; King et al, 2005). Inhibition of NK-1 receptor activity prevents and reverses the development of morphine tolerance (Powell et al., 2003). Here, elimination of NK-1 receptor expressing cells in the spinal cord also prevented expression of morphine tolerance. These observations provide an additional link between mechanisms of hyperalgesia and expression of antinociceptive tolerance. Additionally, the present study extends this interpretation to include descending facilitation from the RVM as a part of a spinal-supraspinal loop which may involve serotonergic systems. This conclusion is also consistent with previous observations in which spinal antinociceptive tolerance to opiates, as well as opiate-induced hyperalgesia, were blocked by microinjection of lidocaine in the RVM, by lesion of mu-opiate receptor expressing cells in the RVM (a possible source of descending facilitation) and by lesion of the dorsolateral funiculus (see Ossipov et al., 2004; Ossipov et al., 2005; Vanderah et al., 2001a for reviews). The finding that spinal ondansetron blocked opiate-induced hyperalgesia and antinociceptive tolerance would be consistent with a role for serotonin in descending facilitation (Suzuki, et al., 2004). We cannot rule out, however, that ondansetron may have direct cellular activities which antagonize the processes by which opiate tolerance occurs. In this regard, it is important to emphasize that while the data in this report demonstrate a link between mechanisms which are pronociceptive and the behavioral expression of spinal opiate antinociceptive actions (i.e., rightward shifts in the antinociceptive dose-response curve or tolerance), our studies do not directly address mechanisms of opiate tolerance which are likely to involve both complex cellular and systems level adaptations.
Our results show strong parallels between the effects of opiates and states of injury-induced central sensitization, the critical role of descending pain facilitation mechanisms, and the engagement of ascending pathways which can trigger such facilitation, all occurring in the absence of tissue injury. Ablation of the lamina I projection neurons that express NK1 receptors abolished the ascending limb of this spinobulbo-spinal loop and the consequent expression of behavioral and neurochemical signs associated with central sensitization as well as the expression of opioid antinociceptive tolerance. Moreover, the observation that the behavioral results obtained with spinal ondansetron were similar to those obtained with SP-SAP are also indicative that these effects are mediated through central sensitization promoted by descending pain facilitatory systems (see Suzuki et al., 2002; 2004; 2005). Importantly, SP-saporin treatment did not alter the upregulation of substance P and CGRP in primary afferent fibers and DRG neurons indicating that loss of sensitization is not the result of a normalization of primary afferent transmitter content.
The present findings offer novel insights into mechanisms by which chronic pain may occur in clinical conditions which are not clearly associated with injury to tissue, including, fibromyalgia, IBS and perhaps migraine. The identification of circuitry in which activation of descending pain facilitation occurs will eventually allow for novel strategies allowing the development of pain relieving therapeutics which do not produce pronociceptive neuroadaptations.
Supplementary Material
Photomicrographs illustrating FOS-immunoreactive labeled profiles in coronal sections (30 µm-thick) of the lumbar cord at L5 are shown for animals implanted with either saline (A, C) or morphine pumps (B,D). The images shown are black and white versions of the original fluorescent images obtained using Adobe Photoshop. Animals with morphine pumps and previous treatment with either saline or SAP (B), demonstrated increased number of FOS cells compared to respective saline pump controls (B). SP-SAP prevented the increase in touch-evoked FOS (D). SP-SAP did not have an effect in the number of FOS cells in animals implanted with saline pumps (C). Scale bar = 100 µm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Besson JM. C-fos expression in rat lumbar spinal cord following peripheral stimulation in adjuvant arthritic and normal rats. Brain Res. 1993;607:195–204. doi: 10.1016/0006-8993(93)91507-o. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in the rat. J Neurosci. 1997;17:8018–8023. doi: 10.1523/JNEUROSCI.17-20-08018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali NM. Hyperalgesic response in a patient receiving high concentrations of spinal morphine. Anesthesiology. 1986;65:449. doi: 10.1097/00000542-198610000-00028. [DOI] [PubMed] [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;3:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1986;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Bian D, Ossipov MH, Ibrahim M, Raffa RB, Tallarida RJ, Malan TP, Jr, Lai J, Porreca F. Loss of antiallodynic and antinociceptive spinal/supraspinal morphine synergy in nerve-injured rats: restoration by MK-801 or dynorphin antiserum. Brain Res. 1999;831:55–63. doi: 10.1016/s0006-8993(99)01393-1. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Honore P, Besson JM. Are there long-term changes in the basal or evoked Fos expression in the dorsal horn of the spinal cord of the mononeuropathic rat? Pain. 1998;80:347–357. doi: 10.1016/s0304-3959(98)00234-6. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Besson JM. Intravenous morphine does not modify dorsal horn touch-evoked allodynia in the mononeuropathic rat: a Fos study. Pain. 2001;92:389–398. doi: 10.1016/S0304-3959(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Childers SR. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Cox BM. Drug tolerance and physical dependence. In: Pratt WB, Taylor P, editors. Principles of Drug Action: The Basis of Pharmacology. New York: Churchill Livingstone; 1990. pp. 639–690. [Google Scholar]
- De Conno F, Caraceni A, Martini C, Spoldi E, Salvetti M, Ventafrida V. Hyperalgesia and myoclonus with intrathecal infusion of high-dose morphine. Pain. 1991;47:337–339. doi: 10.1016/0304-3959(91)90225-M. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Devulder J. Hyperalgesia induced by high-dose intrathecal sufentanil in neuropathic pain. J Neurosurg Anesthesiol. 1997;9:146–148. doi: 10.1097/00008506-199704000-00007. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Suzuki R. Opioids in neuropathic pain: clues from animal studies. Eur J Pain. 2005;9:113–116. doi: 10.1016/j.ejpain.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Bilsky EJ, Ossipov MH, Lai J, Porreca F. Spinal L-type calcium channel blockade abolishes opioid-induced sensory hypersensitivity and antinociceptive tolerance. Anesth Analg. 2005;101:1730–1735. doi: 10.1213/01.ANE.0000184253.49849.B0. [DOI] [PubMed] [Google Scholar]
- Foley KM. Opioids. Neurol Clin. 1993;11:503–522. [PubMed] [Google Scholar]
- Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anti-Cancer Drugs. 1995;6:4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Vanderah TW, Gardell SE, Wang R, Ossipov MH, Lai J, Porreca F. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J Neurosci. 2003;23:8370–8379. doi: 10.1523/JNEUROSCI.23-23-08370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Ibrahim M, Wang R, Wang Z, Ossipov MH, Malan TP, Jr, Porreca F, Lai J. Mouse strains that lack spinal dynorphin upregulation after peripheral nerve injury do not develop neuropathic pain. Neuroscience. 2004;123:43–52. doi: 10.1016/j.neuroscience.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Mol Ther. 2003a;8:367–375. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Goins W, Glorioso JC, Fink DA. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain. 2003b;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the subtance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996;67:307–316. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP. Setting the tone: superficial dorsal horn projection neurons regulate pain sensitivity. Trends Neurosci. 2004;27:582–584. doi: 10.1016/j.tins.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Lu J, Mayer DJ. Antinociceptive tolerance to the mu-opioid agonist DAMGO is dose-dependently reduced by MK-801 in rats. Neurosci Lett. 1998;250:193–196. doi: 10.1016/s0304-3940(98)00472-8. [DOI] [PubMed] [Google Scholar]
- Marshall GE, Shehab SAS, Spike RC, Todd AJ. Neurokinin-1 receptors on lumbar spinothalamic neurons in the rat. Neuroscience. 1996;72:255–263. doi: 10.1016/0306-4522(95)00558-7. [DOI] [PubMed] [Google Scholar]
- Menard DP, van Rossum D, Kar S, St Pierre S, Sutak M, Jhamandas K, Quirion R. A calcitonin gene-related receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–2351. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hubry VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- Polgar E, Puskar Z, Watt C, Matesz C, Todd AJ. Selective innervation of lamina I projections neurones that possess the neurokinin 1 receptor by serotonin-containing axons in the rat spinal cord. Neuroscience. 2002;109:799–809. doi: 10.1016/s0306-4522(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Hosokawa A, Bell A, Sutak M, Milne B, Quirion R, Jhamandas K. Comparative effects of cyclo-oxygenase and nitric oxide systhase inhibition on the development and reversal of spinal opioid tolerance. Br J Pharmacol. 1999;127:631–644. doi: 10.1038/sj.bjp.0702587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonist. Br J Pharmacol. 2000;131:875–884. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KJ, Quirion R, Jhamandas K. Inhibition of neurokinin-1-substance P receptors and prostanoid activity prevents and reverses the development of morphine tolerance in vivo and the morphine-induced increase in CGRP expression in cultured dorsal root ganglion neurons. Eur J Neurosci. 2003;18:1572–1583. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- Rattan AK, Tejwani GA. Effect of chronic treatment with morphine, midazolam and both together on dynorphin(1–13) levels in the rat. Brain Res. 1997;754:239–244. doi: 10.1016/s0006-8993(97)00084-x. [DOI] [PubMed] [Google Scholar]
- Stillman MJ, Moulin DE, Foley KM. Paradoxical pain following high-dose spinal morphine. Pain. 1987;30:S389. [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Hunt SP, Dickenson AH. The coding of noxious mechanical and thermal stimuli of deep dorsal horn neurones is attenuated in NK1 knockout mice. Neuropharmacology. 2003;45:1093–1100. doi: 10.1016/s0028-3908(03)00281-8. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals. 2005;14:175–181. doi: 10.1159/000087656. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 2 ed. New York: Springer-Verlag; 1987. p. 297. [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons on laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance P-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001a;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001b;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang E-T, Ossipov MH, Xie JY, King T, Lai, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- Wei F, Dubner R, Ken K. Dorsolateral funiculus-lesions unmask inhibitory or disfacilitatory mechanisms which modulate the effects of innocuous mechanical stimulation on spinal Fos expression after inflammation. Brain Res. 1999;820:112–116. doi: 10.1016/s0006-8993(98)01359-6. [DOI] [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology. 1986;5:590–597. doi: 10.1097/00000542-198605000-00008. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ. Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. J Pharmacol Exp Ther. 1988;244:510–507. [PubMed] [Google Scholar]
- Yezierski RP, Yu CG, Mantyh PW, Vierck CJ, Lappi DA. Spinal neurons involved in the generation of at-level pain following spinal injury in the rat. Neurosci Lett. 2004;361:232–236. doi: 10.1016/j.neulet.2003.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photomicrographs illustrating FOS-immunoreactive labeled profiles in coronal sections (30 µm-thick) of the lumbar cord at L5 are shown for animals implanted with either saline (A, C) or morphine pumps (B,D). The images shown are black and white versions of the original fluorescent images obtained using Adobe Photoshop. Animals with morphine pumps and previous treatment with either saline or SAP (B), demonstrated increased number of FOS cells compared to respective saline pump controls (B). SP-SAP prevented the increase in touch-evoked FOS (D). SP-SAP did not have an effect in the number of FOS cells in animals implanted with saline pumps (C). Scale bar = 100 µm.