Abstract
Opioids produce analgesic effects and extended use can produce physical dependence in both humans and animals. Dependence to opiates can be demonstrated by either termination of drug administration or through precipitation of the withdrawal syndrome by opiate antagonists. Key features of the opiate withdrawal syndrome include hyperalgesia, anxiety and autonomic signs such as diarrhea. The rostral ventromedial medulla (RVM) plays an important role in the modulation of pain and for this reason, may influence withdrawal-induced hyperalgesia. The mechanisms that drive opiate withdrawal-induced hyperalgesia have not been elucidated. Here, rats made dependent upon morphine received naloxone to precipitate withdrawal. RVM microinjection of lidocaine, kynurenic acid (excitatory amino acid antagonist) or YM022 (CCK2 receptor antagonist) blocked withdrawal-induced hyperalgesia. Additionally, these treatments reduced both somatic and autonomic signs of naloxone-induced withdrawal. Spinal application of ondansetron, a 5HT3 receptor antagonist thought to ultimately be engaged by descending pain facilitatory drive, also blocked hyperalgesia and somatic and autonomic features of the withdrawal syndrome. These results indicate that the RVM plays a critical role in mediating components of opioid withdrawal that may contribute to opioid dependence.
Perspective
Manipulations targeting these descending pathways from the RVM may diminish the consequences of prolonged opioid administration-induced dependence and be useful adjunct strategies in reducing the risk of opioid addiction.
Keywords: naloxone-induced withdrawal, hyperalgesia, RVM, descending facilitation, morphine dependence
1. Introduction
In recent years, the use of opioids for chronic non-malignant pain has increased substantially10. Clinical reports indicate that sustained administration of opioids in persistent pain states can elicit physical dependence, opioid induced hyperalgesia (OIH) and analgesic tolerance in some patients2, 3. After periods of extended use, removal of opiates can result in withdrawal characterized by physical and affective-motivational symptoms that may contribute to continued opiate use and drug seeking behavior53. The severity of opioid withdrawal syndrome is considered a measurement of dependence on opioid drugs. In pre-clinical models, this syndrome is commonly induced by injection of an opioid antagonist in opioid-dependent rats4, 24 resulting in a cluster of withdrawal behaviors including hyperalgesia, wet dog shakes, teeth chattering, writhing, yawning and grooming8. Additionally, autonomic signs of withdrawal are produced including, piloerection, diarrhea and weight loss6. Injection of naloxone in opioid dependent rats also produces aversion41 and withdrawal from opiates has been described as highly aversive in humans15.
The rostral ventromedial medulla (RVM) has a prominent role in the modulation of pain states. Many studies have focused on the descending inhibitory influences arising from this region68. Additionally, the RVM has been demonstrated to exert descending facilitatory influences in pain processing46, 64. Recent evidence has implicated the RVM as a critical component to the maintenance, but not the initiation of pain arising from nerve injury9, 46 and visceral inflammation69. The RVM also has a prominent role in the modulation of opioid-induced hypersensitivity. Microinjection of lidocaine or a cholescystokinin (CCK) receptor antagonist into the RVM can block opioid-induced hyperalgesia resulting from continuous exposure to the opioid67, 73. The RVM appears to also have a role in promoting hyperalgesia during opioid withdrawal. Lidocaine microinjected into the RVM attenuates the hyperalgesia observed during opioid withdrawal26. The hyperalgesia during opioid withdrawal seems to be mediated by activation of facilitatory cells in the RVM5 which utilize cAMP-mediated mechanisms to promote hyperalgesia during opioid withdrawal7. Other studies have implicated the RVM in other homoeostatic functions besides pain modulation12, 51, 57. Lesions in this area blocked the activation of the locus coeruleus (LC) during naloxone-precipitated withdrawal48 indicating its prominent role in activation of supraspinal areas during opioid withdrawal.
The present studies explored the mechanisms that drive opiate-induced hyperalgesia and also determined whether these mechanisms are relevant to other components of the withdrawal syndrome. Activation of descending facilitation from the RVM is believed to promote hyperalgesia by ultimately engaging spinal 5HT3 receptors59, 70. For this reason, we have also used the 5HT3 receptor antagonist ondansetron to investigate whether this pathway is active during opioid-induced withdrawal.
2. Methods
2.1. Experimental Design
For evaluation of hyperalgesia and somatic/autonomic signs of withdrawal, rats were implanted subcutaneously with osmotic minipumps containing either morphine (64 mg/ml) or saline. On day 6 after implantation of minipumps, baseline measurements were taken of hot plate latency and weight. After the baseline measurements, naloxone hydrochloride was injected subcutaneously in a dose of 3 mg/kg, animals were placed in individual observation chambers and withdrawal signs were observed for the following 30 min. At the end of the observation period, measurements of hot plate latency and weight were again taken. Drugs were microinjected into the RVM before systemic naloxone injection at a time so as to coincide with their peak effect. Ondansetron was injected spinally 20 min before naloxone injection. Groups of animals were microinjected in sites outside the RVM as off-site controls. To control for any effect the act of injection may have, animals were microinjected with the vehicle for the respective drugs. To control for the effects of the injection of naloxone, animals were injected systemically with saline. All procedures were approved by the University of Arizona Animal Care and Use committee and conform to the guidelines for the use of laboratory animals of the National Institutes of Health.
2.2. Animals
Male, Sprague-Dawley rats (Harlan-Sprague-Dawley, Indianapolis) were 250–300 g at the time of testing. Animals were maintained in a climate-controlled room on a 12-h light/dark cycle and were allowed to have food and water ad libitum.
2.3. Drug Administration
2.3.1 RVM Drug Administration
All animals were prepared for bilateral microinjections into the RVM as described previously9. The rats were anesthetized with a mixture of intraperitoneal ketamine/xylazine (10/100 mg/kg of ketamine/xylazine, respectively (Sigma-Aldrich, St Louis, MO), and positioned in a stereotaxic head holder. The skull was exposed and two 26 ga guide cannulae separated by 1.2 mm (Plastics One Inc. Roanoke, VA, USA) were directed toward the lateral portions of the RVM (−11.0 mm anteroposterior from Bregma; ± 0.6 mm lateral from midline; −8.5 mm dorsoventral from the cranium, Paxinos and Watson, 1986). The guide cannulas were secured to the skull, and the animals were allowed to recover for 5 days after surgery before osmotic pump implantation. Drug administration into the RVM was performed by slowly expelling 0.5 µl of drug solution through a 33 ga injection cannula inserted through the guide cannula and protruding an additional 1 mm into fresh brain tissue to prevent backflow of the drug into the injection cannula. For off-site control injections, separate groups of animals were implanted with guide cannulae with the following coordinates: −11.0 mm from bregma; ±0.6 lateral and −6.5 mm from the cranium (2 mm above target, Figs 1E, 2E, 3E, X symbols). Other groups of animals were implanted with guide cannulae with the following coordinates: −9.0 mm from bregma, ±0.6 lateral; −8.5 mm from the cranium. The cannulae were left in place to be able to confirm the microinjection sites at the end of the experiments.
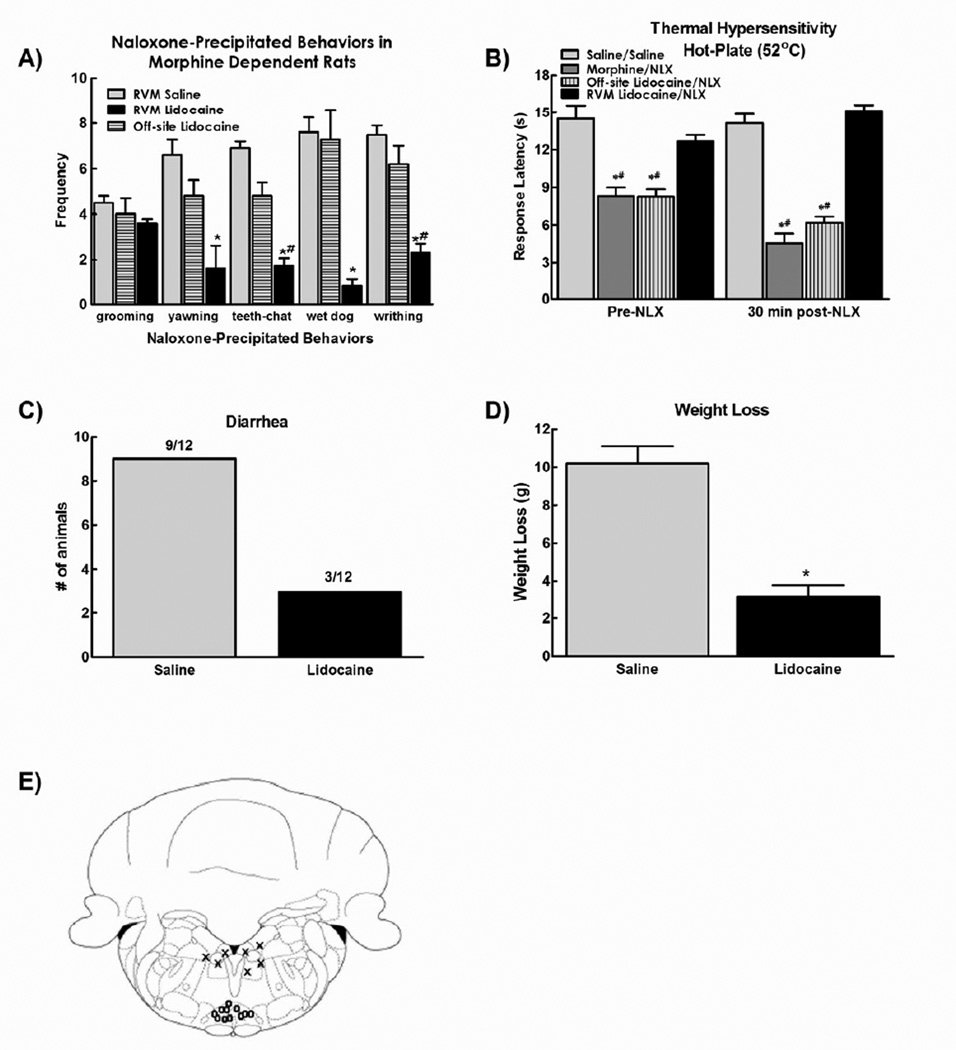
Figure 1.
A) Microinjection of lidocaine (4% in 0.5µl bilaterally) attenuated the naloxone-precipitated withdrawal behaviors in morphine dependent rats. Off-site microinjection of lidocaine did not alter naloxone-precipitated withdrawal behaviors. B). Lidocaine also prevented the reduction in hot plate latency observed 30 minutes after naloxone injection. Off-site microinjection of lidocaine failed to block morphine-induced thermal hypersensitivity or naloxone induced enhanced thermal hypersensitivity. C). Lidocaine reduced the number of animals demonstrating naloxone precipitated diarrhea. Off-site microinjection of lidocaine failed to block naloxone induced diarrhea. D) RVM lidocaine, but not off-site injections, also attenuated the weight loss observed after naloxone injection. (E) Diagram showing site of injection within the RVM (0) and off-site injections (X). Data in all graphs are expressed as mean ± SEM, n=6–12 per group, * p<0.05 compared to RVM saline/systemic naloxone; # p<0.05 compared to RVM saline/systemic saline (control group).
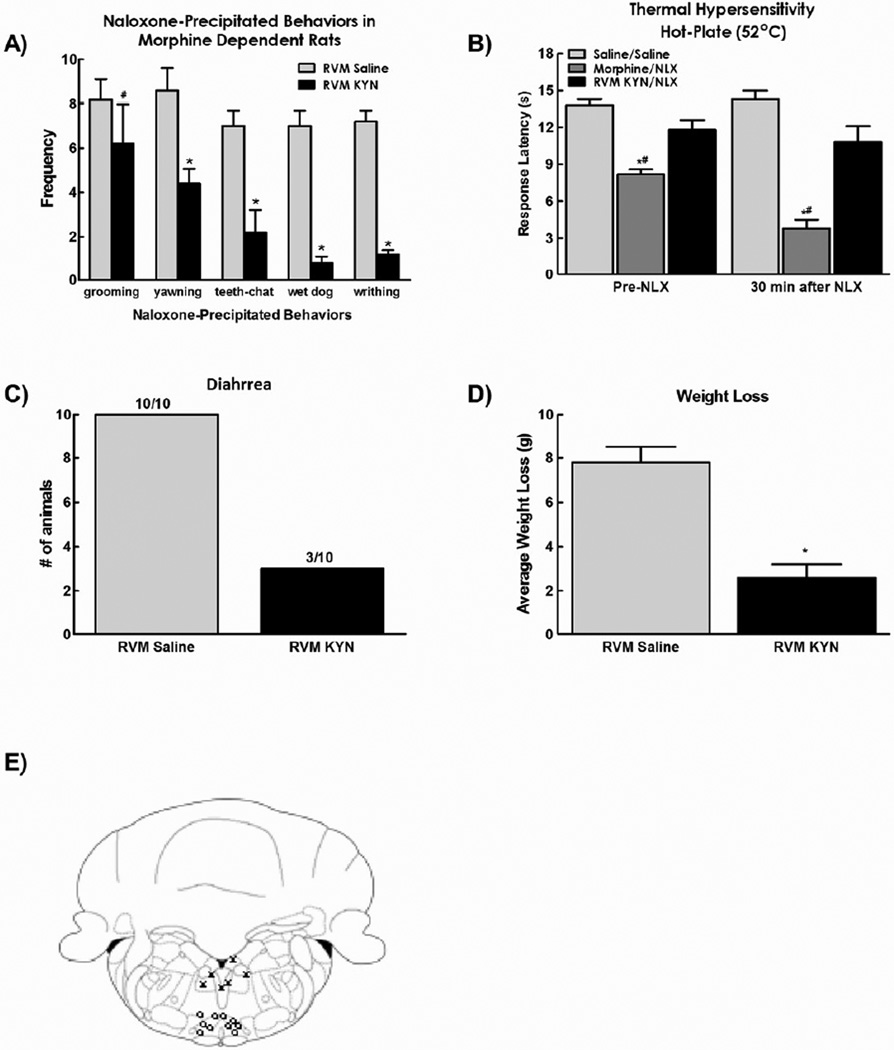
Figure 2.
A) Microinjection of kynurenic acid (1 nmol in 0.5µl bilaterally) attenuated the withdrawal behaviors observed after systemic injection of naloxone in morphine-dependent rats. B). Kynurenic acid also prevented the reduction in hot plate latency observed 30 minutes after naloxone injection C). RVM kynurenic acid reduced the number of animals demonstrating diarrhea and D) also attenuated the weight loss observed after naloxone injection. (E) Diagram showing site of injection within the RVM (0) and off-site injections (X). Data are expressed as mean ± SEM, n=6–10 per group, * p<0.05 compared to RVM saline/systemic naloxone; # p<0.05 compared to RVM saline/systemic saline (control group).
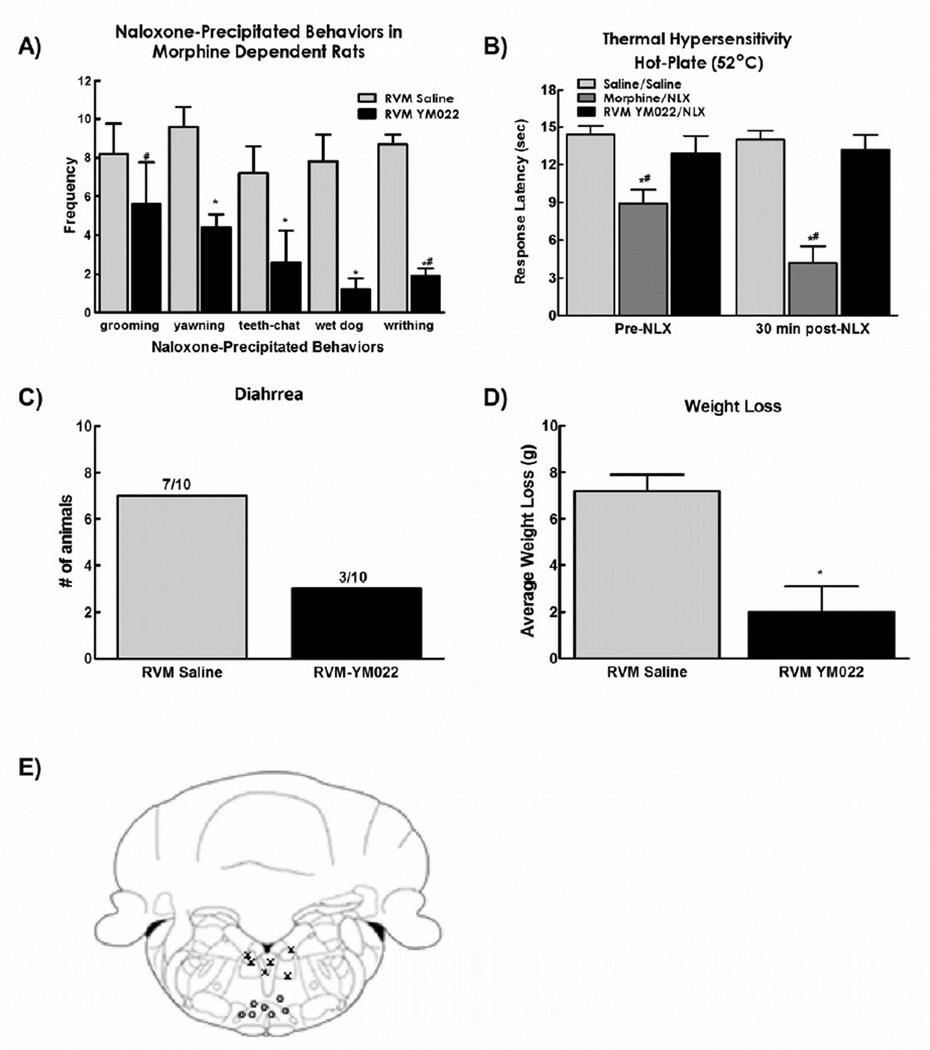
Figure 3.
A) Microinjection of YM022 (0.25 ng in 0.5µl bilaterally) attenuated the withdrawal behaviors observed after systemic injection of naloxone in morphine dependent rats. B). YM022 also prevented the reduction in hot plate latency observed 30 minutes after naloxone injection C). YM022 reduced the number of animals demonstrating diarrhea and D) also attenuated the weight loss observed after naloxone injection. (E) Diagram showing site of injection within the RVM (0) and off-site injections (X). Data are expressed as mean ± SEM, n=6–10 per group, * p<0.05 compared to RVM saline/systemic naloxone; # p<0.05 compared to RVM saline/systemic saline (control group).
2.3.2 Spinal Drug Administration
Rats were anesthetized with the use of intraperitoneal ketamine/xylazine (Sigma, 10/100 mg/kg, respectively) and placed in a stereotaxic holder. The cisternum magnum was exposed, and an 7.8 cm catheter (PE-10) was implanted, terminating in the lumbar region of the spinal cord74. Catheters were sutured into place with the tubing externalized at the back of the neck. Animals with signs of motor weakness or paralysis after surgery were not used (~25%). Animals were allowed to recover for 7 days prior to osmotic pump implantation.
2.3.3. Drugs and Chemicals
Morphine, a generous gift from the National Institute on Drug Abuse Drug Supply Program, was dissolved to 64 mg/kg to fill the osmotic minipumps (Alzet, Mountain View, CA) which were surgically implanted subcutaneously under isofluorane anesthesia. Naloxone (RBI Chemicals, Boston, MA) was dissolved in physiological saline to 3 mg/kg and was given through intraperitoneal injection4. Ondansetron (Zofran purchased from the University Medical Center Pharmacy) was dissolved to 10 µg in 5µl of physiological saline and delivered through the intrathecal cathether59. Kynurenic acid (Tocris, Ellisville, MO) was dissolved to 1 nmol in 0.5µl of physiological saline for bilateral microinjection into the RVM22. YM022 (Tocris, Ellisville, MO) was dissolved in 0.25 ng in 0.5µl of physiological saline for microinjection into the RVM. Lidocaine hydrochloride (4% w/v; Sigma-Aldrich, St Louis, MO) was given by bilateral microinjection (0.5 µl/side) into the RVM. Pilot studies were carried out to determine the best dose of naloxone to induce the opioid withdrawal. The doses used for all drugs either microinjected in the RVM or applied intrathecally are doses used before in previous studies in our laboratory. Injections of all drug either into the RVM or spinally took place 15 minutes prior systemic naloxone.
2.4. Behavioral Measurements
2.4.1. Hot plate latency
Before any drug administrations, rats were placed in the hot plate apparatus set at 52°C to attain baseline latency. The hot plate response latency was measured as the time at which the rat withdraws and shakes either hindpaw from the plate surface. The latency was measured with a timer and approximated to the tenth of second. A cut-off latency of 30-s was used. After baseline, rats received an injection of naloxone and observed for withdrawal behaviors during a 30 min period. At the end of the observation period, rats underwent a final measurement of hot plate latency testing.
2.4.2. Physical signs of morphine withdrawal
Rats were monitored for physical signs of morphine withdrawal as previously described4, 24. Briefly, rats were placed in Plexiglas cylinders (radius 7 cm; height 24 cm) covered with saw dust bedding. Before any drug administrations, rats were observed for a period of 30 min for spontaneous signs of morphine withdrawal. After this observation period, rats were separated in different groups receiving a subcutaneous injection of saline or naloxone (3 mg/kg) and placed back on the cylinders and the frequency of naloxone-induced morphine withdrawal signs such as wet dog shakes, teeth chattering, writhing, yawning and grooming were scored. Frequency of behaviors was quantified instead of duration of behaviors as this methodology has been reported in the literature4, 24, 33. A single observer was trained in the methodology and blinded to the treatment that each rat had received as previously reported4, 24, 32, 34, 44, 49, 50, 54, 76, 77. Additionally, autonomic signs of withdrawal were recorded such as diarrhea (yes/no) and weight loss (weight was recorded before and then 30 min after naloxone injection).
2.5. Data Analysis
Group differences were determined using a 2 way ANOVA, with factors being 1) saline vs. morphine treatment and 2) drug vs vehicle treatment. Post-hoc analysis of selected pairings was done using the Student t test with Bonferroni’s correction. Differences were considered significant if p<0.05. A Fisher’s exact test was run to determine group differences for the diarrhea measure, differences were considered significant if p<0.05.
3. Results
3.1. Inactivation of RVM neuronal transmission blocks somatic signs of naloxone-induced withdrawal
Systemic injection of naloxone (3 mg/kg, s.c.) induced a robust spectrum of withdrawal behaviors including grooming, yawning, teeth chattering, wet dog shakes and writhing behaviors in morphine treated rats. Microinjection of lidocaine into the RVM reduced the incidence of all naloxone-precipitated withdrawal behaviors except for grooming behavior (Fig. 1a). Of note, there was low incidence of these behaviors in saline-treated rats irrespective of RVM treatment, with no yawning, teeth-chattering, wet dog shakes and few instances (< 2) of grooming and writhing behaviors following this dose of naloxone (data not shown).
In rats treated with systemic morphine infusion, pre-lidocaine hot plate latencies were significantly reduced compared to rats treated with systemic saline infusion, consistent with the development of opioid-induced hyperalgesia (OIH) (Fig 1b, #p<0.05). Microinjection of lidocaine reversed the thermal hyperalgesia in the morphine dependent animals, returning hot plate latencies to saline control values. Systemic injection of naloxone further reduced hot plate latencies in morphine-treated rats (Fig 1b, *p < 0.05). Administration of lidocaine 15 minutes prior to systemic naloxone prevented the naloxone-induced reduction in hot plate response latencies, resulting in latencies equivalent to saline-treated control animals (Fig 1b). Administration of lidocaine into the RVM did not alter hot-plate latencies of rats treated with saline infusion (Fig. 1b). Administration of saline into the RVM did not alter hot-plate latencies of saline- or morphine-infused animals prior to systemic administration of naloxone (data not shown).
Naloxone also precipitated autonomic signs of withdrawal, measured as the number of rats that had diarrhea (Fig. 1c) and rapid weight loss within 30 min in morphine-treated rats (Fig. 1d). RVM microinjection of lidocaine significantly reduced the number of rats with naloxone-induced diarrhea and the amount of weight loss in morphine-treated rats (Figs 1c and 1d, respectively). Rats treated with saline infusion did not develop diarrhea or show weight loss following administration of naloxone irrespective of microinjection of saline or lidocaine into the RVM (data not shown).
The effects of lidocaine were specific for the RVM (Fig. 1e). Lidocaine microinjections outside the RVM (Fig 1e, “X” symbols) did not alter the naloxone-induced withdrawal syndrome (Fig. 1A) or hyperalgesia (Fig. 1B).
3.2. Glutamatergic antagonist into the RVM blocks naloxone-induced withdrawal syndrome
It has been shown previously that facilitatory cells in the RVM express glutamate receptors and antagonizing these receptors blocks facilitation of pain transmission20, 22. To determine the role of glutamatergic transmission in the RVM in naloxone-induced withdrawal, the non-selective excitatory amino acid receptor antagonist kynurenic acid was microinjected into the RVM, at a non-neurotoxic dose72 used in electrophysiological and behavioral studies within the RVM18, 19, 22, 15 min prior to systemic naloxone. As described above, saline microinjection into the RVM did not prevent naloxone-induced withdrawal behaviors of grooming, yawning, teeth-chattering, wet dog shakes, or writhing (Fig 2a). Microinjection of kynurenic acid (1 nmol in 0.5 µl, bilaterally) into the RVM prior to systemic injection of naloxone significantly inhibited naloxone-precipitated yawning, teeth-chattering, wet-dog shakes, and writhing in morphine dependent rats (*p < 0.05), but did not alter naloxone precipitated grooming (Fig 2a).
As with lidocaine microinjection, microinjection of kynurenic acid into the RVM reversed morphine induced hyperalgesia, elevating hot-plate latencies to saline control values. Moreover, microinjection of kynurenic acid into the RVM prevented the naloxone-induced enhanced reduction in hot plate latencies in morphine-treated rats with paw withdrawal latencies equivalent to saline control values (Fig 2b).
Autonomic signs of naloxone-induced withdrawal were also prevented by microinjection of kynurenic acid into the RVM. Microinjection of kynurenic acid into the RVM reduced the number of animals demonstrating naloxone precipitated diarrhea (Fig. 2c) and reduced the weight loss in morphine-dependent rats (Fig. 2d). As with lidocaine injections, the effects of kynurenic acid were specific for the RVM, as off-site injections (Fig 2e, “X” symbols) did not have any effects on naloxone-induced withdrawal (data not shown). No signs of neurotoxicity were observed in any animals that had received kynurenic acid injections. Additionally, no signs of neuronal cell loss were observed in histological sections taken from randomized selected rats that had received kynurenic acid.
3.3. CCK receptor antagonist in the RVM blocks naloxone-induced withdrawal syndrome
It has been shown previously that CCK plays a role in the RVM to engage descending facilitation promoting opioid-induced hyperalgesia21, 62. The present study investigated the role of CCK transmission in the RVM in the naloxone-induced withdrawal syndrome. Microinjection of the specific CCK2 receptor antagonist YM022 (0.25 ng in 0.5 µl, bilaterally) 15 min before systemic naloxone injection significantly inhibited naloxone-precipitated yawning, teeth chattering, wet dog shakes, and writhing, but failed to alter grooming (Fig. 3a).
As previously reported62, antagonizing CCK2 receptors in the RVM reversed sustained morphine-induced hyperalgesia, with hot-plate response latencies equivalent to saline control values. Moreover, YM022 microinjection into the RVM prevented the naloxone-induced enhanced reduction in hot plate latencies observed in morphine treated rats, with response latencies returning to saline control values (Fig 3b).
Autonomic signs of naloxone-induced withdrawal were also prevented by microinjection of YM022 into the RVM. Animals receiving control microinjections of saline into the RVM demonstrated signs of diarrhea and greater weight loss than animals receiving YM022 (Figs 3c and 3d, respectively). The effects of YM022 were specific for the RVM, as off-site injections (Fig 3e) did not have any effects on naloxone-induced withdrawal (data not shown).
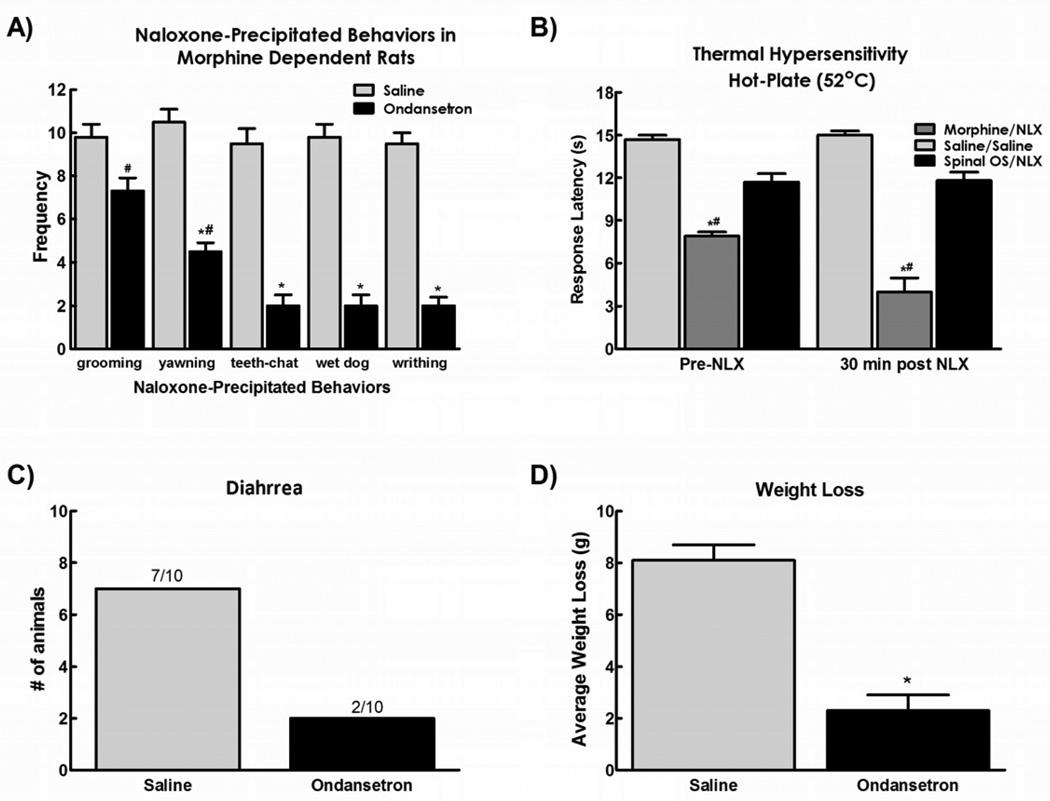
3.4. Antagonizing 5HT3 receptors in the spinal cord blocks naloxone-induced withdrawal syndrome
It has been shown previously that serotonin acting at the 5HT3 receptor can facilitate pain transmission at the spinal cord level58, 59, 61. The present study investigated the role of spinal 5-HT3 receptors in naloxone-induced withdrawal syndrome by spinal application of the 5HT3 receptor antagonist ondansetron (10 µg) 15 min prior to systemic administration of naloxone. Spinal ondansetron attenuated naloxone-induced yawning, teeth chattering, wet dog shakes, and writhing, but did not alter naloxone-induced grooming behavior in morphine dependent rats (Fig. 4a).
Figure 4.
A) Spinal application of ondansetron (30µg in 5µl) attenuated the withdrawal behaviors observed after systemic injection of naloxone in morphine dependent rats B). Ondansetron also prevented the reduction in hot plate latency observed 30 minutes after naloxone injection C). Ondansetron reduced the number of animals demonstrating diarrhea and D) also attenuated the weight loss observed after naloxone injection. Data are expressed as mean ± SEM, n=6–10 per group, * p<0.05 compared to RVM saline/systemic naloxone; # p<0.05 compared to RVM saline/systemic saline (control group).
As previously reported70, spinal administration of ondansetron reversed sustained morphine-induced thermal hypersensitivity, with response latencies equivalent to saline control values (Fig 4b). Administration of naloxone further reduced response latencies in morphine-treated rats given intrathecal saline. Administration of spinal ondansetron prevented the naloxone-precipitated reduction in hot plate latency in morphine-treated rats, with response latencies equivalent to saline-treated animals (i.e. control values). Animals receiving control saline intrathecal injections demonstrated lower hot plate latencies when compared with animals receiving ondansetron (p<0.05).
Autonomic signs of naloxone-induced withdrawal were also prevented by spinal injection of ondansetron (Figs 4d and 4e). Animals, receiving control intrathecal injections of saline, demonstrated signs of diarrhea and greater weight loss than animals receiving ondansetron (p<0.05).
4. Discussion
Opiate use is associated with feelings of well-being and euphoria. The desire to recapture these pleasurable feelings may lead to craving and drug seeking behaviors. Opiates thus are positively reinforcing, which contributes to continued drug use and abuse. It has been suggested that dependence may form part of a continuum that stretches from no drug use through “controlled” drug use and finally to, in extreme cases, compulsive drug use. The transition from “controlled” drug use to compulsive use is considered the initiation phase of drug dependence. Transition to compulsive drug use likely depends, in part, on the ability of drug self-administration to prevent the consequences of drug abstinence. During continual drug use, neuroadaptive changes occur which underlie negative symptoms of withdrawal55. In humans, opiate withdrawal is characterized by somatic and autonomic signs such as pupil dilation, piloerection, shivering, sweating, lacrimation, tremors, muscle pain (myalgia), diarrhea, vomiting, insomnia, and yawning, and affective symptoms including dysphoria or anhedonia, hyper-irritability, anxiety, restlessness, tension, and depression17, 23, 25, 38, 55. Moreover, tolerance to the positive subjective effects of opioids (e.g., euphoria) has been demonstrated in humans, suggesting that a decrease in the positive reinforcing effects of the drug may occur concurrently with neuroadaptive changes that produce negative reinforcing effects55. Thus, it is possible that compulsive, uncontrolled drug use is driven in some individuals by a desire to avoid the negative consequences of opioid withdrawal rather than to derive the positive subjective effects originally associated with drug use during the initiation phase of drug dependence28, 31, 55. However, as some studies indicate that complete tolerance to the euphoric effects of opioids does not occur, pleasure-seeking and withdrawal-avoidance most likely exist as parallel motivational factors in continued drug use37, 55.
The present study demonstrates that the RVM plays an important role in many characteristic behaviors observed during naloxone-precipitated withdrawal syndrome in morphine-dependent rats. Disrupting descending facilitatory signaling from the RVM by microinjection of lidocaine, the EAA antagonist kynurenic acid, or the CCK2 receptor antagonist YM022 not only blocked naloxone-induced thermal hyperalgesia, but also blocked all other measured behavioral signs of the opioid-induced withdrawal syndrome with the exception of increased grooming behaviors. Spinal 5-HT3 receptors have been suggested to be critical in descending facilitatory signaling from the RVM58, 60, 61. We demonstrate that spinal administration of the 5HT3 receptor antagonist ondansetron also blocked all measured signs of naloxone-precipitated withdrawal except for grooming, in a similar manner to disrupting descending facilitatory signaling from the RVM. The present results indicate a prominent role of descending facilitatory signaling from the RVM in mediating naloxone-precipitated symptoms of opioid withdrawal.
A large body of literature describes the role of brainstem structures in the descending inhibition of pain68. In recent years, it has additionally been recognized that there is a descending facilitatory component to this brainstem pathway, which uses the RVM as its major output nuclei46, 64 The descending facilitation component is engaged especially in situations of tissue injury9, 43, 45, inflammation of somatic35, 63 and visceral tissues11, 63 and is likely important even in situations such as opioid infusion induced hyperalgesia, where there is no apparent tissue injury14, 67, 69. This body of evidence indicates that the RVM has a prominent role in maintaining sensitization of neural pathways responsible for pain transmission. Our findings expand the role of descending pathways from the RVM to other physical signs of opioid withdrawal. Interrupting neuronal transmission in the RVM prevented most signs of opioid withdrawal including hyperalgesia and autonomic signs. Previous studies have demonstrated that lidocaine microinjection into the RVM blocked the opioid withdrawal-induced hyperalgesia observed after acute injections of morphine and subsequent naloxone26. It was speculated that blocking of synaptic transmission of “ON” cells was responsible for the reduction in hyperalgesia, since “ON” cells were the only cells active during the hyperalgesic period5. There is evidence that neurons in this area mediate other homoeostatic functions besides pain12, 36, 51, 57, 66. Additionally, the RVM may serve as a relay output for effects of the locus coeruleus47, 52, 65, 75. Indeed, lesions of the locus coeruleus (LC) have been shown to decrease physical signs of opioid withdrawal33, and electrical stimulation of this nucleus or direct injection of opioid antagonists into the locus coeruleus precipitate the expression of opiate withdrawal16, 29. There are reciprocal connections between the RVM and the LC40, 56. Some of these connections are excitatory in nature as activation of LC neurons by nalaxone-precipitated withdrawal is blocked by lesions in the nucleus paragigantocellularis48, or by injection of EAA antagonists into the LC1. It would seem that the RVM, given its central role in modulating the antinociceptive effects of opioids and its multiple connections with other brain structures that have a role in the expression of opiate withdrawal, is a prime candidate to modulate multiple physical symptoms that occur during opioid withdrawal.
In recent years, there has been an effort to identify the neurochemical signature of the pain facilitatory (i.e., presumably “ON”) cells in the RVM. Electrophysiological experiments have elucidated that these cells may express glutamate receptors since microinjection of glutamate antagonists reduces the firing of this specific population of cells20, 22. Recent studies have also suggested that these cells likely express CCK2 receptors. CKK can act as a physiological “anti-opioid” which can work against the antinociceptive effects of opioids71. Injection of CCK into the RVM facilitates nociception13, 30, and this effect can be attenuated by CCK2 antagonists73. CCK levels are elevated in the RVM after spinal nerve injury and sustained exposure to morphine leading to hypersensitivity30, 73. In the present study, antagonizing EAA receptors with the broad EAA antagonist kynurenic acid or blocking CCK2 receptors with the antagonist YM022 was able to reduce behavioral signs of morphine withdrawal. These data suggest that EAA transmission in the RVM is important in the output from the RVM involved with the manifestation of behavioral signs of withdrawal. CCK2 receptors in the RVM seem to be also important in the facilitation of the withdrawal behavior. These data support the possibility that modulation of facilitatory output from the RVM requires activation of EAA and CCK2 receptors to promote features of the opiate withdrawal syndrome.
Previous studies by Suzuki, Dickenson and colleagues have determined that descending facilitation from the RVM uses 5HT acting at 5HT3 receptors to enhance nociceptive transmission at the spinal cord level58, 59, 61. In our study we have taken a similar approach and used the specific 5HT3 receptor antagonist ondansetron in the spinal cord to block the effects of descending facilitation. Ondansetron had similar effects on behavioral signs of opioid withdrawal as the manipulations done in the RVM. These data suggest that serotonin acting at the 5HT3 receptor in the spinal cord ultimately mediate many of the behavioral signs of opioid withdrawal. It should be noted that systemic injection of ondansetron has also been demonstrated to inhibit behavioral signs of opioid withdrawal44 raising the possibility of other sites within the nervous system where 5HT3 receptors may participate36. However, it is not likely that spinal administered ondansetron exerted its effects in supraspinal sites. Several lines of evidence suggest that spinally applied ondansetron does not exert its effects through supraspinal sites including: 1) consistent with previous studies69, the effects of ondansetron are very rapid and last no more than one hour supporting a local (spinal) effect of spinally-administered ondansetron; 2) antagonizing 5HT3 receptors in the medulla blocks the effects of opiate analgesia27 suggesting that supraspinal ondansetron would have a net pro-nociceptive effect, opposite to that observed here; and 3) i.c.v. injection of ondansetron did not produce any changes in baseline thresholds in naïve rat42 suggesting that the effects of spinal administration are likely to be mediated locally.
The RVM thus has a prominent role in the behavioral syndrome observed after injection of naloxone in morphine-dependent rats. This study provides new insights into the mechanisms that drive descending facilitation to elicit opiate-induced withdrawal hyperalgesia and additionally demonstrates a critical role for this circuit in other withdrawal behaviors. It should be noted that throughout our studies, the manipulations made to interfere with descending output from the RVM were effective in reducing multiple aspects of naloxone-precipitated withdrawal but consistently failed to affect grooming behaviors. Grooming has been associated with increased states of anxiety and may reflect the activation of neural circuits that are rostral to the RVM39. As the withdrawal syndrome is known to be aversive, understanding the mechanisms that promote withdrawal through the descending facilitation circuit may be useful in developing adjunct strategies that can diminish drug seeking in order to prevent this unpleasant state. Whether mechanisms that drive descending facilitation also mediate aversiveness is unknown and requires further study.
Acknowledgement
This work was funded by P01 DA 06284.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali NM. Hyperalgesic response in a patient receiving high concentrations of spinal morphine. Anesthesiology. 1986;65:449. doi: 10.1097/00000542-198610000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 4.Aricioglu-Kartal F, Kayir H, Tayfun Uzbay I. Effects of harman and harmine on naloxone-precipitated withdrawal syndrome in morphine-dependent rats. Life Sci. 2003;73:2363–2371. doi: 10.1016/s0024-3205(03)00647-7. [DOI] [PubMed] [Google Scholar]
- 5.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 6.Beubler E, Bukhave K, Rask-Madsen J. Colonic secretion mediated by prostaglandin E2 and 5-hydroxytryptamine may contribute to diarrhea due to morphine withdrawal in the rat. Gastroenterology. 1984;87:1042–1048. [PubMed] [Google Scholar]
- 7.Bie B, Peng Y, Zhang Y, Pan ZZ. cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci. 2005;25:3824–3832. doi: 10.1523/JNEUROSCI.5010-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- 9.Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 12.Dampney RA, Goodchild AK, Robertson LG, Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res. 1982;249:223–235. doi: 10.1016/0006-8993(82)90056-7. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;104:93–101. doi: 10.1016/s0304-3959(02)00469-4. [DOI] [PubMed] [Google Scholar]
- 14.Gardell LR, Burgess SE, Dogrul A, Ossipov MH, Malan TP, Lai J, Porreca F. Pronociceptive effects of spinal dynorphin promote cannabinoid-induced pain and antinociceptive tolerance. Pain. 2002;98:79–88. doi: 10.1016/s0304-3959(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 15.Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD002025.pub4. CD002025. [DOI] [PubMed] [Google Scholar]
- 16.Grant SJ, Huang YH, Redmond DE., Jr Behavior of monkeys during opiate withdrawal and locus coeruleus stimulation. Pharmacol Biochem Behav. 1988;30:13–19. doi: 10.1016/0091-3057(88)90419-4. [DOI] [PubMed] [Google Scholar]
- 17.Haertzen CA, Hooks NT., Jr Changes in personality and subjective experience associated with the chronic administration and withdrawal of opiates. J Nerv Ment Dis. 1969;148:606–614. doi: 10.1097/00005053-196906000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: implications for circuitry. Pain. 1998;75:247–255. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 19.Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 20.Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol. 2001;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- 21.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 22.Heinricher MM, Roychowdhury SM. Reflex-related activation of putative pain facilitating neurons in rostral ventromedial medulla requires excitatory amino acid transmission. Neuroscience. 1997;78:1159–1165. doi: 10.1016/s0306-4522(96)00683-5. [DOI] [PubMed] [Google Scholar]
- 23.Henningfield JE, Nemeth-Coslett R, Katz JL, Goldberg SR. Intravenous cocaine self-administration by human volunteers: second-order schedules of reinforcement. NIDA Res Monogr. 1987;76:266–273. [PubMed] [Google Scholar]
- 24.Higgins GA, Nguyen P, Sellers EM. The NMDA antagonist dizocilpine (MK801) attenuates motivational as well as somatic aspects of naloxone precipitated opioid withdrawal. Life Sci. 1992;50:PL167–PL172. doi: 10.1016/0024-3205(92)90452-u. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe JH. Trivializing dependence. Br J Addict. 1990;85:1425–1427. doi: 10.1111/j.1360-0443.1990.tb01624.x. discussion 9–31. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–1439. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiefel JM, Cooper ML, Bodnar RJ. Serotonin receptor subtype antagonists in the medial ventral medulla inhibit mesencephalic opiate analgesia. Brain Res. 1992;597:331–338. doi: 10.1016/0006-8993(92)91490-6. [DOI] [PubMed] [Google Scholar]
- 28.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 29.Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 30.Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79:101–108. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Maldonado R, Koob GF. Destruction of the locus coeruleus decreases physical signs of opiate withdrawal. Brain Res. 1993;605:128–138. doi: 10.1016/0006-8993(93)91364-x. [DOI] [PubMed] [Google Scholar]
- 33.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- 34.Maldonado R, Valverde O, Derrien M, Tejedor-Real P, Roques BP. Effects induced by BC 264, a selective agonist of CCK-B receptors, on morphine-dependent rats. Pharmacol Biochem Behav. 1994;48:363–369. doi: 10.1016/0091-3057(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 35.Mansikka H, Pertovaara A. Supraspinal influence on hindlimb withdrawal thresholds and mustard oil-induced secondary allodynia in rats. Brain ResBull. 1997;42:359–365. doi: 10.1016/s0361-9230(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 36.Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- 37.McAuliffe WE, Gordon RA. A test of Lindesmith's theory of addiction: the frequency of euphoria among long-term addicts. AJS. 1974;79:795–840. doi: 10.1086/225628. [DOI] [PubMed] [Google Scholar]
- 38.Meyer RE, McNamee HB, Mirin SM, Altman JL. Analysis and modification of opiate reinforcement. Int J Addict. 1976;11:467–484. doi: 10.3109/10826087609056164. [DOI] [PubMed] [Google Scholar]
- 39.Moody TW, Merali Z, Crawley JN. The Effects of Anxiolytics and Other Agents on Rat Grooming Behaviors. Annals of the New York Academy of Sciences. 1988;525:281–290. doi: 10.1111/j.1749-6632.1988.tb38613.x. [DOI] [PubMed] [Google Scholar]
- 40.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 41.Mucha RF. Is the motivational effect of opiate withdrawal reflected by common somatic indices of precipitated withdrawal? A place conditioning study in the rat. Brain Res. 1987;418:214–220. doi: 10.1016/0006-8993(87)90088-6. [DOI] [PubMed] [Google Scholar]
- 42.Nalwalk JW, Svokos K, Leurs R, Hough LB. Absence of 5-HT3 and cholinergic mechanisms in improgan antinociception. Pharmacol Biochem Behav. 2005;80:505–510. doi: 10.1016/j.pbb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Pertovaara A, Wei H, Hamalainen MM. Lidocaine in the rostroventromedial medulla and the periaqueductal gray attenuates allodynia in neuropathic rats. Neurosci Lett. 1996;218:127–130. doi: 10.1016/s0304-3940(96)13136-0. [DOI] [PubMed] [Google Scholar]
- 44.Pinelli A, Trivulzio S, Tomasoni L. Effects of ondansetron administration on opioid withdrawal syndrome observed in rats. Eur J Pharmacol. 1997;340:111–119. doi: 10.1016/s0014-2999(97)01349-6. [DOI] [PubMed] [Google Scholar]
- 45.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 47.Prieto GJ, Cannon JT, Liebeskind JC: N. raphe magnus lesions disrupt stimulation-produced analgesia from ventral but not dorsal midbrain areas in the rat. Brain Res. 1983;261:53–57. doi: 10.1016/0006-8993(83)91282-9. [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–350. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- 49.Rasmussen K, Fuller RW, Stockton ME, Perry KW, Swinford RM, Ornstein PL. NMDA receptor antagonists suppress behaviors but not norepinephrine turnover or locus coeruleus unit activity induced by opiate withdrawal. Eur J Pharmacol. 1991;197:9–16. doi: 10.1016/0014-2999(91)90358-w. [DOI] [PubMed] [Google Scholar]
- 50.Rohde DS, McKay WR, Chang DS, Abbadie C, Basbaum AI. The contribution of supraspinal, peripheral and intrinsic spinal circuits to the pattern and magnitude of Fos-like immunoreactivity in the lumbar spinal cord of the rat withdrawing from morphine. Neuroscience. 1997;80:599–612. doi: 10.1016/s0306-4522(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 51.Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- 52.Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- 53.Savage SR, Joranson DE, Covington EC, Schnoll SH, Heit HA, Gilson AM. Definitions related to the medical use of opioids: evolution towards universal agreement. J Pain Symptom Manage. 2003;26:655–667. doi: 10.1016/s0885-3924(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt BL, Tambeli CH, Levine JD, Gear RW. Adaptations in nucleus accumbens circuitry during opioid withdrawal associated with persistence of noxious stimulus-induced antinociception in the rat. J Pain. 2003;4:141–147. doi: 10.1054/jpai.2003.12. [DOI] [PubMed] [Google Scholar]
- 55.Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochem Res. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- 56.Sim LJ, Joseph SA. Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat. 1991;4:97–109. doi: 10.1016/0891-0618(91)90034-a. [DOI] [PubMed] [Google Scholar]
- 57.Sun MK, Young BS, Hackett JT, Guyenet PG. Rostral ventrolateral medullary neurons with intrinsic pacemaker properties are not catecholaminergic. Brain Res. 1988;451:345–349. doi: 10.1016/0006-8993(88)90781-0. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals. 2005;14:175–181. doi: 10.1159/000087656. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends in Pharmacological Sciences. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Urban MO, Coutinho SV, Gebhart GF. Biphasic modulation of visceral nociception by neurotensin in rat rostral ventromedial medulla. J Pharmacol Exp Ther. 1999;290:207–213. [PubMed] [Google Scholar]
- 63.Urban MO, Coutinho SV, Gebhart GF. Involvement of excitatory amino acid receptors and nitric oxide in the rostral ventromedial medulla in modulating secondary hyperalgesia produced by mustard oil. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 64.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urban MO, Smith DJ. Localization of the antinociceptive and antianalgesic effects of neurotensin within the rostral ventromedial medulla. Neurosci Lett. 1994;174:21–25. doi: 10.1016/0304-3940(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 66.Van Bockstaele EJ, Aston-Jones G. Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clin Exp Hypertens. 1995;17:153–165. doi: 10.3109/10641969509087062. [DOI] [PubMed] [Google Scholar]
- 67.Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 70.Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, Porreca F. Spinal NK 1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129(1–2):35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiesenfeld-Hallin Z, de Arauja Lucas G, Alster P, Xu XJ, Hokfelt T. Cholecystokinin/opioid interactions. Brain Res. 1999;848:78–89. doi: 10.1016/s0006-8993(99)01978-2. [DOI] [PubMed] [Google Scholar]
- 72.Winn P, Stone TW, Latimer M, Hastings MH, Clark AJ. A comparison of excitotoxic lesions of the basal forebrain by kainate, quinolinate, ibotenate, N-methyl-D-aspartate or quisqualate, and the effects on toxicity of 2-amino-5-phosphonovaleric acid and kynurenic acid in the rat. Br J Pharmacol. 1991;102:904–908. doi: 10.1111/j.1476-5381.1991.tb12274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie JY, Herman DS, Stiller C-O, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the Rostral Ventromedial Medulla Mediates Opioid-Induced Hyperalgesia and Antinociceptive Tolerance. J Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 75.Young EG, Watkins LR, Mayer DJ. Comparison of the effects of ventral medullary lesions on systemic and microinjection morphine analgesia. Brain Res. 1984;290:119–129. doi: 10.1016/0006-8993(84)90741-8. [DOI] [PubMed] [Google Scholar]
- 76.Zhang T, Feng Y, Rockhold RW, Ho IK. Naloxone-precipitated morphine withdrawal increases pontine glutamate levels in the rat. Life Sci. 1994;55:PL25–PL31. doi: 10.1016/0024-3205(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Q, Karlsson K, Liu Z, Johansson P, Le Greves M, Kiuru A, Nyberg F. Substance P endopeptidase like activity is altered in various regions of the rat central nervous system during morphine tolerance and withdrawal. Neuropharmacology. 2001;41:246–253. doi: 10.1016/s0028-3908(01)00055-7. [DOI] [PubMed] [Google Scholar]