Abstract
The tumor necrosis factor related apoptosis-inducing ligand (TRAIL) causes cancer cell death, but many cancers, including pancreatic cancer, are resistant to TRAIL therapy. A combination of TRAIL and the diterpene triepoxide, triptolide, is effective in inducing pancreatic cancer cell death. Triptolide increases levels of death receptor DR5 and decreases the pro-survival FLICE-like inhibitory protein (c-FLIP), which contribute to the activation of caspase-8. This combination further causes both lysosomal and mitochondrial membrane permeabilization, resulting in cell death. Our study provides a mechanism by which triptolide sensitizes TRAIL resistant cells, which may become a novel therapeutic strategy against pancreatic cancer.
Keywords: TRAIL, pancreatic cancer, triptolide, apoptosis, death receptor
1. Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States with a five year survival of less than 5% [17]. Over 44,000 cases were diagnosed in 2012, and nearly the same number succumbed to the disease [33]. The poor outcome of this disease is due to late stage diagnosis and lack of effective chemotherapeutic options [10, 14].
TNF-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, selectively kills a wide range of cancer cells, while leaving normal cells unaffected [30]. TRAIL initiates the extrinsic apoptotic pathway by activation of pro-caspase-8 [26]. Cleavage of pro-caspase-8 initiates apoptosis by directly activating the effector caspase-3 or by triggering the intrinsic apoptotic pathway via Bcl-2 family members activation or translocation [8, 24, 25]. Cellular FLICE-inhibitory protein (c-FLIP) is a caspase-8 homologue that lacks caspase activity. c-FLIP competes for the caspase-8 binding site on the receptor, thereby preventing recruitment of pro-caspase-8 to DISC (Death Inducing Signaling Complex) [22].
Triptolide, a diterpene triepoxide extracted from the Chinese herb Tripterygium wilfordii, decreases viability of pancreatic cancer cells in vitro and reduces growth and metastases of tumors in vivo [28]. Previous data from our group has shown that a combination of low doses of TRAIL and triptolide induces significant pancreatic cancer cell death compared with either treatment alone [3]; however, the underlying mechanism by which cell death is induced remains unclear.
Permeabilization of the lysosome induces the cell death pathway in response to a variety of cell death stimuli [4]. We have previously shown that a high dose of triptolide induces lysosomal membrane permeabilization (LMP), resulting in pancreatic cancer cell death. However, the mechanism responsible for apoptosis-associated LMP and the contribution of LMP in TRAIL/triptolide-induced cell death remains to be explored [18].
In this study, we show that a combination of TRAIL and triptolide at low concentrations induces caspase-8 dependent pancreatic cancer cell death. Triptolide treatment sensitizes cells to TRAIL-induced death by down-regulation of c-FLIP and up-regulation of DR5. Finally, in the presence of triptolide, low concentration of TRAIL activated the death receptor pathway, resulting in LMP and MOMP mediated pancreatic cancer cell death. Since TRAIL is already in use against several cancers, understanding the mechanism by which triptolide sensitizes pancreatic cancer cells to TRAIL may result in a novel therapeutic strategy against pancreatic cancer.
2. Materials and Methods
2.1 Cell Culture and viability
MIA PaCa-2 cells derived from a primary pancreatic tumor were obtained from ATCC and cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin. S2-VP10 cells (a gift from Dr. Masato Yamamoto, University of Minnesota) were cultured in RPMI medium (Hyclone) supplemented with 10% Fetal Bovine Serum and 1% penicillin-streptomycin. All cells were maintained at 37°C in a humidified air atmosphere with 5% CO2. Cell viability was measured as previously described [29].
2.2 Transfection With Short Interfering RNA
ON-TARGET plus SMART Pool human caspase-8 short interfering RNA (siRNA) (L-003466-00-0005) and human c-FLIP siRNA (L-003772-00-0005) were purchased from Thermo Scientific. Human Death Receptor 5 siRNA (S100056700) was purchased from QIAGEN. Transfections were performed as previously described [1].
2.3 Chemicals and reagents
Triptolide (>99% pure) was purchased from Calbiochem, dissolved in DMSO, aliquoted, and stored at −20°C. The caspase-8 inhibitor Z-IETD-FMK (cat #550380), caspase-9 inhibitor Z-LEHD-FMK (cat #550381) and negative control caspase inhibitors Z-FA-FMK (cat #550411) were purchased from BD Pharmingen. The Cathepsin B inhibitor CA-074me (620106) was purchased from Peptide Institute, INC. Primary antibodies used for western blots were as follows: Caspase-8, Cleaved-caspase-3, Cleaved-capase-9, PARP, Bcl-2, Bid, JNK, phosphorylated-JNK, DR5, cleaved Caspase-3 (Cell Signaling); BAX (N-20) (Santa Cruz) and c-FLIP (ENZO life science). Primary antibodies against DR4 and DR5 for flow cytometry were purchased from eBioscience.
2.4 Cathepsin B Activity Assay
The cytosolic cathepsin B activity assay was performed as previously described [9]. To measure cytosolic Cathepsin B activity, cells were permeabilized using bacterial toxin Streptolysin O, which preferentially interact and permeabilized plasma membrane. Briefly, cells were suspended in the cytosolic buffer (120mM KCl, 0.15mM CaCl2, 10mM K2HPO4, 25mM HEPES, 2mM EGTA, 5mM MgCl2, 2mM ATP, pH 7.6) containing 20 ug/mL Streptolysin O (Sigma, St. Louis, MI). This was incubated for 20 minutes on ice. Cells were separated, suspended Streptolysin O free buffer and incubated at 37°C for 20 min. The suspension was centrifuged at higher speed to get cytosol and pellet containing cell organelles. The Streptolysin O concentration and treatment times were optimized to result in permeabilization of the cells without disrupting the lysosomes. Cathepsin B activity was determined using N-carbobenzoxy-arginyl-arginine-naphthylamide as the substrate, according to the method of McDonald and Ellis[27].
2.5 Immunofluorescence
To analyze cellular distribution of BAX, Cytochrome c and Cathepsin B, pancreatic cancer cells grown in chamber slides were treated with TRAIL/triptolide for 24 hours. Cells for BAX (N20) or Cytochrome c immunostaining were incubated with 100 nM of Mitotracker Red 30 minutes prior to fixation. Cells were fixed with 2% paraformaldehyde for 10 minutes, and permeabilized with 0.1% Triton X-100 for 10 minutes at room temperature (RT). Cells were blocked with 2% BSA at RT for 1 hour. The cells were then incubated with a 1:800 dilution of mouse monoclonal anti–cytochrome c antibody (BD Pharmingen, 556432) or 1:200 dilution of rabbit polyclonal anti-BAX (N20) (Santa Cruz, sc-493) at 4°C overnight. After three 5-minute washes with phosphate-buffered saline (PBS), cells were incubated with secondary antibodies: 1:200 dilution of Alexa-488–conjugated donkey anti-mouse IgG (Molecular Probes) or 1:1000 dilution of Alexa-488–conjugated donkey anti-rabbit IgG (Molecular Probes) for 1 hour at RT.
Cells for Cathepsin B immunostaining were incubated with 2μM of Lysotracker RED for 2 hours before fixation. Cells were fixed with 2% paraformaldehyde + 0.19% picric acid for 20 minutes at 37°C, and permeabilized with 0.0125% CHAPS in fixation buffer for 10 minutes at 37°C. The cells were blocked with PBS containing 5% FBS+5% glycerol, incubate at 37°C for 1 hour. The cells were then incubated with a 1:100 dilution of mouse monoclonal anti–Cathepsin B antibody (EMD Millipore IM27L) at 4°C overnight. After three 5-minute washes with PBS, cells were incubated with secondary antibodies: 1:1000 dilution of Alexa-488–conjugated donkey anti-mouse IgG (Molecular Probes) for 1 hour at 37°C. All samples were then washed and mounted using Prolong Gold anti-fade agent with DAPI (Molecular Probes).
2.6 Immunoprecipitation of Active BAX
Cells were lysed in RIPA buffer containing protease inhibitor and phosphatase inhibitor. After 1h pre-clean with 40 μl of protein A/G Agarose (Roche), an aliquot of 500 μg total protein was incubated with 2 μg of anti-BAX (clone 6A7) antibody (Exalpha, A105M) for 4 hours at 4°C, followed by another 40 μl of protein A/G Agarose for an additional 2h to precipitate the conformational changed BAX protein. The beads were washed with RIPA buffer three times and were resuspended in Laemmli sample buffer (Sigma Aldrich) and boiled for 5 min. The eluted proteins were subjected to SDS-PAGE immunoblot analysis with anti-BAX (N-20) (Santa Cruz) antibody.
2.7 Western blot assay
Western Blot for protein measurement was performed as previously described [29]. The blots were re-probed with an antibody against β-actin (Santa Cruz) to ensure equal loading of proteins.
2.8 Measurement of Caspase-3, 8, 9 activation
Caspase-3, 8, 9 activity was analyzed using the Caspase-Glo luminescent-based assays (Promega) according to the manufacturer’s instructions. Cells (3× 103) were seeded into 96-well white opaque plates and a corresponding optically clear 96-well plate. After 48 hours, cells were treated at concentrations and times indicated. At the end of the incubation, 100 μl of Caspase-Glo reagent was added to each well containing 100 μl of cells in culture medium. Plates were gently mixed and incubated for 45 minutes at room temperature, and luminescence was then read on a luminometer. The corresponding 96-well clear plates were used to measure the number of viable cells with the CCK-8 reagent, and caspase activity was normalized to these values.
2.9 Statistical Analysis
All values are expressed as the mean +/↕ standard error of the mean. All experiments using cell lines were repeated of a minimum 3 times. Statistical significance was reported if p-value was < 0.05 using an unpaired Student t-test.
3. Results
3.1 Combination of TRAIL and triptolide activates Caspase-8 in pancreatic cancer cells
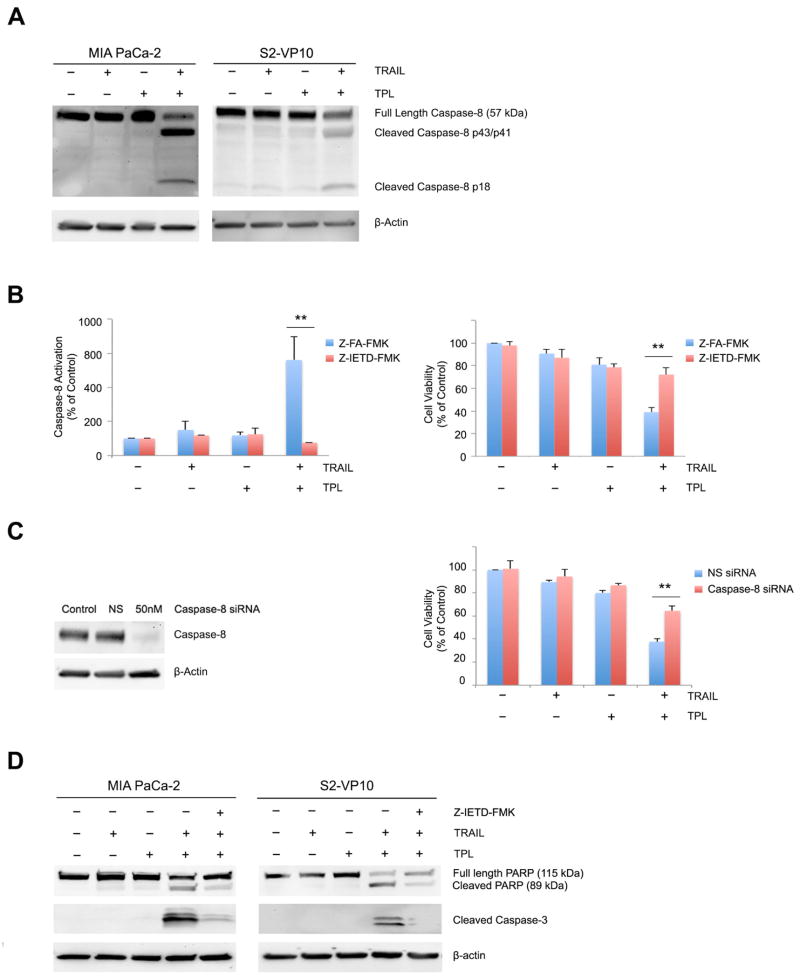
Previous studies from our group have shown that treatment of pancreatic cancer cells with a combination of TRAIL and triptolide at low concentrations results in cell death [3]. Consistent with these results, we have confirmed that cell viability decreased significantly and increase in pro-apoptotic markers were detected in MIA PaCa-2 and S2-VP10 pancreatic cancer cells treated with a combination of 1.25ng/ml TRAIL and 50nM triptolide (Supplemental Figure 1A/1B). However, the viability of primary mouse acinar cells and human pancreatic duct epithelial cells (HPDEC) did not decrease in the presence of a combination of TRAIL and triptolide (Supplemental Figure 1C). Previous studies suggest that caspase-8 is involved in the release of apoptotic factors from mitochondrial or lysosomes [6, 35]. We therefore assessed its activation in the presence of TRAIL or triptolide in pancreatic cancer cells. As shown in Figure 1A, caspase-8 was cleaved only in cells treated with both TRAIL and triptolide, but not with either treatment alone. We further tested whether caspase-8 activation was required for TRAIL/triptolide mediated cell death by the introduction of a caspase-8 specific inhibitor, Z-IETD-FMK [31]. Caspase-8 activation induced by TRAIL and triptolide (711 ± 133.8%) was significantly inhibited by Z-IETD-FMK (76 ± 0.18%), compared to its negative control Z-FA-FMK, demonstrating the efficacy of the inhibitor. Importantly, while the combination of TRAIL and triptolide resulted in decreased cell viability to 38.98 ± 3.93% vs. control untreated cells (100%), nearly 73% cells were viable when a combination of TRAIL and triptolide were used in the presence of the caspase-8 inhibitor (Figure 1B), suggesting that caspase-8 activation causes pancreatic cancer cell death (p<0.01). Since inhibitors may have off-target effects, we decreased caspase-8 expression using siRNA (Figure 1C). While treatment of cells transfected with non-silencing siRNA with a combination of TRAIL and triptolide resulted in a decrease in cell viability to 37.6 ± 1.8%, loss of caspase-8 protected cells against cell death, as indicated by an increase in cell viability to 61.56 ± 5.78% (p<0.01; Figure 1C).
Figure 1. Caspase-8 activation is required for the combination of TRAIL and triptolide to induce cell death. Pancreatic cancer cells were treated with either TRAIL or triptolide or a combination of both for 24h.
A. Caspase-8 cleavage was detected in MIA PaCa-2 and S2-VP10 cells by western blot. β-actin expression was used as loading control.
B. Treatment with specific caspase-8 inhibitor (Z-IETD-FMK, 40μM) in MIA PaCa-2 cells results in a significant decrease in caspase-8 activity (left panel) and increase in viability (right panel) when treated with a combination of TRAIL and triptolide. The bars represent mean ± SEM, n≥3. **p< 0.01.
C. MIA PaCa-2 cells treated with caspase-8 siRNA show a significant decrease of caspase-8 expression and increase in viability when treated with a combination of TRAIL and triptolide. The bars represent mean ± SEM, n≥3. **p< 0.01.
D. PARP and caspase-3 cleavage was assessed in MIA PaCa-2 and S2-VP10 cells using western blots. β-actin expression was used as loading control.
We further evaluated markers of apoptotic cell death in the presence or absence of the caspase-8 inhibitor in MIA PaCa-2 and S2-VP10 treated with TRAIL and triptolide. As shown in Figure 1D, lesser cleavage of both PARP and caspase-3 was observed in TRAIL and triptolide treated cells in the presence of Z-IETD-FMK in both MIA PaCa-2 and S2-VP10 cells. Our data, taken together, show that caspase-8 activation is required for the combination of TRAIL and triptolide to induce apoptotic pancreatic cancer cell death.
3.2 Triptolide-induced increase in Death Receptor 5 expression is required for caspase-8 activation in pancreatic cancer cells
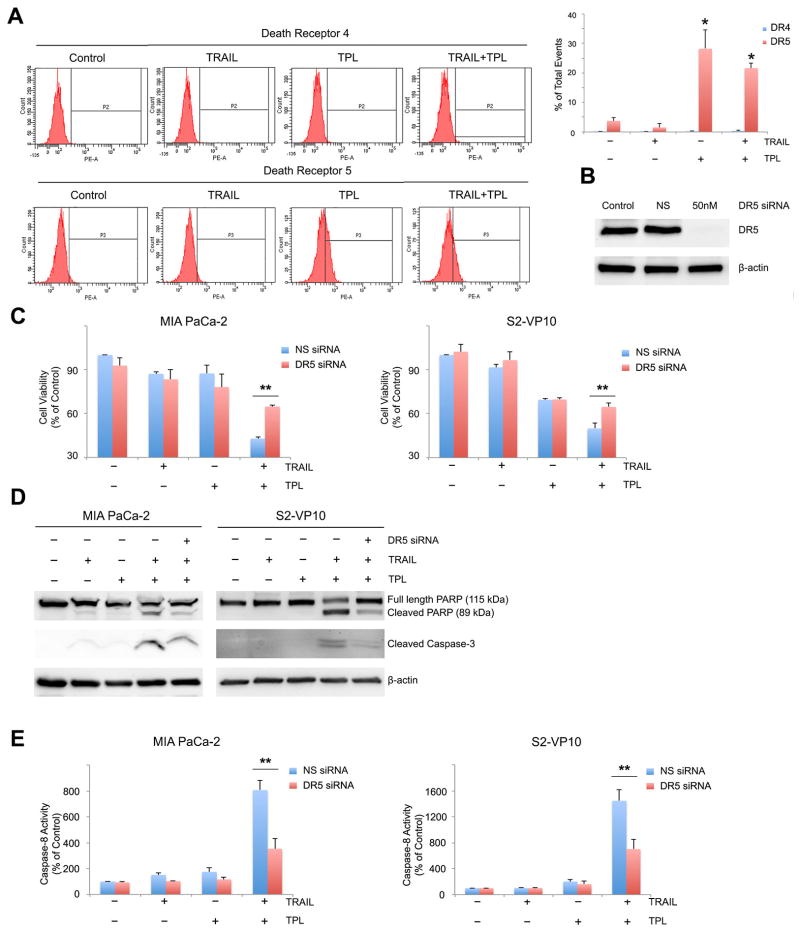
Oligomerization of death receptors by binding of death ligands results in transduction of either apoptotic or survival signals [23]. Using flow cytometry, we assessed the effect of triptolide on the death receptors DR4 and DR5. Treatment of MIA PaCa-2 cells with triptolide for 24 hours led to a significant increase in the number of DR5 positive events (28.2 ± 6.43 %) compared to untreated cells (3.77 ± 1.0 %) (Fig. 2A). This increase in DR5 expression was caused by triptolide treatment alone since no further increase in DR5 expression was observed in the combination group (21.63 ± 1.59 %). However, no significant difference in the number of DR4 positive events were observed in the cells treated with TRAIL or triptolide or a combination of both. Similar results were obtained with S2-VP10 cells (data not shown).
Figure 2. Triptolide induced increase in DR5 expression sensitizes pancreatic cancer cells to TRAIL mediated cell death. MIA PaCa-2 and S2-VP10 cells were treated with TRAIL or triptolide or a combination of both and harvested 24h post treatment.
A. DR4 and DR5 levels were evaluated using flow cytometry.
B. Knockdown of DR5 by DR5 siRNA 24h prior to treatment was confirmed by western blot. β-actin expression was used as loading control.
C. Loss of DR5 rescues cells from TRAIL and triptolide mediated cell death. The bars represent mean ± SEM, n≥3. **p< 0.01.
D. PARP and caspase-3 cleavage was assessed using western blots. β-actin expression was used as loading control.
E. Loss of DR5 decreases caspase-8 activity in TRAIL and triptolide treated cells.
Since triptolide increased levels of DR5 in pancreatic cancer cells, we further tested the importance of this event in the ability of triptolide to sensitize pancreatic cancer cells to TRAIL mediated cell death. DR5 expression was down-regulated using DR5-specific siRNA (Figure 2B) 24h prior to treatment of cells with TRAIL and triptolide. DR5 siRNA transfected cells showed an increase in cell viability (64.81 ± 1.17 %) compared to non-silencing siRNA transfected cells (42.9 ± 0.99%) when treated with TRAIL and triptolide (p<0.01). We therefore concluded that a loss of DR5 resulted in a significant rescue of cell viability in pancreatic cancer cells treated with a combination of TRAIL and triptolide (Figure 2C). This was confirmed by a decrease in levels of cleaved PARP and caspase-3 in cells treated with DR5 siRNA and a combination of TRAIL and triptolide (Fig. 2D). To further determine the effect of loss of DR5 on TRAIL/triptolide induced cell death, we assessed the activation of caspase-8, which was significantly lower in both MIA PaCa-2 and S2-VP10 cells transfected with DR5 siRNA (352.9 ± 80.6 and 701 ± 154.1) compared with those transfected with non-silencing siRNA (805.99 ± 76.8 in MIA PaCa-2 and 1445.75 ± 174.94 in S2-VP10) when treated with a combination of TRAIL and triptolide (Fig. 2E). Our data, taken together, show that triptolide increases levels of DR5 and loss of DR5 decreases levels of downstream effectors and rescues cells from TRAIL/triptolide mediated cell death.
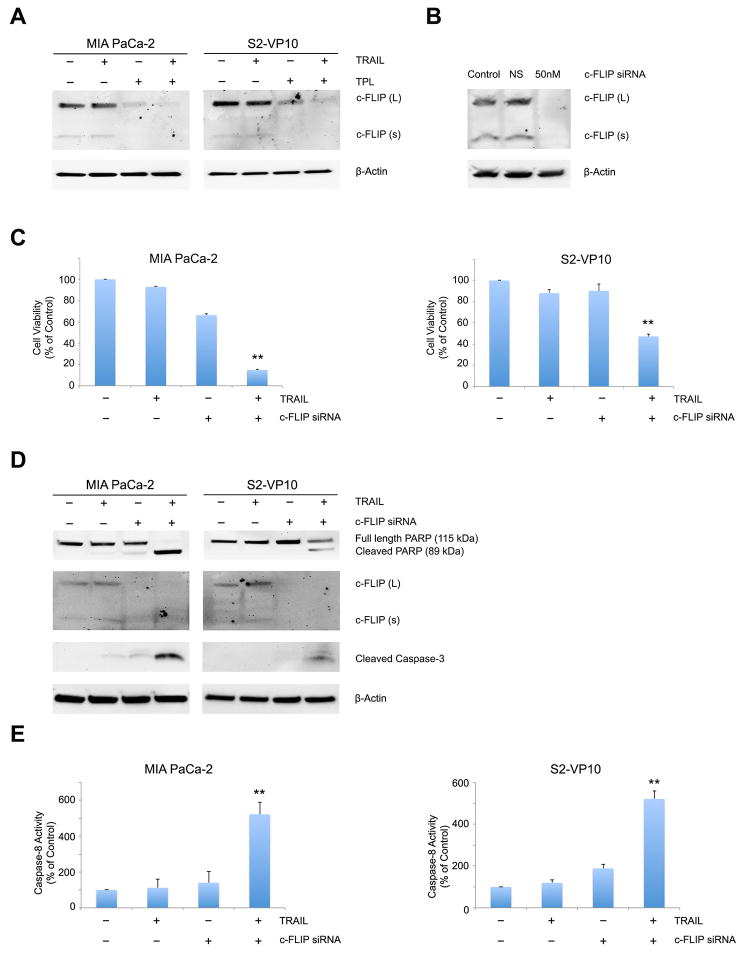
3.3 Triptolide-mediated decrease of c-FLIP is essential for TRAIL induced caspase-8 activation in pancreatic cancer cells
Cleavage of pro-caspase-8 results in activation of caspase-8, ultimately leading to cell death. c-FLIP is an anti-apoptotic factor which competes with pro-caspase 8 in binding to the DISC, thereby preventing caspase-8 activation in malignant cells [32]. We therefore evaluated levels of c-FLIP to explore its role, if any, in TRAIL/triptolide induced pancreatic cancer apoptosis. As shown in Figure 3A, c-FLIP levels were significantly down-regulated by treatment with triptolide in both MIA PaCa-2 and S2-VP10 cells. In order to assess the importance of c-FLIP down-regulation in TRAIL/triptolide mediated cell death, we used c-FLIP specific siRNA, thereby mimicking the effect of triptolide on c-FLIP expression. A significant decrease in c-FLIP levels was observed after c-FLIP specific siRNA transfection in MIA PaCa-2 cells, indicating a total knockdown of c-FLIP (Figure 3B). When c-FLIP siRNA transfected MIA PaCa-2 or S2-VP10 cells were treated with TRAIL, the decrease in cell viability was greater with the combination of c-FLIP siRNA and TRAIL (14.81 ± 0.833 % in MIA Paca-2 and 47.25 ± 2.17 % in S2-VP10 cells) than with either c-FLIP siRNA (66.36 ± 1.3 % in MIA PaCa-2 and 90.2 ± 6.4% in S2-VP10 cells) or TRAIL treatment (MIA PaCa-2 92.95 ± 0.42 %; S2-VP10 88.07 ± 3.02%) alone (p<0.01) (Figure 3C). c-FLIP knockdown cells treated with TRAIL also showed PARP and caspase-3 cleavage, confirming apoptotic cell death (Figure 3D). Since c-FLIP significantly decreases activation of procaspase-8, we further tested if down-regulation of c-FLIP by triptolide could sensitize cells to TRAIL induced caspase-8 activation. Significant caspase-8 activation was observed after treatment with a combination of TRAIL and c-FLIP siRNA in both MIA PaCa-2 (522.4 ± 66.69 %) and S2-VP10 (544 ± 38.9 %) cells compared to either treatment alone. From the data above, we conclude that down-regulation of c-FLIP by triptolide contributes to TRAIL-induced caspase-8 activation, which results in cell death.
Figure 3. Triptolide induced decrease in c-FLIP expression sensitizes pancreatic cancer cells to TRAIL mediated cell death.
A. c-FLIP expression was detected by western blot MIA PaCa-2 and S2-VP10 cells treated with TRAIL or triptolide or a combination of both for 24h. β-actin expression was used as loading control.
B. Knockdown of c-FLIP by siRNA in MIA PaCa-2 and S2-VP10 cells was confirmed by western blot. β-actin expression was used as loading control.
C. Treatment of MIA PaCa-2 and S2-VP10 cells with a combination of TRAIL and c-FLIP siRNA significantly decreases cell viability after 24 hours of treatment. The bars represent mean ± SEM, n≥3. **p< 0.01.
D. PARP and caspase-3 cleavage was assessed in MIA PaCa-2 and S2-VP10 cells after treatment with TRAIL or c-FLIP siRNA or both using western blot. β-actin expression was used as loading control.
E. Treatment of MIA PaCa-2 and S2-VP10 cells with TRAIL or c-FLIP siRNA or a combination of both shows a significant increase in caspase-8 activation 24 hours post treatment. The bars represent mean ± SEM, n≥3. **p< 0.01.
3.4 Combination of TRAIL and triptolide induces lysosomal membrane permeabilization in pancreatic cancer cells
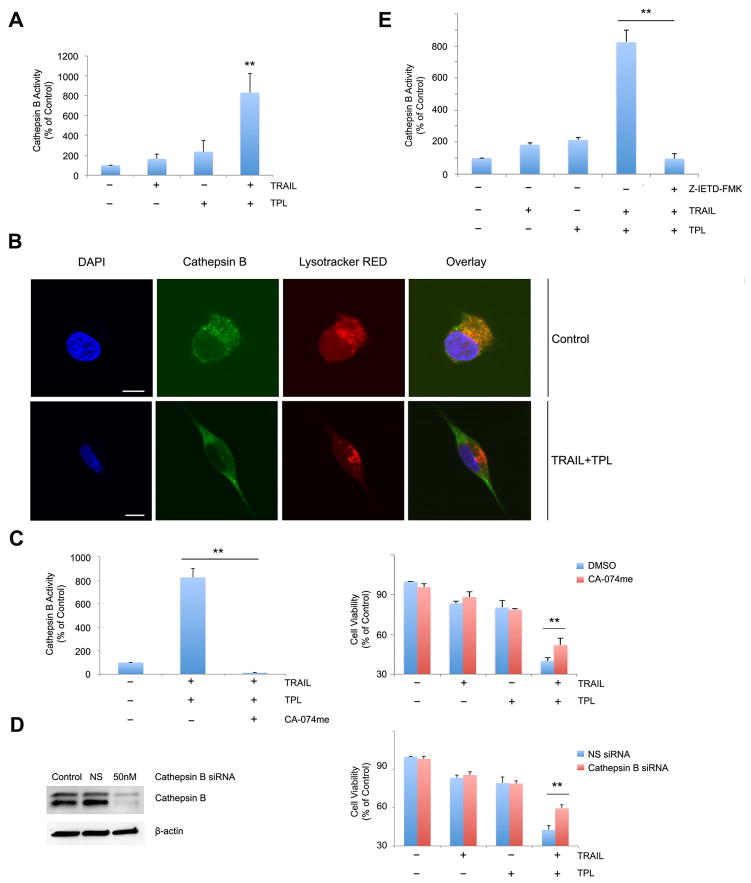
Cell death occurs via activation of several cascades, one of which is events leading to the permeabilization of the lysosome. [4]. Previous data from our group has shown that high concentrations (200nM) of triptolide induce LMP in pancreatic cancer cells [9]. To evaluate if LMP plays a role in TRAIL/triptolide mediated cell death, we measured release of Cathepsin B, an enzyme localized to the lysosome, into the cytosol, as a marker of lysosomal permeabilization. As shown in Figure 4A, treatment of MIA PaCa-2 cells with either TRAIL or triptolide resulted in an increase of cytosolic Cathepsin B activity (163.533 ± 51.48 % and 237.9 ± 109.7 % respectively vs. control (100%)). However, treatment with the combination of TRAIL and triptolide significantly increased cytosolic cathepsin B activity (831.01 ± 193.82 %). The purity of cytosolic and lysosomal fraction was confirmed by isolating the fractions and western blotting with GAPDH (cytosolic marker) and LAMP1/2 (lysosomal markers) (Supplemental Figure 2). To confirm that the combination of TRAIL and triptolide results in LMP, localization of Cathepsin B was observed by immunostaining cells with a Cathepsin B-specific antibody using confocal microscopy. As shown in Figure 4B, cathepsin B (green) shows punctuate staining in control cells that co-localizes with lysosomal (lysotracker Red, a lysosomal marker). However, treatment of cells with a combination of TRAIL and triptolide leads to a diffused cytoplasmic staining of cathepsin B with decreased co-localization with Lysotracker Red, demonstrating that, in treated cells, Cathepsin B is released from the lysosome into the cytosol.
Figure 4. Combination of TRAIL and triptolide increases lysosomal membrane permeabilization in pancreatic cancer cells.
A. MIA PaCa-2 cells treated with either TRAIL or triptolide or their combination show an increase in cytosolic cathepsin B activity, suggesting lysosomal permeabilization. Cytosolic cathepsin B values are expressed as a percentage of control. Data are expressed as the mean ± SEM of 6 independent experiments. **p <0.01.
B. MIA PaCa-2 cells treated with a combination of TRAIL and triptolide were evaluated for presence of cytosolic cathepsin B using confocal microscopy. Cathepsin (green) colocalizes with lysosomal (lysotracker red) in a punctuate fashion in control cells, suggesting intra-lysosomal location. The nuclei have been stained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). Treatment with TRAIL and triptolide results in diffused staining of cathepsin B and reduced co-localization with the lysosome, suggesting release of cathepsin B into the cytosol. Scale bar, 10 micron/L.
C. MIA PaCa-2 cells treated with the cathepsin B specific inhibitor, CA-074me, show a significant decrease in cathepsin B activity (left) and an increase in cell viability (right) when treated with TRAIL and triptolide. Data are expressed as the mean ± SEM of three independent experiments. *p < 0.01.
D. MIA PaCa-2 cells treated with a cathepsin B specific siRNA show a significant decrease in cathepsin B expression (left) and an increase in cell viability (right) when treated with TRAIL and triptolide. Data are expressed as the mean ± SEM of 3 independent. *p < 0.01.
E. MIA PaCa-2 cells treated with a caspase-8 inhibitor, Z-IETD-FMK and TRAIL and triptolide for 24 hours show a significant decrease in Cathepsin B activation when compared to control untreated cells. The bars represent mean ± SEM, n≥3. **p < 0.01.
Once we had established that cathepsin B is released into the cytosol upon treatment of cells with a combination of TRAIL and triptolide, we further investigated if this release was responsible for combination treatment-induced cell death. For this purpose, we down-regulated cathepsin B using either the Cathepsin B inhibitor (CA-074me) or cathepsin B- specific siRNA in pancreatic cancer cells. We confirmed that the Cathepsin B inhibitor was functional by measurement of Cathepsin B activity in the absence (823.32 ± 73.1 %) and presence (12.8 ± 4.6 %) of inhibitor, and siRNA-mediated loss of expression by western blot. We found that down-regulation of Cathepsin B by CA-074me or siRNA significantly reduced cell death induced by TRAIL/triptolide in MIA PaCa-2 cells (p<0.01) (Figure 4C/4D). We further demonstrated that cytosolic cathepsin B activity is significantly down-regulated in the presence of the caspase-8 inhibitor (Z-IETD-FMK) in MIA PaCa-2 cells treated with a combination of TRAIL and triptolide.
Our data, taken together, suggest that the combination of TRAIL and triptolide causes caspase-8 dependent lysosomal membrane permeabilization, which leads to pancreatic cancer cell death.
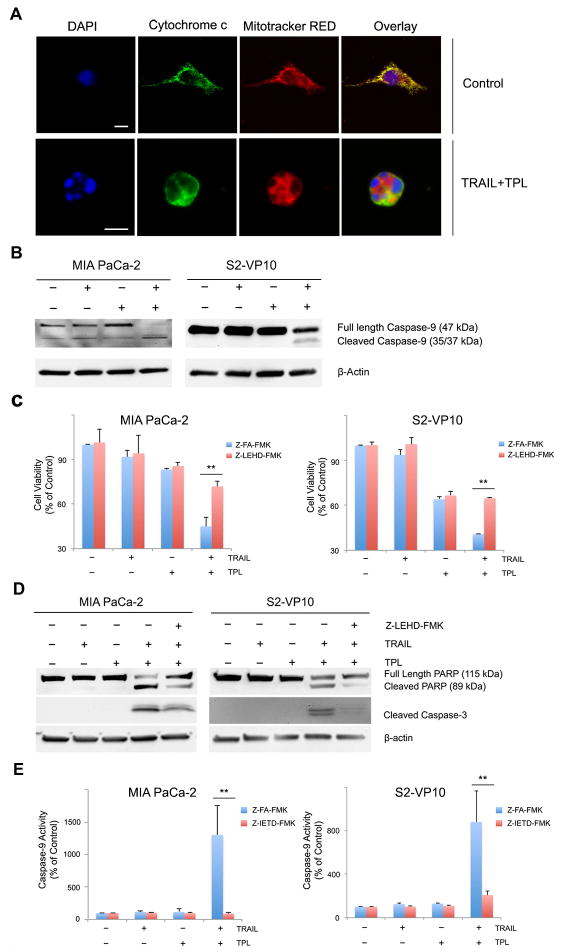
3.5 Mitochondrial outer membrane permeabilization is required for TRAIL/triptolide induced apoptosis
Mitochondrial outer membrane permeabilization (MOMP) is an important component of the apoptotic cell death mechanism [13]. Since inhibition of cathepsin B was unable to completely rescue cells from TRAIL/triptolide mediated cell death, we further tested if MOMP was involved in TRAIL/triptolide-induced apoptosis.
Using release of mitochondrial membrane bound Cytochrome c as a measure of MOMP, we observed Cytochrome c (green) co-localization with Mitotracker Red (red) in untreated MIA PaCa-2 pancreatic cancer cells, represented as a yellow color in the overlay images (Fig. 5A). However, Cytochrome c release from the mitochondria to the cytosol was only observed in cells treated with the combination of TRAIL and triptolide. In agreement with this observation, activation of caspase-9, an event that occurs downstream from MOMP, was present only in cells treated with a combination of TRAIL and triptolide in both MIA PaCa-2 and S2-VP10 cells (Fig. 5B). This finding is also consistent with our previously published data [3]. The importance of MOMP was underscored by inhibition of caspase-9 activity using a caspase-9 specific inhibitor (Z-LEHD-FMK). Cells treated with this inhibitor together with a combination of TRAIL and triptolide showed a significant increase in cell viability (71.85 ± 3.55%) compared to those not treated with inhibitor (45.04 ± 6.224 %) (p<0.01) (Figure 5C). Further analysis of events downstream from death receptor pathway activation showed a decrease in cleavage of both PARP and caspase-3 (Figure 5D) in the presence of the caspase-9 inhibitor in both MIA PaCa-2 and S2-VP10 cells suggesting that caspase-9, a component of MOMP mediated apoptosis, regulates caspase-3 and its downstream targets. Since caspase-8 is also involved in MOMP mediated cell death, we further explored the relationship between caspase-8 activation and the mitochondrial apoptotic pathway by inhibiting caspase-8. Cells treated with caspase-8 inhibitor showed a decrease in TRAIL/triptolide mediated caspase-9 activity in both MIA PaCa-2 (91 ± 14.3% vs. 1302.8 ± 458 % in the absence of inhibitor) and S2-VP10 cells (208 ± 38.89% vs. 881.5 ± 283.6 % in the absence of inhibitor) (Figure 5E).
Figure 5. Combination of TRAIL and triptolide induces mitochondrial membrane permeabilization in pancreatic cancer cells.
A. MIA PaCa-2 cells treated with a combination of TRAIL and triptolide for 24h show release of cytochrome c from mitochondrial into the cytosol as evaluated by confocal microscopy. Cytochrome c (green) colocalizes with mitochondrial (mitochondrial red) in a punctuate fashion in control cells, suggesting intra-mitochondrial location. The nuclei have been stained with DAPI (blue). Scale bar, 10 micron/L.
B. MIA PaCa-2 and S2-VP10 cells treated with either TRAIL or triptolide or a combination of both for 24h show cleavage of caspase-9 as evaluated by western blot. β-actin expression was used a loading control.
C. Treatment of MIA PaCa-2 and S2-VP10 cells with caspase-9 inhibitor (Z-LEHD-FMK) leads to rescue of cells from TRAIL/triptolide mediated cell death compared to those treated with a negative control. The bars represent mean ± SEM, n≥3. **p<0.01.
D. PARP and caspase-3 cleavage was assessed in samples treated as described in (C), using western blots. β-actin expression was used as loading control.
E. Treatment of MIA PaCa-2 and S2-VP10 cells with caspase-8 inhibitor (Z- IETD-FMK) leads to decrease in TAIL/triptolide mediated caspase-9 activation compared to those treated with a negative control. The bars represent mean ± SEM, n≥3. **p<0.01.
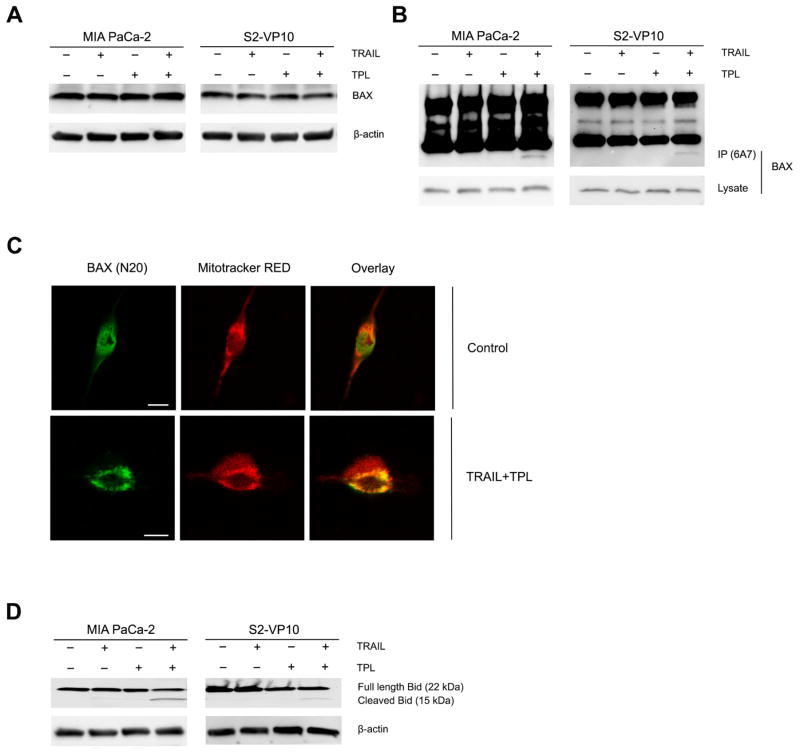
3.6 BAX activation and Bid cleavage is involved in TRAIL/triptolide induced apoptosis
Previous studies show that under certain stimuli, the pro-apoptotic Bcl-2 family protein BAX undergoes a conformational change, detected by an active form-specific antibody (6A7) [5, 37, 38]. Upon treatment of either MIA PaCa-2 or S2-VP10 cells with a combination of TRAIL and triptolide, there was no change in the levels of BAX protein (Fig. 6A), but there was an increase in the active form of BAX. (Fig. 6B). In order to establish that BAX activation was a downstream event linked to the activation of the death receptor pathway, cells were treated with caspase-8 siRNA prior to TRAIL/triptolide treatment. BAX activation was observed in cells containing non-silencing siRNA but not those with caspase-8 siRNA (Supplemental Figure 3A).
Figure 6. Pancreatic cancer cells treated with either TRAIL or triptolide or their combination induce a conformational change in BAX and cleavage of Bid.
A. Total BAX expression was detected by western blot with anti-BAX (N20) antibody in MIA PaCa-2 and S2-VP10 cells treated with TRAIL or triptolide or both. β-actin expression was used as loading control.
B. Conformational change in BAX protein was detected by western blot with anti-BAX (6A7) antibody in MIA PaCa-2 and S2-VP10 cells treated with TRAIL or triptolide or both. β-actin expression was used as loading control.
C. Mitochondrial translocation of BAX was evaluated with confocal microscopy. BAX (N20) (green) colocalizes with mitochondrial (MitoTracker red) in TRAIL/triptolide treated MIA PaCa-2 cells, suggesting a translocation of BAX from cytosol to the mitochondria. Scale bar, 10 micron/L.
D. Bid cleavage was evaluated by western blot with anti-Bid antibody in MIA PaCa-2 and S2-VP10 cells treated with TRAIL or triptolide or both. β-actin expression was used as loading control.
We further examined BAX localization using confocal microscopy. As shown in Figure 6C, BAX was located predominantly in cytosol in untreated MIA PaCa-2 cells. Incubation of cells with a combination of TRAIL and triptolide resulted in colocalization of BAX with mitochondria. Our biochemical and microscopy data support the conclusion that a combination of TRAIL and triptolide leads to a conformational change in BAX and its translocation into the mitochondria.
In order to investigate the mechanism by which BAX is activated during apoptotic events we assessed the cleavage of Bid, a pro-apototic protein known to trigger BAX activation [2, 34]. As shown in Figure 6D, cleavage of Bid was observed in both MIA PaCa-2 and S2-VP10 cells treated with the combination of TRAIL and triptolide. Since activated caspase-8 amplifies the death receptor signal by cleaving Bid [11, 24], we further investigated Bid cleavage in the presence of the caspase-8 inhibitor and found it to be significantly inhibited, suggesting that Bid cleavage is a consequence of the activation of the death receptor pathway (Supplemental Figure 3B).
4. Discussion
Pancreatic cancer, the fourth leading cause of cancer related deaths, has a five-year survival of less than 5% [19]. Resistance to conventional chemotherapy remains a significant obstacle in long-term survival of pancreatic cancer patients. We have previously shown that triptolide, and its water soluble pro-drug, Minnelide, induces pancreatic cancer cell death in vitro. Minnelide also causes tumor regression and prevents tumor progression in several preclinical mouse models [28, 29]. We have also previously shown that low dose of triptolide in combination with TRAIL induces significant pancreatic cancer cell death [3]. However, the mechanism of TRAIL/triptolide combination induced pancreatic cancer cell death remains unclear.
The focus of the present study is therefore to elucidate the mechanism by which triptolide sensitizes pancreatic cancer cells to TRAIL. This is the first study, to our knowledge, that has shown the mechanism of caspase-8 activation in triptolide/TRAIL mediated cell death, thereby suggesting that low doses of triptolide allow TRAIL to activate the death receptor pathways. Since inhibition of caspase-8 prevented TRAIL mediated cell death, we conclude that the death receptor pathway plays a key role in TRAIL/triptolide induced apoptosis.
Initiation of the death receptor pathway occurs when TRAIL binds to the death receptors DR4 and DR5, causing oligomerization of the receptors [15], leading to the recruitment of FADD, followed by binding of procaspase-8, thereby forming the DISC [16]. Cleavage of DISC associated pro-caspase-8 leads to activation of downstream events, and finally, cell death. Since inhibition of caspase-8 prevented TRAIL/triptolide mediated cell death, we hypothesized that the effect of triptolide on TRAIL mediated cell death occurred upstream of caspase-8 activation. We therefore studied the effect of triptolide on levels of DR4 and DR5 and found that DR5, but not DR4, was up-regulated by triptolide in pancreatic cancer cells (Figure 2A), which contributed to the activation of caspase-8 (Figure 2E). Furthermore, an important modulator of caspase-8, c-FLIP, plays a key role in resistance to death receptor-mediated apoptosis in many cancer cells. c-FLIP is over-expressed in pancreatic cancer tissue compared to normal pancreatic ducts [12]. We therefore evaluated the effect of triptolide on c-FLIP and found that triptolide down-regulates c-FLIP in pancreatic cancer cells (Figure 3A). This down-regulation of c-FLIP alone allowed TRAIL to induce caspase-8 activation (Figure 3E).
In the presence of a low dose of TRAIL, the effect of triptolide on c-FLIP and DR5 becomes necessary for the death receptor pathway to result in caspase-8 activation. Previous studies from our lab and others have shown that TRAIL or triptolide induces LMP in cancer cells [1, 36]. In this study, we found that only the combination of TRAIL and triptolide, but neither treatment alone induces LMP in pancreatic cancer cells (Figure 4A/4B). Loss of cathepsin B was able to partially decrease the effect of the TRAIL/triptolide combination on cell death (Figure 4C/4D), which further confirmed that LMP contributes to TRAIL/triptolide induced pancreatic cancer cell death. Further studies also show that TRAIL/triptolide combination induced LMP was caspase-8 dependent in pancreatic cancer cells (Figure 4E).
Inhibition of Cathepsin B did not completely rescue cells from TRAIL/triptolide induced cell death, suggesting that LMP may only be one of the players in causing TRAIL/triptolide mediated apoptosis [7, 28]. Studies from our group and others show that both triptolide and TRAIL independently have an effect on MOMP [7]. Here we found that low doses of TRAIL and triptolide were able to induce Cytochrome c release and caspase-9 activation, suggesting that mitochondrial events are also required to engage the apoptotic program (Figure 5A/5B). Importantly, inhibition of caspase-9 rescued TRAIL/triptolide induced pancreatic cancer cell death (Figure 5C). Furthermore, down-regulation of caspase-8 inhibits TRAIL/triptolide mediated caspase-9 activation; indicating caspase-8 is up-stream of the mitochondrial apoptotic pathway (Figure 5E).
Previous studies show that the pro-apoptotic Bcl-2 family member, Bid, is the link between the death receptor and mitochondrial apoptotic pathways [40], and initiation of mitochondrial dysfunction requires BAX/BAK activation [21]. Activation and oligomerization of BAX results in permeabilization of the mitochondrial membrane [20], inducing cytochrome c release [39]. In accordance with these observations, we observed Bid cleavage and BAX translocation in response to TRAIL/triptolide (Figure 6D).
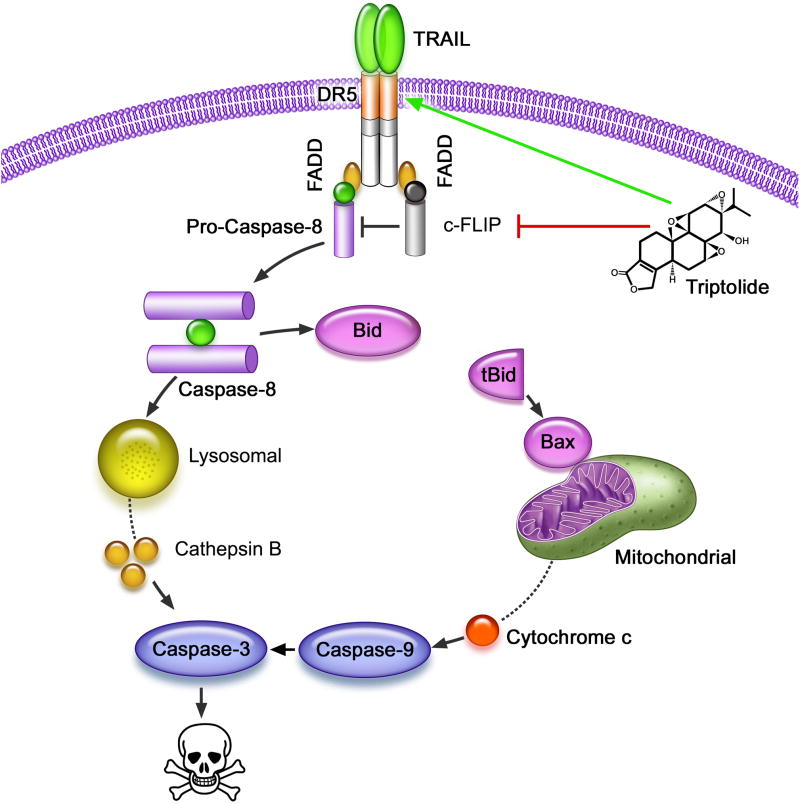
In conclusion, in this study we have shown that triptolide mediated modulation of DR5 and c-FLIP expression contributes to activation of the death receptor pathway as evidenced by caspase-8 activation in pancreatic cancer cells. Collectively, our data show that low doses of triptolide sensitize cancer cells to TRAIL mediated death by various mechanisms (Figure 7). Since both TRAIL and triptolide are already being tested in the clinic, the understanding of TRAIL and triptolide-induced pancreatic cancer cell death holds important implications for the translation of this combination into the clinic.
Figure 7. Schematic diagram of the mechanism by which a combination of TRAIL and triptolide cause cell death in pancreatic cancer cells.
Triptolide treatment sensitizes cells to TRAIL-induced death by down-regulation of c-FLIP and up-regulation of DR5. Finally, in the presence of triptolide, low concentration of TRAIL activated the death receptor pathway, resulting in LMP and MOMP mediated pancreatic cancer cell death.
Supplementary Material
Supplemental Figure 1. Combination of TRAIL and triptolide decreases viability and induces apoptosis in pancreatic cancer cells. MIA PaCa-2 and S2-VP10 cells were treated with either TRAIL or triptolide or a combination of both for 24h and assessed for cell viability and apoptotic markers. To assess the effect of the treatment of TRAIL and triptolide on non-malignant cells, primary mouse epithelial cells or human pancreatic ductal epithelial cells (HPDEC) were treated as indicated and assessed for cell viability.
A. Cell viability. Bars represent mean ± SEM, n≥3. **p< .01.
B. PARP and caspase-3 cleavage was assessed using western blots. β-actin expression was used as loading control.
C. Cell viability. Bars represent mean ± SEM, n≥3.
Supplemental Figure 2. The purity of cytosolic and pellet fractions was tested by immunoblotting protein lysates from the supernantant or pellet with the cytosolic marker, GAPDH, or the lysosomal markers, LMAP1/2.
Supplemental Figure 3. MIA PaCa-2 cells treated with a combination of TRAIL and triptolide for 24h either in the presence or absence of caspase-8 inhibitor (Z-IETD-FMK, 40 μM) show,
A. The BAX conformational change was detected by western blot with anti-BAX (6A7) antibody. Treatment with specific caspase-8 inhibitor (Z-IETD-FMK, 40μM) shows a significant decrease of active BAX expression. β-actin expression was used as loading control.
B. Bid cleavage was evaluated by western blot with anti-Bid antibody. Treatment with specific caspase-8 inhibitor shows a significant decrease of Bid cleavage. β-actin expression was used as loading control.
Acknowledgments
Funding: Masonic Cancer Center, the Department of Surgery, Pancreatic Cancer SPORE grant to the University of Minnesota and University of Alabama, and the National Institute of Health grants R01CA124723, R01 CA170496 (to AKS), the Katherine and Robert Goodale Foundation and the Hirshberg Foundation to AKS.
Abbreviations
- TRAIL
Tumor necrosis factor related apoptosis-inducing ligand
- LMP
Lysosomal Membrane Permeabilization
- MOMP
Mitochondrial Outer Membrane Permeabilization
Footnotes
Conflict of Interest Statement: Nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhiyu Chen, Email: chenz@umn.edu.
Veena Sangwan, Email: sang0001@umn.edu.
Sulagna Banerjee, Email: sbanerje@umn.edu.
Rohit Chugh, Email: chugh012@umn.edu.
Vikas Dudeja, Email: dudej001@umn.edu.
Selwyn M. Vickers, Email: Vickers@umn.edu.
Ashok K. Saluja, Email: asaluja@umn.edu.
References
- 1.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer research. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 2.Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, Bordignon E. Molecular details of Bax activation, oligomerization, and membrane insertion. The Journal of biological chemistry. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borja-Cacho D, Yokoyama Y, Chugh RK, Mujumdar NR, Dudeja V, Clawson KA, Dawra RK, Saluja AK, Vickers SM. TRAIL and triptolide: an effective combination that induces apoptosis in pancreatic cancer cells. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2010;14:252–260. doi: 10.1007/s11605-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Gao N, Zhang Z, Chen G, Budhraja A, Ke Z, Son YO, Wang X, Luo J, Shi X. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of Bax. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:5679–5691. doi: 10.1158/1078-0432.CCR-10-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirman T, Oresic K, Mazovec GD, Turk V, Reed JC, Myers RM, Salvesen GS, Turk B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. The Journal of biological chemistry. 2004;279:3578–3587. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- 7.Crowder RN, El-Deiry WS. Caspase-8 regulation of TRAIL-mediated cell death. Experimental oncology. 2012;34:160–164. [PubMed] [Google Scholar]
- 8.de Vries EG, Gietema JA, de Jong S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:2390–2393. doi: 10.1158/1078-0432.CCR-06-0352. [DOI] [PubMed] [Google Scholar]
- 9.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–1782. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Gu Q, Wang JD, Xia HH, Lin MC, He H, Zou B, Tu SP, Yang Y, Liu XG, Lam SK, Wong WM, Chan AO, Yuen MF, Kung HF, Wong BC. Activation of the caspase-8/Bid and Bax pathways in aspirin-induced apoptosis in gastric cancer. Carcinogenesis. 2005;26:541–546. doi: 10.1093/carcin/bgh345. [DOI] [PubMed] [Google Scholar]
- 12.Haag C, Stadel D, Zhou S, Bachem MG, Moller P, Debatin KM, Fulda S. Identification of c-FLIP(L) and c-FLIP(S) as critical regulators of death receptor-induced apoptosis in pancreatic cancer cells. Gut. 2011;60:225–237. doi: 10.1136/gut.2009.202325. [DOI] [PubMed] [Google Scholar]
- 13.Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. doi: 10.1038/sj.onc.1207534. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo M. Pancreatic cancer. The New England journal of medicine. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Sheikh MS. TRAIL death receptors and cancer therapeutics. Toxicology and applied pharmacology. 2007;224:284–289. doi: 10.1016/j.taap.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Molecular cell. 2009;35:265–279. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 18.Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K, Ollinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis: an international journal on programmed cell death. 2010;15:527–540. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleeff J, Michalski C, Friess H, Buchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111–118. doi: 10.1097/01.mpa.0000229010.62538.f2. [DOI] [PubMed] [Google Scholar]
- 20.Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR, Newmeyer DD. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. The Journal of cell biology. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell death and differentiation. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 22.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Molecular and cellular biology. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavrik I, Golks A, Krammer PH. Death receptor signaling. Journal of cell science. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 25.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 26.Mc Guire C, Beyaert R, van Loo G. Death receptor signalling in central nervous system inflammation and demyelination. Trends in neurosciences. 2011;34:619–628. doi: 10.1016/j.tins.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 27.McDonald JK, Ellis S. On the substrate specificity of cathepsins B1 and B2 including a new fluorogenic substrate for cathepsin B1. Life sciences. 1975;17:1269–1276. doi: 10.1016/0024-3205(75)90137-x. [DOI] [PubMed] [Google Scholar]
- 28.Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM, Saluja AK. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139:598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer research. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 30.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. The Journal of biological chemistry. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 31.Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Current biology: CB. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- 32.Safa AR, Pollok KE. Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy. Cancers. 2011;3:1639–1671. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 34.Sobhan PK, Seervi M, Deb L, Varghese S, Soman A, Joseph J, Mathew KA, Raghu G, Thomas G, SEMSSKR Calpain and reactive oxygen species targets Bax for mitochondrial permeabilisation and caspase activation in zerumbone induced apoptosis. PloS one. 2013;8:e59350. doi: 10.1371/journal.pone.0059350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G. Mitochondrial release of caspase-2 and -9 during the apoptotic process. The Journal of experimental medicine. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. The Journal of biological chemistry. 2007;282:28960–28970. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 37.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. The Journal of cell biology. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi H, Bhalla K, Wang HG. Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer research. 2003;63:1483–1489. [PubMed] [Google Scholar]
- 39.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 40.Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell research. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Combination of TRAIL and triptolide decreases viability and induces apoptosis in pancreatic cancer cells. MIA PaCa-2 and S2-VP10 cells were treated with either TRAIL or triptolide or a combination of both for 24h and assessed for cell viability and apoptotic markers. To assess the effect of the treatment of TRAIL and triptolide on non-malignant cells, primary mouse epithelial cells or human pancreatic ductal epithelial cells (HPDEC) were treated as indicated and assessed for cell viability.
A. Cell viability. Bars represent mean ± SEM, n≥3. **p< .01.
B. PARP and caspase-3 cleavage was assessed using western blots. β-actin expression was used as loading control.
C. Cell viability. Bars represent mean ± SEM, n≥3.
Supplemental Figure 2. The purity of cytosolic and pellet fractions was tested by immunoblotting protein lysates from the supernantant or pellet with the cytosolic marker, GAPDH, or the lysosomal markers, LMAP1/2.
Supplemental Figure 3. MIA PaCa-2 cells treated with a combination of TRAIL and triptolide for 24h either in the presence or absence of caspase-8 inhibitor (Z-IETD-FMK, 40 μM) show,
A. The BAX conformational change was detected by western blot with anti-BAX (6A7) antibody. Treatment with specific caspase-8 inhibitor (Z-IETD-FMK, 40μM) shows a significant decrease of active BAX expression. β-actin expression was used as loading control.
B. Bid cleavage was evaluated by western blot with anti-Bid antibody. Treatment with specific caspase-8 inhibitor shows a significant decrease of Bid cleavage. β-actin expression was used as loading control.