Abstract
Purpose
Non-small-cell lung cancer (NSCLC) is the primary cause of cancer-related death in Western countries. One important approach taken to address this problem is the development of effective chemoprevention strategies. In this study, we examined whether the cyclooxygenase-2 (COX-2) inhibitor celecoxib, as evidenced by decreased cell proliferation, is biologically active in the bronchial epithelium of current and former smokers.
Patients and Methods
Current or former smokers with at least a 20 pack-year (pack-year = number of packs of cigarettes per day times number of years smoked) smoking history were randomized into one of four treatment arms (3-month intervals of celecoxib then placebo, celecoxib then celecoxib, placebo then celecoxib, or placebo then placebo) and underwent bronchoscopies with biopsies at baseline, 3 months, and 6 months. The 204 patients were primarily (79.4%) current smokers; 81 received either low-dose celecoxib or placebo and 123 received either high-dose celecoxib or placebo. Celecoxib was originally administered orally at 200 mg twice daily and the protocol subsequently increased the dose to 400 mg twice daily. The primary endpoint was change in Ki-67 labeling (from baseline to 3 months) in bronchial epithelium.
Results
No cardiac toxicities were observed in the participants. Although the effect of low-dose treatment was not significant, high-dose celecoxib decreased Ki-67 labeling by 3.85% in former smokers and by 1.10% in current smokers—a significantly greater reduction (P = 0.02) than that seen with placebo after adjusting for metaplasia and smoking status.
Conclusion
A 3–6-month celecoxib regimen proved safe to administer. Celecoxib 400 mg bid was biologically active in the bronchial epithelium of current and former smokers; additional studies on the efficacy of celecoxib in NSCLC chemoprevention may be warranted.
INTRODUCTION
Non-small-cell lung cancer (NSCLC) is the leading cause of death from cancer among both men and women in the United States, accounting for approximately 28% of such deaths. Indeed, an estimated 160,000 Americans died of NSCLC in 2007. In recent years, the incidence of NSCLC has begun to decline among men (1). However, smoking-related NSCLC has continued to increase among women, surpassing even breast cancer as the leading cause of cancer death in this group (2). Despite aggressive treatment strategies, the 5-year survival rate for NSCLC remains only about 15% (1). These grim facts underscore the urgent need for a change in our approach to NSCLC.
Smoking prevention and cessation have been emphasized as ways to reduce deaths from cancer. Despite the reduction in NSCLC risk observed with smoking cessation, however, several studies have demonstrated that former smokers still have a higher NSCLC risk than nonsmokers have (3–7) and consequently account for a large proportion of NSCLC patients in this country. Chemoprevention strategies, especially for high-risk populations such as former smokers, are appropriate in NSCLC. However, large-scale NSCLC chemoprevention trials, including the Alpha-Tocopherol Beta-Carotene trial (ATBC), Beta-Carotene and Retinol Efficacy Trial (CARET), and Lung Intergroup Trial (LIT), have yet to show that any agent can reduce lung cancer risk (8–11).
One of the changes identified in premalignant bronchial tissues that has potential therapeutic significance is an increase in expression of cyclooxygenase-2 (COX-2). COX-2 converts arachidonic acid to prostaglandin H2, a precursor of prostaglandin E2 that has been implicated in a variety of biochemical processes, required for cell proliferation and survival, and whose expression increases in response to growth factors, oncogenes, and carcinogens (12–18). COX-2 has been extensively studied in epithelial tumors, including colorectal cancer and NSCLC (19–22). COX-2 overexpression has prognostic value, predicting a worse outcome in NSCLC patients with stage I disease whose tumors have been surgically resected (23, 24) and thus suggesting that COX-2 is an important biologic determinant in NSCLC. Tellingly, COX-2 expression is higher in bronchial premalignant lesions than in adjacent normal lung tissue (22, 25), raising the possibility that COX-2 promotes malignant progression in the lung. Moreover, COX-2 inhibitors have demonstrated efficacy as NSCLC chemopreventive agents in preclinical studies, reducing the size and number of carcinogen-induced NSCLC tumors in mice (26). These findings provide a compelling rationale to investigate the activity of COX-2 inhibitors as chemopreventive agents for lung cancer.
In this study, our goal was to determine whether a 6-month treatment with celecoxib, a COX-2 inhibitor, would be safe and have biologic activity in the lungs of current and former smokers. We performed a randomized, placebo-controlled study to examine the toxicity and efficacy of celecoxib; bronchial epithelial cell proliferation, as +measured by the Ki-67 labeling index after 3 months, was the primary endpoint. We chose this primary endpoint on the basis of evidence that bronchial premalignant lesions increase epithelial cellular proliferation and that COX-2 promotes cellular proliferation and survival (27–29).
PATIENTS AND METHODS
For the present study, we recruited current smokers (those actively smoking or those who had quit within the previous 12 months) and former smokers (those who had quit at least 12 months’ prior to study entry) who had at least a 20 pack-year history of smoking (pack-year = number of packs of cigarettes per day times number of years smoked). Patients could have had prior stage I NSCLC or stage I or II laryngeal cancer but had to have been free of disease for at least 6 months’ prior to study entry. Other exclusion criteria included the chronic use of steroids, the use of H2 blockers for active ulcers, the use of nonsteroidal anti-inflammatory drugs other than low-dose aspirin of ≤ 81 mg/day, and a history of stroke, uncontrolled hypertension, and/or angina pectoris. Patients were recruited through local community groups, health fairs, and advertisements distributed to referring practitioners and patients at The University of Texas M. D. Anderson Cancer Center. The study was approved by the Institutional Review Board (IRB) and by the U. S. Department of Health and Human Services. All patients provided written informed consent before entering the study.
Trial Design and Treatment
The clinical study design was a four-arm, double-blind, placebo-controlled, randomized study to evaluate the biological effects of celecoxib as a chemopreventive agent in current and former smokers. The primary endpoint was modulation of Ki-67 in the bronchial epithelium after a 3-month period of treatment. Patients were treated for up to 6 months and were randomized onto one of four treatment arms: celecoxib daily for 3 months, then placebo daily for 3 months (CCX + PCB); celecoxib daily for 3 months, then celecoxib daily for 3 months (CCX + CCX); placebo daily for 3 months, then celecoxib daily for 3 months, (PCB + CCX); and placebo daily for 3 months, then placebo daily for 3 months, (PCB + PCB). The research pharmacy randomly assigned each patient to one of the four treatment arms and recorded this assignment by using a computer-generated treatment code that was available only to the pharmacist. Pfizer Corporation (New York, NY) provided the 200-mg celecoxib capsules and the matching placebo capsules.
After a bronchoscopy at 3 months, patients received treatment for an additional 3 months. A bronchoscopy was then performed at the 6-month time point.
Upon completing informed consent and enrollment, patients were screened with a chest x-ray and bronchoscopy, which included bronchial washings, brushings, and biopsies from 6 predetermined sites (carina, right lower, middle, and upper lobes and left lower and upper lobe regions) as well as from any abnormal sites suspicious for cancer. Metaplasia indices (MI) were calculated from the biopsies performed at the predetermined sites. The presence of dysplasia was confirmed by histologic evaluation of all biopsy samples. Patients with severe dysplasia at initial or subsequent bronchoscopy were strongly encouraged to undergo additional bronchoscopies at 6-months.
Patients were stratified for randomization according to smoking history (current vs. former), prior cancer (prior vs. no prior), and metaplasia index (MI; <15% vs. ≥15% and/or dysplasia). Toxicity was monitored utilizing the National Cancer Institute Common Toxicity Criteria 2.0, and patients who experienced grade 2 or higher toxicity had their dose reduced. Clinic visits occurred before treatment and during treatment at 1-month intervals. A complete physical examination, which included asking about the patient’s relevant medical history and history of tobacco and alcohol exposure, was performed at each clinic visit. Patients were referred to smoking cessation programs upon request.
Celecoxib Dose
In the original study design, celecoxib was to be administered at 200 mg twice daily. At a scheduled External Advisory Board (EAB) meeting, the committee raised the concern that in a recently completed colon polyp prevention study (20), a 100-mg dose of celecoxib did not differ from placebo in terms of polyp reduction. On the basis of this updated data, the EAB recommended a higher dose of celecoxib (400 mg). Therefore, the starting dose level, effective December 2003 (after 81 patients had been enrolled at the low-dose celecoxib level), was set at 400 mg for new patients randomized to receive celecoxib. The first subject was randomized using the new schedule on January 23, 2004.
On December 17, 2004, reports of cardiovascular toxicity in other COX-2 inhibitor trials were released (30–35). At that point, a total of 150 patients had been registered on the current study and 143 had been randomized to treatment. New subject entry and celecoxib treatment were put on hold by the study’s principal investigator and The M. D. Anderson Cancer Center IRB. During the subsequent months, efforts were made to follow-up with participants, audit clinical data and the laboratory specimen inventories, modify the eligibility criteria to exclude patients with preexisting cardiovascular conditions, and include additional procedures to screen and monitor for cardiovascular toxicities. The protocol was amended to address cardiovascular safety issues to ensure the safety of study patients. The M. D. Anderson IRB approved the amended protocol, the study was reactivated in April 2005, and we began registering new patients; seven of the patients whose treatment was put on hold reentered the trial. We stopped patient accrual for this trial as of January 2007.
Biopsy Specimens
Per the protocol, patients underwent bronchoscopies at the time of enrollment prior to randomization. All evaluable study patients then had repeat bronchoscopies with biopsies, brushings, and washes at the completion of the first stage of treatment (3 months) and again at 6 months. Biopsy, brushing, and wash samples were obtained from the same predetermined sites sampled in the initial bronchoscopy. As noted, these biopsy specimens were taken at six predetermined sites in the bronchial tree: the main carina, the bifurcation of the right upper lobe and the main stem bronchus, the bifurcation of the right middle lobe and right lower lobe, the bifurcation of the left upper lobe and lingula, the medial bronchus of the right lower lobe, and the anterior bronchus of the left lower lobe. We fixed the biopsy specimens in 10% buffered formalin, embedded them in paraffin, and sectioned them. The first two 4-µm tissue sections from each biopsy site were stained with hematoxylin and eosin and evaluated for the presence of squamous metaplasia and dysplasia. We performed histologic assessments to determine whether the MI had changed during the 3-month period. The MI was calculated as the percentage of biopsy sections exhibiting squamous metaplasia out of the total number of sections examined. A single pathologist (X.T.) who was blinded to the study treatment served as the reference pathologist.
We cytologically analyzed sputum samples acquired by sputum induction from all patients prior to treatment and after 3 and 6 months’ of treatment. Additionally, we performed buccal brushings on all patients prior to treatment and after 3 and 6 months of treatment to look for evidence of tobacco-induced histologic and genetic alterations.
Immunohistochemical Analysis of Ki-67
We calculated the fraction of Ki-67–positive cells in the bronchial epithelium, including the basal, para basal, and superficial layers of the biopsy specimens. Ki-67 labeling indices were expressed as the percentage of cells with positive nuclear staining, as detailed in our prior reports (27, 28). Slides that lacked evaluable epithelium were excluded from the analyses. Ki-67 labeling indices were analyzed on a per-biopsy-site basis and on a per-subject basis (the average of all biopsy sites that could be evaluated from a participant at a particular time point.)
The immunohistochemical analysis was performed as follows: one 4-µm tissue section was deparaffinized in xylene and rehydrated through a series of alcohols. Peroxide blocking was done by immersing the section in 3% hydrogen peroxide in methanol for 15 minutes. Antigen retrieval was accomplished by placing slides in a steamer for 10 minutes with 10 mmol/L of sodium citrate (pH 6.0) and washing them in Tris buffer. The slides were then blocked in 10% fetal bovine serum for 35 minutes. The Ki-67 antibody used was MIB-1 (DAKO, Carpinteria, CA, USA), and incubation occurred at room temperature at 1:200 dilution for 65 minutes. Secondary antibody was provided and detection was performed using the DAKO Envision Link+ kit (DAKO) for 30 minutes. Diaminobenzidine chromogen was applied for 5 minutes. The slides were then counter stained with hematoxylin and topped with a cover slip. We used NSCLC cell line pallet sections that had been formalin fixed and paraffin embedded and that evidenced confirmed antigen expressions as the control cell lines
Statistical Design and Analysis
This study was designed as a randomized, double-blinded, placebo-controlled trial to evaluate the efficacy and toxicity of celecoxib as a chemopreventive agent in current and former smokers. The planned duration of treatment was a total of 6 months. The primary endpoint of the study was modulation of Ki-67, measured after a 3-month treatment intervention. The secondary endpoint of the study was the change in Ki-67 labeling at 6 months. The Stratified Z-test was applied for comparing the reduction of Ki-67 from baseline to 3 months between the treatment and placebo groups. The target number of randomized and evaluable patients was 182, which would require a total of 216 randomized patients, allowing for a 15% dropout rate. The study design had at least 80% power to detect a 1.2% difference in the reduction of Ki-67 between the COX-2 inhibitor and placebo, with a two-sided 5% level of significance.
Summary statistics, including frequency, tabulation, mean (and standard deviation), and median (and range), were used to characterize the distribution of Ki-67 labeling indices in the basal layer, para basal layer, and all layers. The mean Ki-67 index across all six potential biopsy sites was computed with the patient used as the analysis unit. The Wilcoxon rank sum test was used to compare continuous variables between two groups. The Kruskal–Wallis test was used to compare continuous variables among three groups. The chi-square test or the Fisher’s exact test was used to test the statistical significance of the association between two categorical variables. The Wilcoxon signed-rank test was used to test changes in Ki-67 labeling indices by patient before and after treatment within each treatment group. To increase the efficiency of the statistical analysis, we also used the biopsy site as a unit of analysis, under the assumption that the site was nested within the patient.
For these analyses, we used a mixed-effect model to test the effect of treatment with celecoxib against placebo on Ki-67 labeling indices, adjusting for covariates that affect Ki-67 levels, such as number of years since smoking cessation (in categories), squamous metaplasia (presence or absence), treatment arm, and time point (0 or 3 months). When the mixed-effect model was used, a logarithmic transformation was applied to Ki-67 labeling indices to satisfy the Gaussian distribution assumption. All statistical tests were two-sided, with a 5% type I error rate. Statistical analysis was performed using standard statistical software, including SAS release 9.1 (SAS Institute, Cary, NC) and S-Plus version 7 (Insightful Inc, Seattle, WA).
RESULTS
Patient Characteristics
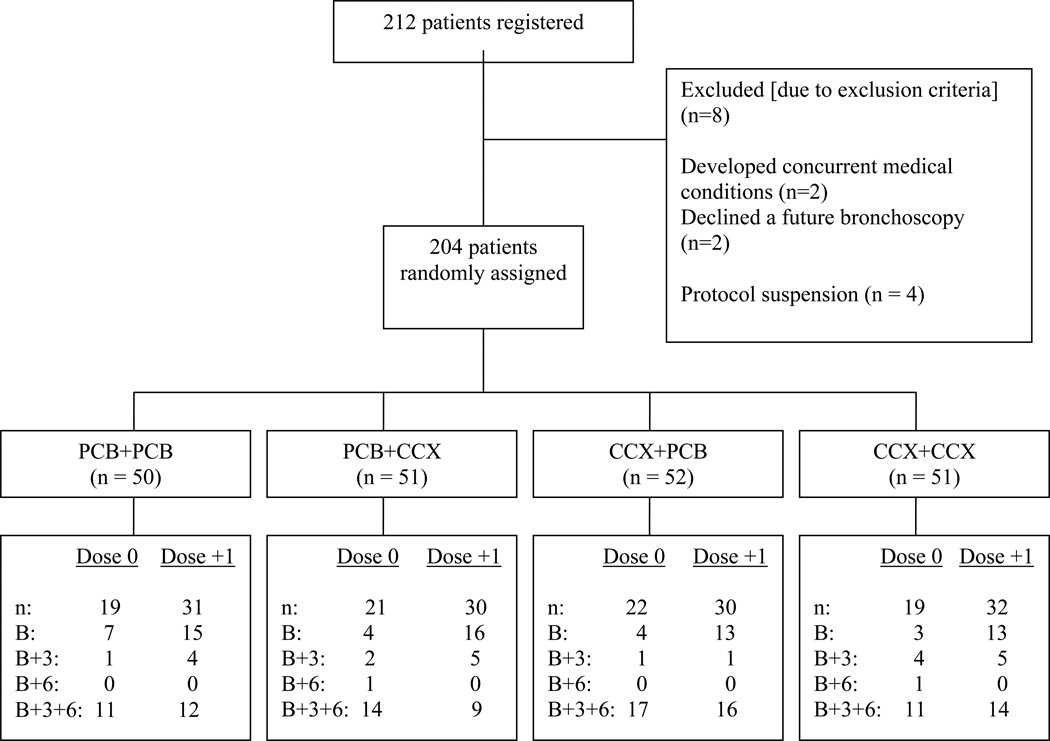
From November 2001 to September 2006, a total of 212 patients registered onto the study, with 204 patients randomized to treatment with either agent or placebo. Eight patients were not randomized to treatment arms; two patients because they declined bronchoscopies, two patients because they had concurrent medical conditions, and four patients because of the temporary protocol suspension on December 17, 2004.
Of the 204 patients randomized to study sections, 127 patients (61 receiving low-dose celecoxib and 66 receiving high-dose celecoxib) received baseline and 3-month bronchoscopies and thus had data evaluable for the primary endpoint analysis (Figure 1). There were 104 patients who received all three (the baseline, 3-month, and 6-month) bronchoscopies. The characteristics of the patients who were randomized to study sections are listed in Table 1. Although the treatment groups generally had similar characteristics, there were fewer women in the arm treated with PCB + CCX (P = 0.52), no hispanic patients in the CCX + PCB arm (P = 0.06), and less dysplasia at baseline in the CCX + PCB and PCB + CCX arms (P = 0.10).
Figure 1.
CONSORT flow diagram of subject accrual into the trial. Patients were randomized to receive the following daily for 3-month intervals: placebo then placebo (PCB + PCB); placebo then celecoxib (PCB + CCX); celecoxib then placebo (CCX + PCB); or celecoxib then celecoxib (CCX + CCX). Number of patients (n) who completed baseline (B), 3-month (B+3), and 6-month (B+6) evaluations are listed. Reasons for leaving the study are also listed.
Table 1.
Characteristics of Randomized Patients by Treatment Arm
| Characteristic | PCB+PCB | PCB+CCX | CCX+PCB | CCX+CCX | Total |
|---|---|---|---|---|---|
| Total Patients Treated | 50 | 51 | 52 | 51 | 204 |
| Age (years): | |||||
| Mean ± STD | 53.6 ± 7.9 | 52.5 ± 9.0 | 54.3 ± 8.4 | 53.0 ± 9.5 | 53.4 ± 8.7 |
| Median (Range) | 52.8 (39.6, 70.4) | 52.4 (32.9, 73.2) | 54.9 (39.9, 73.6) | 52.4 (32.0, 71.8) | 53.3 (32.0, 73.6) |
| Gender: | |||||
| Female | 25 (50.0%) | 20 (39.2%) | 26 (50.0%) | 27 (52.9%) | 98 (48.0%) |
| Male | 25 (50.0%) | 31 (60.8%) | 26 (50.0%) | 24 (47.1%) | 106 (52.0%) |
| Race: | |||||
| Black | 4 (8.9%) | 3 (5.9%) | 4 (7.7%) | 6 (11.8%) | 17 (8.3%) |
| Hispanic | 1 (2.0%) | 3 (5.9%) | 0 | 5 (9.8%) | 9 (4.4%) |
| White | 45 (90.0%) | 44 (86.3%) | 46 (88.5%) | 40 (78.4%) | 175 (85.8%) |
| Other | 0 | 1 (2.0%) | 2 (3.8%) | 0 | 3 (1.5%) |
| Cancer History: | |||||
| No | 46 (92.0%) | 45 (88.2%) | 43 (82.7%) | 48 (94.1%) | 182 (89.2%) |
| Yes | 4 (8.0%) | 6 (11.8%) | 9 (17.3%) | 3 (5.9%) | 22 (10.8%) |
| Smoking Related Cancer: | |||||
| No | 49 (98.0%) | 49 (96.1%) | 51 (98.1%) | 51 (100%) | 200 (98.0%) |
| Yes* | 1 (2.0%) | 2 (3.9%) | 1 (1.9 %) | 0 | 4 (2.0%) |
| Smoking Status: | |||||
| Former Smokers | 8 (16.0%) | 13 (25.5%) | 11 (21.2%) | 10 (19.6%) | 42 (20.6%) |
| Current Smokers | 42 (84.0%) | 38 (74.5%) | 41 (78.8%) | 41 (80.4%) | 162 (79.4%) |
| Pack-Years: | |||||
| Mean ± STD (Range) | |||||
| Former Smokers | 46 ± 24.4 (21.3, 85.2) | 39.8 ± 13.7 (20.6, 61.5) | 39.8 ± 18.7 (20.4, 73.9) | 43.6 ± 24.2 (20.0, 92.6) | 41.9 ± 19.4 (19.9, 92.6) |
| Current Smokers | 44.0± 14.7 (19.7, 81.5) | 38.4 ± 18.2 (20.8, 85.6) | 47.2 ± 17.7 (20.0, 87.9) | 45.0 ± 22.3 (21.3, 132.8) | 43.8 ± 18.5 (19.7, 132.8) |
| Smoking Quit Years: | |||||
| Mean ± STD (Range)** | 9.5± 7.2 (1.5, 18.1) | 7.2 ± 10.0 (1.0, 33.4) | 9.9 ± 9.6 (1.2, 35.3) | 9.0 ± 7.9 (1.3, 23.8) | 8.8± 8.7 (1.0, 35.3) |
| Metaplasia Index: | |||||
| Mean ± STD (Range) | |||||
| Former Smokers | 6.3 ± 12.4 (0, 33.3) | 2.6 ± 6.3 (0, 16.7) | 4.5 ± 7.8 (0, 16.7) | 10.0 ± 14.1 (0, 33.3) | 5.6 ± 10.2 (0, 33.3) |
| Current Smokers | 17.0 ± 24.3 (0, 83.3) | 14.9 ± 18.9 (0, 66.7) | 14.8 ± 19.3 (0, 66.7) | 14.0 ± 18.5 (0, 60.0) | 15.2 ± 20.3 (0, 83.3) |
| Dysplasia: | |||||
| No | 46 (92.0%) | 50 (98.0%) | 51 (98.1%) | 45 (88.2%) | 192 (94.1%) |
| Yes | 4 (8.0%) | 1 (2.0%) | 1 (1.9%) | 6 (11.8%) | 12 (5.9%) |
PCB: Placebo
CCX: Celecoxib
More patients than expected dropped out of the study because of the temporary protocol suspension, which may explain why the accrual goal of 182 patients with evaluable data was not reached. Common reasons for study dropout in the low-dose celecoxib (200 mg) group included personal reasons (10 patients), being lost to follow-up (8 patients), and concurrent medical conditions (5 patients).
During the protocol suspension, treatment was suspended for 29 patients, all of whom subsequently left the study. Four patients were randomized but never started the study drug because of safety concerns.
Common reasons for dropout in the high-dose celecoxib group (400 mg) included nonadherence as judged by pill counts (12 participants), having or developing concurrent medical conditions (10 participants), and personal reasons (5 participants).
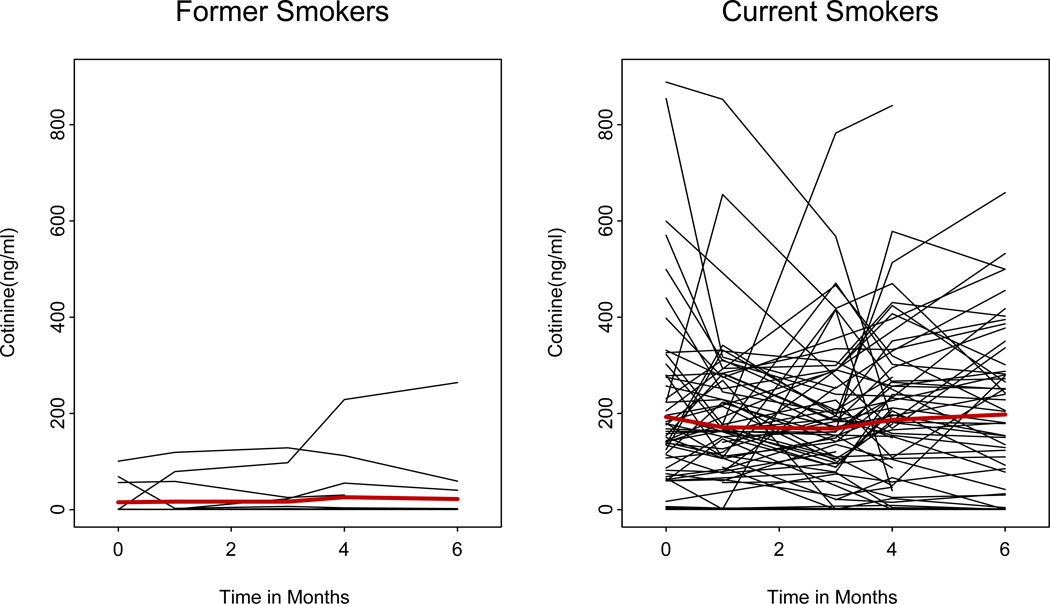
Patients were stratified into statistical groups according to smoking status (current or former smokers). To monitor smoking status, patients were asked at each visit whether they were actively smoking, and serum cotinine levels were measured. In general, serum cotinine values were in agreement with the patient reports, but Fig. 2 shows two patients who reported having stopped smoking but had cotinine levels >20 ng/mL at baseline. An additional two former smokers admitted to resuming smoking during the study.
Figure 2.
Cotinine levels by smoking status over time in both former smokers and current smokers. Each black line represents one participant’s data. The red line represents the average. The Y axis is the measured cotinine level.
Adherence to Treatment
Pill counts were performed on a monthly basis to measure treatment adherence, which was excellent. Based on pill counts over the first 3 months of treatment, participants enrolled in the PCB, CCX0, and CCX1 arms took 93.7% (±15.2), 92.1% (±11.78), and 94.5% (±15.3) of the prescribed doses, respectively. Comparable results were observed at 6 months’ follow-up (data not shown). There were no differences in adherence levels between the treatment groups.
Treatment-related Toxicity
Of the 204 patients who were randomized to study sections, 92 experienced at least one toxic effect, and a total of 196 toxicity episodes were reported. Fifty-eight patients experienced grade 1 toxicities, 28 patients experienced grade 2 toxicities, and 6 patients reported grades 3–4 toxicities (confusion, thrombosis, hyperglycemia, allergic reaction, hypertension, and nausea/abdominal pain), but only hyperglycemia, hypertension and nausea/abdominal pain were considered to be possibly treatment-related (Table 2). No cardiac toxicities were observed in the study. According to protocol guidelines, patients who experienced toxicities of grade 2 or greater had their dose levels reduced to the -1 dose level (100 mg).
Table 2.
Toxic Effects of Protocol Treatment over Time Period
| Treatment Group | Time Period | Number of Patients Experiencing Each Grade of Toxicity |
|||||
| 1 | 2 | 3 | 4 | ||||
| PCB+PCB | (n=50) | All | 17 | 7 | 1 | 0 | |
| PCB+CCX | Dose 0 | (n=21) | 1st 3-month | 6 | 0 | 0 | 0 |
| 2nd 3-month | 4 | 1 | 0 | 0 | |||
| Dose +1 | (n=30) | 1st 3-month | 10 | 2 | 1 | 0 | |
| 2nd 3-month | 1 | 3 | 2 | 0 | |||
| CCX+PCB | Dose 0 | (n=22) | 1st 3-month | 2 | 0 | 0 | 0 |
| 2nd 3-month | 7 | 1 | 0 | 0 | |||
| Dose +1 | (n=30) | 1st 3-month | 5 | 6 | 0 | 0 | |
| 2nd 3-month | 2 | 4 | 0 | 0 | |||
| CCX+CCX | Dose 0 | (n=19) | All | 4 | 0 | 0 | 1 |
| Dose +1 | (n=32) | All | 5 | 4 | 1 | 0 | |
| Treatment Group | Time Period |
Number of Patients Experiencing Each Grade of Toxicity |
|||||
| 1 | 2 | 3 | 4 | ||||
| Placebo-Placebo | (n=50) | All | 17 | 7 | 1 | 0 | |
| Placebo-Celecoxib, | Dose 0 | (n=21) | 1st 3-month | 6 | 0 | 0 | 0 |
| 2nd 3-month | 4 | 1 | 0 | 0 | |||
| Dose +1 | (n=30) | 1st 3-month | 10 | 2 | 1 | 0 | |
| 2nd 3-month | 1 | 3 | 2 | 0 | |||
| Celecoxib-Placebo, | Dose 0 | (n=22) | 1st 3-month | 2 | 0 | 0 | 0 |
| 2nd 3-month | 7 | 1 | 0 | 0 | |||
| Dose +1 | (n=30) | 1st 3-month | 5 | 6 | 0 | 0 | |
| 2nd 3-month | 2 | 4 | 0 | 0 | |||
| Celecoxib-Celecoxib, | Dose 0 | (n=19) | All | 4 | 0 | 0 | 1 |
| Dose +1 | (n=32) | All | 5 | 4 | 1 | 0 | |
| Treatment Group | Time Period |
Number of Patients Experiencing Each Grade of Toxicity |
|||||
| 1 | 2 | 3 | 4 | ||||
| Placebo-Placebo | (n=50) | All | 17 | 7 | 1 | 0 | |
| Placebo-Celecoxib, | Dose 0 | (n=21) | 1st 3-month | 6 | 0 | 0 | 0 |
| 2nd 3-month | 4 | 1 | 0 | 0 | |||
| Dose +1 | (n=30) | 1st 3-month | 10 | 2 | 1 | 0 | |
| 2nd 3-month | 1 | 3 | 2 | 0 | |||
| Celecoxib-Placebo, | Dose 0 | (n=22) | 1st 3-month | 2 | 0 | 0 | 0 |
| 2nd 3-month | 7 | 1 | 0 | 0 | |||
| Dose +1 | (n=30) | 1st 3-month | 5 | 6 | 0 | 0 | |
| 2nd 3-month | 2 | 4 | 0 | 0 | |||
| Celecoxib-Celecoxib, | Dose 0 | (n=19) | All | 4 | 0 | 0 | 1 |
| Dose +1 | (n=32) | All | 5 | 4 | 1 | 0 | |
PCB: Placebo
CCX: Celecoxib
Serum Celecoxib Levels
Serum celecoxib levels were collected via blood draw and measured at baseline and at defined treatment time points (1, 3, 4, and 6 months) prior to the patient taking the morning dose. At 3 months, mean celecoxib levels were generally in the low micromolar range (Table 3). Serum celecoxib levels were dose-dependent in current smokers (2.71±1.34 with high dose versus 2.11±3.40 with low dose, P = 0.004, Wilcoxon rank sum test) but not in former smokers (Table 3). On the basis of findings from an in vitro study (29), low-micromolar celecoxib levels would be sufficient to have biological effects on NSCLC cells.
Table 3.
Celecoxib Levels at 3 Months by Smoking Status
| Celecoxib levels | |||||||
|---|---|---|---|---|---|---|---|
| Smoking status |
Treatment | N Obs | Mean (uM) | Std Dev | Minimum | Median | Maximum |
| CURRENT | CCX: 0 | 23 | 2.11 | 3.40 | 0.00 | 1.26 | 16.53 |
| CCX: +1 | 17 | 2.71 | 1.34 | 0.00 | 2.43 | 5.16 | |
| FORMER | CCX: 0 | 7 | 2.86 | 0.79 | 1.92 | 2.62 | 3.98 |
| CCX: +1 | 4 | 3.23 | 3.08 | 0.64 | 2.38 | 7.50 | |
Wilcoxon rank sum test, P = 0.78 and P = 0.004, comparing celecoxib levels between dose levels in former (2.86 ± 0.79 vs 3.23 ± 3.08) and current (2.11 ± 3.40 vs 2.71 ± 1.34) smoker groups, respectively.
Wilcoxon rank sum test, P = 0.008 and P = 0.89, comparing celecoxib levels between smoking status for dose 0 (2.11 ± 3.40 vs 2.86 ± 0.79) and +1 (2.71 ± 1.34 vs 3.23 ± 3.08) levels, respectively.
CCX: 0; low-dose celecoxib
CCX: +1; high-dose celecoxib
Squamous Metaplasia and Dysplasia in the Bronchial Epithelium
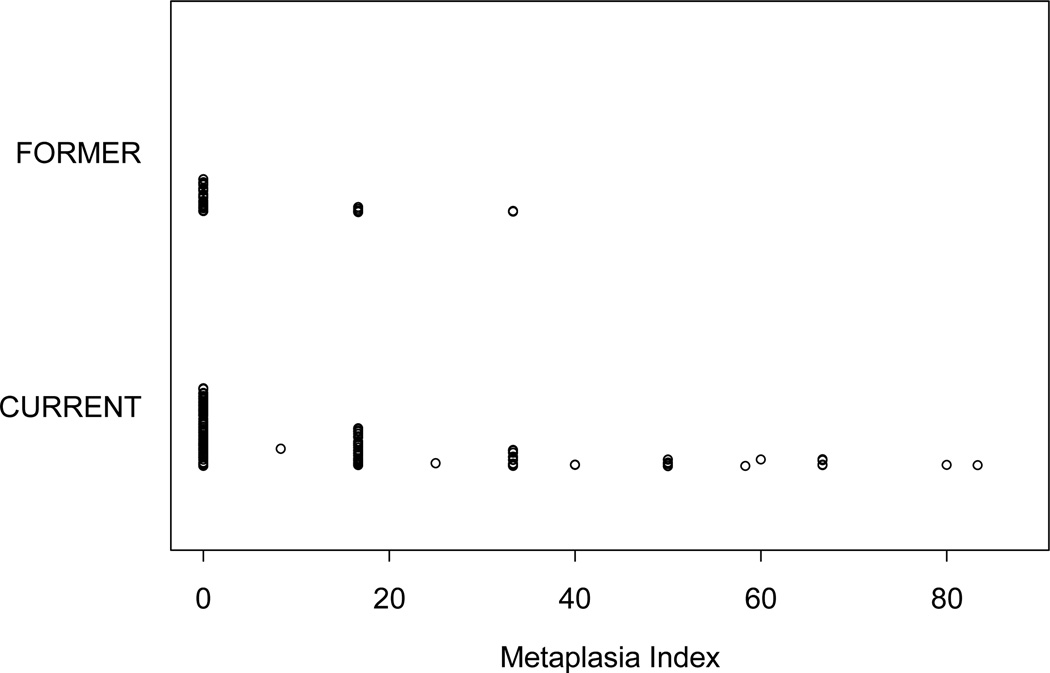
A total of 212 patients underwent at least one bronchoscopic procedure each, adding up to a sum of 443 bronchoscopic procedures generating 2,658 biopsy samples. Among them, 1,272 biopsy samples were performed at baseline, 762 at 3 months’ time, and 624 at 6 months’ time. Eighteen baseline biopsy samples were inadequate for histologic interpretation. Of the remaining 1,254 baseline samples, 1,086 (86.6%) had normal histology, 152 (12.1%) had squamous metaplasia, and 16 (1.3%) had dysplasia. Squamous metaplasia or dysplasia was detected in 15.5% (148 of 958) of the samples obtained from current smokers and 5.7% (14 of 248) of the samples obtained from former smokers. The corresponding MI was higher in current smokers (15.2 [±20.3], n=162) than in former smokers (5.6 [±10.2], n=42) (P = 0.004, Wilcoxon rank sum test) (Fig. 3). There were no differences in MI values among the treatment groups (Table 4).
Figure 3.
Baseline squamous metaplasia. Current smokers had a higher percentage of squamous metaplasia than former smokers. Each dot represents one participant’s information in relation to metaplasia index.
Table 4.
Modulation of Metaplasia Index (MI) from Baseline to 3 Months by Treatment Arm at Each Dose Level, by Smoking Status#
| Smoking status | Treatment | Variable | N | Mean | Std Dev | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|---|
| FORMER | CCX, Dose 0 | MI0 | 8 | 6.25 | 8.63 | 0.00 | 0.00 | 16.67 |
| MI3 | 8 | 6.25 | 12.40 | 0.00 | 0.00 | 33.33 | ||
| MI30 | 8 | 0.00 | 12.60 | −16.67 | 0.00 | 16.67 | ||
| CCX, Dose +1 | MI0 | 7 | 4.76 | 8.13 | 0.00 | 0.00 | 16.67 | |
| MI3 | 7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| MI30 | 7 | −4.76 | 8.13 | −16.67 | 0.00 | 0.00 | ||
| Placebo | MI0 | 13 | 5.13 | 10.51 | 0.00 | 0.00 | 33.33 | |
| MI3 | 13 | 1.28 | 4.62 | 0.00 | 0.00 | 16.67 | ||
| MI30 | 13 | −3.85 | 12.08 | −33.33 | 0.00 | 16.67 | ||
| CURRENT | CCX, Dose 0 | MI0 | 25 | 15.00 | 21.97 | 0.00 | 0.00 | 66.67 |
| MI3 | 25 | 16.13 | 18.95 | 0.00 | 16.67 | 66.67 | ||
| MI30 | 25 | 1.13 | 14.87 | −33.33 | 0.00 | 33.33 | ||
| CCX, Dose +1 | MI0 | 29 | 12.87 | 14.93 | 0.00 | 16.67 | 50.00 | |
| MI3 | 29 | 15.92 | 17.88 | 0.00 | 16.67 | 66.67 | ||
| MI30 | 29 | 3.05 | 18.44 | −33.33 | 0.00 | 33.33 | ||
| Placebo | MI0 | 44 | 15.45 | 23.18 | 0.00 | 0.00 | 83.33 | |
| MI3 | 44 | 16.06 | 22.45 | 0.00 | 8.33 | 100.00 | ||
| MI30 | 44 | 0.61 | 22.43 | −50.00 | 0.00 | 66.67 |
CCX, Dose 0; low-dose celecoxib
CCX, Dose +1; high-dose celecoxib
MI0; metaplasia index baseline
MI3; metaplasia index at 3 months
MI30; metaplasia index difference (MI3 – MI0)
one patient did not have a 3-month metaplasia index reading from the biopsy
Effect of Treatment on Ki-67 Labeling Index
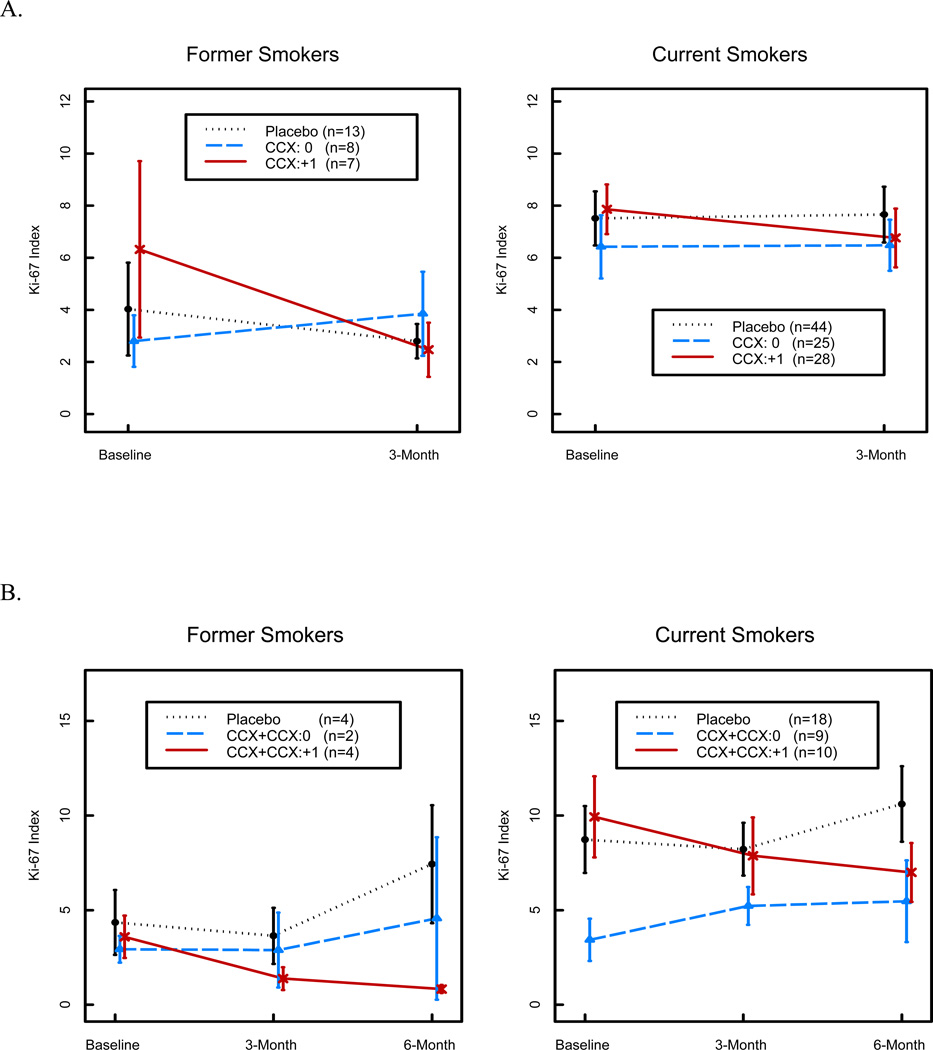
The primary endpoint of the study was modulation of the Ki-67 index from the baseline level after 3 months’ of treatment. Ki-67 values were measurable in 2,202 biopsy samples (1,069 at baseline, 627 at 3 months’ time, and 506 at 6 months’ time) obtained from 200 patients randomized to one of the four study groups (Table 5). Wilcoxon rank sum test shows that baseline Ki-67 expression was significantly higher in current than in former smokers among all epithelial layers (6.15±6.01% versus 3.86±5.56%, P = 0.002), the basal layer (6.07±6.77% versus 3.49±4.69%, P =0.003), and the Para basal layer (9.23±10.42% versus 6.31±12.43%, P = 0.009). Other variables that affected Ki-67 labeling were the presence of squamous metaplasia (P < 0.0001) and the number of quit-years (1–<5, P = 0.0004; >5, P <0.0001). We first examined the effect of celecoxib treatment on Ki-67 labeling in all epithelial layers, which was the primary study endpoint, by combining the low- and high-dose treatment cohorts. Mixed model analysis revealed that Ki-67 labeling was not significantly different between the celecoxib and placebo groups (P = 0.12). However, although the effect of low-dose treatment was not significant (P = 0.79), 3 months of high-dose treatment decreased Ki-67 labeling in all epithelial layers in both former smokers (3.85% decrease) and current smokers (1.10% decrease), which was a significantly greater reduction in both groups (P = 0.02, Mixed Model Analysis) than that in the placebo group after adjusting for metaplasia and smoking status (Table 6 and Fig. 4A). This treatment effect persisted at the 6-month time point (Fig. 4B), which further supports the idea that there was a biological effect resulting from high-dose treatment. Additional analysis was then performed to examine the effect of high-dose treatment on specific epithelial layers. Although changes in the Para basal layer did not reach significance, Ki-67 labeling decreased in the basal layer by 4.14% in former smokers and 1.41% in current smokers, which was a significantly greater reduction than that observed in the placebo arm (P = 0.008, Mixed Model Analysis).
Table 5.
Distribution of Ki-67 Index (All Layers) in Patients by Smoking Status and Treatment at Baseline, 3 Months, and Difference from Baseline to 3 Months (n=200)#
| Smoking Status |
Treatment | Variable | N | Mean | Std Dev | Minimum | Median | Maximum |
P value* |
|---|---|---|---|---|---|---|---|---|---|
| FORMER | CCX, Dose 0 | Baseline | 11 | 2.90 | 2.36 | 0.50 | 2.69 | 8.63 | 0.55 |
| 3-Month | 8 | 3.85 | 4.57 | 0.17 | 1.31 | 11.06 | |||
| Difference | 8 | 1.04 | 5.43 | −8.19 | 0.55 | 9.88 | |||
| CCX, Dose +1 | Baseline | 10 | 4.89 | 7.68 | 0.93 | 1.87 | 26.24 | 0.38 | |
| 3-Month | 7 | 2.47 | 2.75 | 0.10 | 1.62 | 8.18 | |||
| Difference | 7 | −3.85 | 10.13 | −25.6 | −1.79 | 6.32 | |||
| Placebo | Baseline | 18 | 3.88 | 5.77 | 0.08 | 1.50 | 23.81 | 0.95 | |
| 3-Month | 13 | 2.80 | 2.38 | 0.14 | 2.23 | 7.45 | |||
| Difference | 13 | −1.23 | 6.16 | −20.1 | 0.03 | 6.89 | |||
| CURRENT | CCX, Dose 0 | Baseline | 30 | 6.62 | 7.37 | 0.38 | 4.16 | 30.89 | 0.97 |
| 3-Month | 25 | 6.48 | 4.89 | 0.25 | 5.46 | 16.23 | |||
| Difference | 25 | 0.06 | 5.22 | −11.5 | −0.68 | 12.97 | |||
| CCX, Dose +1 | Baseline | 50 | 6.03 | 4.84 | 0.35 | 4.59 | 23.66 | 0.27 | |
| 3-Month | 29 | 6.76 | 5.88 | 0.00 | 5.43 | 19.82 | |||
| Difference | 28 | −1.10 | 6.91 | −13.5 | −1.71 | 14.12 | |||
| Placebo | Baseline | 80 | 6.05 | 6.18 | 0.00 | 4.65 | 32.13 | 0.89 | |
| 3-Month | 44 | 7.66 | 7.10 | 0.00 | 6.15 | 38.10 | |||
| Difference | 44 | 0.15 | 8.58 | −26.4 | −0.15 | 34.42 |
Wilcoxon signed rank test comparing modulation of Ki-67 index within each subgroup.
CCX: Celecoxib
one patient did not have a baseline Ki-67 reading due to inadequate tissue
Table 6.
Mixed Model Analysis on the Effects of Ki-67 (n=200)
| Covariates | Estimate | SE | P value | |
|---|---|---|---|---|
| SQM (+ vs −) | 0.62 | 0.05 | <0.0001 | |
| Quit-years | (1 – <5 Yrs vs Current Smokers) | −0.21 | 0.06 | 0.0004 |
| (>=5 Yrs vs Current Smokers) | −0.37 | 0.06 | <0.0001 | |
| Treatment (CCX, Dose 0 vs Placebo) | 0.09 | 0.05 | 0.12 | |
| (CCX, Dose +1 vs Placebo) | 0.10 | 0.05 | 0.03 | |
| Time (3-Month vs Baseline) | 0.09 | 0.05 | 0.049 | |
| Treatment*Time (CCX, Dose 0 vs Placebo at 3-Month) | −0.02 | 0.08 | 0.79 | |
| (CCX, Dose +1 vs Placebo at 3-Month) | −0.17 | 0.07 | 0.02 | |
SQM; any squamous metaplasia
CCX, Dose 0; low-dose celecoxib
CCX, Dose +1; high-dose celecoxib
Figure 4.
Mean Ki-67 over time in all layers.
A. Baseline and 3-month time points show decreasing expression of Ki-67 with high-dose celecoxib over time in both current and former smokers who had both baseline and 3-month Ki-67 measurements. Total evaluable patients are 28 in former smokers group and 97 in current smoker group. Y-axis represents Ki-67 index.
B. Baseline and 3- and 6-month time periods show a similar trend for Ki-67 expression with high-dose celecoxib in both current and former smokers who had baseline, 3-month and 6-month Ki-67 measurements. Total evaluable patients are 10 in former smokers group and 37 in current smoker group. Placebo and low-dose celecoxib follow similar patterns, especially in current smokers. Y-axis represents Ki-67 index.
DISCUSSION
In this first-ever randomized clinical trial of a 6-month celecoxib regimen in current and former smokers, we found that celecoxib is safe to administer and biologically active in the bronchial epithelium. The effects of treatment on the primary endpoint, bronchial epithelial proliferation after 3 months’ time, are noteworthy given that the participant accrual goal was not reached. Moreover, the biological activity and safety of celecoxib in this cohort warrant additional studies on the efficacy of celecoxib in NSCLC chemoprevention.
Problems encountered during the conduct of this trial highlight several important feasibility issues in planning lung chemoprevention studies. The unanticipated cardiac toxicities reported in large trials examining the efficacy of celecoxib and other nonsteroidal anti-inflammatory drugs in colon cancer chemoprevention (30–35) negatively impacted the conduct of this study in several respects. First, participant accrual was interrupted for 6 months. Second, data from patients who had been actively receiving treatment at the time of protocol suspension were deemed inevaluable due to early treatment cessation. Third, patient accrual after the trial reopened proceeded at a slower rate than it had prior to trial suspension, which suggests that the negative publicity associated with the cardiac toxicity reports adversely affected patient accrual. In fact, we did not observe any cardiovascular toxicity in this cohort. This may have been related to the short duration of celecoxib treatment in this study relative to that of the trials reporting these toxicities, which required treatments of more than 12 months’ duration (20, 30, 36–38).
The findings reported here on Ki-67 labeling in the bronchial epithelium are noteworthy for several reasons. First, Ki-67 labeling decreased in participants treated with high-dose but not low-dose celecoxib. Similarly, celecoxib is more efficacious in colon cancer chemoprevention when administered at 400 mg twice daily than at 200 mg twice daily (20, 30, 38). These findings suggest that, although it may have less treatment-related toxicity, low-dose (200 mg) celecoxib has no efficacy in NSCLC chemoprevention and argue against such a trial design.
Second, Ki-67 levels decreased more prominently in former smokers than in current smokers, especially in those patients who completed baseline and 3-month bronchoscopies (Figure 4A). This effect was also observed in patients who completed baseline, 3 and 6 month bronchoscopies (Figure 4B) and received high-dose celecoxib. It is important to note that these are subset analyses and the number of patients is low, especially in the former smokers. Serum celecoxib levels did not differ in current versus former smokers treated with high-dose celecoxib (data not shown), but detailed pharmacokinetic studies were not performed, so we cannot exclude the possibility that celecoxib’s pharmacokinetics contributed to this outcome. Current and former smokers may differ with respect to the role that COX-2 plays in maintaining bronchial epithelial proliferation. In fact, other studies have reported differences between these two groups with respect to bronchial epithelial biology (28, 39, 40).
Third, the reduction in Ki-67 labeling was not accompanied by a decrease in MI, and the effect of celecoxib on Ki-67 did not vary on the basis of histology, indicating that the decrease in Ki-67 was not due to a reduction in bronchial metaplasia, which has been reported to increase Ki-67 labeling (28, 41–43). Dysplasia was uncommon in this cohort, so no conclusions can be made about the effect of celecoxib on this histologic abnormality.
Fourth, Ki-67 decreased more prominently in the basal layer than it did in the Para basal layer, a strikingly different finding from those reported in chemoprevention studies utilizing retinoids, which reduce bronchial metaplasia and are active primarily in the Para basal layer of the bronchial epithelium (27, 28, 44). Collectively, these findings suggest that the basal and Para basal compartments of the bronchial epithelium are biologically distinct, which is consistent with evidence that cells in the basal layer have a low proliferation rate, express progenitor cell markers, and have multipotent differentiation potential (45), whereas cells in the Para basal layer have a higher proliferation rate and have undergone differentiation into mucous-secreting and other epithelial cell types.
Progress in the field of NSCLC chemoprevention research will require the ability to identify individuals at high risk for the development of NSCLC, a way to isolate premalignant bronchial epithelial cells in danger of malignant progression, and a method to elucidate the mechanisms by which these premalignant cells maintain their proliferation and survival. With respect to the latter, findings presented here and elsewhere (46) raise the possibility that COX-2 is one mediator of bronchial epithelial proliferation in current and former smokers. Additional studies are warranted to examine the importance of COX-2 in NSCLC development and explore NSCLC prevention with COX-2 inhibitors.
Supplementary Material
ACKNOWLEDGMENTS
Funding Sources
This work was supported by grants from the National Cancer Institute [CA 091844, CA 16672 to JMK] and from the Department of Defense [W81XWH-04-0142 to WKH] and Pfizer Pharmaceuticals.
Footnotes
AUTHOR’S DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors have disclosed no potential conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. The health benefits of smoking cessation: a report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 1990 DHHS Publication No. (CDC) 90-8416. [Google Scholar]
- 3.Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of NSCLC mortality in former smokers. J Natl Cancer Inst. 1993;85:457–464. doi: 10.1093/jnci/85.6.457. [DOI] [PubMed] [Google Scholar]
- 4.Burns DM. Primary prevention, smoking, and smoking cessation: implications for future trends in NSCLC prevention. Cancer. 2000;89(11 Suppl):2506–2509. doi: 10.1002/1097-0142(20001201)89:11+<2506::aid-cncr33>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on NSCLC risk. JAMA. 2005;294(12):1505–1510. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- 6.Lubin JH, Blot WJ. NSCLC and smoking cessation: patterns of risk. J Natl Cancer Inst. 1993;85:422–423. doi: 10.1093/jnci/85.6.422. [DOI] [PubMed] [Google Scholar]
- 7.Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78(5):1004–1010. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim ES, Hong WK, Khuri FR. Chemoprevention of aero digestive tract cancers. Annu Rev Med. 2002;53:223–243. doi: 10.1146/annurev.med.53.082901.104015. [DOI] [PubMed] [Google Scholar]
- 9.The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of NSCLC and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 10.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on NSCLC and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 11.Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, Goodman GE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I NSCLC. J Natl Cancer Inst. 2001;93(8):605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 12.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 13.Subbaramiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, et al. Transcription of cyclooxygenase 2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- 14.Kelly DJ, Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, et al. Benzo[a]pyrene upregulates cyclooxygenase-2 gene expression in oral epithelial cells. Carcinogenesis. 1997;18:795–799. doi: 10.1093/carcin/18.4.795. [DOI] [PubMed] [Google Scholar]
- 15.Kutchera W, Jones DA, Matsunami N, Groden J, McIntyre TM, Zimmerman GA, et al. Prostaglandin H synthase-2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 17.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 18.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, et al. Increased expression of cyclooxygenase 2 occurs frequently in human NSCLCs, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 19.Oshima M, Dinchuk SL, Kargman H, Oshima H, Hancock E, Kwong E, et al. Suppression of intestinal polyposis in Apc Γ176 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 20.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 21.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 22.Koki AT, Khan NK, Woerner BM, Seibert K, Harmon JL, Dannenberg AJ, et al. Characterization of cyclooxygenase-2 (COX-2) during tumorigenesis in human epithelial cancers: evidence for potential clinical utility of COX-2 inhibitors in epithelial cancers. Prostaglandins Leukot Essent Fatty Acids. 2002;66(1):13–18. doi: 10.1054/plef.2001.0335. Review. [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Soria JC, Tang X, Xu XC, Wang L, Mao L, et al. Prognostic factors in resected stage I non-small-cell NSCLC: a multivariate analysis of six molecular markers. J Clin Oncol. 2004;22(22):4575–4583. doi: 10.1200/JCO.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 24.Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell NSCLC. Clin Cancer Res. 2001;7(4):861–867. [PubMed] [Google Scholar]
- 25.Hosomi Y, Yokose T, Hirose Y, Nakajima R, Nagai K, Nishiwaki Y, et al. Increased cyclooxygenase-2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. NSCLC. 2000;30:73–81. doi: 10.1016/s0169-5002(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 26.Rioux N, Castonguay A. Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998;58:5354–5360. [PubMed] [Google Scholar]
- 27.Hittelman WN, Liu DD, Kurie JM, Lotan R, Lee JS, Khuri F, et al. Proliferative changes in the bronchial epithelium of former smokers treated with retinoids. J Natl Cancer Inst. 2007;99(21):1603–1612. doi: 10.1093/jnci/djm205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JJ, Liu D, Lee JS, Kurie JM, Khuri FR, Ibarguen H, et al. Long-term impact of smoking on lung epithelial proliferation in current and former smokers. J Natl Cancer Inst. 2001;93(14):1081–1088. doi: 10.1093/jnci/93.14.1081. [DOI] [PubMed] [Google Scholar]
- 29.Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell NSCLC cell lines. Clin. Cancer Res. 2000;6:2006–2011. [PubMed] [Google Scholar]
- 30.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. APC Study Investigators. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 31.Solomon SD, McMurray JV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 32.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 33.Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 35.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cross Trial Safety Assessment Group. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 37.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 38.Bertagnolli MM, Zauber AG, Hawk ET. The Adenoma Prevention with Celecoxib (APC) trial: five-year efficacy and safety results; Proceedings of the 99th Annual Meeting of the American Association for Cancer Research, abstr; 2008. LB-141. [Google Scholar]
- 39.Spira A, Beane J, Shah V, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Lee JJ, Tang H, Fan YH, Xiao L, Ren H, et al. Impact of smoking cessation on global gene expression in the bronchial epithelium of chronic smokers. Cancer Prev Res. 2008;1(2):112–118. doi: 10.1158/1940-6207.CAPR-07-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert AP, et al. Ki-67 expression and patients’ survival in NSCLC: systematic review of the literature with meta-analysis. Br J Cancer. 2004;91(12):2018–2025. doi: 10.1038/sj.bjc.6602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller YE, Blatchford P, Hyun DS, Keith RL, Kennedy TC, Wolf H, et al. Bronchial epithelial Ki-67 index is related to histology, smoking, and gender, but not NSCLC or chronic obstructive pulmonary disease. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2425–2431. doi: 10.1158/1055-9965.EPI-07-0220. [DOI] [PubMed] [Google Scholar]
- 43.Szabo E. Lung Epithelial Proliferation: a Biomarker for Chemoprevention Trials? J Natl Cancer Inst. 2001;93(4):1042–1043. doi: 10.1093/jnci/93.14.1042. [DOI] [PubMed] [Google Scholar]
- 44.Kurie JM, Lotan R, Lee JJ, Lee JS, Morice RC, Liu DD, et al. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-beta expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003;95(3):206–214. doi: 10.1093/jnci/95.3.206. [DOI] [PubMed] [Google Scholar]
- 45.Vaughan MB, Ramirez RD, Wright WE, et al. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74(4):141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 46.Mao JT, Fishbein MC, Adams B, Roth MD, Goodglick L, Hong L, et al. Celecoxib decreases Ki-67 proliferative index in active smokers. Clin Cancer Res. 2006;12(1):314–320. doi: 10.1158/1078-0432.CCR-05-1440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.