Abstract
Rationale: Introduction of sedation protocols has been associated with improved patient outcomes. It is not known if an update to an existing high-quality sedation protocol, featuring increased patient assessment and reduced benzodiazepine exposure, is associated with improved patient process and outcome quality metrics.
Methods: This was an observational before (n = 703) and after (n = 780) cohort study of mechanically ventilated patients in a 24-bed trauma-surgical intensive care unit (ICU) from 2009 to 2011. The three main protocol updates were: (1) requirement to document Richmond Agitation Sedation Scale (RASS) scores every 4 hours, (2) requirement to document Confusion Assessment Method-ICU (CAM ICU) twice daily, and (3) systematic, protocolized deescalation of excess sedation. Multivariable linear regression was used for the primary analysis. The primary outcome was the duration of mechanical ventilation. Prespecified secondary endpoints included days of delirium; the frequency of patient assessment with the RASS and CAM-ICU instruments; benzodiazepine dosing; durations of mechanical ventilation, ICU stay, and hospitalization; and hospital mortality and ventilator associated pneumonia rate.
Results: Patients in the updated protocol cohort had 1.22 more RASS assessments per day (5.38 vs. 4.16; 95% confidence interval [CI], 1.05–1.39; P < 0.01) and 1.15 more CAM-ICU assessments per day (1.49 vs. 0.35; 95% CI, 1.08–1.21; P < 0.01) than the baseline cohort. The mean hourly benzodiazepine dose decreased by 34.8% (0.08 mg lorazepam equivalents/h; 0.15 vs. 0.23; P < 0.01). In the multivariable model, the median duration of mechanical ventilation decreased by 17.6% (95% CI, 0.6–31.7%; P = 0.04). The overall odds ratio of delirium was 0.67 (95% CI, 0.49–0.91; P = 0.01) comparing updated versus baseline cohort. A 12.4% reduction in median duration of ICU stay (95% CI, 0.5–22.8%; P = 0.04) and a 14.0% reduction in median duration of hospitalization (95% CI, 2.0–24.5%; P = 0.02) were also seen. No significant association with mortality (odds ratio, 1.18; 95% CI, 0.80–1.76; P = 0.40) was seen.
Conclusions: Implementation of an updated ICU analgesia, sedation, and delirium protocol was associated with an increase in RASS and CAM-ICU assessment and documentation; reduced hourly benzodiazepine dose; and decreased delirium and median durations of mechanical ventilation, ICU stay, and hospitalization.
Keywords: critical care, protocol, delirium; quality improvement
Mechanical ventilation is common in the intensive care unit (ICU) and is associated with pain, delirium, and agitation (1, 2). Sedative medications are often provided for comfort in the mechanically ventilated patient but have been associated with harm, including occurrence of delirium, ventilator-associated pneumonia (VAP), and prolonged mechanical ventilation (1, 3). Over the past 20 years, a number of sedation practices, including bolus sedation and daily sedation interruptions, have been shown to improve patient outcomes, including decreased duration of mechanical ventilation, in some but not all studies (4–9). Protocol-directed sedation and analgesia assessment and treatment, including protocolized weaning of sedation, also have been associated with improved patient outcomes in many, but not all, studies in a variety of ICU populations (1, 9–18).
Frequently, clinical trial evidence is synthesized into consensus guidelines that are then incorporated into order sets and protocols at the hospital or unit level to help guide the implementation of care but across medicine guidelines are not uniformly implemented (7, 19–23). Protocols can be conceptualized as structure elements in a structure-process-outcomes model of quality improvement, which states that the structure in which care is delivered impacts the process of delivery of care, which in turn impacts care outcomes (24). Much of the previous work has focused on the implementation of analgesia, sedation, and delirium protocols into ICUs that did not have them, implementation of a structure element in an environment that previously lacked a structure element. As the uptake of order sets has increased, hospitals are now facing decisions about order set maintenance and updates (25). Less attention has been paid to how incorporating new clinical trial evidence and clinical guidelines into existing protocols can further improve the structure (and thereby the process and outcome) of ICU care (26). Protocol dissemination and adoption could be a strategy to improve innovation in health care (27).
There is a clear association between decreased exposure to sedative medications, particularly benzodiazepines, and improved patient outcomes (8, 13, 28, 29). Based on the association between benzodiazepines and harm, we undertook a quality improvement to improve the structure of our ICU by introducing an updated pain, sedation, and delirium protocol.
We tested the hypothesis, based in the structure-process-outcome model of quality improvement, that implementation of an updated protocol emphasizing patient assessment and reducing the targeted level of sedation (a structure quality element) would be associated with practice changes reflected by decreased benzodiazepine exposures and lightened Richmond Agitation Sedation Scale (RASS) scores (process elements) (30). We further hypothesized that these sedation practice changes would be associated with decreased burden of delirium, as assessed by the Confusion Assessment Method–ICU (CAM-ICU), duration of mechanical ventilation, and decreased duration of ICU and hospital stay (outcomes) (31).
Methods
As part of ongoing quality-improvement initiatives in an academic health system, a sedation protocol task force, composed of direct-care nurses, nurse educators, nurse managers, pharmacists, respiratory therapists, and physicians from three university-affiliated hospitals, was convened, systematically reviewed the evidence, and designed an updated analgesia, sedation, and delirium protocol (see online supplement). Our trauma-surgical ICU’s first sedation protocol was implemented in 1999 and had undergone periodic revision since then. Previously, the most recent update was in 2004, and it lacked several features preferred for optimal analgesia, sedation, and delirium care, including regular pain and agitation score documentation, assessment of delirium, and protocolized deescalation of sedative medications (7). Additionally, it did not include elements supported by more recent randomized controlled trials, specifically the pairing of a daily spontaneous awakening trial and a daily spontaneous breathing trial (5, 7). These elements were all included in the updated, sedation-reducing protocol. Other important protocol elements aiming at reduction of sedation included lightening the sedation goal on the RASS, providing sedation after assessing and treating pain, offering adjunct pain medications, and regularly assessing delirium with avoidance of benzodiazepines in delirium-positive patients. A complete list of changes is shown in Table E1 in the online supplement.
The primary intent of the protocol update was to increase patient assessment and decrease the amount of sedative medications given to our patients, particularly benzodiazepines. The baseline protocol called for hourly sedation assessment but did not specify an interval at which those assessments should be documented. We believed that by explicitly requiring documentation of the RASS score every 4 hours, the patient would be more likely to be assessed. Similarly, we introduced a requirement to document CAM-ICU scores on a twice-daily basis. Based on previous work, we believed that more regular patient assessment could lead to decreased benzodiazepine dosing and improved patient outcomes (3, 12). Based on the workgroup’s clinical experience, it was believed that despite the requirement to assess sedation on an hourly basis, we were perhaps missing an opportunity to decrease sedative doses based on those reassessments, as sedation interruption was only mandated once daily, and there was provision for reducing sedation when the patient was not on target due to oversedation. Therefore, to further increase the chances for deescalation of sedative infusions, we empirically chose to protocolize 25% reduction in sedative dose in patients with RASS scores of −2 to −3 or deeper who were meeting ventilator-tolerance goals, an internal scale created to assess the patient interaction with the ventilator (see online supplement), for the past 4 hours.
The new protocol was implemented in the Trauma and Surgical ICU at Harborview Medical Center in July 2010. During this time, academic detailing with the various stakeholders, including the direct care nursing staff and the house staff, was performed, with a number of sessions for both the daytime and nighttime staff. House staff education was performed by lecture and during daily rounds. We also created educational materials, including posters, detailing the protocol change. Widespread use of the RASS and CAM-ICU patient assessment instruments predated the updated protocol implementation and was not assessed in our study.
The updated, sedation-reducing protocol was the only protocol available for analgesia, delirium, and sedation treatment of mechanically ventilated patients during the entire study period. Sedation and analgesia medications could be prescribed outside of the protocol. No other analgesia, sedation, and delirium or mechanical ventilation quality-improvement projects, including changes in the weaning strategy, were undertaken during this period.
We took advantage of the implementation of the updated, sedation-reducing protocol to perform a natural history experiment (before and after) to test associations of a new assessment-increasing and sedation-reducing protocol as a whole on duration of mechanical ventilation but did not seek to understand the contributions of the individual protocol elements (24). The study design was reviewed by the Human Subjects Division of the University of Washington and found not to require Institutional Review Board oversight, as it was considered a quality-improvement activity, and the requirement for informed consent did not apply (#36066).
Patient Population
All patients admitted to the Trauma and Surgical ICU at Harborview Medical Center who received mechanical ventilation in the ICU, regardless of the admitting service, were included in the study. Patients who died before arrival in the ICU and patients who did not require mechanical ventilation were excluded. The baseline protocol cohort included patients admitted from July 1, 2009 through June 30, 2010, and the updated protocol cohort included patients from September 1, 2010 until August 30, 2011. A 2-month “wash-in” phase during which the updated protocol was introduced was chosen to allow time for full protocol implementation and uptake.
Data Sources
Data were collected via electronic abstraction from the electronic medical record. VAP data were obtained from the hospital infection prevention team using National Healthcare Safety Network surveillance definitions.
Study Endpoints
The study endpoints were chosen using a structure-process-outcome model. The duration in hours of the total time of mechanical ventilation was chosen a priori as the primary endpoint (outcome). Prespecified secondary outcome endpoints included occurrence of delirium, the duration of ICU stay, 28-day ventilator-free survival, the duration of hospitalization, the incidence and rate of VAP per 1,000 ventilator-days, and hospital mortality. Prespecified secondary process endpoints to document protocol uptake included the number of RASS assessments documented per patient day, the mean RASS score over the period of mechanical ventilation, and the hourly dose of benzodiazepine equivalents administered during a patient’s ICU stay. As the study institution used paper order entry during this timeframe, order set usage could not be captured by our electronic chart abstraction; however, the process measures reflect protocol adherence. Benzodiazepine doses were all converted into milligrams of lorazepam equivalents, and opiate doses were converted into milligrams of morphine equivalents (32, 33). We measured potential confounders including age, sex, weight, and severity of illness, captured by the Simplified Acute Physiology Score II (SAPS II) at the time of ICU admission (34).
Statistical Analysis
To test the association between cohort and duration of mechanical ventilation, a multivariable linear regression model was constructed after adjustment for the a priori identified potential confounders, including injury severity as captured by the SAPS II score, sex, age, and weight. The duration of mechanical ventilation had a skewed distribution and was log-transformed. Separate multivariable models were constructed to evaluate the relationship between cohort membership and the prespecified secondary process and outcome endpoints using the same a priori identified potential confounders. To investigate the relationship between cohort and risk of VAP over time, a Cox proportional hazard model was constructed with the same prespecified potential confounders. All P values were two-sided, and P < 0.05 was considered statistically significant. All analyses were conducted with STATA 11.2 (StataCorp, College Station, TX).
Results
Patient Characteristics
A total of 1,483 intubated patients were included; 703 patients were included in the baseline cohort, and 780 were included in the updated protocol cohort (Table 1). Patients in the updated protocol cohort had a lower Injury Severity Score than the baseline cohort (24.7 vs. 29.4, P < 0.01) and a trend toward a lower SAPS II (42.2 vs. 43.8, P = 0.06). The cohorts were otherwise similar in other demographic and clinical characteristics.
Table 1.
Patient characteristics by order set cohort
| Baseline (n = 703) | Sedation-Reducing (n = 780) | P Value* | |
|---|---|---|---|
| Age, mean (SD), yr | 48.1 (18.7) | 49.3 (19.7) | 0.2 |
| Male, n (%) | 489 (69.4) | 560 (70.8) | 0.6 |
| Weight, mean (SD), kg | 85.2 (23.3) | 85.1 (23.9) | 0.9 |
| Admission SAPS II, mean (SD) | 43.8 (16.3) | 42.2 (15.0) | 0.06 |
| Trauma service patients, n (%) | 549 (78.1) | 611 (78.4) | 0.9 |
| Admission ISS, trauma patients, mean (SD) | 29.4 (15.5) | 24.7 (16.3) | <0.01 |
Definition of abbreviations: ISS = Injury Severity Score; SAPS = Simplified Acute Physiology Score.
P value determined by Chi-square or Student t test.
Process Results
There was an increase in the mean number of RASS assessments per 24 hours for patients in the updated cohort compared with the baseline cohort (5.38 vs. 4.16, P < 0.01; Table 2, Figure 1). The average RASS score was 0.31 higher in the updated compared with the baseline protocol period (−0.99 vs. −1.30, respectively; P < 0.01), indicating a decreased level of sedation.
Table 2.
Daily analgesia, sedation, and delirium assessments and pharmacologic treatment by order set cohort
| Baseline (n = 4,977 Patient Days) | Sedation-Reducing (n = 5,095 Patient Days) | P Value* | |
|---|---|---|---|
| No. RASS assessments per 24 h, mean (SD) | 4.16 (0.059) | 5.38 (0.063) | <0.01 |
| 24-h Weighted average RASS score, mean (SD) | −1.30 (0.026) | −0.99 (0.023) | <0.01 |
| No. CAM-ICU assessments per 24 h, mean (SD) | 0.35 (0.015) | 1.49 (0.030) | <0.01 |
| Days with positive CAM-ICU score when CAM-ICU was assessed, n (%) | 172 (25.1) | 455 (21.2) | <0.01 |
| Patient ever CAM-ICU positive, n (%) | 75 (10.7) | 176 (22.6) | <0.01 |
| No. of pain assessments per 24 h, mean (SD) | 1.47 (0.041) | 2.13 (0.048) | <0.01 |
| No. “Unable to Assess” pain assessments per 24 h, mean (SD) | 4.10 (0.073) | 0.20 (0.020) | <0.01 |
| 24-h Weighted average NRS pain score, mean (SD) | 2.74 (0.074) | 2.86 (0.060) | 0.2 |
| Hourly benzodiazepine dose,† mean (SD), mg | 0.23 (0.018) | 0.15 (0.011) | <0.01 |
| Total benzodiazepine dose,† mean (SD), mg | 49.2 (156.5) | 17.2 (53.6) | <0.01 |
| Hourly propofol dose, mean (SD), mg | 24.3 (1.68) | 23.4 (1.53) | 0.7 |
| Hourly dexmedetomidine dose, mean (SD), mg | 0.23 (0.095) | 0.30 (0.080) | 0.6 |
| Daily opiate dose,‡ mean (SD), mg | 1.24 (0.065) | 1.13 (0.052) | 0.2 |
| Daily haloperidol dose, mean (SD), mg | 2.72 (0.24) | 1.01 (0.11) | <0.01 |
| Daily quetiapine dose, mean (SD), mg | 5.66 (1.19) | 7.84 (3.25) | 0.5 |
Definition of abbreviations: CAM-ICU = Confusion Assessment Method–Intensive Care Unit; NRS = Numerical Rating Scale; RASS = Richmond Agitation Sedation Scale.
P value determined by Chi-square or Student t test.
mg Lorazepam equivalents (does not include propofol).
mg Morphine equivalents.
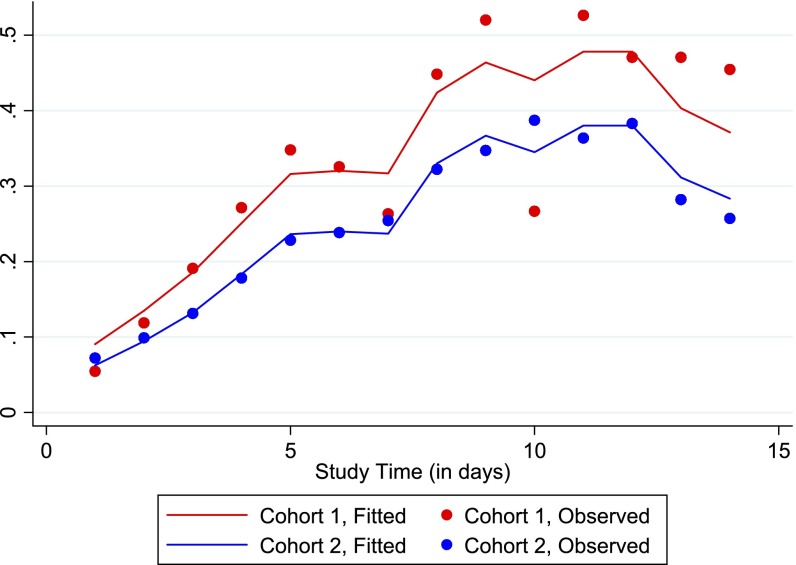
Figure 1.
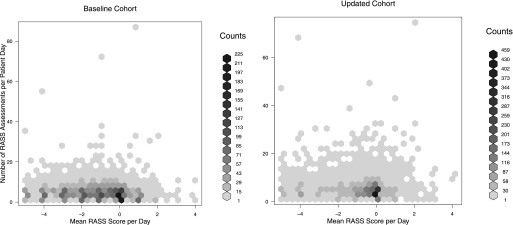
Hex plot of mean number of Richmond Agitation Sedation Scale (RASS) assessments per day versus mean RASS score per day, in baseline versus updated, sedation-reducing cohort. The darker hexes denote more observations. The updated cohort features a greater number of RASS assessments as well as a higher (less sedated) mean RASS score (P < 0.01 for both). Note: Both the y-axes scales and the number of observations per hex shade are different in the baseline and updated cohort figures.
Similarly, significant increases were also seen in the mean number of CAM-ICU assessments per 24 hours, which increased by 1.14 (updated vs. baseline cohorts: 1.49 vs. 0.35; P < 0.01).
The mean number of pain assessments increased by 0.66 per 24 hours (2.13 vs. 1.47, P < 0.01), and the mean pain score remained unchanged (2.86 vs. 2.74, P = 0.2). Of note, the mean number of “Unable to Assess” pain assessments fell dramatically by 3.90 per 24 hours (updated vs. baseline: 0.20 vs. 4.10; P < 0.01).
The updated, sedation-reducing protocol was also associated with a 34.7% (0.08 mg/h) reduction in the mean hourly benzodiazepine dose (0.15 vs. 0.23, P < 0.01). The mean doses of the other sedatives and analgesics remained unchanged (Table 2). The mean daily haloperidol dose decreased by 1.71 mg/d (1.01 vs. 2.72, P < 0.01), and the mean daily quetiapine dose did not significantly change.
The association between cohort and mean benzodiazepine dose persisted after adjusting for severity of illness, male sex, age, and body weight. In our multivariable model, implementation of the updated protocol was associated with a 0.11 mg/h decrease in the mean benzodiazepine (95% confidence interval [CI], 0.14–0.07 mg/h; P < 0.01).
Clinical Outcome Results
In unadjusted analysis, patients in the updated protocol cohort had a 4-hour reduction in median duration of mechanical ventilation (16 vs. 20 h, P < 0.01) and a 1-day increase in the median number of ventilator-free days (25 [interquartile range, 1–26] vs. 26 [interquartile range, 20–26] d, P < 0.01) (Table 3).
Table 3.
Patient outcomes by order set cohort
| Baseline (n = 703) | Sedation-Reducing (n = 780) | P Value* | |
|---|---|---|---|
| Discharged alive, n (%) | 607 (86.3) | 673 (86.3) | 1.0 |
| Discharged home, n (%) | 373 (53.1) | 386 (49.6) | 0.2 |
| Discharged to SNF only, n (%) | 146 (20.8) | 161 (20.6) | 1.0 |
| Discharged to SNF, LTAC, or other institution, n (%) | 230 (32.7) | 282 (36.2) | 0.2 |
| 28-day ventilator-free survival, median (IQR), d | 25 (17–26) | 26 (20–26) | <0.01 |
| Duration of mechanical ventilation, median (IQR), h | 20 (7–61) | 16 (6–44) | 0.01 |
| Duration of ICU stay, median (IQR), d | 3 (1–7) | 3 (1–6) | 0.03 |
| Duration of hospitalization, median (IQR), d | 11 (5–21) | 10 (4–18) | 0.02 |
| VAP, n (%) | 46 (6.5) | 36 (4.6) | 0.08 |
| VAP rate (n/1,000 ventilator-days), mean (SD) | 11.7 (16.7) | 9.7 (16.3) | 0.40 |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; LTAC = long-term acute care; SNF = skilled nursing facility; VAP = ventilator-associated pneumonia.
P value determined by Chi-square or Wilcoxon rank-sum test.
Patients in the updated protocol cohort also had a shorter median duration of ICU stay (P = 0.03) and hospitalization (P = 0.02) in bivariable analysis. These effects persisted in multivariable analysis, where the updated protocol cohort had a 12.4% shorter median duration of ICU stay (95% CI, 0.5–22.8%; P = 0.04) and a 14.0% shorter median duration of hospitalization (95% CI, 2.0–24.5%; P = 0.02).
Analysis of changes in delirium between the two cohorts is complex. The key factor is significant increase in CAM-ICU assessment by an average of 1.14 (1.49 vs. 0.35, P < 0.01) assessments per day. This increase in assessment drove an apparent increase in delirium. The percentage of patients who were ever CAM-ICU positive increased by 11.9% (22.6 vs. 10.7%, P < 0.01). However, if we restrict our analysis of delirium to the periods in which the CAM-ICU score was measured, delirium actually decreased by 3.9% (21.2 vs. 25.1, P < 0.01). Indeed, the overall odds ratio of delirium on per 12-hour basis in the first 16 days of ICU stay was 0.67 (95% CI, 0.49–0.91; P = 0.01) comparing updated versus baseline cohort (Figure 3).
Figure 3.
Plot of the probability of delirium over time if a Confusion Assessment Method–Intensive Care Unit (CAM-ICU) score was measured in the baseline cohort (red) and in the updated cohort (blue). The overall odds ratio of delirium in the first 16 days of ICU stay was 0.67 (95% confidence interval, 0.49–0.91; P = 0.01) comparing updated versus baseline cohort.
In the multivariable model of our primary outcome, duration of mechanical ventilation, patients in the updated protocol cohort had a 17.6% reduction in the median duration of mechanical ventilation (95% CI, 0.6–31.7%; P = 0.04), after adjusting for their admission SAPS II score, age, male sex, and weight (Table 4, Figure 2). The multivariable models of two of our secondary endpoints—ICU and hospital stays—also showed significant associations with the intervention. The implementation cohort had a 12.4% reduction in median duration of ICU stay (95% CI, 0.5–22.8%; P = 0.04) and a 14.0% reduction in median duration of hospitalization (95% CI, 2.0–24.5%; P = 0.02). We did not find a significant association of the intervention with in-hospital mortality (odds ratio, 1.18; 95% CI, 0.80–1.76; P = 0.40).
Table 4.
Multivariable linear regression model of the association between sedation cohort and the duration of mechanical ventilation
| Hospital/Patient Characteristic | Ratio of Medians exp β (95% CI) | P Value |
|---|---|---|
| Cohort, sedation-reducing protocol | 0.82 (0.68–0.99) | 0.04 |
| Admission SAPS II | 1.04 (1.04–1.05) | <0.01 |
| Male sex | 0.83 (0.67–1.03) | 0.09 |
| Age, yr | 0.99 (0.99–0.997) | <0.01 |
| Weight, kg | 1.01 (1.002–1.01) | <0.01 |
Definition of abbreviations: CI = confidence interval; SAPS = Simplified Acute Physiology Score.
Duration of mechanical ventilation log-transformed to make the distribution of the variable more symmetric. The β coefficients have been exponentiated and represent the ratio of median duration of mechanical ventilation. Errors were computed using robust variance estimation.
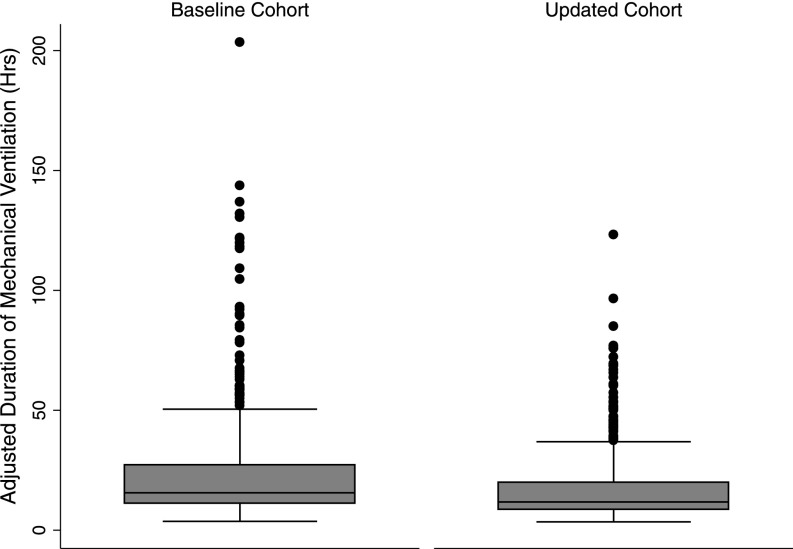
Figure 2.
Box plot of the adjusted duration of mechanical ventilation (hours) adjusted for SAPS, age, weight, and male sex in the baseline versus sedation-reducing protocol cohorts. The horizontal line at the center of the box denotes the median. The upper and lower boundaries signify the 75th and 25th percentiles, respectively. Outlying values are denoted with the box whiskers and dots. The updated cohort features a 17.6% reduction in the median duration of mechanical ventilation (95% confidence interval, 0.6–31.7%; P = 0.04).
Rates of VAP were similar between the groups, with a nonsignificant 2.0 case/1,000 ventilator-days decrease in the rate of VAP (95% CI, 2.6 to −6.6; P = 0.40). Similarly, a Cox proportional hazards model showed a nonsignificant 23% lower rate of VAP (P = 0.3) in the sedation-reducing protocol cohort. There were no differences in hospital mortality or in the proportion of patients discharged home between the baseline and the updated protocol cohorts (Table 3).
Discussion
Implementation of an updated pain, agitation, and delirium protocol in a trauma-surgical ICU, designed to decrease exposure to benzodiazepines by increasing patient assessment and protcolized deescalation of sedative medication, was associated with an increased frequency of sedation assessment, a higher RASS score (lighter sedation), decreased mean benzodiazepine dose, a significant reduction of the burden of delirium, and decreased median durations of mechanical ventilation, ICU stay, and hospital stay.
Using a structure-process-outcome model of quality improvement, we have shown that implementation of an improved sedation and delirium protocol (a structure element of quality) is associated with process improvements in care: an increase in RASS and CAM-ICU documentation frequency, RASS scores reflective of lighter sedation, and a decrease in benzodiazepine dosing. Implementation of the improved protocol was also associated with improved outcomes: decreased burden of delirium, decreased median duration of mechanical ventilation, decreased median duration of ICU stay, and decreased median duration of hospitalization.
As there appears to be clear association between benzodiazepine exposure and duration of mechanical ventilation and delirium, we sought to update our protocol in a way that decreased the total exposure to benzodiazepines (28, 35, 36). Even though the specified frequency of patient sedation assessment was actually lower, the number of documented sedation assessments significantly increased, likely as a consequence of the education process during the protocol implementation phase as well as the content of the protocol itself. The requirement to document the pain, RASS, and CAM-ICU scores before initiating appropriate treatment was likely an additional powerful lever to decrease exposure to benzodiazepine sedative medications and their attendant consequences and a potential unit-level marker of process of care quality.
Indeed, the effects on process and outcomes metrics of an updated protocol are key. Some studies have demonstrated an association between protocol implementation and an unchanged or increased duration of mechanical ventilation. However, in many of these studies, sedative drug dosing and levels of sedation have actually increased, and duration of mechanical ventilation also increased (11, 15, 37). A protocol may well modify care process in a way that is not associated with improved patient outcomes. If the goal of quality improvement is to decrease unwanted variation, order sets and protocols represent a potentially powerful and measurable tool to decrease variation and improve quality (38, 39). One of the challenges for clinical leaders is to understand the potential structure-process-outcome effects of a given protocol update and to measure those effects.
Many previous sedation and analgesia studies have involved the implementation of a pain, agitation, and/or delirium protocol into an environment where no such protocol was being previously used (4, 12, 13). One particular strength of our study is that we implemented our updated protocol in an environment where an evidence-based analgesia, sedation, and delirium protocol already existed. As the use of pain, agitation and delirium order sets has increased over time, the question facing many ICUs is when and how to update their order sets. This task competes for organizational bandwidth and resources with a number of other important priorities.
In order for organizations and institutions to allocate resources efficiently, the clinical and financial potential benefits of the effort necessary to convene and support a protocol update must be understood. For example, our protocol redesign efforts included a series of multidisciplinary meetings spanning over months as well as involving academic detailing with day and night shift nurses and the house staff. More research into the optimal mechanisms of protocol updates and revision is needed (40). However, we have shown that the effort necessary to update an order can have significant clinical and operational return on investment. It is possible that a more real-time incorporation of evolving evidence could further decrease the innovation cycle time necessary to bring care innovation to the patient’s bedside and increase the value of care delivered.
There is also an opportunity for professional societies or regional cooperatives to lead this effort. Previous work has shown that although both high- and low-performing hospitals often have protocols, there is significant heterogeneity in protocol quality across a geographic area (25, 41). Lower-volume hospitals and hospitals that are not part of hospital networks have lower-quality pain, agitation, and delirium order sets (25). This variation of order set quality across a geographic area likely represents unwanted variation in the structure of care. As such, it presents a potential target for quality-improvement programs, especially if the variation is common (as it is in sedation practices), measurable, and amenable to modification (22, 38, 42–44). Given significant variation in protocol quality and an association between protocol presence and quality and clinical processes and outcomes, this is an area in need of further study and professional activity (45–47).
Our study has several limitations. First it is a single-institution, retrospective cohort study involving a trauma and surgical patient population. After implementation in the trauma-surgical ICU, the protocol was disseminated more broadly. However, because patients, house staff, and attendings move from unit to unit, we designed our study to capture the initial implementation of the updated protocol at our institution. The results might not be generalizable to other institutions or other populations, and unmeasured confounders could exist.
Second, the pre–post design of our study is unable to account for secular trends, such as the decreased use of benzodiazepines or lighter level of target sedation. However, the entire study extended over a relatively narrow time period, and no other sedation-related quality-improvement interventions were introduced during the study period. Third, there was only one analgesia, sedation, and delirium order set available during the study periods, but we were not able to capture data on how frequently orders for analgesia, sedation, or antipsychotics, were written outside the specifications of the protocol. However, we accounted for all sedatives, opioids, and antipsychotics administered. If nonprotocolized prescription occurred, this could lead to underestimation of the real magnitude of the effect of an updated sedation-reducing protocol and as such would bias the results toward the null. We also were not able to feedback data on protocol adherence to ordering providers, including data on the paired spontaneous awakening trial–spontaneous breathing trial.
Finally, as all the new components of the updated protocol were included simultaneously, it is not possible to measure the effects of any individual element, nor was that the purpose of the study. Rather, by using the structure-process-outcome model, we demonstrated a plausible casual pathway where the aggregate effects of the protocol implementation could be measured. This meets a key stakeholder need to understand the clinical and operational return on investment of the effort necessary to update the protocol. In a time of limited resources, clinical leaders must be able to clearly articulate the value of their activities.
Conclusions
Using a structure-process-outcome model of quality improvement, we have demonstrated that implementation of an updated benzodiazepine-reducing pain, agitation, and delirium protocol, a structure element of quality, was associated with significant improvements in process measures of quality, including an increase in the documented frequency of patient assessment, lighter sedation as measured by the RASS score, and reduced administration of benzodiazepines. Protocol implementation was also associated with outcome improvements, including a reduced burden of delirium, decreased median duration of mechanical ventilation, decreased median duration of ICU stay, and decreased median duration of hospitalization. Broader implementation of updated, assessment-increasing, sedation-reducing, guideline-concordant protocols might be one way to improve the structure of ICUs and thereby improve the care processes that produce outcomes of importance to patients and their caregivers and thereby enhance the value of ICU care.
Acknowledgments
Acknowledgment
The authors thank the nurses and patients of Harborview Medical Center.
Footnotes
Supported by the National Institutes of Health grant T32 HL07287 (to C.R.D.).
Author contributions: C.R.D.: Served as primary author, designed the study protocol, obtained the data, analyzed all the data, and wrote the manuscript and its revisions and approved the final version of the manuscript. D.A.K.: Designed the study protocol, obtained the data, assisted with its analysis, reviewed the manuscript, and approved the final version. V.S.F.: Analyzed the data, reviewed the manuscript, and approved the final version. S.L.D.: Designed the study protocol, obtained the data, reviewed the manuscript, and approved its final version. S.D.: Designed the study protocol, reviewed the manuscript, and approved the final version. N.D.Y.: Analyzed the data, reviewed the manuscript, and approved the final version. C.L.H.: Assisted with analysis of the data, reviewed the manuscript, and approved the final version. T.H.D.: Designed the study protocol, obtained the data, reviewed the manuscript, and approved its final version. M.M.T.: Conceived and designed the study protocol, analyzed study data, reviewed the manuscript, and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jackson DL, Proudfoot CW, Cann KF, Walsh T. A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care. 2010;14:R59. doi: 10.1186/cc8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson DL, Proudfoot CW, Cann KF, Walsh TS. The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care. 2009;13:R204. doi: 10.1186/cc8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treggiari MM, Romand J-A, Yanez ND, Deem SA, Goldberg J, Hudson L, Heidegger C-P, Weiss NS. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37:2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 4.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 5.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 6.Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, et al. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists (ASHP), American College of Chest Physicians. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous I.V. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 9.Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, Herridge M. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308:1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 10.Williams TA, Martin S, Leslie G, Thomas L, Leen T, Tamaliunas S, Lee KY, Dobb G. Duration of mechanical ventilation in an adult intensive care unit after introduction of sedation and pain scales. Am J Crit Care. 2008;17:349–356. [PubMed] [Google Scholar]
- 11.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med. 2008;36:1444–1450. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 12.Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. BMJ. 2002;324:1386–1389. doi: 10.1136/bmj.324.7350.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degrado JR, Anger KE, Szumita PM, Pierce CD, Massaro AF. Evaluation of a local ICU sedation guideline on goal-directed administration of sedatives and analgesics. J Pain Res. 2011;4:127–134. doi: 10.2147/JPR.S18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive Care Med. 2006;32:1506–1514. doi: 10.1007/s00134-006-0309-0. [DOI] [PubMed] [Google Scholar]
- 16.De Jonghe B, Bastuji-Garin S, Fangio P, Lacherade J-C, Jabot J, Appéré-De-Vecchi C, Rocha N, Outin H. Sedation algorithm in critically ill patients without acute brain injury. Crit Care Med. 2005;33:120–127. doi: 10.1097/01.ccm.0000150268.04228.68. [DOI] [PubMed] [Google Scholar]
- 17.Robinson BRH, Mueller EW, Henson K, Branson RD, Barsoum S, Tsuei BJ. An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. J Trauma. 2008;65:517–526. doi: 10.1097/TA.0b013e318181b8f6. [DOI] [PubMed] [Google Scholar]
- 18.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi D-K, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111:451–463. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 19.Prasad M, Christie JD, Bellamy SL, Rubenfeld GD, Kahn JM. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25:610–619. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scales DC, Dainty K, Hales B, Pinto R, Fowler RA, Adhikari NKJ, Zwarenstein M. A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA. 2011;305:363–372. doi: 10.1001/jama.2010.2000. [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GCG, Yancy CWC, Hernandez AFA, Peterson EDE, Spertus JAJ, Heidenreich PAP.Potential impact of optimal implementation of evidence-based heart failure therapies on mortality Am Heart J 20111611024–1030.e3 [DOI] [PubMed] [Google Scholar]
- 24.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44:166–206. [PubMed] [Google Scholar]
- 25.Dale CR, Hayden SJ, Treggiari MM, Curtis JR, Seymour CW, Yanez ND, III, Fan VS. Association between hospital volume and network membership and an analgesia, sedation and delirium order set quality score: a cohort study. Crit Care. 2012;16:R106. doi: 10.1186/cc11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor M, Bucknall T, Manias E. International variations in outcomes from sedation protocol research: where are we at and where do we go from here? Intensive Crit Care Nurs. 2010;26:189–195. doi: 10.1016/j.iccn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 28.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 29.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur CJ, Seppelt IM, Webb SA, Weisbrodt L Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators; ANZICS Clinical Trials Group. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 30.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 32.Wilson WC, Smedira NG, Fink C, McDowell JA, Luce JM. Ordering and administration of sedatives and analgesics during the withholding and withdrawal of life support from critically ill patients. JAMA. 1992;267:949–953. [PubMed] [Google Scholar]
- 33.Patanwala AE, Duby J, Waters D, Erstad BL. Opioid conversions in acute care. Ann Pharmacother. 2007;41:255–266. doi: 10.1345/aph.1H421. [DOI] [PubMed] [Google Scholar]
- 34.Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, Rocco M, Maviglia R, Pennisi MA, Gonzalez-Diaz G, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35:18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 35.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 36.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 37.MacLaren R, Plamondon JM, Ramsay KB, Rocker GM, Patrick WD, Hall RI. A prospective evaluation of empiric versus protocol-based sedation and analgesia. Pharmacotherapy. 2000;20:662–672. doi: 10.1592/phco.20.7.662.35172. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Cook DJ, Wall RJ, Angus DC, Bion J, Kacmarek R, Kane-Gill SL, Kirchhoff KT, Levy M, Mitchell PH, et al. Intensive care unit quality improvement: a “how-to” guide for the interdisciplinary team. Crit Care Med. 2006;34:211–218. doi: 10.1097/01.ccm.0000190617.76104.ac. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor C, Adhikari NKJ, DeCaire K, Friedrich JO. Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4:81–89. doi: 10.1002/jhm.399. [DOI] [PubMed] [Google Scholar]
- 40.McGreevey JD. Order sets in electronic health records: principles of good practice. Chest. 2013;143:228–235. doi: 10.1378/chest.12-0949. [DOI] [PubMed] [Google Scholar]
- 41.Curry LA, Spatz E, Cherlin E, Thompson JW, Berg D, Ting HH, Decker C, Krumholz HM, Bradley EH. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? A qualitative study. Ann Intern Med. 2011;154:384–390. doi: 10.7326/0003-4819-154-6-201103150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amalberti R, Auroy Y, Berwick D, Barach P. Five system barriers to achieving ultrasafe health care. Ann Intern Med. 2005;142:756–764. doi: 10.7326/0003-4819-142-9-200505030-00012. [DOI] [PubMed] [Google Scholar]
- 43.Pronovost PJ, Jenckes MW, Dorman T, Garrett E, Breslow MJ, Rosenfeld BA, Lipsett PA, Bass E. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281:1310–1317. doi: 10.1001/jama.281.14.1310. [DOI] [PubMed] [Google Scholar]
- 44.Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: a systematic review. JAMA. 2000;283:1451–1459. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen Y-L, Wunsch H, Angus DC. Critical care: the impact of organization and management on outcomes. Curr Opin Crit Care. 2010;16:487–492. doi: 10.1097/MCC.0b013e32833d9180. [DOI] [PubMed] [Google Scholar]
- 46.Kahn JM, Linde-Zwirble WT, Wunsch H, Barnato AE, Iwashyna TJ, Roberts MS, Lave JR, Angus DC. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177:285–291. doi: 10.1164/rccm.200708-1214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwashyna TJ, Christie JD, Kahn JM, Asch DA. Uncharted paths: hospital networks in critical care. Chest. 2009;135:827–833. doi: 10.1378/chest.08-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]