Abstract
Rationale: Muscle wasting in chronic obstructive pulmonary disease (COPD) is associated with a poor prognosis and is not readily assessed by measures of body mass index (BMI). BMI does not discriminate between relative proportions of adipose tissue and lean muscle and may be insensitive to early pathologic changes in body composition. Computed tomography (CT)–based assessments of the pectoralis muscles may provide insight into the clinical significance of skeletal muscles in smokers.
Objectives: We hypothesized that objective assessment of the pectoralis muscle area on chest CT scans provides information that is clinically relevant and independent of BMI.
Methods: Data from the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) Study (n = 73) were used to assess the relationship between pectoralis muscle area and fat-free mass. We then used data in a subset (n = 966) of a larger cohort, the COPDGene (COPD Genetic Epidemiology) Study, to explore the relationship between pectoralis muscle area and COPD-related traits.
Measurements and Main Results: We first investigated the correlation between pectoralis muscle area and fat-free mass, using data from a subset of participants in the ECLIPSE Study. We then further investigated pectoralis muscle area in COPDGene Study participants and found that higher pectoralis muscle area values were associated with greater height, male sex, and younger age. On subsequent clinical correlation, compared with BMI, pectoralis muscle area was more significantly associated with COPD-related traits, including spirometric measures, dyspnea, and 6-minute-walk distance (6MWD). For example, on average, each 10-cm2 increase in pectoralis muscle area was associated with a 0.8-unit decrease in the BODE (Body mass index, Obstruction, Dyspnea, Exercise) index (95% confidence interval, –1.0 to –0.6; P < 0.001). Furthermore, statistically significant associations between pectoralis muscle area and COPD-related traits remained even after adjustment for BMI.
Conclusions: CT-derived pectoralis muscle area provides relevant indices of COPD morbidity that may be more predictive of important COPD-related traits than BMI. However, the relationship with clinically relevant outcomes such as hospitalization and death requires additional investigation. Pectoralis muscle area is a convenient measure that can be collected in the clinical setting in addition to BMI.
Keywords: COPD, wasting, pectoral muscle area, imaging
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States (1). Approximately 20% of patients with COPD develop a wasting phenotype, which is highly correlated with increased morbidity and mortality and is associated with the term cachexia (2). Although the etiology of this process may include disuse atrophy (3), low tissue oxygen tension (4), hormonal insufficiency (5), and inflammation (6, 7), care must be taken when defining it (8). Body habitus alone may not reflect this process because the pathologic loss of skeletal muscle mass is not always accompanied by a similar loss in adipose tissue (2, 8). For this reason, muscle wasting is not adequately characterized by measures of body mass index (BMI).
Several clinical, epidemiologic, and genetic studies of COPD have used computerized tomography (CT) scans to assess the severity of lung disease (9, 10). Although many of these investigations have focused on the airway and parenchymal manifestations of this process, use of the additional imaging data available external to the lungs to assess muscle wasting has been more limited. Güerri and colleagues reported that smaller cross-sectional area of the intercostal and abdominal muscles was associated with a history of more frequent acute exacerbations of COPD (AECOPD) (11). In a larger cohort, Marquis and colleagues demonstrated that mid-thigh cross-sectional area was independently predictive of death in 142 smokers (12). Although these investigations provide compelling data justifying the usefulness of CT-based assessments of muscle area, they were either small (n = 10 case subjects and 10 control subjects for the AECOPD Study) or in the case of the mid-thigh cross-sectional area study, used data not commonly obtained in a standard clinical CT scan of the chest.
Our goal was to develop and investigate a measure that may be applied to existing, clinically acquired CT scans of the chest to assess skeletal muscle area. We focused on the pectoralis muscle area at the level of the aortic arch because both the muscles and aorta are easy to identify and measurement could be standardized across one or more cohorts. We hypothesized that pectoralis muscle area may provide clinically relevant insight into smoking-related COPD and that these associations would be both independent from and stronger than BMI. Some of the results detailed in this manuscript have been previously reported in the form of an abstract (13).
Methods
We used two research cohorts for this investigation. We began by examining the association of pectoralis muscle area and measures of fat-free mass at baseline in a subset of 73 subjects enrolled in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study (10). Bioelectrical impedance analysis (BIA) was performed on each subject, using the Bodystat 1500 (Bodystat Ltd, Isle of Man, UK), and the resistance was used to calculate fat-free mass (14) as previously described (15). The goal of this analysis was to establish the correlation between fat-free mass and pectoralis muscle area; therefore we investigated only that subset of the total ECLIPSE population that had pectoralis muscle area, bioimpedance, and CT measures at baseline. We then explored the clinical associations of pectoralis muscle area in 966 subjects enrolled in the COPD Genetic Epidemiology (COPDGene) Study (9). COPDGene subject data reported in this article are from the first 1,000 subjects enrolled in the COPDGene Study, which was the first, predefined stopping point for analysis for the project. Approximately 3% (n = 34) of the CT scans were unreadable for pectoralis muscle area. Additional descriptions of the study participants are available in the online supplement.
CT Scan Analysis
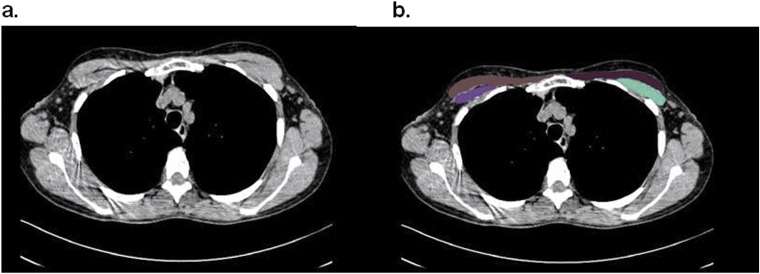
Densitometric measures of lung parenchyma were performed with Slicer (www.slicer.org) as described previously (16). Low-attenuation areas thought to represent emphysema were defined as those having a Hounsfield unit (HU) attenuation of less than –950 (16). Quantitative assessments of pectoralis muscle area were performed with in-house software with readers blinded to case–control status. Quantitative assessment of pectoralis muscle area was performed on a single axial slice of the CT scan above the aortic arch. The user visually identified the superior aspect of the aortic arch and then scrolled toward the apex of the lungs to identify the first axial image above the arch. This slice was selected because it was easily identifiable and could be replicated across a large cohort of subjects. The left and right pectoralis major and minor muscles were then identified on the anterior chest and measures of area were performed (Figures 1a and 1b). Muscles were manually shaded, using a predefined attenuation range of –50 and 90 HU, although this was modified on a case-by-case basis when the user found excluded muscle regions. These measures were performed for each subject, and pectoralis muscle area was presented as the aggregate area (in cm2) of the right and left pectoralis major and minor assessed in this axial plane.
Figure 1.
Sample computed tomography (CT) scans used to determine muscle area in chronic obstructive pulmonary disease (COPD) case subjects and control subjects. (a) CT image used for pectoralis muscle imaging. (b) Pectoralis muscles shaded in green and purple.
Statistical Analysis
Interreader assessments of pectoralis muscle area reproducibility were performed by repeating the assessment of the same CT scan for 40 subjects, by two independent readers, at two time points separated by approximately 4 months. We reported R2, the adjusted multiple square correlation, between the two sets of readings. Bland–Altman plotting was also used to check for systematic bias across the range of pectoralis muscle area values, between pectoralis muscle area and fat-free mass, and also between pectoralis muscle area and BMI. To facilitate interpretation of the Bland–Altman plots, all measures were mean-centered to 0 and scaled to have a standard deviation of 1. We reported the adjusted multiple square correlation (R2) between fat-free mass and pectoralis muscle area adjusted for age, sex, and height. Next, analysis was performed on 484 case subjects (GOLD [Global Initiative for Chronic Obstructive Pulmonary Disease] stage ≥ 2) and 482 control subjects (smokers with normal lung function) with nonmissing pectoralis muscle area. The t test was used to assess the difference between continuous variables and the chi-square test was used to test the difference in categorical variables between case subjects and control subjects. We investigated the following lung function traits: FEV1 % pred (percentage of the predicted value), FVC % pred, and the ratio of FEV1 to FVC. Additional clinical traits of interest included the St. George’s Respiratory Questionnaire (SGRQ) total score; SGRQ active score; oxygen saturation as measured by pulse oximetry (SpO2); 6-minute-walk distance (6MWD); BODE (Body mass index, Obstruction, Dyspnea, Exercise) Index and Modified Medical Research Council (MMRC) scores; and history of exacerbation. For pectoralis muscle area and BMI, all linear or logistic regression analyses with clinical traits were adjusted for confounders including age, sex, height, current smoking, and pack-years of smoking. Sex-stratified regression models were performed in male and female COPD case subjects adjusting for age, height, current smoking, and pack-years of smoking. Regression models of SpO2 were also adjusted for center, as a dichotomous variable, to account for the effect of altitude on oxygen tension at the National Jewish Health clinical center in Denver, Colorado (17). Model diagnostics, including the visual inspection of normal probability plots of residuals and plots of residuals against predictor variables to ensure assumptions of normality, independence, and constancy of error variance, held. A P value less than 0.05 was considered significant. All statistical analyses were performed with R version 2.15.1 (http://cran.r-project.org/).
Results
Descriptive Characteristics of Study Populations Used in the Analyses
Table 1 describes the baseline characteristics of the 58 COPD case subjects and 15 control subjects from ECLIPSE and the 484 COPD case subjects and 482 smoking control subjects in the COPDGene Study included in the analyses. Case subjects and control subjects in both studies differed significantly with respect to lung function measures and smoking exposures. The control subjects were slightly younger in both studies.
Table 1.
Baseline characteristics of chronic obstructive pulmonary disease (COPD) case subjects and of normal spirometry control subjects included in the analyses from the ECLIPSE and COPDGene studies
| ECLIPSE |
COPDGene |
|||||
|---|---|---|---|---|---|---|
| Control Subjects | Case Subjects | P Value | Control Subjects | Case Subjects | P Value | |
| n | 15 | 58 | 482 | 484 | ||
| Male, % | 33 | 58 | 0.14 | 50 | 49 | 0.8 |
| Age, yr | 57.3 (7.4) | 62.7 (6.1) | 0.017 | 60.3 (8.6) | 64.6 (8.1) | <0.001 |
| BMI, kg/m2 | 27.2 (4.3) | 26.6 (4.4) | 0.6 | 28.6 (5.7) | 28.1 (6.3) | 0.24 |
| Height, cm | 167.3 (10.1) | 167.9 (8.4) | 0.82 | 169.8 (9.2) | 169.3 (9.7) | 0.39 |
| Smoking, pack-years | 23.3 (21.3) | 49.7 (28.8) | 0.006 | 38.4 (20.4) | 54.9 (26.9) | <0.001 |
| Current smoking, % | 13.3 | 34.5 | <0.001 | 34.6 | 30.2 | 0.16 |
| FEV1, % pred | 114.5 (14.8) | 43.3 (14.9) | <0.001 | 98.1 (11.2) | 48.7 (18.5) | <0.001 |
| FEV1/FVC | 0.80 (0.05) | 0.41 (0.11) | <0.001 | 0.78 (0.05) | 0.48 (0.13) | <0.001 |
| Pectoralis muscle area, cm2 | 35.7 (12.5) | 31.5 (10.7) | 0.25 | 34.9 (12.1) | 29.7 (11.1) | <0.001 |
Definition of abbreviations: % pred = percentage of the predicted value; BMI = body mass index; COPDGene = COPD Genetic Epidemiology Study; ECLIPSE = Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Study.
Means and standard deviation in parentheses are reported unless otherwise noted.
Correlation between Pectoralis Muscle Area and Fat-Free Mass and Examination of Interreader Pectoralis Muscle Area
In the ECLIPSE Study, pectoralis muscle area was correlated with fat-free mass as demonstrated by an adjusted model R2 of 0.76. Bland–Altman plots of fat-free mass and pectoralis muscle area and of BMI and pectoralis muscle area did not indicate a systematic bias across the range of pectoralis muscle area in the analysis (see Figures E1a and E1b in the online supplement). The interreader pectoralis muscle area R2 correlation was 0.73, and the Bland–Altman plot (Figure E1c) did not indicate a systematic bias across the range of pectoralis muscle area values between readers.
Pectoralis Muscle Area Sample Characteristics and Bivariate Analyses
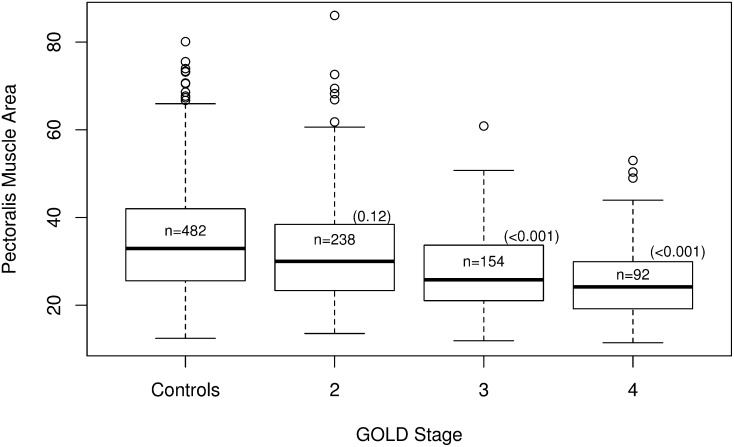
In both studies, pectoralis muscle area was lower in COPD case subjects than in control subjects (Table 1). However, this trend reached statistical significance only in the larger COPDGene Study. Furthermore, in COPDGene case subjects, pectoralis muscle area was also lower in females and older subjects, but it was associated with increasing subject height (Table 2). BMI was significantly associated with increasing pectoralis muscle area. When examined by GOLD stage, pectoralis muscle area was significantly lower in GOLD stage 3 (mean difference, –5 cm2; 95% confidence interval [CI], –6.2 to –3.5; P < 0.001) and GOLD stage 4 (mean difference, –9.2 cm2; 95% CI, –11.1 to –7.3; P < 0.001) case subjects in comparison with smoking control subjects (Figure 2). Pectoralis muscle area was also lower in GOLD stage 2 case subjects compared with control subjects, but this was not statistically significant.
Table 2.
Bivariate assessment of covariates with pectoralis muscle area in COPDGene case subjects
| Predictor | Mean Difference per cm2 Increment in Pectoralis Muscle Area | 95% CI | P Value |
|---|---|---|---|
| Sex, female | −13.8 | –15.4 to –12.2 | <0.001 |
| Height, cm | 0.58 | 0.49 to 0.67 | <0.001 |
| Age, yr | −0.17 | –0.29 to –0.05 | <0.001 |
| BMI, kg/m2 | 0.65 | 0.50 to 0.80 | <0.001 |
| Smoking, pack-years | 0.032 | –0.005 to 0.069 | 0.09 |
| Current smoking | 1.9 | –0.3 to 4.1 | 0.08 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; COPDGene = COPD Genetic Epidemiology Study.
Figure 2.
Distribution of pectoralis muscle area (in cm2) stratified by GOLD stage in the CODPGene Study (13). Sample sizes for each group are listed within their respective box plots. The P values for each group compared with the control subjects are reported in parentheses. COPDGene = COPD Genetic Epidemiology; GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease.
Pectoralis Muscle Area Relationship with Lung Function Measurements in COPD
A COPD case-only analysis was performed to compare pectoralis muscle area and BMI with spirometry and CT measures of emphysema and to determine potential relationships among those groups. In general, those subjects with greater pectoralis muscle area had significantly higher FEV1, higher FVC, and less airflow obstruction as assessed by the FEV1/FVC ratio. For example, in Table 3, on average a 1-cm2 increase in pectoralis muscle area was significantly associated with an increase of 0.81% predicted FEV1 (95% CI, 0.63 to 0.99; P < 0.001) when adjusted for age, sex, height, cigarette pack-years, and current smoking status. Most interestingly, a 1-cm2 increase in pectoralis muscle area was significantly associated with 0.63% less emphysema (95% CI, –0.75 to –0.51; P < 0.001). These observations were also consistent when examining BMI, although the relationship between BMI and FVC did not reach statistical significance (Table 3).
Table 3.
Relationship between pectoralis muscle area and body mass index to clinical and computed tomography scan traits in chronic obstructive pulmonary disease case subjects
| Trait | Pectoralis Muscle Area (Case Subjects) |
BMI (Case Subjects) |
||||
|---|---|---|---|---|---|---|
| Mean Difference per cm2 Increment in Pectoralis Muscle Area | 95% CI | P Value | Mean Difference per kg/m2 Increment in Body Mass Index | 95% CI | P Value | |
| Lung function | ||||||
| FEV1, % pred | 0.81 | 0.63 to 0.99 | <0.001 | 0.84 | 0.59 to 1.09 | <0.001 |
| FVC, % pred | 0.37 | 0.19 to 0.55 | <0.001 | 0.13 | –0.12 to 0.38 | 0.3 |
| FEV1/FVC | 0.0062 | 0.0042 to 0.0082 | <0.001 | 0.0082 | 0.0064 to 0.0100 | <0.001 |
| CT emphysema, % | −0.63 | –0.75 to –0.51 | <0.001 | −1.01 | –1.17 to –0.85 | <0.001 |
| Clinical | ||||||
| SGRQ total | −0.44 | –0.64 to –0.24 | <0.001 | −0.037 | –0.33 to 0.26 | 0.8 |
| SGRQ active | −0.58 | –0.83 to –0.33 | <0.001 | 0.045 | –0.308 to 0.398 | 0.81 |
| SpO2 | 0.038 | 0.009 to 0.067 | 0.01 | −0.038 | –0.0792 to 0.0032 | 0.073 |
| 6-Minute-walk distance, m | 8.3 | 4.2 to 12.4 | <0.001 | −6.3 | –12.2 to –0.4 | 0.03 |
| BODE | −0.082 | –0.104 to –0.060 | <0.001 | −0.076 | –0.105 to –0.047 | <0.001 |
| MMRC | −0.029 | –0.043 to –0.015 | <0.001 | 0.00055 | –0.01905 to 0.02015 | 0.96 |
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | ||
|---|---|---|---|---|---|---|---|
| History of severe exacerbation | 0.97 | 0.94 to 1.0 | 0.05 | 0.97 | 0.94 to 1.0 | 0.2 |
Definition of abbreviations: % pred = percentage of the predicted value; BMI = body mass index; BODE = Body mass index, Obstruction, Dyspnea, Exercise; CI = confidence interval; CT = computed tomography; MMRC = Modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire; SpO2 = oxygen saturation as measured by pulse oximetry.
Models with pectoralis muscle area were adjusted for age at enrollment, sex, height, current smoking, and cigarette pack-years. Models with BMI were adjusted for the same variables with the exception of height. Regression models of SpO2 were also adjusted for center.
Pectoralis Muscle Area Relationship with Clinically Relevant Traits in COPD Case Subjects
The associations of CT measures of the pectoralis muscles with SGRQ total and active scores, SpO2 levels, 6MWD, BODE Index score, MMRC dyspnea score, and history of exacerbation in the year before enrollment were examined (Table 3). In multivariate models adjusted for age, sex, height, cigarette pack-years, and current smoking status, pectoralis muscle area was significantly and negatively associated with SGRQ total and active scores as well as the MMRC dyspnea score and 6MWD. On average, those subjects with greater pectoralis muscle area had less disease-related impact as assessed by the SGRQ total and active scores, less dyspnea, and greater exercise capacity as reflected by their 6MWD. In contrast, only the 6MWD was statistically significantly associated with BMI; there was no statistically significant association between BMI and either the SGRQ or MMRC dyspnea score, although there was a statistically significant association between greater BMI and a shorter 6MWD.
Sex-Stratified Analyses
We performed a sex-stratified analysis for both pectoralis muscle area and BMI (Tables E1 and E2). When we analyzed pectoralis muscle area in men and in women separately, the direction of effect was similar to that of the combined analysis. However, when we analyzed BMI separately by sex, we noted that the direction of effect was opposite in men and women for FVC, SGRQ active, and MMRC. However, none of these associations for BMI reached statistical significance.
Pectoralis Muscle Area Associations with Lung Function Measurements and Clinical Traits Controlling for BMI
Last, we examined linear regression models of pectoralis muscle area with lung function measurements and clinical traits adjusted for BMI in addition to the above-described covariates (Table 4). Overall, pectoralis muscle area remained statistically significant for its association with all lung function measurements and with clinical traits when BMI was also included in the model. With the exception of BODE, the statistical significance increased as did the magnitude of the effect, as described by mean difference, for pectoralis muscle area with all clinical traits when the association was controlled for BMI. In particular, from Table 3, each 1-cm2 increase in pectoralis muscle area was associated with a 8.3-m increase in 6MWD (95% CI, 4.2 to 12.4; P < 0.001) after adjusting for age, sex, height, cigarette pack-years, and current smoking status. When the model also controlled for BMI (Table 4), a 1-cm2 increase in pectoralis muscle area was associated with an average increase of 12.5 m walked (95% CI, 8.0 to 17.0; P < 0.001).
Table 4.
Relationship of pectoralis muscle area to clinical and computed tomography scan traits in chronic obstructive pulmonary disease case subjects, adjusting for body mass index in models
| Trait | Mean Difference per cm2 Increment in Pectoralis Muscle Area | 95% CI | P Value |
|---|---|---|---|
| Lung function | |||
| FEV1, % pred | 0.67 | 0.47 to 0.87 | <0.001 |
| FVC, % pred | 0.4 | 0.20 to 0.60 | <0.001 |
| FEV1/FVC | 0.0044 | –0.3092 to 0.3180 | <0.001 |
| CT emphysema, % | −0.4 | –0.52 to –0.28 | <0.001 |
| Clinical | |||
| SGRQ total | −0.52 | –0.76 to –0.28 | <0.001 |
| SGRQ active | −0.73 | –1.00 to –0.46 | <0.001 |
| SpO2 | 0.061 | 0.028 to 0.094 | <0.001 |
| 6-Minute walk distance, m | 12.5 | 8.0 to 17.0 | <0.001 |
| BODE | −0.072 | –0.096 to –0.048 | <0.001 |
| MMRC | −0.036 | –0.052 to –0.020 | <0.001 |
| Odds Ratio per cm2 Increment in Pectoralis Muscle Area | 95% CI | P Value | |
|---|---|---|---|
| History of severe exacerbation | 0.98 | 0.94 to 1.0 | 0.1 |
Definition of abbreviations: % pred = percentage of the predicted value; BMI = body mass index; BODE = Body mass index, Obstruction, Dyspnea, Exercise; CI = confidence interval; CT = computed tomography; MMRC = Modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire; SpO2 = oxygen saturation as measured by pulse oximetry.
In addition to adjustment for BMI, models with pectoralis muscle area were adjusted for age at enrollment, sex, height, current smoking, and cigarette pack-years. Regression models of SpO2 were also adjusted for center.
Discussion
Overall, we have demonstrated that a new CT-derived anthropometric measure, pectoralis muscle area, is correlated with fat-free mass and is associated with the presence of COPD. We have also provided evidence that pectoralis muscle area is associated with higher GOLD stage and is more statistically significantly associated with measures of COPD disease severity than BMI. Our analyses adjusting for BMI also demonstrate that these associations are independent of BMI.
In our study, we found that a higher pectoralis muscle area was associated with male sex, taller stature, younger age, and current smoking status. These results are not surprising and are consistent with prior studies of lean body mass (7, 18–20). Pectoralis muscle area also was lower in subjects with worse lung function (more severe airflow limitation) and in those with lower resting oxygen saturations, a key indicator of poor prognosis (17). Further work is needed to refine our understanding of these observations.
A notable finding in our investigation is the inverse association between pectoralis muscle area and CT emphysema. Our results are consistent with previous studies that have reported that those subjects with a higher BMI tended to have less emphysema on their CT scan (21–24). A similar link between bone density and CT emphysema has also been described. Engelen and colleagues (25) also observed that patients with emphysema had lower values for BMI, lower lean mass, and lower bone mineral content. Further work is needed to understand the biologic link between these two processes.
Shoup and colleagues (26) have previously examined the relationship of weight and lean body mass with measures of health-related quality of life as determined by the SGRQ. In their cohort of 50 patients with expiratory airflow obstruction, low lean body mass (as assessed by dual-energy X-ray absorptiometry) was associated with greater activity and total SGRQ scores (26), whereas both underweight and overweight patients experienced higher total SGRQ scores. In our much larger cohort, we found similar results with pectoralis muscle area being significantly inversely associated with MMRC and SGRQ. We, however, did not find an association between BMI and either MMRC or the SGRQ, possibly because of the previously documented nonlinear relationship between weight and health-related quality of life measures (26). This is in keeping with findings from Mostert and colleagues (27), who found that patients with COPD who lost fat-free mass irrespective of body weight had greater impairment in 12MWD, handgrip strength, and SGRQ. This finding in conjunction with results from observational research reporting lower mortality in obese critically ill patients (the obesity paradox) (28) highlights the need to include measures of muscle mass when assessing body composition.
We also observed that those patients with COPD with better exercise capacity as assessed by 6MWD tended to have higher pectoralis muscle area but lower BMI. When we assessed the effect of pectoralis muscle area on 6MWD while adjusting for BMI, we observed a stronger relationship, both in effect size and statistical significance, between pectoralis muscle area and 6MWD. Given the relationship between lean body mass and adipose tissue in determining BMI (e.g., in our study pectoralis muscle area and BMI were correlated [R2 = 0.13]), our results are consistent with the hypothesis that it is not the muscle mass that is driving the inverse association between BMI and the 6MWD, but rather the adipose tissue in excess of the lean body mass that is detrimental to exercise capacity in smokers.
However, we must note that neither BMI nor pectoralis muscle area was significantly associated with a history of severe exacerbations. This analysis may have been limited by the small sample of 484 COPD case subjects in which we performed the analyses. Also, the more relevant question regarding the influence of pectoralis muscle area on exacerbations is whether pectoralis muscle area can be used to predict exacerbation frequency and clinical outcomes such as mortality. This requires implicit knowledge that the exposure (e.g., decrease in pectoralis muscle area) occurred in advance of the subsequent development of the trait or outcome (e.g., death), and therefore it is more appropriately investigated using a longitudinal study design (29). Fortunately, the COPDGene Study is currently enrolling participants in a second 5-year study that will provide longitudinal data to address these sorts of questions. It is important to note that case–control studies have many other advantages over longitudinal studies including typically being less expensive to conduct, requiring less time to begin investigating research questions, and offering a convenient approach for investigating many exposures (29). Further, the analysis of a case–control study within an ongoing longitudinal study is an efficient use of resources. The COPDGene Study has harnessed these advantages with the initial recruitment criteria including a 10+ pack-year smoking history in both case subjects and control subjects. Thus at the 5-year visit, a significant proportion of the control subjects are expected to have developed COPD, which would allow investigators the opportunity to calculate incidence rates or risks.
Finally, it is interesting that pectoralis muscle area was more significantly associated with BODE score than with BMI despite the fact that BMI is one of the four components of BODE. An explanation for this may be found by examining three determinants of BODE (airflow limitation, dyspnea, and exercise capacity). For all three components, pectoralis muscle area was more statistically significantly associated with these traits than BMI, which in aggregate likely resulted in pectoralis muscle area being more highly associated with BODE. Further work is needed to determine whether CT-based measures of pectoralis muscle area may outperform BODE in predicting mortality in COPD.
Our findings dovetail with previous well-established reports that quantitative assessments of both the intercostal muscles and quadriceps are associated with exacerbations and mortality in COPD (11, 12). The uniqueness of our study lies in the ease and practicality of obtaining additional clinically relevant information pertaining to skeletal muscle size from readily available CT scans of the chest. Given the increased interest in phenotyping COPD patients with CT scan (21), the usefulness of imaging to assess eligibility for lung volume reduction procedures (22, 23), and the potential reduction in mortality associated with CT screening for lung cancer (24), it is likely such imaging will be widely employed as a clinical tool. Our technique can be applied to such images at no added cost of acquisition or burden of radiation exposure to the patient.
There are limitations to this investigation. As detailed in Methods, we elected to perform an analysis of the cross-sectional area of the pectoralis muscles in a single axial slice through the chest. Our intent was to develop and validate a measure that may be applied to existing clinically acquired imaging data. We did not assess muscle area on multiple slices or take advantage of the nature of the research-acquired CT scans to measure muscle volume. Further, it was not possible to completely blind readers to BMI as adipose tissue is readily visible on chest CTs. However, it is not likely that this limitation influenced our overall conclusions as our results held even in models adjusting for BMI (Table 4). Our goal was to explore the usefulness of a tool that would be simple to use and could be readily disseminated or replicated on existing radiology viewing stations (and even freely available DICOM [Digital Imaging and Communications in Medicine] viewing software) without training or computer science support. This would allow clinicians and researchers to explore these measures in their existing patient and investigational cohorts (pulmonary fibrosis, lung cancer, lung transplantation, etc.). Further, we compared pectoralis muscle area with fat-free mass as measured by BIA. Measurement of fat-free mass by BIA is an indirect method of body composition and is not recommended in the clinical setting (30). However, body composition as assessed by BIA has been related to COPD-related traits and outcomes, including mortality, indicating that the method has use in the research setting (15, 18, 27, 30).
We anticipate that our approach will not provide a comprehensive assessment of body composition and cannot fully assess disease-specific regional differences in body composition. To do so would require CT imaging of all body regions of interest, which is beyond the scope of clinical practice. We cannot, for example, comment on upper or lower extremity musculature or the relationship of our pectoralis muscle area measure with prior reports of mid-thigh cross-sectional area or strength (12, 25, 31). Engelen and colleagues (25) observed that whole-body, extremity, and trunk fat-free mass were significantly different in 49 patients with emphysema compared with 28 control subjects. The group observed that fat-free mass was significantly different between both COPD case subjects with emphysema and bronchitis compared with control subjects but could not distinguish between the two COPD subtypes. They also observed that skeletal muscle weakness was associated with extremity wasting. In a cancer population, Mourtzakis and colleagues (32) found that fat-free mass in the limbs did not necessarily correlate with whole-body fat-free mass, but changes in trunk fat-free mass had a strong influence. Given our findings that pectoralis muscle area is associated with clinically relevant COPD traits, it is possible that it provides more valuable information than whole-body fat-free mass measured by dual-energy X-ray absorptiometry for COPD. In our analysis of pectoralis muscle area adjusting for BMI in Table 4, we demonstrated that pectoralis muscle area provides information regarding COPD severity while controlling for BMI. Thus, despite our study’s shortcomings, we have demonstrated that pectoralis muscle area is associated with fat-free mass and offers unique insight into clinical manifestations of smoking-related lung disease independent of what is provided by BMI.
In summary, using clinical, epidemiologic, and radiologic data from two study populations including patients with COPD with a range of airflow limitation, we found that measures of the cross-sectional area of the pectoralis muscles demonstrated interesting clinically relevant associations with COPD disease severity. Those with lower pectoralis muscle area tended to have more severe expiratory airflow obstruction, lower quality of life scores, and diminished exercise capacity. Further work is needed to explore the clinical relevance of pectoralis muscle area with markers of systemic inflammation and their prognostic value for morbidity and mortality in COPD. More specifically, the predictive value of pectoralis muscle area with clinically relevant outcomes such as hospitalization and death requires additional investigation.
Acknowledgments
COPDGene Investigators: Core Units
Administrative Core: James Crapo, M.D. (PI), Edwin Silverman, M.D., Ph.D. (PI), Barry Make, M.D., Elizabeth Regan, M.D., Ph.D., Rochelle Lantz, Lori Stepp, Sandra Melanson
Genetic Analysis Core: Terri Beaty, Ph.D., Barbara Klanderman, Ph.D., Nan Laird, Ph.D., Christoph Lange, Ph.D., Michael Cho, M.D., Stephanie Santorico, Ph.D., John Hokanson, M.P.H., Ph.D., Dawn DeMeo, M.D., M.P.H., Nadia Hansel, M.D., M.P.H., Craig Hersh, M.D., M.P.H., Peter Castaldi, M.D., M.Sc., Merry-Lynn McDonald, Ph.D., Jin Zhou, M.D., Ph.D., Manuel Mattheisen, M.D., Ph.D., Emily Wan, M.D., Megan Hardin, M.D., Jacqueline Hetmanski, M.S., Margaret Parker, M.S., Tanda Murray, M.S.
Imaging Core: David Lynch, M.B., Joyce Schroeder, M.D., John Newell, Jr., M.D., John Reilly, M.D., Harvey Coxson, Ph.D., Philip Judy, Ph.D., Eric Hoffman, Ph.D., George Washko, M.D., Raul San Jose Estepar, Ph.D., James Ross, M.Sc., Mustafa Al Qaisi, M.D., Jordan Zach, Alex Kluiber, Jered Sieren, Tanya Mann, Deanna Richert, Alexander McKenzie, Jaleh Akhavan, Douglas Stinson
PFT QA Core, LDS Hospital, Salt Lake City, UT: Robert Jensen, Ph.D.
Biological Repository, Johns Hopkins University, Baltimore, MD: Homayoon Farzadegan, Ph.D., Stacey Meyerer, Shivam Chandan, Samantha Bragan
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, Ph.D., Andre Williams, Ph.D., Carla Wilson, M.S., Anna Forssen, M.S., Amber Powell, Joe Piccoli
Epidemiology Core, University of Colorado School of Public Health, Denver, CO: John Hokanson, M.P.H., Ph.D., Marci Sontag, Ph.D., Jennifer Black-Shinn, M.P.H., Gregory Kinney, M.P.H., Ph.D., Sharon Lutz, M.P.H., Ph.D.
COPDGene Investigators: Clinical Centers
VA Ann Arbor Health Care, Ann Arbor, MI: Jeffrey Curtis, M.D., Ella Kazerooni, M.D.
Baylor College of Medicine, Houston, TX: Nicola Hanania, M.D., M.S., Philip Alapat, M.D., Venkata Bandi, M.D., Kalpalatha Guntupalli, M.D., Elizabeth Guy, M.D., Antara Mallampalli, M.D., Charles Trinh, M.D., Mustafa Atik, M.D., Hasan Al-Azzawi, M.D., Marc Willis, D.O., Susan Pinero, M.D., Linda Fahr, M.D., Arun Nachiappan, M.D., Collin Bray, M.D., L. Alexander Frigini, M.D., Carlos Farinas, M.D., David Katz, M.D., Jose Freytes, M.D., Anne Marie Marciel, M.D.
Brigham and Women’s Hospital, Boston, MA: Dawn DeMeo, M.D., M.P.H., Craig Hersh, M.D., M.P.H., George Washko, M.D., Francine Jacobson, M.D., M.P.H., Hiroto Hatabu, M.D., Ph.D., Peter Clarke, M.D., Ritu Gill, M.D., Andetta Hunsaker, M.D., Beatrice Trotman-Dickenson, M.B.B.S., Rachna Madan, M.D.
Columbia University, New York, NY: R. Graham Barr, M.D., DrPH, Byron Thomashow, M.D., John Austin, M.D., Belinda D’Souza, M.D.
Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., M.D., Lacey Washington, M.D., H. Page McAdams, M.D.
Fallon Clinic, Worcester, MA: Richard Rosiello, M.D., Timothy Bresnahan, M.D., Joseph Bradley, M.D., Sharon Kuong, M.D., Steven Meller, M.D., Suzanne Roland, M.D.
Health Partners Research Foundation, Minneapolis, MN: Charlene McEvoy, M.D., M.P.H., Joseph Tashjian, M.D.
Johns Hopkins University, Baltimore, MD: Robert Wise, M.D., Nadia Hansel, M.D., M.P.H., Robert Brown, M.D., Gregory Diette, M.D., Karen Horton, M.D.
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Los Angeles, CA: Richard Casaburi, Ph.D., M.D., Janos Porszasz, M.D., Ph.D., Hans Fischer, M.D., Ph.D., Matt Budoff, M.D., Mehdi Rambod, M.D.
Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, M.D., Charles Trinh, M.D., Hirani Kamal, M.D., Roham Darvishi, M.D., Marc Willis, D.O., Susan Pinero, M.D., Linda Fahr, M.D., Arun Nachiappan, M.D., Collin Bray, M.D., L. Alexander Frigini, M.D., Carlos Farinas, M.D., David Katz, M.D., Jose Freytes, M.D., Anne Marie Marciel, M.D.
Minneapolis VA Health Care System, Minneapolis, MN: Dennis Niewoehner, M.D., Quentin Anderson, M.D., Kathryn Rice, M.D., Audrey Caine, M.D.
Morehouse School of Medicine, Atlanta, GA: Marilyn Foreman, M.D., M.S., Gloria Westney, M.D., M.S., Eugene Berkowitz, M.D., Ph.D.
National Jewish Health, Denver, CO: Russell Bowler, M.D., Ph.D., David Lynch, M.B., Joyce Schroeder, M.D., Valerie Hale, M.D., John Armstrong II, M.D., Debra Dyer, M.D., Jonathan Chung, M.D., Christian Cox, M.D.
Temple University, Philadelphia, PA: Gerard Criner, M.D., Victor Kim, M.D., Nathaniel Marchetti, D.O., Aditi Satti, M.D., A. James Mamary, M.D., Robert Steiner, M.D., Chandra Dass, M.D., Libby Cone, M.D.
University of Alabama, Birmingham, AL: William Bailey, M.D., Mark Dransfield, M.D., Michael Wells, M.D., Surya Bhatt, M.D., Hrudaya Nath, M.D., Satinder Singh, M.D.
University of California, San Diego, CA: Joe Ramsdell, M.D., Paul Friedman, M.D.
University of Iowa, Iowa City, IA: Alejandro Cornellas, M.D., John Newell, Jr., M.D., Edwin J. R. van Beek, M.D., Ph.D.
University of Michigan, Ann Arbor, MI: Fernando Martinez, M.D., MeiLan Han, M.D., Ella Kazerooni, M.D.
University of Minnesota, Minneapolis, MN: Christine Wendt, M.D., Tadashi Allen, M.D.
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D., Joel Weissfeld, M.D., M.P.H., Carl Fuhrman, M.D., Jessica Bon, M.D., Danielle Hooper, M.D.
University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, M.D., Sandra Adams, M.D., Carlos Orozco, M.D., Mario Ruiz, M.D., Amy Mumbower, M.D., Ariel Kruger, M.D., Carlos Restrepo, M.D., Michael Lane, M.D.
Principal investigators and centers participating in ECLIPSE:
Steering Committee: H. Coxson (Canada), C. Crim (GlaxoSmithKline, USA), L. Edwards (GlaxoSmithKline, USA), D. Lomas (UK), W. MacNee (UK), E. Silverman (USA), R. Tal Singer (Co-Chair, GlaxoSmithKline, USA), J. Vestbo (Co-Chair, Denmark), J. Yates (GlaxoSmithKline, USA)
Scientific Committee: A. Agusti (Spain), P. Calverley (UK), B. Celli (USA), C. Crim (GlaxoSmithKline, USA), B. Miller (GlaxoSmithKline, USA), W. MacNee (Chair, UK), S. Rennard (USA), R. Tal-Singer (GlaxoSmithKline, USA), E. Wouters (The Netherlands), J. Yates (GlaxoSmithKline, USA)
Footnotes
Author Contributions: Conception and design: M.-L.N.M., A.A.D., J.C.R., R.S.J.E., E.A.R., R.H.C., E.E., C.E.C., M.H.C., C.P.H., E.W., H.O.C., W.M., S.I.R., D.A.L., A.A., B.R.C., J.L.B.-S., J.E.H., E.K.S., G.R.W. Data collection: A.A.D., J.C.R., L.Z., R.S.J.E., E.E., N.M., G.R.W. Data analysis: M.-L.N.M., M.H.C., C.P.H., C.L., J.L.B.-S. G.L.K., S.M.L., J.E.H., G.R.W. Statistical support: M.-L.N.M., C.L., S.M.L. All authors contributed to and approved the final draft of the manuscript, the conception, and the design.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: preliminary data for 2010. National Vital Stat Rep. 2012;60:1–52. [PubMed] [Google Scholar]
- 2.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 3.Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31:492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 4.Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-α system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 5.Creutzberg EC, Wouters EFM, Mostert R, Pluymers RJ, Schols AMWJ. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124:1733–1742. doi: 10.1378/chest.124.5.1733. [DOI] [PubMed] [Google Scholar]
- 6.Langen RCJ, Haegens A, Vernooy JHJ, Wouters EFM, de Winther MPJ, Carlsen H, Steele C, Shoelson SE, Schols AMWJ. NF-κB activation is required for the transition of pulmonary inflammation to muscle atrophy. Am J Respir Cell Mol Biol. 2012;47:288–297. doi: 10.1165/rcmb.2011-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, Shale DJ. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 8.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 9.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, Hagan G, Knobil K, Lomas DA, MacNee W, et al. ECLIPSE Investigators. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 11.Güerri R, Gayete A, Balcells E, Ramirez-Sarmiento A, Vollmer I, Garcia-Aymerich J, Gea J, Orozco-Levi M. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD. Respir Med. 2010;104:378–388. doi: 10.1016/j.rmed.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 13.McDonald M-LN, Diaz AA, Ross JC, San Jose Estepar R, Regan EA, Eckbo E, Muralidhar N, Come CE, Cho MH, Hersh CP, et al. Pectoralis muscle area is more highly associated than BMI with COPD severity [abstract]. Poster board presentation at the American Thoracic Society International Conference, Philadelphia, PA, 2013. Am J Respir Crit Care Med. 2013;620:A5454. [Google Scholar]
- 14.Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19:626–631. doi: 10.1183/09031936.02.00279602. [DOI] [PubMed] [Google Scholar]
- 15.Rutten EP, Calverley PM, Casaburi R, Agusti A, Bakke P, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, et al. Changes in body composition in patients with chronic obstructive pulmonary disease: do they influence patient-related outcomes? Ann Nutr Metab. 2013;63:239–247. doi: 10.1159/000353211. [DOI] [PubMed] [Google Scholar]
- 16.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DK, Jacobson FL, Washko GR, Casaburi R, Make BJ, Crapo JD, Silverman EK, Hersh CP. Clinical and radiographic correlates of hypoxemia and oxygen therapy in the COPDGene study. Respir Med. 2011;105:1211–1221. doi: 10.1016/j.rmed.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schols AMWJ, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 21.Han HJ, Park SJ, Min KH, Kim SR, Lee MH, Chung CR, Choi KH, Rhee YK, Jin GY, Chung MJ, et al. Whole-body magnetic resonance imaging for staging metastatic thymic carcinoma. Am J Respir Crit Care Med. 2011;183:1573–1574. doi: 10.1164/ajrccm.183.11.1573. [DOI] [PubMed] [Google Scholar]
- 22.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 23.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 24.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelen MP, Schols AM, Does JD, Wouters EF. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–738. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 26.Shoup R, Dalsky G, Warner S, Davies M, Connors M, Khan M, Khan F, ZuWallack R. Body composition and health-related quality of life in patients with obstructive airways disease. Eur Respir J. 1997;10:1576–1580. doi: 10.1183/09031936.97.10071576. [DOI] [PubMed] [Google Scholar]
- 27.Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:859–867. doi: 10.1053/rmed.2000.0829. [DOI] [PubMed] [Google Scholar]
- 28.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98:944–948. doi: 10.1016/j.amjcard.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ.Epidemiology: an introduction. New York: Oxford University Press; 2002 [Google Scholar]
- 30.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19:433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 31.Bernard S, LeBlanc P, Whittom F, Carrier G, Jobin J, Belleau R, Maltais F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 32.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]