Abstract
Background
Cyclospora is an uncommon pathogen. The diagnosis of Cyclospora infection can be difficult because of its scarcity in developed countries, intracellular mode of life, small size of the parasite and its inability to take up routine microscopic stains. However, it is endemic in many countries in Asia, Africa, Central and South America. With the increase in travels to these areas, the number of cases is expected to increase. Moreover, it is found to be associated with numerous food-borne outbreaks.
Case presentation
We encountered a patient with human immunodeficiency virus presented with 6 months of diarrhoea. The initial investigation was unrevealing. The diagnosis of Cyclospora infection was finally made on the histological sample obtained by colonoscopy. Moreover, the initial therapy with ciprofloxacin was not effective, while trimethoprim/sulfamethoxazole resulted in final cure of the disease.
Conclusion
Travel and food histories are important for the suspicion of Cyclospora infection. Histological examination is more sensitive in making a diagnosis of Cyclospora infection of the gut than fecal microscopic examination. Trimethoprim/sulfamethoxazole is a more reliable therapy for Cyclospora infection in patients with human immunodeficiency virus.
Keywords: Cyclospora, Protozoa, HIV, Prolonged diarrhoea
Background
Cyclospora is a rare pathogen even in human immunodeficiency virus (HIV)-infected population. Cyclospora species belong to the subphylum Apicomplexa, subclass Coccidiasina, and family Eimeriidae. It was first described to be a coccidian parasite causing diarrhoea in patients of Papua New Guinea in 1979 by Ashford though it was thought to be the parasite of the genus Isospora at that time [1]. The parasite was finally named Cyclospora cayetanensis when it was noted to be a distinct organism by morphological and serological examinations [2]. C. cayetanensis has been identified only in humans to date [3]. It is endemic in many regions including India, Indonesia, Thailand, some parts of Africa, Central and South America [4]. It is a significant culprit for travelers’ diarrhoea in developed countries and has been found to be associated with numerous food-borne outbreaks. Food items typically noted to be associated with the infection are fresh fruits and vegetables like raspberries, blackberries, basil and lettuce [4]. The prevalence in developed countries like the United States can vary from 0.01 cases per 100,000 persons to 0.07 cases [5]. In endemic areas, the prevalence is found to be high in children. In a 2-year cross-sectional study of 5836 Peruvian children, the overall prevalence of Cyclospora infection by examining their fecal samples for oocysts, was 1.1% with a peak in those aged 2 to 4 years old (2%) [6]. Another possible risk factor for Cyclospora infestation is HIV infection. An Indian case–control study demonstrated that the fecal positive rate of Cyclospora in HIV positive patients is much higher than that of the HIV negative control (20.44% vs 1.5%, respectively) [7]. We present here an HIV patient with Cyclospora infection and highlight some of the difficulties in the diagnosis and treatment of this disease.
Case presentation
A 38-year-old lady who was otherwise healthy presented with 6-month history of intermittent diarrhoea since her trip to Vietnam 2 weeks before the onset of symptoms. She had no fever, vomiting or abdominal pain. Her stool was watery with no blood or mucus. Her symptoms did not subside despite treatment prescribed by general practitioners. She also had severe weight loss of 5 kg over 6 months. She claimed that she had taken some homemade salad during her stay in Vietnam for her holiday. She worked as a dental nurse in Hong Kong while her husband stayed in Vietnam and used to visit her twice yearly. She did not take any long-term medications before the onset of symptoms nor have any sign and symptoms suggestive of hyperthyroidism. Her blood counts including hemoglobin, white blood cells and platelet counts were normal when she presented to us 6 months after onset of symptoms. The renal and liver functions tests were also unremarkable expect for a low albumin level of 33 g/L. Her thyroid function tests, fasting glucose, calcium and phosphate levels were all normal.
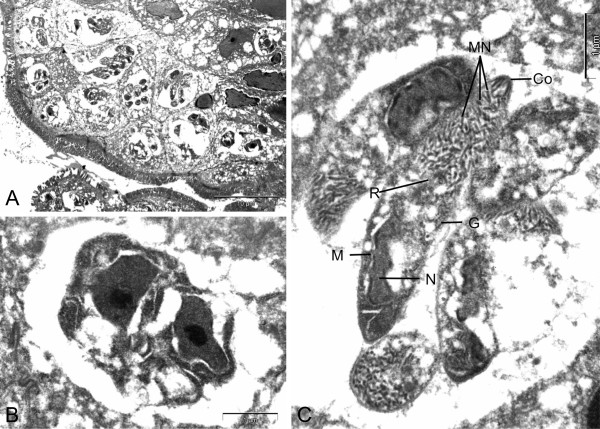
Microscopic examination and culture on repeated stool samples collected within 6 months’ time did not yield any bacteria, virus, or parasites initially. In view of the prolonged diarrhoea, an upper gastrointestinal endoscopy (UGIE) and a colonoscopy were performed. The UGIE only identified mild gastritis and the duodenum appeared normal. The colonoscopy showed only a small area of erythema over the descending colon with normal terminal ileum and other parts of colon. However, numerous intracellular protozoa could be identified at the apical half of the enterocytes of both the terminal ileum and the duodenum. Villus blunting with non-specific inflammatory cells infiltration of the terminal ileum was also noted (Figure 1). The electron microscopy of the parasite demonstrated a typical morphology of an Apicomplexa organism. The merozoites were surrounded by layers of thick parasitophorous vacuoles (Figure 2, Panel A). A binucleated schizont could be seen inside a parasitophorous vacuole (Figure 2, Panel B). Mature merozoites could also be identified (Figure 2, Panel C). Further stool samples using modified acid-fast staining showed typical Cyclospora oocyst (Figure 3, Panel A). An unstained oocyst, the “ghost” cell, could also be found (Figure 3, Panel B).
Figure 1.
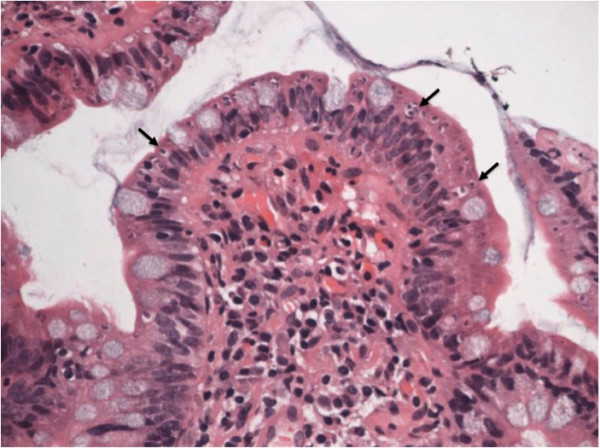
Histology of terminal ileum showing villus blunting and the presence of multiple intracellular protozoa (arrows) at the apical half of the enterocytes of the terminal ileum.
Figure 2.
Electron microscopy of the terminal ileum. Panel A: The Cyclospora merozoites were surrounded by layers of thick parasitophorous vacuoles at the apical end of the enterocytes. Panel B: A binucleated schizont can be seen inside a parasitophorous vacuole. Panel C: Cyclospora merozoites showing typical features of an Apicomplexa organism: Co: Conoid with polar rings, G: Golgi body, MN: Micronemes, N: Nucleus, R: Rhoptry, M: Mitochondria.
Figure 3.
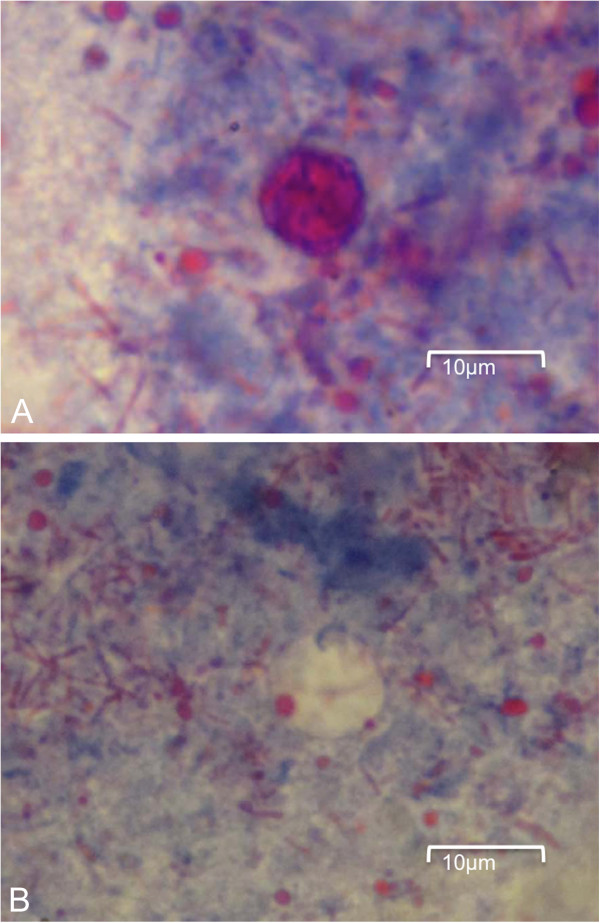
Stool microscopy (modified Ziehl-Neelsen acid-fast stain). Panel A: Circular Cyclospora oocyst of size about 9 microns. Panel B: An unstained Cyclospora oocyst, the “ghost” cell.
Further history revealed that her husband had multiple sexual partners in Vietnam. The lady was subsequently diagnosed to be HIV positive and the baseline CD4 count was only 98 cells/μL with HIV ribonucleic acid (RNA) of 420,000 copies/mL. She was initially treated with 2 weeks of ciprofloxacin 500 mg twice daily. However, her response was suboptimal. Trimethoprim/sulfamethoxazole (TMP-SMX) 960 mg twice daily was then introduced and her symptoms totally subsided after two weeks. Anti-retroviral therapy (ART) was also prescribed and her CD4 count went up to 153 cells/μL in half-year’s time. Her HIV RNA had become undetectable after 3 months of ART and she remained well. She also had weight gain of 5 kg after 6 months of ART.
Discussion
Diagnosis of Cyclospora infection can be difficult because of its scarcity in developed countries, intracellular mode of life, small size of the parasite and its inability to take up routine microscopic stains. Difficulties in making a diagnosis of Cyclospora infection in our patient, especially in non-endemic areas, are illustrated. However, history of travelling to Vietnam with fresh fruit or vegetable exposure can be the clues, since the Cyclospora oocysts can be identified in vegetables, environmental water samples and patients suffering from diarrhoeal diseases in Vietnam [8-10]. In fact, the Vietnam coriander, which is commonly used in the Southeast Asian cooking, was found to be the most frequent vegetable contaminated with Cyclospora oocysts (11.6%) [8]. The symptoms of Cyclospora infection vary widely, depending on the immune status, age of the patient, and the infecting dose of the parasites. In endemic areas, the symptoms of infection are generally milder as a result of the presence of partial immunity. The vulnerable groups are usually the elderly and young children [11,12]. In non-immune individuals, the symptoms are more severe and usually come after a median incubation period of about 7 days. Typically they present with nausea, abdominal cramp, watery diarrhoea, low-grade fever and weight loss, lasting for weeks and even months [3,13]. Just as the case in our patient, the symptoms are more severe and prolonged in the HIV population [14,15]. Extra-intestinal forms of Cyclospora infection including biliary disease, acalculous cholecystitis or Reiter syndrome may occur, especially in the HIV population [15-17].
Cyclospora is an uncommon pathogen and many laboratories may not be experienced enough in making a diagnosis. Therefore routine stool examination for ova or cysts may not be adequate to pick up the Cyclospora oocysts. This was probably one of the reasons for the delayed diagnosis in our patient. Examining the fresh stool specimen for oocysts by using modified Ziehl-Neelsen acid-fast staining (MAFS) is helpful. However, the Cyclospora oocysts take up MAFS quite variably. The use of strong decolorizing agent in the MAFS may remove too much color in the Cyclospora oocysts, and it may then appear as unstained oocysts (ghost cells). In view of the small number of oocysts passed by the infected cases, the sensitivity for diagnosis may be enhanced by concentration and purification techniques [2,18]. Increasing the number of fecal samples for examination may further enhance the detection rate despite the absence of studies in this area. It is equally important to distinguish the oocysts of Cyclospora from those of Cryptosporidium, which is an even more common protozoa causing enteritis in HIV patients. In the past, the Cyclospora oocysts have been misdiagnosed as the “Big Crypto” since both oocysts are acid-fast [19]. However, the sizes of the oocysts can help to distinguish the two species. Cyclospora oocysts have a diameter of about 8–10 microns while those of Cryptosporidium measure about 5 microns [3,4]. As a result of the autofluorescence nature of the Cyclospora oocysts, the diagnosis can also be secured by examining the specimen under the fluorescence microscope. However, the sensitivity for microscopic detection may drop even with the concentration technique if the oocysts load is low, especially in patients with mild symptoms. Polymerase chain reaction may be helpful in this regard for its high sensitivity and specificity, though it is not widely available [3,20]. Unfortunately no serologic tests for Cyclospora are available so far. In highly suspected cases with negative fecal investigation, early enteroscopy with biopsy over small intestine may be imperative in making a diagnosis. Similar to our patient, there may be minimal or no sign of inflammation over the duodenum or terminal ileum under the endoscopic view [21,22]. However, typical histological findings of the small intestinal biopsy can be identified in our patient. They include blunting of the villi due to edema and infiltration by a mixture of inflammatory cells, vascular dilatation and congestion of the villous capillaries, and parasitophorous vacuoles containing sexual and asexual forms of Cyclospora protozoa [21,22]. Moreover, the parasites are usually present in the supranuclear area of the enterocytes and absent in the crypts [23].
Cyclospora infection is usually self-limiting in immunocompetent individuals, though relapse can occur. Seven to ten days of oral double strength TMP-SMX is the standard therapy [3,4,12]. The duration of treatment can be longer if symptoms persist. Many alternative medications have failed to demonstrate efficacies similar to that of TMP-SMX in eradicating the protozoa. A randomized trial in HIV patients has suggested that ciprofloxacin 500 mg twice daily for 7 days is an acceptable alternative to TMP-SMX, especially in patients with sulfa allergy [24]. However, the use of ciprofloxacin in our patient was not effective. Since recurrence can occur in up to 43% of Cyclospora infected HIV patients after effective treatment, secondary prophylaxis with double strength TMP-SMX three times weekly can be considered [14]. Nitazoxanide, a thiazolide agent with activities against many parasites, may also be considered in cases who have sulfa allergy or fail to respond to sulfa or ciprofloxacin [25,26].
Conclusions
In summary, Cyclospora is an uncommon pathogen and may be difficult to be diagnosed. Meticulous history taking with special focus on travel and food intake is imperative. Microscopic examination of the concentrated fecal specimen using MAFS and /or immunofluorescence staining helps to identify the Cyclospora oocysts. Early enteroscopy with small bowel biopsy may be required in some cases and the typical histological findings can help to coin the diagnosis. TMP-SMX remains to be the standard therapy and quinolones appear to be less useful in some cases.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
OTYT drafted and wrote the manuscript and provided therapy for the patient. RWCW performed histological examination, electron microscopy and contributed figures. JMCC, KYT and WSL were involved in the clinical guidance of the patient. BHSL performed the microbiological examinations and contributed figures. All authors read and approved the final manuscript.
Contributor Information
Owen Tak-yin Tsang, Email: tyotsang@yahoo.com.hk.
Richard Wing-cheuk Wong, Email: richardwcwong@yahoo.com.hk.
Bosco Hoi-shiu Lam, Email: lamhsb@ha.org.hk.
Jacky Man-chun Chan, Email: jacky_chanmc@yahoo.com.hk.
Kay-yan Tsang, Email: tsangky1@ha.org.hk.
Wai-shing Leung, Email: leungws4@ha.org.hk.
References
- Ashford RW. Occurrence of an undescribed coccidian in man in Papua New Guinea. Ann Trop Med Parasitol. 1979;6:497–500. doi: 10.1080/00034983.1979.11687291. [DOI] [PubMed] [Google Scholar]
- Ortega YR, Sterling CR, Gilman RH, Cama VA, Diaz F. Cyclospora species—a new protozoan pathogen of humans. N Engl J Med. 1993;6:1308–1312. doi: 10.1056/NEJM199305063281804. [DOI] [PubMed] [Google Scholar]
- Ortega YR, Sanchez R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Microbiol Rev. 2010;6:218–234. doi: 10.1128/CMR.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Olson BH. Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int J Parasitol. 2003;6:371–391. doi: 10.1016/S0020-7519(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Hall RL, Jones JL, Herwaldt BL. Surveillance for laboratory-confirmed sporadic cases of cyclosporiasis–United States, 1997–2008. MMWR Surveill Summ. 2011;6:1–11. [PubMed] [Google Scholar]
- Madico G, McDonald J, Gilman RH, Cabrera L, Sterling CR. Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin Infect Dis. 1997;6:977–981. doi: 10.1093/clinids/24.5.977. [DOI] [PubMed] [Google Scholar]
- Tuli L, Singh DK, Gulati AK, Sundar S, Mohapatra TM. A multiattribute utility evaluation of different methods for the detection of enteric protozoa causing diarrhea in AIDS patients. BMC Microbiol. 2010;6(10):11. doi: 10.1186/1471-2180-10-11. doi: 10.1186/1471-2180-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tram NT, Hoang LM, Cam PD, Chung PT, Fyfe MW, Isaac-Renton JL, Ong CS. Cyclospora spp. in herbs and water samples collected from markets and farms in Hanoi, Vietnam. Trop Med Int Health. 2008;6:1415–1420. doi: 10.1111/j.1365-3156.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- Uga S, Hoa NT, le Thuan K, Noda S, Fujimaki Y. Intestinal parasitic infections in schoolchildren in a suburban area of Hanoi, Vietnam. Southeast Asian J Trop Med Public Health. 2005;6:1407–1411. [PubMed] [Google Scholar]
- Miegeville M, Koubi V, Dan LC, Barbier JP, Cam PD. Cyclospora cayetanensis presence in aquatic surroundings in Hanoi (Vietnam). Environmental study (well water, lakes and rivers) Bull Soc Pathol Exot. 2003;6:149–152. [PubMed] [Google Scholar]
- Behera B, Mirdha BR, Makharia GK, Bhatnagar S, Dattagupta S, Samantaray JC. Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig Dis Sci. 2008;6:672–679. doi: 10.1007/s10620-007-9927-9. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis. 2000;6:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- Huang P, Weber JT, Sosin DM, Griffin PM, Long EG, Murphy JJ, Kocka F, Peters C, Kallick C. The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Ann Intern Med. 1995;6:409–414. doi: 10.7326/0003-4819-123-6-199509150-00002. [DOI] [PubMed] [Google Scholar]
- Pape JW, Verdier RI, Boncy M, Boncy J, Johnson WD Jr. Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment, and prophylaxis. Ann Intern Med. 1994;6:654–657. doi: 10.7326/0003-4819-121-9-199411010-00004. [DOI] [PubMed] [Google Scholar]
- Sifuentes-Osornio J, Porras-Cortes G, Bendall RP, Morales-Villarreal F, Reyes-Teran G, Ruiz-Palacios GM. Cyclospora cayetanensis infection in patients with and without AIDS: biliary disease as another clinical manifestation. Clin Infect Dis. 1995;6:1092–1097. doi: 10.1093/clinids/21.5.1092. [DOI] [PubMed] [Google Scholar]
- de Go’rgolas M, Fortes J, Fernandez Guerrero ML. Cyclospora cayetanensis cholecystitis in a patient with AIDS. Ann Intern Med. 2001;6:166. doi: 10.7326/0003-4819-134-2-200101160-00021. [DOI] [PubMed] [Google Scholar]
- Connor BA, Johnson EJ, Soave R. Reiter syndrome following protracted symptoms of Cyclospora infection. Emerg Infect Dis. 2001;6:453–454. doi: 10.3201/eid0703.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Kumar RS, Takemasa K, Ishibashi Y, Kawabata M, Belosevic M, Uga S. Comparison of three microscopic techniques for diagnosis of Cyclospora cayetanensis. FEMS Microbiol Lett. 2004;6:263–266. doi: 10.1016/j.femsle.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Henriksen SA, Pohlenz JFL. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;6:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein EM, El-Moamly AA, Dawoud HA, Fahmy H, El-Shal HE, Sabek NA. Real-time PCR and flow cytometry in detection of Cyclospora oocysts in fecal samples of symptomatic and asymptomatic pediatrics patients. J Egypt Soc Parasitol. 2007;6:151–170. [PubMed] [Google Scholar]
- Ortega YR, Nagle R, Gilman RH, Watanabe J, Miyagui J, Quispe H, Kanagusuku P, Roxas C, Sterling CR. Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J Infect Dis. 1997;6:1584–1589. doi: 10.1086/514158. [DOI] [PubMed] [Google Scholar]
- Connor BA, Reidy J, Soave R. Cyclosporiasis: clinical and histopathologic correlates. Clin Infect Dis. 1999;6:1216–1222. doi: 10.1086/514780. [DOI] [PubMed] [Google Scholar]
- Sun T, Ilardi CF, Asnis D, Bresciani AR, Goldenberg S, Roberts B, Teichberg S. Light and electron microscopic identification of Cyclospora species in the small intestine. Evidence of the presence of asexual life cycle in human host. Am J Clin Pathol. 1996;6:216–220. doi: 10.1093/ajcp/105.2.216. [DOI] [PubMed] [Google Scholar]
- Verdier RI, Fitzgerald DW, Johnson WD, Pape JW. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. Ann Intern Med. 2000;6:885–888. doi: 10.7326/0003-4819-132-11-200006060-00006. [DOI] [PubMed] [Google Scholar]
- Diaz E, Mondragon J, Ramirez E, Bernal R. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg. 2003;6:384–385. [PubMed] [Google Scholar]
- Zimmer SM, Schuetz AN, Franco-Paredes C. Efficacy of nitazoxanide for cyclosporiasis in patients with sulfa allergy. Clin Infect Dis. 2007;6:466–467. doi: 10.1086/510744. [DOI] [PubMed] [Google Scholar]