Abstract
Introduction
Apical periodontitis is an inflammatory disease of the periradicular tissues caused by the host’s immune response to infection of the root canal system. microRNAs (miRNAs) have been shown to play an important role in the regulation of inflammation and the immune response; however, their role in the pathogenesis of endodontic periapical disease has not been explored. The purpose of this study was to examine the differential expression of miRNAs in diseased periapical tissues as compared to healthy controls.
Methods
We first compared miRNA profiles in diseased periapical tissues collected from patients undergoing endodontic surgery to that of healthy pulps using microarray analyses. The target genes of the differentially expressed miRNAs were identified using miRWalk and PUBMED. Selected miRNAs linked to inflammation and the immune response were then confirmed in a separate cohort of diseased and healthy tissues using quantitative RT-PCR. Healthy pulps and periodontal ligaments were used as controls. Data was normalized to the level of SNORD 44 which served as an endogenous control.
Results
Of the 381 miRNAs identified using microarray, 24 miRNAs were down-regulated in diseased periapical tissues compared to controls (n=13) (P<0.003). The down-regulation of 7 miRNAs was confirmed from 9 selected miRNAs using qRT-PCR (n=19) (P<0.05). Target genes of these miRNAs include key mediators in the immune and inflammatory response such as of IL-6, MMP-9 and TGF-β.
Conclusions
These findings offer new insight into the pathogenesis of endodontic disease and have the potential to impact the development of new methods for prevention, diagnosis, and treatment of apical periodontitis.
Keywords: microRNA, periapical, microarray, bioinformatics, non-coding RNA
Introduction
The discovery of microRNAs (miRNAs) is one of the major scientific breakthroughs in recent years and has dramatically changed the view of a linear relationship between gene and protein expression (1). miRNAs are short, non-coding, single stranded RNA molecules that mediate RNA-interference through post-transcriptional modulation of gene expression. They silence genes by binding to complementary sequences of their respective target messenger RNAs either inhibiting their translation into proteins or initiating cleavage of the messenger RNA leading to its degradation (2).
miRNAs play a fundamental role in mediating biological events and are involved in virtually all physiologic processes (1). They have also been implicated in a multitude of pathologic states such as inflammatory diseases, cancer, developmental abnormalities, cardiovascular diseases and neurodegenerative disorders (3–7). miRNAs are emerging as novel biomarkers of disease, prognostic indicators, and targets for drug therapy. Their high sequence conservation across species and tissue specificity make them ideal biomarkers (8). Stable miRNAs have recently been identified in many body fluids including saliva and plasma, which allows for a non-invasive means to measure miRNA profiles (9, 10). Functional miRNAs have been discovered in exosomes, which presents a novel strategy to deliver RNA therapeutic agents (11). miRNA-based technology is currently being implemented in a wide range of applications such as cancer diagnosis and prognosis, predicting risk of transplant rejection, determining the quality of stored blood, and prenatal diagnostics (12).
The role of miRNAs in orofacial inflammation is just beginning to be explored. Altered miRNA expression levels have been demonstrated in periodontal disease by comparing healthy and inflamed gingival tissues (13–15). The first miRNA study in the field of endodontics found significant differential expression of several miRNAs between healthy and diseased pulps (16). Although some insight has been gained on the role of miRNAs in endodontic pulpal disease, its role in endodontic periapical pathogenesis has not been explored. The purpose of this study is to determine the differential expression of miRNAs in diseased periapical tissues by comparing the miRNA profiles of diseased periapical tissues and healthy control tissues.
Materials and Methods
Study participants and sample collection
This study was approved by our Institutional Review Board and written informed consent was obtained from all study participants. The inclusion criteria were patients of age ≥12 years old and American Society of Anesthesiologists class I or II. Patients who were immune compromised or currently taking antibiotics or other medications known to influence the immune response were excluded from the study.
Diseased periapical tissues were collected from teeth undergoing surgical endodontic treatment (apicoectomy). These teeth had previous non-surgical endodontic treatment and were associated with a non-healing periapical lesion. The majority of these cases were associated with asymptomatic chronic lesions with the exception of two cases, in which pain symptoms were associated with the tooth from which the sample was collected. During the apicoectomy procedure, granulation tissue from the periapical lesion was curetted from the bony cavity prior to root end resection. Two different types of tissues were used as controls: normal periodontal ligament (PDL) and pulp tissues. These were collected from extracted non-carious third molars or premolars. Healthy PDL was collected immediately following extraction using a scaling instrument to separate the tissues from the surface of the root. Pulp tissue was extirpated using sterilized barbed broaches immediately after extraction. Tissues samples were placed in a sterile eppendorf tube with 0.5ml RNAsafer Stabilizer Reagent (VWR, Bridgeport, NJ) and stored at −80°C until processing. Thirteen samples (eight diseased periapical tissues and five healthy pulps) were used for the microarray experiment and 19 samples (eight diseased periapical tissues, eight periodontal ligaments and three healthy pulps) were used for qRT-PCR.
RNA isolation and miRNA microarray
Samples were thawed on ice and centrifuged at 4°C for 2 minutes at 12,000 rpm to remove the stabilizer reagent. Total RNA was extracted using the miRNeasy Mini kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. The RNA was quantitated using the NanoDrop (Thermo Scientific, Wilmington, DE) and RNA integrity assessed using the 2100 Bioanalyzer (Agilent, Foster City, CA). The miRNA expression profiles were interrogated using Human miRNA Microarrays (V3) and the miRNA Complete Labeling and Hyb Kit (both from Agilent Technologies, Santa Clara, CA). The microarrays consist of glass slides containing 8 identical 15K oligonucleotide microarrays incorporating probes for 866 human miRNAs represented from the Sanger miRBase 12.0. The procedure was performed as described previously (16). Slides were scanned using the Agilent Microarray Scanner and the Agilent Feature Extraction Software version 10.5.1.1 (both from Agilent, Foster City, CA).
Bioinformatics miRNA analysis and target selection
Potential mRNA target genes for differentially expressed miRNAs in diseased periapical tissues were identified using miRWalk (http://www.rna.uni-heidelberg.de/apps/zmf/mirwalk/index_html). miRWalk is a comprehensive database that provides information on human and murine miRNAs on their predicted and validated targets associated with genes, pathways, diseases, organs, cell lines and transcription factors. It is based on a comparison of computed mRNA 3′ UTRs miRNA binding sites with 8 miRNA-target prediction programs. Candidate mRNAs were selected if they were identified as validated miRNA targets in at least 5 out of 8 databases and were linked to immunity, inflammation and pain by GO Biological Process (www.geneontology.org). Results from miRWalk and PUBMED search were integrated to reach our final results.
Quantitative RT-PCR
9 miRNAs that demonstrated significant differential expression in the microarray analysis and are linked to inflammation and the immune response were selected for further validation using qRT-PCR. miScript primers and miScript II RT Kit were purchased from Qiagen (Germantown, MD, USA). Total RNA (450ng) was reverse transcribed using cDNA synthesis kit according to manufacturer’s instructions. The PCR reactions were run using miRNA specific primers and the miScript universal primer (Qiagen, Catalogue #: MS00006692, MS00006699, MS00008841, MS00031500, MS00007588, MS00009744, MS00031878, MS00010906, MS00003570, MS00007518). Reaction mixes were prepared using 2X EvaGreen Master Mix (Biotium, Hayward, CA, USA), 2 μl of 1:10 diluted cDNA, and 10 pmoles of each forward and reverse primer (total 20 μl). The real-time PCR was carried out in the StepOne 7500 thermocycler (Applied Biosystems, Carlsbad CA, USA). SNORD 44 served as an internal control and all reactions were run in triplicates. SNORD 44 was selected from a total of 3 endogenous controls due to its’ relatively constant expression in the tissues used for this study.
Statistical analysis
For microarray data analysis, any expression value that was lower than the reported error for that particular gene (which includes negative expression values) was set to be equal to the estimated error rate. Quantile normalization was applied to the expression data. To identify genes that were differentially expressed in each group, we applied a permutation test to test the null hypothesis that the mean expression of each gene was the same in both groups. An exact hypothesis test was used since the sample size was small. We used the resulting p-values to estimate the false discovery rate q-value when the differential expression of each miRNA is called “significant”. For each resulting p-value, we computed the q-value, which is defined to be the false discovery rate when all tests with a p-value less than or equal to the given p-value are called “significant.” For qRT-PCR analysis, the relative expression of miRNA as compared SNORD44 was computed using the 2(−ΔΔCt) method (17). Significance was determined by applying Welch’s t-test to the relative fold changes of periapical tissues and control tissues. Differences were considered significant when the probability value was less than 5% (P<0.05).
Results
No significant differences were noted in gender distribution between experimental and control groups. However, there was a significant difference in age between subjects from which periapical tissue were collected and subjects from which healthy PDL and pulp were collected (P < 0.05). The mean age of the periapical group was 53 yrs. (±15), while that of the PDL controls was 28 yrs. (±16), and that of the pulp group was 18 yrs. (±3).
Microarray results
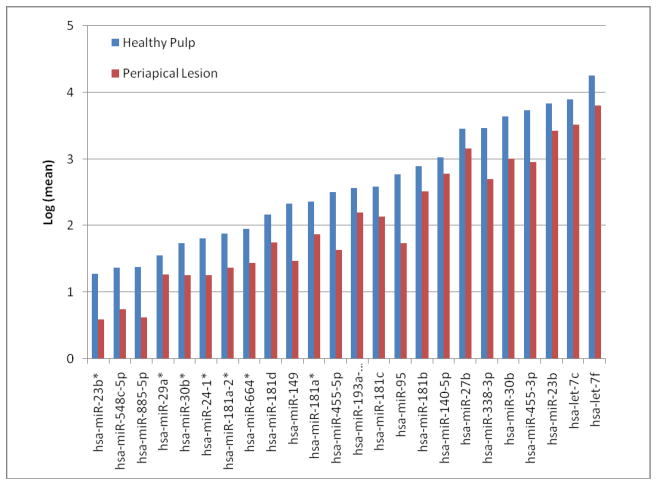
Three hundred and eighty one miRNAs were identified in periapical tissues, of which 24 miRNAs were significantly down-regulated in diseased periapical tissues compared to healthy pulp tissues (P < .003, q < .08). Fifteen miRNAs showed a fold regulation of −2 to −5 and six miRNAs had more than a -5fold regulation (Fig. 1). Of the 24 down-regulated miRNAs identified, nine miRNAs that are linked to inflammation and immunity were selected for further analysis with qRT-PCR.
Figure 1.
Differential expression of miRNAs in healthy pulp (n = 5) and diseased periapical tissues (n = 8) evaluated by microarray. Twenty-four miRNAs were significantly down-regulated in diseased periapical tissues (P < .003, q < .08). Data was analyzed using an exact hypothesis test and expressed as log values.
qRT-PCR results
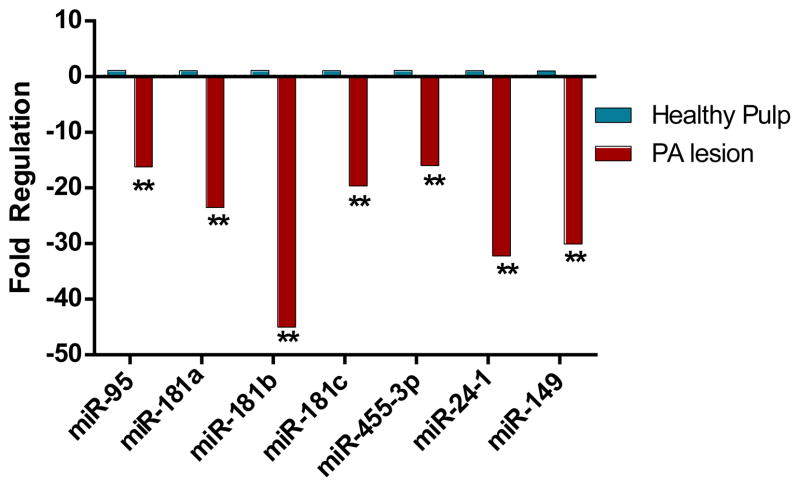
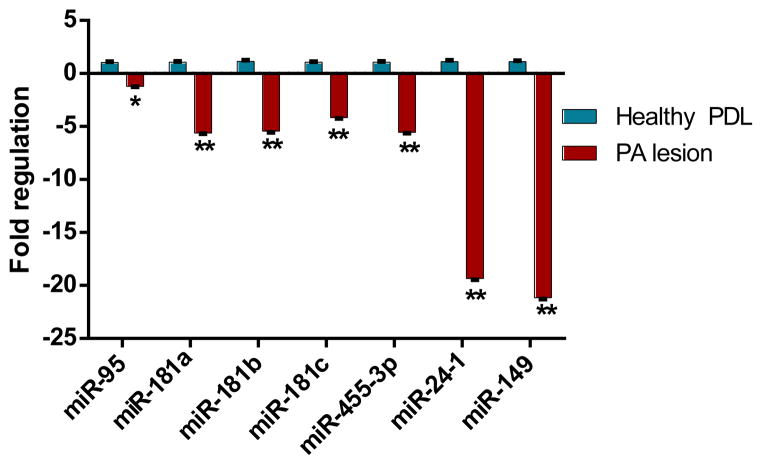
The expression of the nine miRNAs examined did not differ between healthy pulps and PDL. Seven of the nine miRNAs examined were downregulated in diseased periapical tissues as compared to healthy pulp tissues (P ≤ .001) (Fig 2). These same seven miRNAs were also down-regulated when comparing diseased periapical tissues to healthy PDL (P ≤ .005) (Fig. 3). On comparing the relative fold change in miR-95 expression in diseased periapical lesions as compared to normal PDL, the levels were not altered (1.2 fold) to the extent observed for other miRNAs. Nonetheless, the expression pattern is qualitatively similar to data comparing the diseased samples to healthy pulps as well as the microarray data.
Figure 2.
Relative fold changes of seven miRNAs in diseased periapical and healthy pulp tissues analyzed by qRT-PCR. Data was normalized to expression of endogenous control, SNORD44. Relative expression computed using the 2(−ΔΔCt) method. **P ≤ .001
Figure 3.
Relative fold changes of seven miRNAs in diseased periapical and healthy periodontal ligament tissues analyzed by qRT-PCR. Data was normalized to expression of endogenous control, SNORD44. Relative expression computed using the 2(−ΔΔCt) method. * P ≤ 0.05, * *P ≤ .001
Bioinformatics data
The potential targets of the differentially expressed miRNAs identified include key cytokines involved in inflammation (IL-6, IL-10), chemokines (CCL8), pathogen recognition receptors (TLR-4), growth factors (TGF-β1, VEGF-α), and proteins of the matrix metalloproteinase (MMP) family (MMP-9).”(Table 1).
Table 1.
Potential miRNA target genes identified with PUBMED and miRWalk with gene product functions determined by GO biologic process.
| miRNA | Target gene | Gene product function | GO Term (Accession, Ontology) |
|---|---|---|---|
| hsa-miR-181a | TLR-4 | Toll-like receptor-4 (TLR) plays a fundamental role in pathogen recognition and activation of innate immunity | GO:0035662, Molecular Function |
| IL-6 | Acute and chronic inflammation and the maturation of B cells; T helper (Th)17 differentiation | GO:0019981, Molecular Function | |
| DUSP5 DUSP6 PTPN11 PTPN22 |
Regulation of early B/T cell development; Positive regulation of T Cell Receptor (TCR)-signalling; Determination of T helper (Th) subset differentiation; Establishment of central tolerance/autoimmunity | GO:0070373, Biological Process | |
| hsa-miR-181b | IL-6 | Negative regulation of cytokine secretion; negative regulation of collagen biosynthetic process; positive regulation of acute inflammatory response; response to cold, heat and mechanical stimuli; T Helper (Th)17 differentiation | GO:0005138, Molecular Function |
| CCL8 | Immune response, inflammatory response, chemokine activity, phospholipase activator | ||
| MMP9 | Cell response to IL-1, LPS; macrophage differentiation; response to heat and mechanical stimuli; positive regulation of apoptosis and angiogenesis | GO:0004229, Molecular Function | |
| TGFB1 | Adaptive immune response; positive regulation of collagen biosynthesis, chemotaxis, fibroblasts migration, and ondontogenesis; T helper (Th)17 differentiation, T regulatory (Treg) differentiation | GO:0034713, Molecular Function | |
| hsa-miR-181c | SOCS1 | Cytokine mediated signaling pathway; negative regulator of JAK-STAT pathway; LPS response | |
| IL-2 | Cytokine produced by T-cells in response to antigen or mitogen stimulation | GO:0005134, Molecular Function | |
| hsa-miR-24 | MAPK14 | MAP kinase activated by various environmental stresses and proinflammatory cytokines, act as an integration point for multiple biochemical signals | GO:0051403, Biological Process |
| hsa-miR-95 | EIF2C2 | Eukaryotic translation initiation factor interacted with dicer1 in short- interfering-RNA-mediated gene silencing | |
| hsa-miR-149 | VEGFA | Increased vascular permeability, angiogenesis, vasculogenesis and endothelial cell growth, promoting cell migration, and inhibiting apoptosis | GO:0035924, Biological Process |
| has-miR-455 | IL-10 | Pleiotropic effects in immunoregulation and inflammation, B cell survival, proliferation, and antibody production, block NF-kappa B activity | GO:0019969, Molecular Function |
| TLR-4 | Toll-like receptor-4 (TLR) plays a fundamental role in pathogen recognition and activation of innate immunity | GO:0035662, Molecular Function |
Discussion
In this study, multiple miRNAs from the miR-181 family (miR-181a*, miR-181b and miR-181c) were demonstrated to be significantly down-regulated in diseased periapical tissues compared to healthy controls. Down-regulation of miRNAs, which are negative regulators themselves, results in an increase in their respective target messenger RNAs. The targets of miR-181a* include toll-like receptor-4 (TLR-4) (18), which plays a key role in pathogen recognition and activation of the innate immune response, and IL-6, which stimulates neutrophil production and supports B-cell maturation (19). miR-181b targets CCL-8, MMP-9 and TGF-β1, which are involved in a wide range of inflammatory pathways. For example, CCL-8 is chemotactic for and activates several immune cells including monocytes, T cells and NK cells (20). MMP-9 is closely associated with macrophage differentiation and TGF-β1 increases collagen biosynthesis and fibroblast proliferation (21, 22). The targets of miR-181c include SOCS1, which is involved in the LPS response, and IL-2, which plays an essential role in the immune response to antigenic stimuli and is important for the proliferation of T and B lymphocytes (23). The miR-181 family is also notable for altering T-cell receptor signaling and increasing IFN-γ/IL-17 production by Th1/17 cells (24).
Increasing evidence supports the role of the miR-181 family in inflammatory pathologies. Circulating levels of miR-181a, miR-181a-2* and miR181c in whole blood were significantly lower in patients diagnosed with complex regional pain syndrome, a disorder in which neurogenic inflammation plays a key role (25). Blood plasma levels of miR-181b was found to be lower in patients with sepsis (a whole body inflammatory condition due to infection) and in animal models of sepsis (26). Data from our previous study show that members of the miR-181 family are significantly down-regulated in inflamed human pulps as compared to normal pulps (16). Conversely, miR-181c is upregulated in inflamed gingival tissues (15). These differences in expression of miR-181c in distinct inflammatory pathologies could possibly be due to differences in the type of infection or the host tissue or the sampling point after initiation of inflammation. Thus, despite the similarities in expression of this miRNA family with other inflammatory conditions, it is important to note that a direct comparison is to be avoided due to the diversity of the complex regulatory mechanisms across different pathologic processes and different tissues.
In addition to the miR-181 family, miR-24-1*, miR-95, miR-149 and miR-455-3p were significantly down-regulated in diseased periapical tissues. These miRNAs also have a variety of targets that are implicated in the immune and inflammatory response. For example, miR-149 targets VEGF-α, which acts on endothelial cells to mediate increased vascular permeability and promote cell migration to the site of inflammation (27). miR-455-3p targets TLR-4 as well as IL-10, a cytokine produced primarily from monocytes that serve to enhance B cell survival, proliferation and antibody production (28).
A limited number of studies have examined miRNA expression in inflammation related to endodontic infection. Our previous study examined the differential expression of miRNAs in inflamed and healthy pulps using microarray techniques (16). When comparing our microarray data to that of our previous study, we see that there is significant differential expression of 13 of the same miRNAs in both periapical and pulp tissues. These include: miR-29a*, miR-30b*, miR-181a-2*, miR-181d, miR-455-5p, miR199-5p and miR-664. All seven of the significantly down-regulated miRNAs identified in diseased periapical tissues were also shown to be down-regulated in inflamed pulp tissue compared to healthy pulp tissue. The six of which showed significant down-regulation in both tissues include: miR-24-1*, miR-95, miR-181a*, miR-181b, miR-181c and miR-455-3p. The extent of this cross-over suggests some common miRNA regulatory network in pulpal and periapical disease pathogenesis. This could be due to similar inflammatory processes occurring in both tissues. However, the tissue specificity of miRNAs is apparent in the difference between the types of miRNAs identified in pulp and periapical tissues using microarray. Although some overlap exists, it is possible that the other miRNAs identified in this study could be unique to the periapical disease process.
In the present study we first identified differentially expressed miRNAs in diseased periapical tissues as compared to healthy pulps using microarray analysis. We then collected a separate cohort of healthy and diseased samples to validate these findings using qRT-PCR. For this second experiment both pulp and PDL were used as control tissues. We included PDL as a control as the normal periapex consist of PDL and bone tissue. Bone tissue was not used as its removal is not recommended in apicoectomy procedures. It is also relatively acellular. Despite using a separate cohort of samples for the qRT-PCR, the results confirmed the down-regulation of the same miRNAs identified using microarray. Also, the same miRNAs were consistently down-regulated in diseased periapical tissues when comparing to both pulp and PDL control tissues. This correlation strengthens our findings and supports the inclusion of the selected control tissues.
The limitations of this study include the absence of age matched controls. Participants in the control groups consisted of individuals undergoing extraction of third molars or premolars for orthodontic purposes. They were significantly younger than participants in the periapical group who were patients undergoing apicocectomy surgery. We did not differentiate between periapical cysts and granulomas nor did we differentiate between symptomatic and asymptomatic teeth. Studies that implement in situ hybridization techniques could be used to determine the cellular sources of the identified miRNAs and at the same time differentiate between periapical cysts and granulomas. Future studies that include a larger number of teeth with periapical lesions are needed to identify miRNAs that correlate with odontogenic pain.
This study explores the role of miRNAs in endodontic disease and provides new insight into the genetic regulation of endodontic periapical pathogenesis. This study offers potential candidates for further investigation of miRNAs in endodontic disease. These findings could facilitate the development of potential biomarkers and possible therapeutic targets for the treatment of endodontic disease.
Acknowledgments
This work was supported by the School of Dentistry, University of North Carolina-Chapel Hill and NIH, NIDCR R01 DE021052.
Footnotes
The authors deny any conflicts of interest.
References
- 1.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–40. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Ryan B, Robles A, Harris C. Genetic variation in microRNA networks: The implications for cancer research. Nat Rev Cancer. 2010;10(6):389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Thum T, Catalucci D, Bauersachs J. MicroRNAs: Novel regulators in cardiac development and disease. Cardiovascular Research. 2008;79(4):562–70. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- 6.Zovoilis A, Agbemenyah HY, Agis-Balboa R, Stilling RM, Edbauer D, Rao P, et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30(20):4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SMV, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthritis and Cartilage. 2009;4;17(4):464–72. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: A new source of biomarkers. Mutation Research. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Ladomery MR, Maddocks DG, Wilson ID. MicroRNAs: Their discovery, biogenesis, function and potential use as biomarkers in non-invasive prenatal diagnostics. Int J Mol Epidemiol Genet. 2011;2:253–260. [PMC free article] [PubMed] [Google Scholar]
- 13.Stoecklin-Wasmer C, Guarnieri P, Celenti R, Demmer R, Kebschull M, Papapanou P. MicroRNAs and their target genes in gingival tissues. Journal of Dental Research. 2012;91(10):934–40. doi: 10.1177/0022034512456551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. Journal of Dental Research. 2012;91(1):33–8. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Shu R, Jiang S, Liu D, Zhang X. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral Sci. 2011;3:134. doi: 10.4248/IJOS11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong S, Zhang S, Bair E, Nares S, Khan A. Differential expression of MicroRNAs in normal and inflamed human pulps. Journal of Endodontics. 2012;38(6):746–52. doi: 10.1016/j.joen.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Naeem A, Zhong K, Moisá SJ, Drackley JK, Moyes KM, Loor JJ. Bioinformatics analysis of microRNA and putative target genes in bovine mammary tissue infected with Streptococcus uberis. J Dairy Sci. 2012 Nov;95(11):6397–408. doi: 10.3168/jds.2011-5173. [DOI] [PubMed] [Google Scholar]
- 19.Meng F, Glaser SS, Francis H, DeMorrow S, Han Y, Passarini JD, Stokes A, Cleary JP, Liu X, Venter J, Kumar P, Priester S, Hubble L, Staloch D, Sharma J, Liu CG, Alpini G. Functional analysis of microRNAs in human hepatocellular cancer stem cells. J Cell Mol Med. 2012 Jan;16(1):160–73. doi: 10.1111/j.1582-4934.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dave RS, Khalili K. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem. 2010 Jul 1;110(4):834–45. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Ning Q, Wang J. Angiotensin II induced differentially expressed microRNAs in adult rat cardiac fibroblasts. J Physiol Sci. 2013 Jan;63(1):31–8. doi: 10.1007/s12576-012-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. Oncogene. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. 2010 Mar 25;29(12):1787–97. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009 Aug 21;31(2):220–31. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limón P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013 May 23;38(5):984–97. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, et al. MicroRNA modulation in complex regional pain syndrome. J Transl Med. 2011 Nov 10;9:195. doi: 10.1186/1479-5876-9-195. 5876-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J Clin Invest. 2012 Jun 1;122(6):1973–90. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Brannon AR, Reddy AR, Alexe G, Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, Ganesan S, Levine AJ, Rathmell WK, Bhanot GV. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC Syst Biol. 2010 Apr 27;4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monk CE, Hutvagner G, Arthur JS. Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One. 2010 Oct 27;5(10):e13669. doi: 10.1371/journal.pone.0013669. [DOI] [PMC free article] [PubMed] [Google Scholar]