Abstract
Objective
Many U.S. providers remain reluctant to prescribe intrauterine devices (IUDs) to teenagers due to concerns about serious complications. This study examined whether 15–19 year-old IUD users were more likely to experience complications, failure, or early discontinuation than adult users aged 20–24 and 25–44 years and whether there were differences in between users of levonorgestrel-releasing intrauterine systems and copper IUDs.
Methods
A retrospective cohort study was conducted using health insurance claims obtained from a private insurance company of 90,489 women who had an IUD inserted between 2002 and 2009. Logistic regression models were used to estimate the odds of experiencing complications, method failure, or early discontinuation within 12 months of insertion by age group and type of IUD inserted.
Results
Serious complications, including ectopic pregnancy and pelvic inflammatory disease, occurred in less than 1% of patients regardless of age or IUD type. Women aged 15–19 years were more likely than those aged 25–44 years to have a claim for dysmenorrhea OR=1.4, CI=1.1, 1.6), amenorrhea (OR=1.3, CI=1.1, 1.5), or normal pregnancy (OR=1.4, CI=1.1, 1.8). Overall, early discontinuation did not differ between teenagers and women aged 25–44 years (13% vs. 11%, p>.05). However, use of the levonorgestrel-releasing intrauterine system was associated with fewer complications and less early discontinuation than the copper IUD in all age groups.
Conclusions
The IUD is as appropriate for teenagers to use as it is for older women, with serious complications occurring infrequently in all groups. The levonorgestrel-releasing intrauterine system may be a better choice than copper IUD due to lower odds of complications, discontinuation, and failure.
Introduction
Two types of intrauterine devices (IUDs) are currently available in the United States: the levonorgestrel-releasing intrauterine system (Mirena) and intrauterine copper contraceptive (ParaGard). Both are highly effective and provide long-term protection against unintended pregnancy (1). Moreover, they are cost effective with savings surpassing $7 for every $1 spent in services and supplies (2, 3). These devices were once considered too risky for teenagers, but several organizations,(4-6) including the Centers for Disease Control, have recently published guidelines stating they are safe to use in women under 20 years of age (6).
In spite of this, IUD use among 15–24 year-olds has failed to keep pace with that of women over age 24 (7). This may be due to the reluctance of U.S. providers to prescribe this contraceptive to teenagers or nulliparous women, due to lingering concerns about the risk of pelvic inflammatory disease associated with earlier devices. For example, in a California study of 816 clinicians, only 46% considered IUDs appropriate for nulliparous women and only 39% endorsed it for teenagers (8). In addition, only 19% of obstetrician-gynecologists surveyed in a 2010 study stated that they would offer an IUD to an unmarried 17 year-old who had never been pregnant (9).
To address these concerns, information about continuation and complications associated with IUDs currently available among teenage women is needed. However, according to two Cochrane Reviews, few studies have been published on this topic (10, 11). Furthermore, those that have been published found conflicting results. One secondary data analysis reported that 85% of teenagers who had levonorgestrel-releasing intrauterine system inserted continued its use for 12 months or more (n =179) (12). However, this study may not be applicable to the general population as 19% of participants had an intellectual disability. A randomized study of U.S. adolescents using copper IUDs and levonorgestrel-releasing intrauterine systems also found favorable continuation rates among 14–18 year olds, but was limited by a small sample size (n=23) (13). In contrast, a 2012 publication on 136 mothers up to 22 years of age reported a continuation rate of only 55% after 12 months and higher pregnancy rates than expected with both devices (6.2% and 3.7% for copper IUDs and levonorgestrel-releasing intrauterine systems, respectively)(14). Similarly, another study of 89 women 16–22 years of age noted a mean duration of use less than 12 months (15). In addition to their relatively small sample sizes, all four of these studies were limited by a lack of comparison to women from other age groups. Thus, they cannot be used to accurately assess if continuation or complication rates within a population are the same or higher among teenagers compared with women in their early 20s or those 25 years of age or older.
The purpose of this study was to examine the frequency of complications, failure and discontinuation within the first year among a national sample of women 15–19 years of age who had an IUD inserted between 2002 and 2009 as compared to 20–24 and 25–44 year-olds. Differences in complications and continuation between the levonorgestrel-releasing intrauterine system and copper IUD were also estimated.
Materials and Methods
This retrospective cohort study used electronic health insurance claims made between 2002 and 2010 from enrollees in a commercially-available nationwide U.S. health insurance program. The entire dataset contains information on more than 45 million individuals, of which approximately 80% purchase their health insurance through their employer and have at least one medical claim recorded. The de-identified dataset does not contain any information about participants’ socioeconomic status, race, or ethnicity (16). Data for this dataset is collected from records of claims paid by the insurance company, based on submissions from providers across the United States. Studies using this dataset have appeared in prominent journals (17-19). Geographic and gender information are available and are similar in distribution to the U.S. population. However, it has been estimated that more whites, young, and middle-aged adults are included in the dataset than the overall U.S. population. This study was exempted from full review by the University of Texas Medical Branch institutional review board.
First, we identified 156,727 women 15–44 years of age with a claim for an IUD insertion between 2002 and 2009. This was done by identifying those with a healthcare common procedure code (HCPCS) for insertion (levonorgestrel-releasing intrauterine system=J7302, S4989, S4981; copper IUD=J7300) and a current procedural terminology (CPT) code (58300) or international classification of diseases (ICD-9) code (69.7, V25.1, V25.42, V25.11) for insertion on the same date. Of these, 90,489 had 12 months or more of continuous insurance coverage after IUD insertion. A total of 69 of the 90,489 women had codes for intellectual disabilities or autism (ICD-9 codes 042, 043, 044, 279.0, 279.1, 279.2, 279.3, 795.71, V08). These women were not excluded from the study due to their very low number. Of the total sample, 19,904 received a copper IUD and 70,585 received a levonorgestrel-releasing intrauterine system. In this sample, 96% were commercial payers, and the remainder had Medicaid.
Data on age, year of insertion, type of provider that inserted the IUD and whether it was inserted after delivery of a child was obtained from the database. To determine age, the year of birth was subtracted from the year of IUD insertion. The exact date of birth was not available, so year of birth served as a proxy. Age ranges (15–44 years old) and categories (15–19, 20–24, and 25–44 years old) were chosen based on methods from other national studies that examined birth control methods among women by age group (20, 21). Provider specialties were categorized as obstetricians-gynecologists, pediatricians, family practitioner or internal medicine or general practitioners, clinics, and non-physician providers or specialists. A post-partum insertion was defined as one that occurred 8 weeks or fewer after a normal delivery.
Claims for complications that could be associated with IUD use occurring within 12 months of insertion were examined. For women who discontinued IUD use within 12 months, complications that occurred between insertion and discontinuation were evaluated. Outcomes included pain associated with female genital organs (dyspareunia, dysmenorrheal, or premenstrual tension syndrome), disorders of menstruation (absence of menstruation, scanty or infrequent menstruation, excessive or frequent menstruation, irregular menstrual cycles, metrorrhagia, poistcoital bleeding, dysfunctional or functional uterine hemorrhage, or unspecified), inflammation/infection (inflammatory diseases of the ovary, fallopian tube, pelvic cellular tissue, and peritoneum; inflammatory disease of the uterus except cervix; as well as cervicitis and endocervicitis), uterine perforation, and mechanical complications of IUDs. In addition, the frequency of a normal intrauterine pregnancy and abnormal pregnancy that occurred between IUD insertion and discontinuation was examined (ectopic pregnancy, molar pregnancy, abnormal products of conception, missed abortion, spontaneous abortion; ICD-9 codes included in Table footnotes).
IUD discontinuation within 12 months of insertion was determined by the presence of an ICD-9 or CPT code that indicated IUD removal (ICD-9 codes: 97.71, V25.12, V25.13; CPT codes: 58301, 58562). The relationship between IUD discontinuation and complications experienced in the last 30 days was also examined.
Descriptive statistics were used to describe the distributions of patient age and provider specialty by type of IUD inserted and to evaluate the frequencies of complications, failure, and discontinuation within 1 year of IUD insertion or within 30 days of a claim for a complication or failure by age group. The ratio of levonorgestrel-releasing intrauterine system to copper IUD insertion was examined across time. Univariable logistic regression models were used to estimate the associations of the type of IUD inserted (levonorgestrel-releasing intrauterine system compared with copper IUD placement) with age, year of insertion, and physician specialty. Multivariate logistic regression models were used to evaluate 1) the effects of age, type of IUD, and their interaction on outcomes (complications, discontinuation, and failure), 2) the effects of age group, with teenagers being the group of interest and the other two groups acting as the referents, and 3) the association of complications and pregnancy with discontinuation. All multivariate logistic regression models controlled for age at insertion, IUD type, provider type, and year of IUD insertion. All statistical analyses were done using SAS version 9.2 (SAS® Institute, Cary, NC).
Results
The levonorgestrel-releasing intrauterine system was inserted more often than the copper IUD in women of all ages (Table 1). Women 15–19 years of age who requested an IUD were 1.4 times more likely than those 25–44 years old to receive the levonorgestrel-releasing intrauterine system rather than a copper IUD. Gynecologists inserted 91% of all IUDs. All health providers were more likely to insert a levonorgestrel-releasing intrauterine system than a copper IUD.
Table 1.
Intrauterine Device Insertion by Age and Provider Specialty (2002-2009)
| LNG-IUS | Copper IUD | OR (95% CI)* | |
|---|---|---|---|
| Total | 70,585 (78.0) | 19,904 (22.0) | NA |
| Year of insertion, by age | |||
| Age 15-19 | |||
| 2002-2003 | 53 (53.0) | 47 (47.0) | Ref |
| 2004-2005 | 115 (70.1) | 49 (29.9) | 2.08 (1.24, 3.49) |
| 2006-2007 | 396 (82.5) | 84 (17.5) | 4.18 (2.65, 6.61) |
| 2008-2009 | 964 (88.4) | 127 (11.6) | 6.73 (6.36, 10.39) |
| Age 20-24 | |||
| 2002-2003 | 432 (51.4) | 409 (48.6) | Ref |
| 2004-2005 | 1011 (68.6) | 463 (31.4) | 2.07 (1.74, 2.46) |
| 2006-2007 | 2266 (81.6) | 512 (18.4) | 4.19 (3.55, 4.95) |
| 2008-2009 | 4151 (86.6) | 643 (13.4) | 6.11 (5.22, 7.16) |
| Age 25-44 | |||
| 2002-2003 | 3365 (50.7) | 3267 (49.3) | Ref |
| 2004-2005 | 8233 (68.2) | 3832 (31.8) | 2.09 (1.96, 2.22) |
| 2006-2007 | 18256 (80.0) | 4577 (20.0) | 3.87 (3.65, 4.10) |
| 2008-2009 | 31343 (84.2) | 5894 (15.8) | 5.16 (4.88, 5.46) |
| Provider specialty | |||
| OB-GYN | 64,950 (78.9) | 17,344 (21.1) | Ref |
| Pediatrician | 42 (67.7) | 20 (32.3) | 0.56 (0.33, 0.96) |
| FP, IM or GP | 3,290 (66.7) | 1,640 (33.3) | 0.54 (0.50, 0.57) |
| Clinics | 544 (71.9) | 213 (28.1) | 0.68 (0.58, 0.80) |
| Non-physician | 646 (68.5) | 297 (31.5) | 0.58 (0.51, 0.67) |
| Specialist | 308 (73.0) | 114 (27.0) | 0.72 (0.58, 0.90) |
| Missing | 805 | 276 |
Data are n (%).
LNG-IUS, levonorgestrel-releasing intrauterine system; OR, odds ratio; CI, confidence interval; NA, no reference category for comparison; Ref, reference category; OB-GYN, obstetrician gynecologist; FP, family practice; IM, internal medicine; GP, general practitioner.
Unadjusted OR and 95% CI of receiving a LNG-IUS vs copper IUD.
Between 2002 and 2009, there was an increase in the frequency of IUD use among women of all three age groups. In 2002, only 3,200 women had an IUD inserted. Of these, 1.5% were 15–19 years old, 11.7% were 20–24 years old, and 86.8% were 25–44 years old. By 2009, the total number increased to 21,727, with 2.7% among15–19 year-olds, 11.7% in 20–24 year-olds, and 86.3% in 25–44 year-olds.
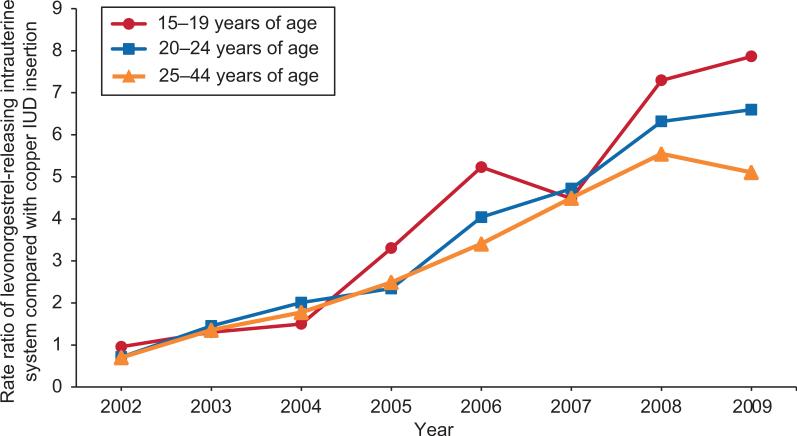
Levonorgestrel-releasing intrauterine system insertions increased more than copper IUD insertions among women in all three age groups from 2002 to 2009 (Figure 1). In 2002, about the same number of each type was inserted. Within 3 years, the levonorgestrel-releasing intrauterine system was being inserted twice as often as the copper IUD in all three age groups. By 2009, the levonorgestrel-releasing intrauterine system was being inserted 5, 7, and 8 times for every copper IUD in the 25–44 , 20–24, and 15–19 age groups, respectively. Younger women (15–19 and 20– 24 year-olds) were more likely to have their IUD inserted after delivery of an infant as compared with those 25–44 years of age (OR = 1.76, CI = 1.54 – 1.97 and OR = 1.63, CI = 1.54-1.72, respectively).
Figure 1.
Ratio of levonorgestrel-releasing intrauterine system compared with copper intrauterine device (IUD) insertion by age and year of insertion (2002–2009).
The frequency of complications within the first year of use was low among users of both types of IUDs in all age groups (Table 2). The most frequent complications were disorders of menstruation. The most serious complications associated with IUD use, ectopic pregnancy and pelvic inflammatory disease, were observed in less than 1% of all patients, regardless of age or IUD type. Uterine perforation was rare.
Table 2.
Frequency of Outcome Measures in the First Year of Use (Complications, Failure, and Discontinuation), by Age and Type of Intrauterine Device (2002-2009)
| Age 15-19 |
Age 20-24 |
Age 25-44 |
||||
|---|---|---|---|---|---|---|
| Outcome* |
LNG-IUS n=1,528 |
Copper IUD n=307 |
LNG-IUS n =7,860 |
Copper IUD n=2,027 |
LNG-IUS n=61,197 |
Copper IUD n=17,570 |
| Dyspareunia | 22 (1.4) | 5 (1.6) | 154 (2.0) | 45 (2.2) | 736 (1.2) | 215 (1.2) |
| Dysmenorrhea | 37 (2.4) | 20 (6.2) | 172 (2.2) | 76 (3.7) | 921 (1.5) | 457 (2.6) |
| Premenstrual tension | 3 (0.2) | 1 (0.3) | 34 (0.4) | 14 (0.7) | 480 (0.8) | 187 (1.1) |
| Excessive menstruation | 49 (3.2) | 15 (4.9) | 220 (2.8) | 119 (5.9) | 2,235 (3.7) | 1,075 (6.1) |
| Uterine hemorrhage | 72 (4.7) | 18 (5.9) | 310 (3.9) | 112 (5.5) | 2,381 (3.9) | 796 (4.5) |
| Post coital bleeding | 5 (0.3) | 0 (0.0) | 40 (0.5) | 10 (0.5) | 179 (0.3) | 66 (0.4) |
| Metrorrhagia | 30 (2.0) | 9 (2.9) | 138 (1.8) | 50 (2.5) | 1,137 (1.9) | 369 (2.1) |
| Irregular menstrual cycle | 84 (5.5) | 16 (5.2) | 380 (4.9) | 122 (6.1) | 2,809 (4.6) | 823 (4.7) |
| Absence of menstruation | 59 (3.9) | 19 (6.2) | 288 (3.7) | 90 (4.4) | 1,346 (2.2) | 473 (2.7) |
| Infrequent menstruation | 5 (0.3) | 4 (1.3) | 23 (0.3) | 11 (0.5) | 176 (0.3) | 67 (0.4) |
| Pelvic inflammatory disease | 3 (0.2) | 1 (0.3) | 9 (0.1) | 3 (0.1) | 38 (0.1) | 23 (0.1) |
| Inflammatory disease of uterus except cervix | 4 (0.3) | 1 (0.3) | 48 (0.6) | 14 (0.7) | 190 (0.3) | 98 (0.5) |
| Cervicitis and endocervicitis | 43 (2.8) | 4 (1.3) | 196 (2.5) | 53 (2.6) | 921 (1.5) | 337 (1.9) |
| Uterine perforation | 0 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 29 (0.0) | 7 (0.0) |
| Mechanical complication within 1 year | 24 (1.6) | 6 (2.0) | 116 (1.5) | 34 (1.7) | 726 (1.2) | 231 (1.3) |
| Ectopic pregnancy | 1 (0.1) | 0 (0.0) | 7 (0.1) | 3 (0.1) | 42 (0.1) | 21 (0.1) |
| Abnormal pregnancy or spontaneous abortion | 2 (0.1) | 2 (0.7) | 16 (0.2) | 14 (0.7) | 92 (0.2) | 47 (0.3) |
| Normal pregnancy | 28 (1.8) | 11 (3.6) | 117 (1.5) | 44 (2.2) | 485 (0.8) | 231 (1.3) |
| IUD discontinuation | 178 (11.8) | 62 (20.2) | 963 (12.3) | 322 (15.9) | 6,471 (10.6) | 2,209 (12.6) |
| Discontinuation within 30 days of any complication | 45 (2.9) | 16 (5.2) | 205 (2.6) | 81 (4.0) | 1,466 (2.4) | 631 (3.6) |
Data are n (%).
IUD, intrauterine device; LNG-IUS, levonorgestrel-releasing intrauterine system.
Classification of each complication was determined by the presence of an International Classification of Diseases, 9th revision (ICD-9) corresponding with each complication, including: dysparunia (625.0), dysmenorrhea (625.3), premenstrual tension syndrome (625.4), excessive or frequent menstruation (626.2), dysfunctional or functional uterine hemorrhage NOS (626.2), post coital bleeding (626.7), metrorrhagia (626.6), irregular menstrual cycle (626.4), absence of menstruation (626.0), scanty or infrequent menstruation (626.1), pelvic inflammatory disease: inflammation/infection inflammatory disease of ovary, fallopian tube, pelvic cellular tissue, and peritoneum (614.0-614.2), inflammatory disease of the uterus except cervix (615, 615.0, 615.1, 615.9), Cervicitis and endocervicitis (616.0), cystitis (595.0-595.4, 595.81-595.89, 595.9), Uterine perforation (998.2), mechanical complication of intrauterine device (996.32), discontinuation (97.71, V25.12, V25.13 + Current Procedural Terminology code 58301 or 58562), ectopic pregnancy (633.10, 633.11, 633.20, 633.21, 633.80, 633.81, 633.90, 633.91, 761.4), molar pregnancy, abnormal products of conception, missed abortion, spontaneous abortion (630-634, 634.0, 634.00-634.02, 634.1, 634.10- 634.12, 634.2, 634.20-634.22, 634.3,634.30, 634.31, 634.4, 634.40-634.42, 634.5, 634.50- 634.52, 634.6, 634.60-634.62, 634.7, 634.70-634.72, 634.8, 634.80-634.82, 634.9, 634.90- 634.92), and normal pregnancy (V22, V22.0, V22.1, V22.2).
Comparison of complications by age group demonstrated few differences (Table 3). Women 15–19 years of age were more likely than 25–44 year olds to have a claim for dysmenorrhea, or absence of menstruation within a year of insertion. Failure of the IUD to prevent a normal pregnancy was observed more often among 15–19 year-olds than 25–44 year-olds. Comparison of complications by type of IUD demonstrated that overall, use of the levonorgestrel-releasing intrauterine system was associated with fewer observed complications than the copper IUD. Levonorgestrel-releasing intrauterine system had reduced odds of seven complications as well as reduced odds of abnormal pregnancy and normal pregnancy compared to the copper IUD. For all other complications, the levonorgestrel-releasing intrauterine system had similar odds compared to the copper IUD. In analyses stratified by age group, levonorgestrel-releasing intrauterine system users in each age group were less likely to have a diagnosis of dysmenorrhea compared to copper IUD. However, the odds of all other complications from the two IUDs were equivalent (analyses not shown).
Table 3.
Association of Age and Type of Intrauterine Device with Complications and Failure (2002-2009)
| OR (95% CI)† |
|||
|---|---|---|---|
| Outcome* | LNG-IUS vs Copper IUD (ref) | Age 15-19 vs 20-24 (ref) | Age 15-19 vs 25-44 (ref) |
| Dyspareunia | 0.98 (0.91, 1.05) | 0.75 (0.50, 1.12) | 0.98 (0.75, 1.27) |
| Dysmenorrhea | 0.76 (0.72, 0.80) | 1.30 (0.97, 1.75) | 1.35 (1.12, 1.62) |
| Premenstrual tension | 0.88 (0.81, 0.96) | 0.52 (0.19, 1.44) | 0.53 (0.27, 1.02) |
| Excessive menstruation | 0.76 (0.73, 0.78) | 1.01 (0.76, 1.33) | 0.94(0.79, 1.12) |
| Uterine hemorrhage | 0.93 (0.90, 0.97) | 1.18 (0.93, 1.50) | 1.14 (0.98, 1.32) |
| Post coital bleeding | 0.91 (0.79, 1.04) | 0.56 (0.22, 1.40) | 0.80 (0.44, 1.44) |
| Metrorrhagia | 0.97 (0.91, 1.02) | 1.15 (0.81, 1.64) | 1.10 (0.88, 1.38) |
| Irregular menstrual cycle | 0.98 (0.94, 1.02) | 1.06 (0.84, 1.33) | 1.08 (0.94, 1.24) |
| Absence of menstruation | 0.97 (0.92, 1.02) | 1.16(0.90, 1.50) | 1.32 (1.12, 1.54) |
| Infrequent menstruation | 0.90 (0.78, 1.03) | 1.17 (0.52, 2.64) | 1.15 (0.69, 1.92) |
| Pelvic inflammatory disease | 0.68 (0.53, 0.86) | 1.36 (0.38, 4.82) | 1.44 (0.65, 3.15) |
| Inflammatory disease of the uterus except cervix | 0.81 (0.72, 0.92) | 0.48 (0.19, 1.19) | 0.74 (0.41, 1.33) |
| Cervicitis and endocervicitis | 0.90 (0.84, 0.95) | 1.03 (0.75, 1.42) | 1.19(0.97, 1.46) |
| Uterine perforation | 1.18(0.77, 1.80) | NA | NA |
| Mechanical complication within 1 year | 0.98 (0.91, 1.05) | 1.07 (0.72, 1.61) | 1.13 (0.88, 1.45) |
| Ectopic pregnancy | 0.76 (0.59, 0.98) | 0.57 (0.07, 4.48) | 0.76 (0.20, 2.85) |
| Abnormal pregnancy or spontaneous abortion | 0.70 (0.60, 0.83) | 0.86 (0.30, 2.46) | 1.05 (0.54, 2.06) |
| Normal pregnancy | 0.80 (0.74, 0.86) | 1.28 (0.89, 1.86) | 1.42 (1.13, 1.78) |
OR, odds ratio; CI, confidence interval; LNG-IUS, levonorgestrel-releasing intrauterine system; Ref, reference category; NA, group size too small for meaningful comparison.
Classification of each complication was determined by the presence of an International Classification of Diseases, 9th revision (ICD-9) corresponding with each complication, including: dysparunia (625.0), dysmenorrhea (625.3), premenstrual tension syndrome (625.4), excessive or frequent menstruation (626.2), dysfunctional or functional uterine hemorrhage NOS (626.2), post coital bleeding (626.7), metrorrhagia (626.6), irregular menstrual cycle (626.4), absence of menstruation (626.0), scanty or infrequent menstruation (626.1), pelvic inflammatory disease: inflammation/infection inflammatory disease of ovary, fallopian tube, pelvic cellular tissue, and peritoneum (614.0-614.2), inflammatory disease of the uterus except cervix (615, 615.0, 615.1, 615.9), Cervicitis and endocervicitis (616.0), cystitis (595.0-595.4, 595.81-595.89, 595.9), Uterine perforation (998.2), mechanical complication of intrauterine device (996.32), ectopic pregnancy (633.10, 633.11, 633.20, 633.21, 633.80, 633.81, 633.90, 633.91, 761.4), molar pregnancy, abnormal products of conception, missed abortion, spontaneous abortion (630-634, 634.0, 634.00-634.02, 634.1, 634.10- 634.12, 634.2, 634.20-634.22, 634.3,634.30, 634.31, 634.4, 634.40-634.42, 634.5, 634.50- 634.52, 634.6, 634.60-634.62, 634.7, 634.70-634.72, 634.8, 634.80-634.82, 634.9, 634.90- 634.92), and normal pregnancy (V22, V22.0, V22.1, V22.2).
OR and 95% CI were estimated using logistic regression models that controlled for age, intrauterince device type, provider specialty, region and year of intrauterine device insertion.
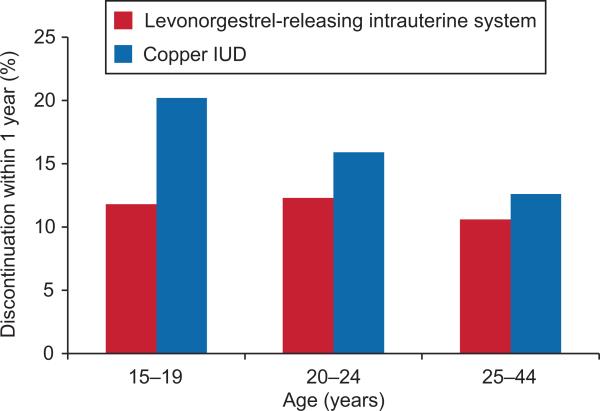
Overall, early discontinuation rates did not differ between women 15–19 years of age and those 25–44 years of age (13% compared with 11% respectively, p>.05). However, IUD type was an important factor in continuation rates. Women of all ages who received the levonorgestrel-releasing intrauterine system were less likely to discontinue its use within 12 months as compared to those who received the copper IUD (Figure 2). Moreover, the proportion of copper IUD users that discontinued was markedly higher among teenagers as compared to other age groups. Although it cannot be determined whether an IUD was removed due to a complication, this study also examined removal of an IUD within 30 days after a complication to try to estimate the frequency with which IUDs were being removed as a result of complications or failure rather than some other reason. In this study, the copper IUD device was more likely to be removed within 30 days of a complication or failure than the levonorgestrel-releasing intrauterine system (OR: 1.34; 95% CI: 1.21-1.48). In particular, women who reported abnormal menstrual bleeding (OR: 1.42, 95% CI: 1.27-1.59) or pregnancy (OR: 4.42; 95% CI: 2.98-6.56) in the last month were more likely to discontinue the copper IUD than the levonorgestrel-releasing intrauterine system (analyses not shown). Age was not significantly associated with discontinuation of the IUD within 30 days of a complication or failure.
Figure 2.
Intrauterine device (IUD) discontinuation within 1 year, by age (2002–2009). Copper IUD users in the 15–19 age group were more likely to discontinue early compared with levonorgestrel-releasing intrauterine system users in all age groups and compared with users of the copper IUD aged 20–24 years and 25–44 years (interaction of type of IUD and age group: P=.01). Odds ratios (95% confidence intervals [CI]) of levonorgestrel-releasing intrauterine system users compared with copper IUD discontinuation within 1 year: Age 15–19 years, 0.77 (0.65–0.91); age 20–24 years, 0.86 (0.80–0.93); age 25–44 years, 0.90 (0.88–0.93).
Discussion
The percentage of reproductive age women who ever used an IUD for birth control steadily declined in the United States from 18% to 6% between 1982 and 2002. However, an increase in insertion rates was observed among insured women from 2 per 1,000 in 2002 to 8 per 1,000 in 2007 (7, 20). By 2008, 5.5% of all contraceptive patients reported using an IUD (22). We also observed an increase in IUD insertions between 2002 and 2009. Together, these studies demonstrate resurgence in the popularity of this contraceptive method among insured U.S. women.
Our study also demonstrated significant growth in the frequency of teenagers using an IUD for contraception. However, many teenagers in this study received their device after delivery of an infant, suggesting that this method may not be prescribed as often to nulliparous teenagers. Thus, educational programs still may be needed to communicate the safety of IUD use in adolescents who have not given birth.
Amenorrhea and dysmenorrhea, which occurred more frequently among teenagers than adults, is not a major issue as neither places the patient at risk of harm. Many young women actually consider amenorrhea a benefit,(23) and dysmenorrhea may be treated with non-steroidal anti-inflammatory agents. The complication of greatest concern to most healthcare providers, pelvic inflammatory disease (PID), occurred in less than 1% of users, regardless of age or IUD type. This finding is in agreement with a prior study demonstrating that the risk of developing PID with an IUD in place is similar to the risk of developing PID without an IUD (24).
Overall, copper IUD users were more likely to experience failure of the device to prevent pregnancy than levonorgestrel-releasing intrauterine system patients. This difference could have occurred by chance because few pregnancies were detected. Similar to our study, a report on 136 adolescent mothers up to 22 years of age showed that pregnancy rates with the copper IUD were 6.2% compared to 3.7% among levonorgestrel-releasing intrauterine system users(14). The authors acknowledged that their pregnancy rates were higher than reported in adult patients, but could offer no explanation, as preexisting pregnancies due to luteal phase placements had been ruled out and the IUD's presence in the fundus had been confirmed in all patients. We were not able to do this, so it is possible that some pregnancies in our study were due to luteal phase placements or spontaneous expulsions that were not coded. Additionally, our observation of elevated odds of pregnancy in younger women (15–19 year olds) compared to those 25 years and older may be due to differences in fertility between the younger and older groups, leading to the observed differences.
This study sheds important light on premature discontinuation of IUDs among young women. We observed that copper IUD discontinuation rates were markedly higher among 15–19 year olds than women in the other age groups while discontinuation rates among levonorgestrel-releasing intrauterine system users were similar among all three age groups. Furthermore, among females who discontinued IUD use due to complications or pregnancy, copper IUDs were more likely to be removed than levonorgestrel-releasing intrauterine systems. These data suggest that the levonorgestrel-releasing intrauterine system may be a better choice for most women than the copper IUD, when available, as it may result in fewer side effects and lower discontinuation rates. However, physicians should consider the needs of each individual patient when discussing contraceptive options, as not all women may desire amenorrhea, which is common among levonorgestrel-releasing intrauterine system users. Overall, this study supports the use of both IUD types as the higher likelihood of discontinuation observed with the copper IUD is still favorable when compared with the high discontinuation rates of other reversible contraceptives (21, 25). To confirm our findings, further prospective studies on complications of both types of IUDs should be conducted in nulliparous adolescent patients in the U.S.
This study has several limitations. Its observational nature could have introduced selection bias with regards to type of IUD inserted. Second, it should also be noted that the women in this sample were all insured, so the results may not be applicable to lower income women. In addition, we used a claims database which likely resulted in an under reporting of some complications. Claims data are intended for billing, not research purposes, so we did not have information on women who experienced a complication, but did not notify their provider. However, there is no reason why women who experienced a complication with one IUD type would be more likely than those with the other type to report the same complication to their provider. Furthermore, use of a national database allows information to be collected on a large sample which may not otherwise be possible. Finally, we could not confirm the diagnosis because charts were not available. This is of special concern regarding pregnancy. We used claims codes that indicated pregnancy for the outcome “normal pregnancy,” but could not confirm the pregnancy in many cases. In addition, it was not always possible to determine whether pregnancy occurred due to spontaneous expulsion, because a claim may not have been made. Miscoding or lack of coding for spontaneous expulsion may also have led to an underestimate of the number of patients who discontinued IUD use during the 1 year follow-up period.
Overall, these data indicate that the IUD is an appropriate contraceptive for younger women, and is not likely to cause serious side effects among teenagers. Physician recommendations play an important role in their patients’ decision making, and their recommendation of the IUD could increase the use of this cost-effective and safe method of birth control among teenagers. Thus, physicians should include information about this highly effective method when they counsel young patients on their contraceptive options to help reduce the unintended pregnancy rate among teenagers in the United States.
Acknowledgments
Funded by the Society of Family Planning (SFP4-1, PI: Berenson). Supported by the Institute for Translational Sciences at the University of Texas Medical Branch, which is partially funded by a Clinical and Translational Science Award (UL1RR029876) from the National Center for Research Resources, National Institutes of Health, and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH/NICHD K24 HD04365, PI: Berenson). Dr. Hirth is currently supported by ORWH (NICHD K12HD052023, PI: Berenson) and was supported at the time of initial submission by the NICHD through an institutional training grant (National Research Service Award T32HD055163, PI: Berenson). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, the NIH, or the SFP.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Contributor Information
Abbey B. Berenson, Center for Interdisciplinary Research in Women's Health University of Texas Medical Branch 301 University Blvd Rte 0587 Galveston, TX 77573 Phone: 409-772-2417 abberens@utmb.edu Fax: 409-747-5129.
Alai Tan, Department of Preventive Medicine Senior Biostatistician, Sealy Center on Aging University of Texas Medical Branch.
Jacqueline M. Hirth, Center for Interdisciplinary Research in Women's Health Department of Obstetrics and Gynecology University of Texas Medical Branch.
Gregg S. Wilkinson, Departments of Preventive Medicine & Community Health and Family Medicine University of Texas Medical Branch.
References
- 1.Sivin I, Stern J, Coutinho E, Mattos CER, Mahgoub SE, Diaz S, et al. Prolonged intrauterine contraception: a seven-year randomized study of the Levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDs. Contraception. 1991;44(5):473–80. doi: 10.1016/0010-7824(91)90149-a. [DOI] [PubMed] [Google Scholar]
- 2.Foster DG, Rostovtseva DP, Brindis CD, Biggs A, Hulett D, Darney PD. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Public Health. 2009;99(3):446–51. doi: 10.2105/AJPH.2007.129353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trussell J, Lalla AM, Daon QV, Reyes E, Pinto L, Gricar J. Cost effectiveness of contraceptives in the United States. Contraception. 2009;79(1):5–14. doi: 10.1016/j.contraception.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Medical eligibility criteria for contraceptive use. 3rd ed. Geneva, Switzerland: 2004. [Google Scholar]
- 5.ACOG Committee Opinion Intrauterine device and adolescents. Obstet Gynecol. 2007;110(6):1493–5. doi: 10.1097/01.AOG.0000291575.93944.1a. [DOI] [PubMed] [Google Scholar]
- 6.Committee on Gynecologic Practice Lon-Acting Reversible Contraception Working Group Understanding and using the US Medical Eligibility Criteria for Contraceptive Use, 2010. Obstet Gynecol. 2011;118(3):754–60. doi: 10.1097/AOG.0b013e3182310cd3. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Macaluso M, Ouyang L, Kulczycki A, Grosse S. Revival of the IUD: Increased use among US women with employer-sponsered insurance, 2002-2007. 2010 doi: 10.1016/j.contraception.2011.06.007. http://wwwacademyhealthorg/files/2010/saturday/xupdf. [DOI] [PubMed]
- 8.Harper CC, Blum M, Thiel de Bocanegra T, Darney PD, Speidel JJ, Policar M, et al. Challenges in translating evidence to practice: the provision of intrauterine contraception. Obstet Gynecol. 2008;111(6):1359–69. doi: 10.1097/AOG.0b013e318173fd83. [DOI] [PubMed] [Google Scholar]
- 9.Madden T, Allsworth JE, Hladky KJ, Secura GM, Peipert JF. Intrauterine contraception in Saint Louis: a survey of obstetrician and gynecologists' knowledge and attitudes. Contraception. 2010;81(2):112–6. doi: 10.1016/j.contraception.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulier R, O'Brien PA, Helmerhorst FM, Usher-Patel M, d'Arcangues C. Copper containing, framed intrauterine devices for contraception. Cochrane Database of Systematic Reviews. 2007;4(4) doi: 10.1002/14651858.CD005347.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French R, Sorhaindo AM, Huib AM, Mansour DD, Robinson AA, Logan S, et al. Progestogen-releasing intrauterine systems versus other forms of reversible contraceptives for contraception. Cochrane Database of Systematic Reviews. 2010;(2) [Google Scholar]
- 12.Paterson H, Ashton J, Harrison-Woolrych M. A nationwide cohort study of the use of the levonorgestrel intrauterine device in New Zealand adolescents. Contraception. 2009;79(6):433–8. doi: 10.1016/j.contraception.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey EM, Memmel LM, Neustadt A, Shah M, Nicosia A, Moorthie M, et al. Intrauterine contraception for adolescents aged 14-18 years: a multicenter randomized pilot study of levonorgestrel-releasing intrauterine system compared to the Copper T380A. Contraception. 2010;81(2):123–7. doi: 10.1016/j.contraception.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Teal SB, Sheeder J. IUD use in adolescent mothers: retention, failure, and reasons for discontinuation. Contraception. 2012;85(3):270–4. doi: 10.1016/j.contraception.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Torre E, Spotswood L, Correia N, Weiss PM. Intrauterine contraception in adolescents and young women: a descriptive study of use, side effects, and compliance. J Pediatr Adolesc Gynecol. 2011;24(1):39–41. doi: 10.1016/j.jpag.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Albright D. Clinformatics Data Mart training conducted at the University of Texas Medical Branch; August 21, 2012. [Google Scholar]
- 17.Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. NEJM. 2011;365(20):1896–904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673–83. doi: 10.1001/jama.2011.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruzikas D, Smith JS, Harley C. Costs associated with management of cervical human papillomavirus-related conditions. Cancer Epidemiol Biomarkers Prev. 2012;21:1469–78. doi: 10.1158/1055-9965.EPI-11-1019. [DOI] [PubMed] [Google Scholar]
- 20.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the US: 1982-2002. Adv Data. 2004;350 [PubMed] [Google Scholar]
- 21.Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: Results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31(2):64–72. [PubMed] [Google Scholar]
- 22.Hubacher D, Finer LB, Espey E. Renewed interest in intrauterine contraception in the United States: evidence and explanation. Contraception. 2011;83(4):291–4. doi: 10.1016/j.contraception.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Glasier AF, Smith KB, van der Spuy ZM, Ho PC, Cheng L, Dada K, et al. Amenorrhea associated with contraception-an international study on acceptability. Contraception. 2003;67(1):1–8. doi: 10.1016/s0010-7824(02)00474-2. [DOI] [PubMed] [Google Scholar]
- 24.Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356(9234):1013–9. doi: 10.1016/S0140-6736(00)02699-4. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan B, Trussell J, Kost K, Singh S, Jones R. Discontinuation and resumption of contraceptive use: Results from the 2002 National Survey of Family Growth. Contraception. 2008;78(4):271–83. doi: 10.1016/j.contraception.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]