Abstract
Rabies viruses, negative-strand RNA viruses, infect neurons through axon terminals and spread transsynaptically in a retrograde direction between neurons. Rabies viruses whose glycoprotein (G) gene is deleted from the genome cannot spread across synapses. Complementation of G in trans, however, enables transsynaptic spreading of G-deleted rabies viruses to directly-connected, presynaptic neurons. Recombinant rabies viruses can encode genes of interest for labeling cells, controlling gene expression, and monitoring or manipulating neural activity. Cre-dependent or bridge-protein-mediated transduction and single-cell electroporation via EnvA/TVA or EnvB/TVB system allow cell-type-specific or single-cell-specific targeting. These rabies virus-based approaches permit the linking of connectivity to cell morphology and circuit function for particular cell types or single cells. Here we describe methods for construction of rabies viral vectors, recovery of G-deleted rabies viruses from cDNA, amplification of the viruses, pseudotyping them with EnvA or EnvB, and concentration and titration of the viruses. The entire protocol takes 6–8 weeks.
INTRODUCTION
The mammalian brain is assembled from thousands of neuronal cell types, which are organized in distinct circuits to perform computations that give rise to perception, cognition, and behavior. Understanding how neural circuits process and represent information is central to understanding how the brain functions and the causes of neurological disorders, and has attracted the interest of neuroscientists over many decades. While technological advances have been providing enormous opportunities to understand the principles organizing complex neural circuits at multiple levels using various models including invertebrates, mice and primates1–5, a central problem is to identify the structural and functional links between component neurons.
One important strategy for elucidating how specific neural components contribute to the computations that generate perception, cognition, and behavior will be to resolve connectivity with high resolution, and then to correlate the function of circuit components with their connectivity. From these results, it will be possible to generate hypotheses that can be tested by manipulating the activity of selected components, monitoring the changes in the activity of other components within the networks, and assessing the behavioral outcomes. The use of G-deleted rabies virus systems, as described here, will enable these strategies by facilitating the studies of the structure and function of complex neural circuits at the level of resolution of specific cell types and even single neurons6–29.
Tracing circuits with rabies virus – Relative utility and experimental strategies
Methods for elucidating connectivity across the whole brain at the level of resolution of specific cell types or single neurons can add considerably to our understanding of neural circuits. The use of such methods to elucidate circuits in living animals can reveal direct correlations between circuit structure and function, and allow perturbation of connectionally-defined neural populations.
To facilitate such studies, we have developed genetically engineered rabies virus systems6, 7, 10–13. Rabies virus is a naturally occurring tracer that travels exclusively in the retrograde direction30–32 and only between neurons connected by conventional synaptic contacts7, 31, 32. Rabies enveloped glycoprotein allows retrograde infection through axon terminals, so that long-distance projection neurons can be selectively labeled on the basis of their connectivity to infected postsynaptic neurons6–14, 16–28, 33. Rabies glycoprotein (G) is essential for virus packaging and transsynaptic spreading, but not for the transcription of viral genes or for the replication of the viral genome6, 7, 34, 35. The deletion of G from the viral genome can restrict transsynaptic spread in the nervous system6, 7, 35. The G-deleted rabies viruses cannot spread following initial infection unless an alternative source of G is present. This results in the complete filling of axons and dendrites with fluorescent proteins (GFP, mCherry, DsRed or BFP), which allows detailed anatomical and morphological investigations6, 8, 9, 15. Importantly, supplying the missing glycoprotein in trans within cells that are infected with G-deleted rabies virus allows the virus to spread to monosynaptic input cells that are directly connected to the initially infected neurons without further spread across additional synapses7, 11, 13, 14, 16–21, 23, 24, 26, 36. To introduce and complement G in starter cells (post-synaptic neurons), various viral vectors such as AAV, lentiviruses, and HSV and genetically engineered mice have been proven effective for transcomplementation transsynaptic tracing in vivo11, 14, 16–19, 21, 23, 24, 36. Some of the published plasmids for these helper viruses are available from Addgene (http://www.addgene.org/).
The recombinant G-deleted rabies viruses can encode any genes of interest13. Therefore, cell labeling, gain-of-function and loss-of-function analyses of a particular gene, activation and silencing of particular neural circuits, and monitoring of neural activity are all possible in connectionally-defined neuronal networks13. For instance, genetically encoded calcium indicators (GCaMP, RCaMP and TNXXL)37–39, voltage sensors (VSFP and Arch)40, 41, or glutamate sensors (iGluSnFR)42 enable the monitoring of neural activity in connectionally-defined neurons and/or genetically-targeted neurons. Ligand-gated (Allatostatin receptor, DREADD and PSAM)43–46 or light-gated ion channels or pumps (Channelrhodopsin, Halorhodopsin and Archaerhodopsin)47–49 can reversibly control neural activity to address causal relationships. In addition, photoactivation of Channelrhodopsin-expressing neurons can reveal the synaptic outputs of the infected neurons both in slice preparations and in vivo50, 51. Cre, FLP, tTA, and rtTA derived from the rabies viral genome can control gene expression when used with genetically engineered animals or in combination with helper viruses13. Conditional expression of genes facilitates experiments aimed at gain-of-function and loss-of-function and circuit tracing. Temporally-controlled tracing across multiple synapses should also be possible by interfacing G-deleted rabies viruses with the increasing number of mouse lines and viral vectors that express rabies glycoprotein in a Cre-, FLP-, tTA-, or rtTA-dependent manner13, 25, 29, 36. These G-deleted rabies virus systems enable us to relate connectivity to detailed cell morphology6, 7, 10–12, 15 and relate connectivity to circuit function13, 22, 25, 27.
While there are numerous advantages of using G-deleted rabies virus-based methods for the study of neural circuits, it is also important to consider the limitations. Most notably, rabies virus infection will eventually induce cytotoxicity in infected neurons6. A working time window, during which functional studies of neurons infected by G-deleted rabies viruses are feasible, is typically between about 4 and 11 days postinfection13. Reduction of cytotoxicity is one of the hurdles to be resolved for long-term experiments. Another limitation is that the rabies virus cannot use promoter-specific expression of transgenes in contrast to DNA viruses because rabies virus is an RNA virus and never produces DNA at any stage of its lifecycle.
To achieve cell-type-specific targeting, we have developed TVA/EnvA and TVB/EnvB systems7, 10–13. EnvA and EnvB are envelope proteins from the avian sarcoma leucosis viruses, and specifically bind to TVA and TVB receptors, respectively, which are not expressed in mammals52. Chimeric glycoproteins that consist of the ectodomain of EnvA or EnvB and the cytoplasmic domain of the rabies glycoprotein can pseudotype G-deleted rabies viruses7, 10. By taking advantage of this specificity and the lack of native receptors for EnvA or EnvB in mammalian cells, virus infection of EnvA- or EnvB-pseudotyped rabies can be limited to those genetically targeted neurons that express TVA or TVB7, 11–13, 17, 19, 20, 23, 24, 26, 36. In particular, transgenic or Knock-In mice expressing Cre, FLP or rtTA under the control of a cell type-specific promoter can be used to obtain expression of TVA selectively in specific cell types53, allowing specific infection with EnvA-pseudotyped G-deleted rabies viruses11, 17, 23, 54–56. Newly generated connections of adult neural stem cell-derived neurons or embryonic stem cell-derived neurons can be also detected by introduction of TVA and G followed by infection with EnvA-pseudotyped G-deleted rabies viruses24, 26. In addition, bridge proteins, which are fusion proteins of a ligand with TVB, are useful for cell-type specific transduction10, 14. Co-injection of EnvB-pseudotyped rabies viruses with the neuregulin-TVB bridge protein can preferentially target ErbB4+ inhibitory cortical neurons. Moreover, electroporation and targeted patching of single cells of interest in vivo to introduce TVA, followed by infection of the TVA-expressing cells with EnvA-pseudotyped rabies viruses, allows for analyzing single neuronal networks with single-cell resolution12, 20.
In summary, monosynaptically restricted labeling systems with G-deleted rabies virus make it possible to:
Identify neurons projecting to the injection site7–9, 13, 15, 22, 27, 28.
Identify neurons that are directly presynaptic to specific types of projection neurons16, 18, 21, 25.
Identify neurons that are directly presynaptic to specific genetically defined cell types11, 14, 17, 19, 23, 24, 26.
Identify neurons that are directly presynaptic to single, functionally-characterized neurons12, 20.
Relate neuronal connectivity to detailed cell morphology6, 7, 10–12, 15.
Rabies Virology
To fully understand the advantages and disadvantages of rabies viruses, as well as the factors that need to be considered when designing rabies viral vectors, producing G-deleted rabies viruses at high titer, pseudotyping rabies viruses with other envelope proteins targeting rabies virus infection to particular cell types, and troubleshooting during the development of experimental procedures (Fig. 1), it is important to understand rabies virology.
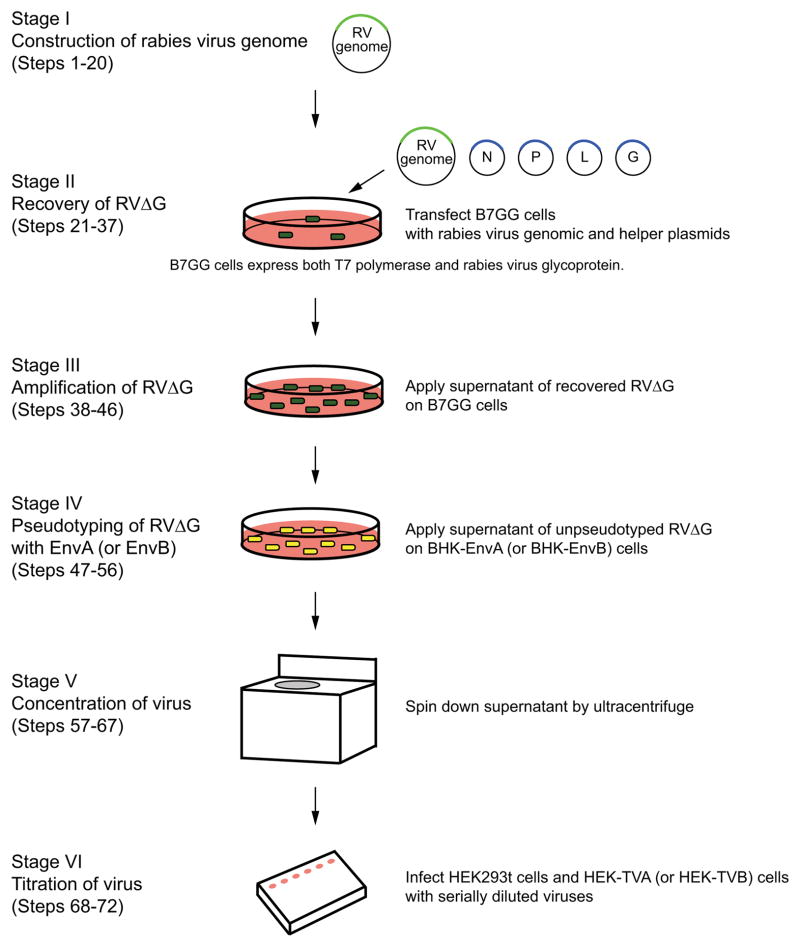
Figure 1. Flowchart and timeline for G-deleted rabies virus production.
Generation of G-deleted rabies virus (RVΔG) consist of 6 stages: (a) construction of rabies viral genome, (b) recovery of G-deleted rabies virus from plasmids, (c) amplification of G-deleted rabies virus, (d) pseudotyping with EnvA or EnvB, (e) concentration of virus, and (f) titration of virus.
Rabies virus is a non-segmented negative-strand RNA virus belonging to Rhabdoviridae57. The virion structure is a bullet-shaped particle, approximately 250 nm long and 70 nm in diameter. The rabies virus genome is composed of single-stranded, linear, negative-sense RNA and has a length of 11–12 kb. The five virus proteins N, P, M, G, and L are encoded in the genome in the order 3′-N-P-M-G-L-5′ (Fig. 2a). Transcription starts with a short uncapped leader RNA from the 3′ end of the genomic RNA. The viral genome RNA serves as a template for transcription by the RNA-dependent RNA polymerase complex, composed of the large protein (L) and the phosphoprotein (P), which results in the synthesis of mRNA for N, P, M, G and L. The replication of negative strand genomes produces full-length positive strand RNA, or antigenomes. The antigenomes serve as templates for the synthesis of progeny negative strand genomes. The genomic RNA and the complementary antigenome RNA are tightly encapsidated by the nucleoprotein (N) to form the helical ribonucleoprotein (RNP). Only RNP is suitable as a template for replication and transcription, not free RNA. The viral capsid is surrounded by the host cell-derived membrane that interacts with two viral proteins, the matrix protein (M) and the glycoprotein (G). The G is an envelope protein and essential for budding and for transsynaptic spread of infectious virion7, 34. The M interfaces between RNP and G and is essential for virus packaging and the regulation of replication and transcription58.
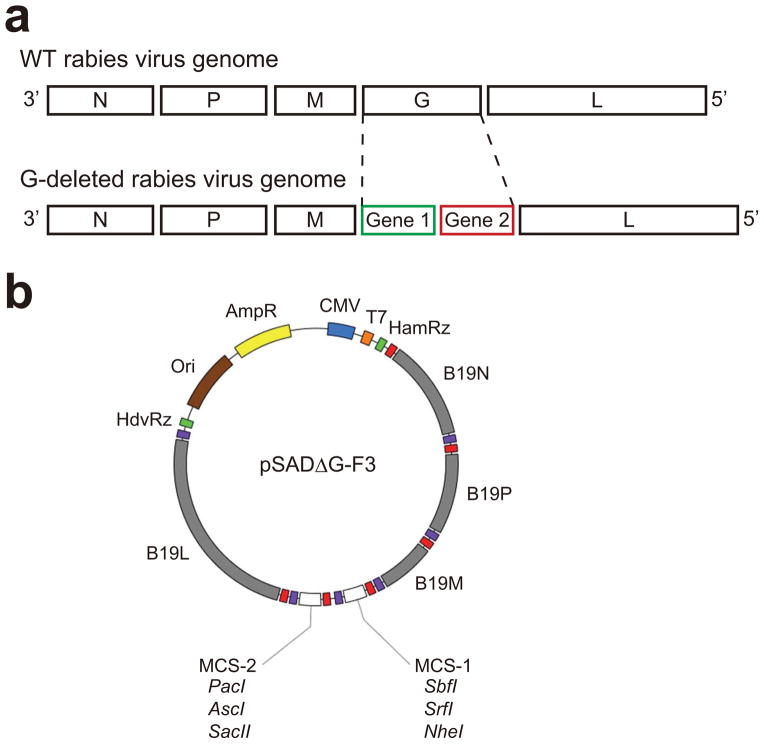
Figure 2. Rabies virus genome and G-deleted rabies virus vector.
(a) Genomes of wild type rabies virus and G-deleted rabies virus. N: nucleoprotein. P: phosphoprotein. M: matrix protein. G: glycoprotein. L: large protein.
(b) Map of the G-deleted rabies virus genomic vector pSADΔG-F3. All unique cloning sites are indicated. Red and purple boxes indicate transcription start and stop sequences, respectively. Further information on pSADΔG-F3 is provided in a previous publication by Osakada et al.13.
It should be noted that the transcriptional machinery for rabies virus genome-derived RNA is completely different from transcription in host cells using DNA-dependent RNA polymerases such as polII. The life cycle of rabies virus is confined to the cytoplasm and rabies viruses do not have reverse transcriptases which generate DNA from RNA. As a result of these characteristics, rabies viruses pose no risks of chromosomal insertion in the host cells, in contrast to retroviruses such as HIV and MoMLV, which pose the danger of insertional mutagenesis in the host genome, leading to unpredictable gain- or loss-of-function, such as tumor formation and loss of physiological properties59, 60.
In 1994, Schnell et al. succeeded in cloning of cDNAs of each rabies virus gene and recovering rabies virus from DNA61. This landmark study was the first successful recovery of negative-strand RNA virus from the DNA. Although reverse genetics systems have become more reliable and several improved methods have been reported, the efficiency of virus recovery is still lower than with positive-strand RNA viruses or DNA viruses. Practical applications for neuroscience and biomedical research were considerably limited. Recovery of G-deleted rabies viruses was particularly inefficient34, 62. We have also cloned viral cDNA by reverse genetics and developed new G-deleted rabies viral vectors and packaging cells in order to efficiently develop useful tools for neural circuit research13. All of the plasmids we generated for G-deleted rabies virus production are available at Addgene (http://www.addgene.org/). Cells for virus production and G-deleted rabies virus starter stocks are also available from the Callaway lab at the Salk Institute for Biological Studies (http://www.salk.edu/).
Each junction between the coding sequences for rabies viral genes within the genome contains a sequence specifying the end of the upstream gene, an intergenic sequence, and the start sequence for a downstream gene. These sequences function as a signal for polyadenylation and termination of the upstream RNA and also as a signal for the initiation, capping and methylation of the downstream RNA. Intergenic regions of rabies virus are variable in length and nucleotide composition; intergenic regions of N-P, P-M, M-G and G-L are 2, 5, 5, and 24 nucleotides, respectively63. Expression of two foreign genes from our G-deleted rabies vectors (pSADΔG-F3) is achieved by joining them with a stop sequence, a two-nucleotide intergenic sequence, and a start sequence derived from between the N and P coding sequences that are presumably more efficient than other longer junctions13, 64. In some plasmids, this stop and start cassette is flanked with two multiple-cloning sites to facilitate insertion of one or two transgenes of interest13 (Fig. 2b).
Because rabies virus is an enveloped virus, G-deleted rabies virus can be pseudotyped to produce particles incorporating non-native envelope proteins and alter its tropism7, 14, 65. The native envelope G of rabies viruses is able to facilitate infection of neurons through axon terminals66, 67. Although the G receptors responsible for retrograde infection have not been fully understood, the putative receptors include nicotinic acetylcholine receptors, NCAM and p75 neurotrophin receptors, but their expression are likely to vary depending on neuron types68. This feature of G allows retrograde infection and retrograde transsynaptic spreading of rabies virus to presynaptic neurons6–14, 16–21, 23, 33. In addition, G-deleted rabies viruses can be pseudotyped with the glycoprotein of vesicular stomatitis virus (VSV-G) and used as an anterograde tracer. VSV-G-pseudotyped G-deleted rabies viruses infect neurons around an injection site and make it possible to visualize axonal projections of neurons locally infected by the virus. On the other hand, to selectively target desired neuronal subsets in the mammal, G-deleted rabies viruses can be pseudotyped with envelope proteins from the avian sarcoma leucosis viruses EnvA and EnvB, which specifically bind to avian-specific receptors TVA and TVB, respectively7, 10, 52. Targeted expression of TVA or TVB in particular cell types or single neurons permits specific infection by rabies viruses pseudotyped with EnvA or EnvB, respectively and subsequent labeling of the monosynaptically restricted inputs to the population by G complementation7, 10, 11, 13, 14, 17, 19, 20, 23.
Experimental design
The following is an overview of the protocols we have developed for the production of G-deleted rabies viruses. While our protocols follow the same basic principals established in previous recovery systems34, 62, there are several specific changes that we have found lead to greater success in viral recovery and in the ability to more routinely produce high titer G-deleted viruses. The production of rabies virus consists of 6 main stages: (I) to design and construct a rabies viral genome plasmid encoding genes of interest, (II) to recover the novel rabies virus from the plasmid DNA, (III) to amplify the rabies virus to high titers, (IV) to pseudotype the virus with EnvA or EnvB, (V) to concentrate the virus, and (VI) to titer the virus (Figs. 1 and 3). In the event that an available starter stock for a particular rabies virus already exists, stages I and II are not necessary. Stage IV is only required if making pseudotyped virus.
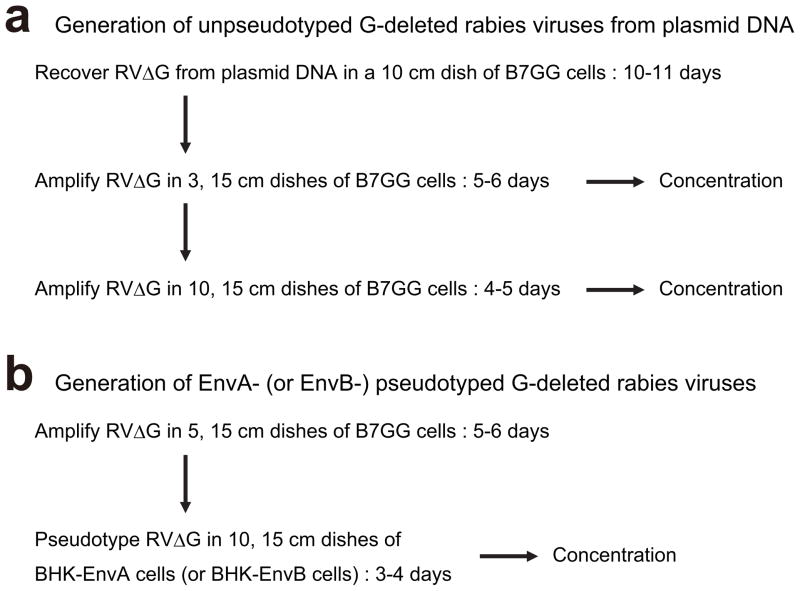
Figure 3. Typical scheme for G-deleted rabies virus production.
(a) Generation of G-deleted rabies virus from plasmid DNA. Recovery of G-deleted rabies virus from plasmids takes 10–12 days. Virus can be concentrated and frozen at each step. Recovered virus is typically amplified two times for concentration to get enough aliquots of the same batch. Recovery, amplification and concentration of virus correspond to steps 21–36, 37–46, and 57–67, in the text, respectively.
(b) Pseudotyping of G-deleted rabies virus with EnvA or EnvB. Pseudotyping requires preparing unpseudotyped G-deleted rabies virus in advance. Pseudotyping and concentration correspond to steps 47–56 and 57–67 in the text, respectively.
Regarding biosafety, G-deleted rabies viruses are unable to spread beyond initially infected cells, and there are no reports of recombination of the viral genome of Rhabdoviridae with DNA or RNA. The production of recombinant G-deleted rabies virus should be performed in a laboratory operating at Biosafety Level 2 (BSL-2), as approved by the researcher’s institutional biosafety committee. These requirements include the use of biosafety cabinet hoods, the establishment of proper procedures for decontamination and disposal of liquid and solid wastes and the disinfection of contaminated surfaces and equipment. For virus injection in animals, animal protocols should be approved by an institutional committee.
Stage I: Design and construction of rabies virus vector
We have created a plasmid that is broadly useful and convenient for cloning novel genes into the rabies viral genome (pSADΔG-F3, Fig. 2b)13. Several strategies are available to ligate foreign DNA to plasmid vectors. We prefer not to carry extra sequences before and after the ORF because viral vectors have a limited capacity in the genome size. Thus, we amplify the ORF containing restriction enzymes sites at both ends by PCR with high-fidelity polymerase, allowing us to clone the ORF without extra sequences. A list of restriction enzymes which produce compatible ends for efficient cloning of foreign DNA is in Table 1. We also use the In-Fusion system, a new cloning technology from Clontech, which is also versatile for directional cloning because it does not require digestion of PCR products, filling with Klenow, nor dephosphorylation with CIP or BAP. For details on general molecular biology techniques such as PCR, DNA ligations, E. coli transformations, E. coli culture, purification of plasmid DNA, restriction enzyme analysis, and DNA sequencing, we recommend to refer to “Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press)”.
Table 1.
Restriction enzyme producing compatible cohesive ends
| Enzyme | Isoschizomer | Ligated to |
|---|---|---|
| SbfI | SdaI, Sse8387I | SbfI, PstI, NsiI, BsiHKAI, Bsp1286I/SduI, BseSI |
| SrfI | --- | SrfI |
| NheI | --- | NheI, AvrII, SpeI, StyI, XbaI, BlnI |
| PacI | --- | PacI, BsiEI, PvuI, SfaAI |
| AscI | PalAI, SgsI | AscI, MluI, BssHII, MauBI |
| SacII | Cfr42I, KspI, Sfr303I, SgrBI, SstII | SscII, BsiEI |
For the expression of multiple genes from the rabies viral genome, the use of rabies virus transcription start and stop cassette is most reliable13. Alternatively, a 2A system can express multiple transgenes via a translation skip mechanism69. However, this can result in the incomplete separation of two up- and downstream proteins. A functional examination of the transgene products, especially upstream proteins, should be performed when using the 2A system because several amino acids derived from the 2A segment are attached to the carboxyl end of the upstream protein and one amino acid is attached to the amino end of the downstream protein. Targeting signals can also be hindered in the 2A system depending on transgenes. Although internal ribosomal entry site (IRES)-mediated multicistronic expression enables the production of two untagged proteins70, the translation of a downstream gene is much lower than that of an upstream gene71, 72. Another good option is expressing fusion proteins as a single ORF by introducing a fluorescent reporter tag (e.g. GFP and mCherry) or an epitope tag (e.g. c-Myc and HA) that are as efficient as ChR2-mCherry and mCherry-Myc13.
We have worked with transgenes of sizes between 0.7 kb and 3.7 kb, and have found that there was no consistent relationship or apparent effects of size on the viral titer13. The largest insert that we have made for our rabies viral vector is 3.7 kb, consisting of GFP (0.7 kb), ERT2CreERT2 (2.9 bp), a transcription stop sequence and a transcription start sequence. The total genome size is 13.6 kb (two transgenes (GFP and ERT2CreERT2) and four native viral genes (N, P, M, and L)), which is 1.7 kb larger than the native SAD-B19 genome of 11.9 kb63. The SADΔG-GFP-ERT2CreERT2 virus can grow to a high titer and infect neurons in a retrograde direction in vivo as other G-deleted rabies viruses do13.
Stage II: Recovery of G-deleted rabies virus from cDNA
The rabies virus genomic sequence in the SADΔG-F3 is flanked by hammerhead ribozyme (HamRz)73 and hepatitis delta ribozyme (HdvRz)74 for the processing of both 5′- and 3′-ends of the viral genome13. For initiation of an infectious cycle during recovery, full-length positive-sense RNA has to be transcribed from a rabies virus genomic plasmid by T7 polymerase and then immediately encapsidated in the transfected cells. Accordingly, the viral RNA polymerase complex (L and P proteins) and nucleocapsid (N proteins) are provided by simultaneously transfecting producer cells with the genomic plasmid (pSADΔG) and plasmids encoding these proteins (pcDNA-SADB19N, pcDNA-SADB19P and pcDNA-SADB19L) to allow the initiation of replication and subsequent transcription. The encapsidated virus genome and B7GG cells express M and G, respectively, which are also required to produce new virus particles. The key point of efficient recovery is the generation of positive-strand antigenome RNA from DNA61. Otherwise, the RNA derived from helper plasmids encoding N, P and L could hybridize to negative-strand genomic RNA and interfere with the initial assembly of RNPs.
We have established packaging cells, called B7GG cells, expressing T7 RNA polymerase, rabies virus G and histone2B-tagged GFP, to efficiently recover G-deleted rabies viruses from plasmid DNA13. New virions produced by B7GG cells will be released in to the media and infect adjacent cells, resulting in formation of fluorescence-positive clusters (Fig. 4). A single particle of G-deleted rabies virus is sufficient for the recovery and amplification because the virion infects an adjacent B7GG cell, which transcomplements G and amplifies the virus more. In cases where there are large numbers of fluorescence-positive clusters, the virus recovery is successful. Although Mebatsion et al. showed that non-infectious spikeless virions can bud out even in the absence of G34, we confirmed that G-deleted rabies viruses expressing GFP or mCherry are unable to produce any functional, infectious virions in the absence of G by monitoring fluorescence derived from the rabies virus. Many reports support this feature of the G-deleted rabies viruses6–27.
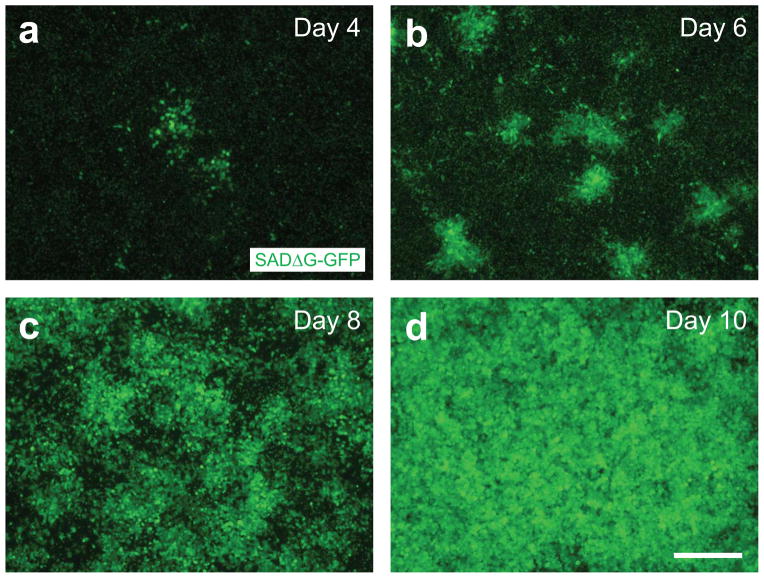
Figure 4. Recovery of G-deleted rabies viruses from DNA plasmid.
Photomicrographs represent typical examples of recovery of SADΔG-GFP on day 4 (A), day 6 (B), day 8 (C) and day 10 (D). Nuclear GFP is derived from B7GG cells, whereas cytosolic GFP is derived from SADΔG-GFP. Scale bar, 300 μm.
Stage III: Amplification of G-deleted rabies virus
The quality of the virus depends on the condition of the producer cells. It is important to keep producer cells healthy for 5–6 days during the virus production. This can be achieved by maintaining them in a humidified atmosphere of 3% CO2 at 35°C. Although rabies viruses can grow at 37°C, cells overgrow at 37°C when cultured for longer periods and start to die before the viruses have sufficient time to amplify. Culturing cells at 35°C reduces cell growth and metabolism, permitting longer maintenance of B7GG cells. It is also important to keep culture media neutral because acidic conditions induce conformation changes of rabies G, fusion of rabies G-expressing cells and loss of a regular virion shape75, 76. Longer culture times make medium acidic, but keeping cells at 3% CO2 slows this process. If the G-deleted rabies virus being produced expresses fluorescence, amplification of the G-deleted rabies virus can be monitored in live cells with fluorescence microscopy (Fig. 5). Because new virions produced by B7GG cells will infect adjacent cells, the formation of fluorescent clusters of infected cells is a good indication of the progress of virus amplification. During the production of G-deleted rabies virus that does not express fluorescence, viral production must be monitored by the collection of supernatant and subsequent staining of fixed cells with an antibody against either one of the viral genes or a transgene expressed from the viral genome. When following the detailed protocol below, it is expected that 90–100% of B7GG cells will be infected and express viral genes 5–6 days after inoculation (Fig. 3a).
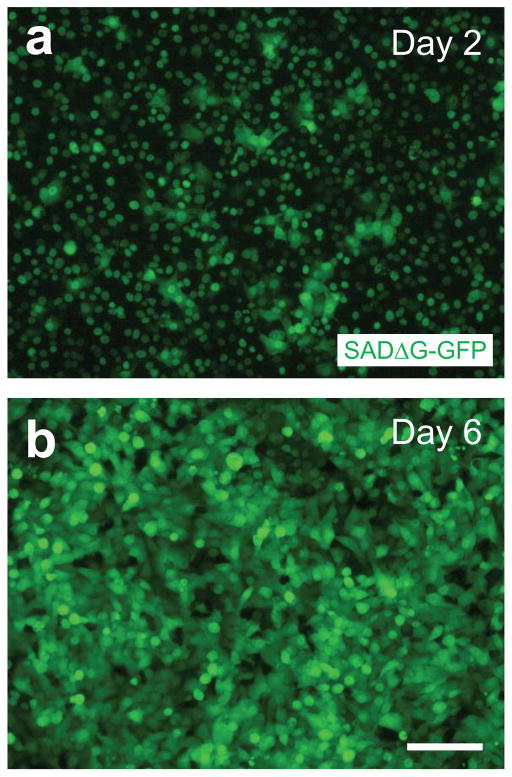
Figure 5. Amplification of G-deleted rabies viruses in B7GG cells.
Photomicrographs represent typical examples of amplification of SADΔG-GFP on day 2 (A), and day 6 (B). Nuclear GFP is derived from B7GG cells, whereas cytosolic GFP is derived from SADΔG-GFP. Scale bar, 100 μm.
Stage IV: Pseudotyping of rabies virus with EnvA or EnvB
To pseudotype G-deleted rabies viruses with EnvA or EnvB, it is necessary to use unpseudotyped rabies virus to infect cells that will produce the pseudotyped virus (Fig. 3b). This is required because EnvA-pseudotyped rabies viruses must be grown in BHK-EnvA cells, but EnvA-pseudotyped rabies viruses cannot infect the BHK-EnvA cells because they do not express a receptor for EnvA (e.g. TVA). The lack of receptors for EnvA in the producer cells impacts the way that virus grows during production and also impacts procedures for generation of high quality and pure preparations of virus. First, unlike during the amplification of unpseudotyped G-deleted rabies virus using B7GG cells, cluster formation is not observed during the production of EnvA-pseudotyped rabies viruses because the EnvA-pseudotyped virus that is produced is not able to infect adjacent cells.
More importantly, because the production of pseudotyped viruses begins with the addition of unpseudotyped viruses, this creates the potential for the final preparation to be contaminated with the unpseudotyped rabies viruses23. It is necessary to take steps to minimize this possibility. To avoid contamination by unpseudotyped G-deleted rabies viruses following the initial infection of the BHK-EnvA cells, we remove the unpseudotyped G-deleted rabies virus-containing media, wash the infected BHK-EnvA cells with PBS, react the cells with trypsin, spin down the cells, remove the media, resuspend the cells with fresh media, replate the cells onto new dishes and change the media. These steps are crucial for the generation of high-quality pseudotyped viruses.
Stage V: Concentration of virus
To concentrate the virus supernatant, we perform two rounds of ultracentrifugation to increase the virus titer and remove the culture medium components. After the collection of the supernatant, we strongly recommend that centrifugation is performed as soon as possible because the virus titer decreases over time, even at 4°C. Freezing and storing the supernatant at −80°C can maintain high virus titer, but the freeze-and-thaw cycle reduces the titer.
Stage VI: Titration of virus
To determine the infectious titer of unpseudotyped rabies virus, HEK293t cells are used. In contrast, to titer pseudotyped rabies viruses, both HEK293t cells and HEK293t-TVA800 cells or HEK293t-TVB cells are necessary. The HEK293t-TVA800 cells or HEK293t-TVB cells are used for the titration of EnvA-pseudotyped, or EnvB-pseudotyped rabies viruses, respectively, while the HEK293t cells are used to check for contamination by unpseudotyped rabies viruses. If infection is observed in HEK293t cells, the preparation is contaminated and should be discarded. Experimental use will likely result in non-specific infection of neurons that do not express TVA or TVB. Measuring infectious titers on cells is preferable to using genomic titers because qPCR is not as accurate. This is because qPCR cannot distinguish between infectious virus particles and non-infectious particles. Also, genomic titers cannot distinguish EnvA-pseudotyped viruses from unpseudotyped viruses that might be present in contaminated preparations.
Comparison with other protocols
Our protocol13 differs from that to produce G-deleted rabies virus previously published by Wickersham et al.7, 34, 62. As mentioned above, we use different plasmids (pSADΔG-F3), different cell lines (B7GG) and different conditions (35°C and 3% CO2). Our system can express two transgenes from the viral genome, recover G-deleted rabies viruses at higher efficiency and produce pseudotyped rabies virus at higher purity13, 23, 62. In particular, the B7GG cells we generated enable significantly higher success of G-deleted rabies virus recovery than the transient transfection of G that was used in the protocol by Wickersham et al.62. In the previous system, when a single particle of G-deleted rabies virus is recovered by transient transfection with G, the virus cannot amplify if the particle goes on to infect non-transfected cells. Another important difference is that the Wickersham protocol62 often produces EnvA-pseudotyped rabies virus that is contaminated with unpseudotyped rabies virus23. This has been reported by Watabe-Uchida et al.23 and is confirmed in our lab. The use of contaminated virus for circuit tracing can lead to misleading results and conclusions or data that must be discarded. To obtain pure preparations of pseudotyped viruses, it is important to follow the steps mentioned above to remove unpseudotyped G-deleted rabies virus. Another limitation of the previously published method by Wickersham et al.62 is that it uses plasmids and reagents which are not as readily available as those described here. In contrast, our plasmids, virus supernatants, and cell lines are freely accessible.
MATERIALS
REAGENTS
Cells
B7GG cells: B7GG cells express T7 RNA polymerase, rabies virus G and histone-tagged GFP. Their origin is BHK-21 cells. B7GG cells can be requested from the authors.
BHK-EnvA cells: BHK-EnvA cells are derived from BHK-21 cells. They express a chimeric envelope protein consisting of the extracellular and transmembrane domains of EnvA fused to the cytoplasmic domain of rabies virus G. These cells are used for pseudotyping G-deleted rabies viruses with EnvA. BHK-EnvA cells can be requested from the authors.
BHK-EnvB cells: BHK-EnvB cells are derived from BHK-21 cells. They express a chimeric envelope protein consisting of the extracellular and transmembrane domains of EnvB fused to the cytoplasmic domain of rabies virus G. These cells are used for pseudotyping rabies virus with EnvB. BHK-EnvB cells can be requested from the authors.
HEK293t cells: (ATCC, cat. no. CRL-11268). These cells are used to titer unpseudotyped G-deleted rabies viruses.
HEK293t-TVA800 cells: HEK293t-TVA800 cells are derived from HEK293t cells and express TVA. These cells are used to titer unpseudotyped G-deleted rabies viruses. HEK293t-TVA800 cells can be requested from the authors.
HEK293t-TVB cells: HEK293t-TVB cells are derived from HKE293t cells and express TVB. These cells are used to titer unpseudotyped G-deleted rabies viruses. HEK293t-TVB cells can be requested from the authors.
SC33: E. coli chemically competent cells (Allele Biotech, cat. no. ABP-CE-CC01020)
Plasmids
pSADΔG-F3 (Addgene, ID. 32634)
pcDNA-SADB19N (Addgene, ID. 32630)
pcDNA-SADB19P (Addgene, ID. 32631)
pcDNA-SADB19L (Addgene, ID. 32632)
-
pcDNA-SADB19G (Addgene, ID. 32633)
Critical: If you would like to recover G-deleted rabies viruses from DNA to produce the same rabies virus variants, produced and characterized in our previous publication, the relevant plasmids are described in Osakada et al.13 and can also be obtained from Addgene (www.addgene.org). It should be noted that it would typically be more efficient to obtain viral supernatant from the authors and to use this virus to propagate, pseudotype, and/or concentrate the virus, as described above. However, from time to time it might be desirable to recover these viral variants de novo, for example if gradual mutations in the viral genome during repeated passaging result in undesirable traits or if import or export restrictions prevent the transfer of rabies virus.
Critical: Endotoxin-free preparation of plasmids is not essential for virus production. But endotoxins in DNA preparation potentially reduce transfection efficiency and viral titer. In addition, endotoxins will induce inflammation, glial activation and malfunction of neural responses following virus injection in the brain. Thus, we recommend endotoxin-free preparation of plasmids.
Water, DNase-free, RNase-free (Invitrogen, cat. no. 10977-015)
TAE buffer x50 (Invitrogen cat. no. 24710-030)
SOC medium (Invitrogen, cat. no. 15544-034)
LB Broth (EMC, cat. no. 1.00547.0500)
LB plate
LB medium
Agarose HS, molecular biology grade (Denville, cat. no. CA3510-8)
Restriction enzymes: i.e. Sbf1 (NEB, cat. no. R0642S), SrfI (Toyobo, cat. no. SRF-101), NheI (NEB, cat. no. R0131S), PacI (NEB, cat. no. R0547S), AscI (NEB, cat. no. R0558S), SacII (NEB, cat. no. R0157S)
Phusion high-fidelity DNA polymerase (Finnzymes, cat. no. F-549S)
Quick ligation kit (NEB, cat. no. M2200S)
In-fusion HD cloning kit (Clontech, cat. no. 639649)
Alkaline Phosphatase, Calf Intestinal (CIP) (NEB, cat. no. M0290S)
Ethidium bromide (Invitrogen, cat. no. 15585-011) CAUTION: Powerful mutagenic and toxic reagent. Wear appropriate gloves.
Carbenicillin (Mediatech, cat. no. MT46100RG) (final concentration:100 μg/ml)
Note that Carbenicillin is more stable than ampicillin and results in less satellite colonies on selection plates.
DMEM with high glucose, L-glutamine and sodium pyruvate (Invitrogen, cat. no. 11995-065)
Opti-MEM I Reduced Serum Medium, GlutaMAX (Invitrogen, cat. no. 51985-034)
DPBS (Invitrogen, cat. no. 14190-144)
Hanks’ Balanced Salt Solution (HBSS) (Invitrogen, cat. no. 14175-095)
0.25% Trypsin-EDTA (Invitrogen, cat. no. 25200)
FBS (Hyclone, cat. no. SH30070.03) CRITICAL: Lot check is essential. The lot of FBS affects efficiency of cell growth and virus production. Several different lots of FBS should be tested to find those that are optimal for high-titer viral preparation. Among companies we have tested, the top grade from Hyclone tends to provide the best results.
DMSO (Sigma, cat. no. D8418) Caution: Harmful by inhalation and skin absorption. Wear appropriate gloves.
Lipofectamine 2000 (Invitrogen, cat. no. 11668-027)
EQUIPMENT
Plastic disposable transfer pipettes (5, 10, 25 and 50 ml) (Corning Costar, cat. nos. 4487, 4488, 4489 and 4490, respectively)
14 ml tube (BD Falcon, cat. no. 35-2059)
Tubes (15 ml and 50 ml) (Falcon, cat. nos. 352095 and 352070, respectively)
5 ml cryogenic tube (Corning, cat. no. 430656)
Culture dish (60 mm, 100 mm and 150 mm) (BD Falcon, cat. nos. 35-3002, 35-3003 and 35-3025, respectively)
24-well culture plate (BD Falcon, cat. no. 35-3047)
-
Steriflip-HV filter unit with cap (Durapore low protein binding, 0.45 μm pore size, 50 ml) (Millipore, cat. no. SE1M 003 M00)
Critical: Filtration through membranes with high affinity to proteins can collapse virions because the rabies virus envelope is derived from cell membrane. Filtration using membrances of 0.45 μm pore size causes less loss of rabies virus particles than filtration with smaller pore size.
Stericup filtration system (Durapore low protein binding, 0.45 μm pore size, 250 ml) (Millipore, cat. no. SCHV U02 RE)
Parafilm (Pechiney, cat. no. PM-996)
Hemacytometer
Agarose gel electrophoresis apparatus
Water bath
Shaker
Vortex
Biosafety cabinet
Two incubators: 37°C and 5% CO2 conditions for cell maintenance and 35°C and 3% CO2 for virus production
Phase contrast microscope (Carl Zeiss, Olympus, or equivalent)
Fluorescence inverted microscope (Carl Zeiss, Olympus, or equivalent)
Appropriate fluorescence filters (i.e. GFP, RFP, BFP) (Chroma Technology or equivalent)
Centrifuge (Eppendorf, Biorad or equivalent)
Ultracentrifuge (Beckman, cat. no. Optima L-100)
Rotor with buckets (Beckman, cat. no. SW28)
Rotor with buckets (Beckman, cat. no. SW55)
Centrigfuge tube, large (Beckman, cat. no. 326823)
Centrigfuge tube, small (Beckman, cat. no. 326819)
PCR thermal cycler (ABI, Biorad, Takara or equivalent)
Glass Flask, 1 L (Pyrex, cat. no. 4446-1L)
Plasmid Mini kit (QIAGEN, cat. no. 12123)
HiSpeed Plasmid Maxi kit (QIAGEN, cat. no. 12663)
QIAquick Gel Extraction kit (QIAGEN, cat. no. 28704)
REAGENT SETUP
Culture medium
Add 50 ml of FBS to 500 ml of DMEM, and store at 4 °C for up to 2 weeks.
CRITICAL: The efficiency of rabies virus production depends on the quality of FBS. We recommend testing several lots in advance and then investing in large quantities from the best lot to assure consistent future production.
Critical: We use culture medium without antibiotics because any contamination will be immediately apparent, thus saving time by avoiding continuation of the protocol with a preparation in which contamination will become apparent only later. Because rabies virus amplification is based on passage of culture supernatant, once supernatant becomes contaminated, the supernatant keeps carrying contamination to subsequent dishes. It is better to identify the contamination as soon as possible and discard the preparation immediately if it is contaminated.
Cell freezing solution
Mix 8 ml of DMEM, 1 ml of FBS, and 1 ml of DMSO. Prepare freshly and cool at 4°C until use. For larger volumes, increase the component amounts proportionally.
20% sucrose/HBSS
Add 20 g sucrose with 10 ml of 10x HBSS, adjust the total volume to 100 ml with distilled water, and sterilize by passing the solution thorough a 0.22 μm filter. For larger volumes, increase the component amounts proportionally. The solution can be stored at 4°C for several months.
PROCEDURE
CRITICAL: In the event that there already exists an available starter stock for a particular rabies virus13, stages I-II are not necessary. Stage IV is only required in the event that pseudotyped virus is required.
Stage I. Design, construct and prepare rabies viral genome plasmid encoding genes of interest
-
1)
Select the transgenes of interest.. The description here is based on the use of pSADΔG-F3 as a starting plasmid that contains the G-deleted SAD-B19 rabies viral genome and convenient restrictions sites to facilitate the incorporation of one or two genes of interest. Choose unique restriction enzyme sites in the pSADΔG-F3 plasmid sequence to enable cloning of your transgenes. Prepare the rabies virus vector backbone such as pSADΔG-F3 and digest the plasmid with chosen restriction enzyme(s) to obtain a backbone fragment.
CRITICAL STEP: If you use two different restriction enzyme sites which produce non-compatible ends, it is possible to clone a fragment unidirectionally into the rabies virus genomic vector. But if you use one restriction enzyme site, ligated products have two directions of insertion and it is therefore essential to verify that the direction of the inserted gene is the desired one.
-
2)
Perform electrophoresis of your digested plasmid on a 0.7% (wt/vol) agarose gel in 1× TAE buffer.
-
3)
Cut the gel containing the desired backbone fragment under illumination of longer wave length UV (360 nm) as quickly as possible, gel-purify it with the QIAquick Gel Extraction Kit according to the manufacturer’s instructions and elute it in water.
Critical: Minimize UV exposure to reduce DNA damage. Exposure to short wave length UV for long period induces mutations in DNA.
-
4)
Obtain an insert DNA fragment. If you have more than one unique restriction site in your transgene follow option A. If you find only one unique restriction enzyme site in your transgene follow option B; the cloning will require extra steps. If there are no unique restriction enzyme sites in your transgene follow option C; you will need extra steps after digesting your insert. Option D is another option for cloning transgenes into the rabies virus genome using the In-fusion system (Clontech). The In-fusion system allows directional cloning in a single reaction without restriction digestion of inserts, phosphatase treatment of vectors, or ligation. Digestion of PCR-generated inserts with restriction enzymes is not required for In-Fusion cloning because In-Fusion enzyme fuses DNA fragments (PCR-generated sequences and linearized vectors) by recognizing a 15 bp overlap at their ends.
-
If using two different restriction enzyme ends to produce an insert.
-
Design a set of primers containing the restriction enzyme sites. Decide on two restriction enzyme sites matching your target ORF. The forward primer needs to contain an additional 2–5 bp, a restriction enzyme site, a Kozak consensus sequence (GCCACC: 6 bp), and a sequence for the initiation of the ORF from 5′ to 3′ (18–25 bp). The reverse primer needs to contain an additional 2–5 bp, a restriction enzyme site, and a terminal sequence for the ORF (18–25 bp). The lengths of forward primers and reverse primers are generally 34–40 bp and 30–36 bp, respectively.
Critical: Virus packaging capacity is limited. PCR is ideal to minimize the insert size.
Critical: The Kozak consensus sequence plays a major role in the initiation of the translation process.
Critical: In general, to cleave efficiently, 6 bp should be added at the end of the recognition site. Table 1 and the NEB website (http://www.neb.com/) provide an enzyme list showing the number of base pairs required for efficient cleavage. Critical: Avoid dimerization of primers and secondary structure such as hairpin structure.
Critical: If you cannot use the same restriction enzyme sites for the backbone vector and the insert, please check different enzymes which produce compatible ends (Table 2). For example, the XbaI end of the insert can be ligated with the NheI end of the backbone vector.
-
Amplify a DNA fragment containing restriction enzyme sites by PCR. Set 25 cycles of PCR using a high-fidelity polymerase such as Fusion polymerase.
Critical: A proofreading high-fidelity polymerase that has a 3′ to 5′ exonuclease activity is essential to avoid PCR errors.
Critical: To reduce mutation, keep the number of amplification cycles to less than 25.
Critical: It is better to add DMSO or other similar agents to weaken hydrogen bonding and prevent formation of hairpin structure, although this depends on the sequence of the ORF and the designed primers.
-
Perform electrophoresis on 0.7 – 1.0 % (wt/vol) agarose gel in 1× TAE buffer.
Critical: The agarose concentration depends on the product size.
-
Cut the gel containing the desired size fragment under illumination of longer wave length UV (360 nm) as quickly as possible, gel-purify it with the QIAquick Gel Extraction Kit according to the manufacturer’s instructions and elute it in water.
Critical: Exposure to short wavelength UV for long period induces mutations in DNA. Minimize UV exposure to reduce DNA damage.
-
-
If using only one unique restriction enzyme site.
-
Treat the backbone vector with CIP at 0.5 units of CIP/μg DNA for 1 hour at 37 °C, and then purify the vector by spin-column purification or phenol extraction.
CRITICAL: If you use a single restriction enzyme site for cloning, recirculation of the cut plasmid frequently occurs. Treatment with the alkaline phosphatase CIP dephosphorylates termini and prevents vector self-ligation.
CRITICAL: CIP cannot be heat-inactivated and requires purification before ligation.
CRITICAL STEP: After ligation, verification of the direction of the inserted gene is essential because this strategy produces two types of clones that have two different direction of the insert.
To obtain an insert, design a set of primers, amplify the transgene by PCR and purify the PCR product as described in option A.
-
-
If your ORF does not have any unique restriction enzyme sites that exist in the pSADΔG-F3 vector.
-
Digest the backbone vectors with restriction enzymes (choose enzyme(s) from SbfI SrfI and NheI to insert the first ORF, enzyme(s) from PacI, AscI, and SacII to insert the second ORF, or choose one enzyme from SbfI, Srf1 and NheI and one enzyme from PacI, AscI and SacII to clone a single ORF), treat with Klenow to produce blunt ends, and ligate with the PCR product with blunt ends.
Critical: Any combination of restriction enzyme sites will work for cloning, but if your transgene(s) is larger, it would be better to choose enzyme sites which make the rabies viral genome smaller.
CRITICAL STEP: You need to check the direction of insert DNA because this strategy produces two types of clones that have two different directions of the insert.
-
To obtain an insert, design a set of primers, amplify the transgene by PCR using a high-fidelity polymerase such as Fusion polymerase, and then purify the PCR product.
CRITICAL: A high-fidelity polymerase that possesses a 3′ to 5′ exonuclease proofreading activity generates blunt-ended PCR products.
-
-
If using the In-fusion system.
Design a forward primer containing sequences of 15 bp extensions homologous to the linearized rabies viral vector end, a Kozak consensus sequence (GCCACC: 6 bp), and initiation of the ORF (18–25 bp). Design a reverse primer containing sequences of 15 bp extensions homologous to the linearized rabies viral vector end and terminal of ORF (18–25 bp). If necessary, the Primer Design tool for the In-Fusion system is available at https://www.takara-bio.co.jp/infusion_primer/infusion_primer_form.php or http://bioinfo.clontech.com/infusion/convertPcrPrimersInit.do
-
Perform PCR with primers designed in step (i), gel-purify the PCR product, mix it with a linearized rabies viral vector (for example, digested with SbfI and NheI to insert the first ORF, PacI and SacII to insert the second ORF, or SbfI and SacII to clone a single ORF) and the In-Fusion enzyme mixture, and let them react at 50°C for 15 min.
Critical: Set the PCR using a high-fidelity polymerase such as Fusion polymerase.
Critical: A proofreading high-fidelity polymerase is essential to avoid PCR errors.
Critical: To reduce mutation, keep the number of amplification cycles to less than 30.
Critical: It is better to add DMSO or other similar agents to weaken hydrogen bonding and prevent formation of hairpin structure, although this depends on the sequence of the ORF and the designed primers.
-
Perform electrophoresis on 0.7 – 1.0 % (wt/vol) agarose gel in 1× TAE buffer.
Critical: The agarose concentration depends on the product size.
-
Cut the gel containing the desired size fragment under illumination of longer wave length UV (360 nm) as quickly as possible, gel-purify it with the QIAquick Gel Extraction Kit according to the manufacturer’s instructions and elute it in water.
Critical: Exposure to short wave length UV for long period induces mutations in DNA. Minimize UV exposure to reduce DNA damage.
Mix the insert, a linearized rabies viral vector and the In-Fusion enzyme mixture, and let them react at 50°C for 15 min.
Table 2.
The number of base pairs to add to the primer end for efficient cutting of the PCR products.
| Enzyme | The number of base pairs to add the primer end |
|---|---|
| Sbf1 | 3 |
| Srf1 | 6 |
| NheI | 3 |
| PacI | 2 |
| AscI | 1 |
| SacII | 2 |
| PstI | 2 |
| NsiI | 3 |
| SpeI | 5 |
| XbaI | 5 |
| PvuI | 2 |
| MluI | 3 |
| BsiEI | 5 |
-
5)
If you chose option A or B for step 4, proceed with the following steps. If you chose option C, proceed to step 9. If you chose option D, proceed to step 10.
-
6)
Digest the purified PCR product with the restriction enzyme(s) which you used for designing primers.
-
7)
Perform electrophoresis on 0.7 – 1.0 % (wt/vol: the concentration depends on the product size) agarose gel in 1× TAE buffer.
Critical: Please confirm that there is a single band of the desired size.
-
8)
Excise the band of the desired size, gel-purify it with the QIAquick Gel Extraction Kit according to the manufacturer’s instructions and elute it in water.
-
9)
Ligate the rabies viral backbone vector with the insert DNA fragment according to the manufacturer’s instructions. A vector/insert ratio at 1:3 – 1:10 should be ideal, but we recommend mixing vectors and inserts at a several different ratios.
-
10)
Transform chemically competent SC33 cells with the ligation reaction mix. Apply 1–2 μl of the reaction mix to SC33 cells that are thawed on ice.
-
11)
Incubate the SC33 cells on ice for 10 minutes.
-
12)
Incubate the SC33 cells at 42°C for 45 seconds. Incubate the cells on ice for 2 min.
-
13)
Add 250 μl of SOC medium to the cells and shake the cells at 37°C for 45 min at 225 rpm in a shaking incubator.
-
14)
To select and collect transformants, spread 20 μl and 200 μl of the cells onto a Carbenicillin (100 μg/ml)- or Ampiliciln (100 μg/ml)-containing LB agar plates. Invert the plates and incubate at 37°C overnight.
-
15)
Check colony formation on the plate. Pick up the resulting colonies to screen for the correct plasmid DNA. Start mini cultures in LB media containing Carbenicillin (100 μg/ml) or Ampiliciln (100 μg/ml).
Critical: We typically pick 8 colonies to assure that at least one will be correct.
Critical: Colony PCR is also effective for screening positive colonies. In brief, pick up a single colony with a micropipette tip, make a replicate of the clone in a new LB plate or LB media, and then pipette up and down in PCR mixture in the PCR tube. Perform PCR followed by agarose gel electrophoresis. If you get positive clones, start mini cultures for positive clones.
-
16)
Extract plasmid DNA from the mini cultures using a Plasmid Mini Prep Kit according to the manufacturer’s instructions.
-
17)
Check the plasmids by diagnostic digestions with appropriate restriction enzymes to confirm the insert, run the digests on agarose gel using electrophoresis and confirm the digestion pattern to obtain a correct clone.
Critical: If you use a single restriction enzyme site for cloning, confirm that your cloned transgene(s) is (are) inserted in the proper orientation.
-
18)
Start Maxi culture of the expected clone in 250 ml of LB media containing Carbenicillin (100 μg/ml) or Ampiliciln (100 μg/ml) by transforming SC33 competent cells and picking up a single colony as described in steps 10–14.
-
19)
Extract the plasmid using a Plasmid Maxi Prep Kit.
-
20)
Sequence the plasmid to check that the clone has the correct sequence.
Crtical: If the clone that you sequenced has mutations, try another clone in step 17.
Stage II. Recover G-deleted rabies virus from plasmid DNA
-
21)
Prepare one 10 cm dish containing 3.3 × 106 B7GG cells according to the procedure of BOX 1.
Maintain the dish at 37°C, 5% CO2 atmosphere.
Critical: Plate the B7GG cells just one day before transfection.
-
22)
The next day, transfect the B7BB cells with the rabies virus genomic vector, pcDNA-SADB19N, pcDNA-SADB19P, pcDNA-SADB19L and pcDNA-SADB19G (This day is defined as stage II day 0). First replace the medium with 7 ml of OPTI-MEM.
Then prepare the transfection mixes as follows:
Tube A: Add 30 μg of pSADΔG genomic vectors, 15 μg of pcDNA-SADB19N, 7.5 μg of pcDNA-SADB19P, 7.5 μg of pcDNA-SADB19L and 5 μg of pcDNA-SADB19G and then add 1.25 ml of Opti-MEM in the tube. Vortex the tube 3 seconds, 4 times.
Tube B: Add 1.25 ml of Opti-MEM and then 112.5 μl of Lipofectamine 2000. Vortex the tube 3 seconds, 4 times.
Critical: When preparing transfection mixes, use polypropylene tubes rather than polystyrene tubes. Direct contact between Lipofectamine 2000 and tubes or pipette could result in reduced transfection efficiency, as the Lipofectamine 2000 will adhere to these plastics preferentially. See the details in the manufacturer’s manual.
-
23)
Let the tubes A and B stand at room temperature (~25°C) for 5 min.
-
24)
Add tube A to tube B, and vortex the tube for 3 seconds 5 times
-
25)
Let the tube stand at room temperature for 15 min.
-
26)
Add 1.0 ml of the AB mixture evenly in a dropwise manner onto the 10 cm dish of B7GG cells. The total volume of the medium will be around 8 ml. Incubate the dish at 5% CO2 and 37°C.
-
27)
Six hours later, remove the media, wash with 10 ml of 10%FBS/DMEM, and add 10 ml of 10%FBS/DMEM. Incubate the dish at 5% CO2 and 37°C overnight.
-
28)
On day 1, transfer the dish to incubate at 3% CO2 and 35°C overnight.
-
29)
On day 2, wash with PBS, add 4 ml of 0.25% trypsin-EDTA and incubate the dish for 4 min at 37°C.
-
30)
Detach the cells from the 10 cm dish and dissociate the cells into single cells by pipetting with a P1000 micropipette. Add 5 ml of 10% FBS/DMEM and collect the cell suspension to a conical tube. Add an additional 10 ml of 10% FBS/DMEM to the dish to collect any remaining cells, and transfer this rinse to the tube containing the cells.
-
31)
Centrifuge the tube at 200 g for 3 min and remove the supernatant. Gently tap the bottom of the tube to loosen the cell pellet, add 10 ml of 10% FBS/DMEM, and gently resuspend the pellet by pipetting up and down with a P1000 micropipette.
-
32)
Plate the cell suspension to a new 150 mm dish. Incubate the dish at 3% CO2 and 35°C overnight.
-
33)
On day 3, remove the supernatant and add 24 ml of fresh 10% FBS/DMEM. Incubate the dish at 3% CO2 and 35°C for 2 days.
-
34)
On day 5, add 5 ml of fresh 10%FBS/DMEM in the 150 mm dish and incubate the dish for 2 days.
-
35)
On day 7, collect the supernatant and filtrate the collected supernatant with 0.45 μm filter. Add 24 ml of fresh 10% FBS/DMEM in the 150 mm dish and incubate the dish at 3% CO2 and 35°C for 2 days. CRITICAL STEP: The timing of the collection of supernatant depends on the speed at which the virus spreads, as well as the condition of the cells. The cells and their GFP (or RFP) signals should be checked under a microscope every day.
-
36)
On day 9, add 5 ml of fresh 10%FBS/DMEM in the 150 mm dish and incubate for 2 days.
-
37)
On day 11, collect and filtrate the 2nd supernatant with a 0.45 μm filter.
TROUBLESHOOTING
BOX 1. Maintenance of B7GG cells.
The quality of G-deleted rabies virus depends on the condition of B7GG cells. It is also important to generate and store a large number of frozen stocks of B7GG cells at a low-passage number. Here we describe how to prepare B7GG cells.
-
Prepare a sub-confluent dish of B7GG cells.
CRITICAL STEP: Do not let them grow up to 100% confluency. When the dish becomes sub-confluent (80–90% of the dish), it is time to split the cells.
-
Aspirate the media from the dish, wash the dish with 10 ml of PBS, add 4 ml of pre-warmed 0.25% (wt/vol) trypsin-EDTA and incubate the dish at 37°C for 5–6 min.
Critical: Confirm that the cells are detached from the dish by tapping the dish. If cells are still attached on the dish, incubate it for an additional 1–2 min.
-
Gently dissociate the cells with a P1000 micropipette.
CRITICAL STEP: Pipetting at this step is important. B7GG cells must be single, not in clumps (3–5 cells). Clumps of B7GG cells do not grow well on the dish.
Add 4 ml of media, and collect the cell suspension in a 50 ml conical tube.
-
To collect the remaining cells on the dish, apply an additional 5 ml of media, and transfer the cell suspension into the 50 ml conical tube.
Critical: Collect all of the cells. If you do not collect all of them, the split ratio will be decreased.
Centrifuge the 50 ml tube for 3 min at 200 g at room temperature.
Aspirate the supernatant carefully, apply 10 ml of pre-warmed media and dissociate into single cells by pipetting with a P1000 micropipette.
-
To split the cells at 1:5, mix 20 ml of pre-warmed media and 2 ml of the cell suspension in a 50 ml tube, and plate 22 ml of the cell suspension to a 150 mm dish.
Critical: Keep the split ratio at 1:5 – 1:10. Culturing B7GG cells at a lower cell-density causes changes in cell morphology and rate of proliferation that appear to be detrimental to efficient viral production.
Maintain the dishes in a humidified atmosphere of 5% CO2 at 37°C. One day after plating B7GG cells at 1:5, the cells will typically be ready for rabies virus amplification.
Stage III. Amplify G-deleted rabies virus
-
38)
If you performed stage II, follow option A. If you have a frozen stock of amplified virus supernatant (from Box 2), follow option B. BOX 2 describes the procedure for making frozen stocks of virus supernatant.
BOX 2. Production of frozen seed stocks of G-deleted rabies virus.
G-deleted rabies viruses can grow in B7GG cells. If you make frozen seed stocks of viruses, you do not need to recover G-deleted rabies viruses from plasmid DNA every time. G-deleted rabies viruses that we have already recovered and characterized are also available13. To avoid accumulation of mutations in the rabies viral genome, it is ideal to generate as many frozen stocks of low passage as possible.
Recover a novel G-deleted rabies virus from plasmid DNAs according to the procedure described in stage II.
Mix the 1st supernatant and 2nd supernatant and split the mixed supernatant into 3 parts and amplify the G-deleted rabies virus in 3, 15 cm dishes of B7GG cells. After the inoculation, change medium with 24 ml of 10% FBS/DMEM on day 0 and then apply additional 4 ml of 10% FBS/DMEM on day 3. Collect and filtrate the supernatant on day 5. The total volume of the supernatant is approximately 80 ml.
Make 4 ml aliquots in 5 ml cryogenic tubes and store them at −80°C. The total number of frozen stocks is approximately 20 tubes.
When you initiate amplification from this frozen stock, prepare a 15 cm dish and apply 4 ml of supernatant to the 15 cm dish. If the titer of the stocks is low, use a 10 cm dish to amplify the virus.
-
Using recovered virus from stage II.
Prepare 3 60% confluent 15 cm dishes of B7GG cells. Aspirate and discard medium from the dish and wash with 10ml PBS.
Mix the 1st supernatant and 2nd supernatant (from steps 35 and 37), split the mixed supernatant into three parts and apply it (~18 ml) to each 150 mm dish and add 7 ml of fresh 10% FBS/DMEM. Maintain the dishes in a humidified atmosphere of 3% CO2 at 35°C.
-
Using a frozen stock of amplified virus supernatant.
-
Prepare a 60% confluent 15 cm dish of B7GG cells. Aspirate and discard medium from the dish and wash with 10 ml PBS.
Critical: For more amplification, increase the numbers of B7GG cells and virus stocks proportionally.
-
Add medium and virus supernatant to the dish to an MOI at 0.1–0.3. For example, add 16 ml of fresh 10% FBS/DMEM and 4 ml of virus supernatant. Maintain the dish in a humidified atmosphere of 3% CO2 at 35°C.
CRITICAL STEP: 35°C and 3% CO2 are better than 37°C and 5% CO2 during virus production steps. Cell growth and metabolism decrease and medium tends not to turn acidic under the 35°C and 3% CO2 condition.
-
-
39)
Five to six hours later, aspirate the media from the dish, and add 24 ml of pre-warmed medium. This day is defined as stage III day 0 (day numbering is independent of that in stage II). Incubate the dish at 3% CO2 and 35°C for 4 days.
Critical: Acidic medium is detrimental to rabies virus production. Glycoproteins change their conformation according to pH. If the color of medium turns yellow, the medium is acidic because the medium contains the pH indicator phenol-red. Before the medium turns yellow on day 2 or day 3, it is better to add extra fresh medium (3–5 ml).
-
40)
On day 4, collect the supernatant from the rabies virus-infected 150 mm dish, and filtrate it with a 0.45 μm filter. Store the supernatant at 4°C for less than 2 days or −80°C for years. Add 24 ml of 10% FBS/DMEM to the 150 mm dish and incubate the dish at 3% CO2 and 35°C for additional 2 days.
-
41)
On day 6, collect the supernatant from the rabies virus-infected 150 mm dish, and filtrate it with a 0.45 μm filter. Store the supernatant at 4°C for less than 2 days or −80°C for years.
CRITICAL STEP: The timing of collection of the supernatant depends on the speed at which the virus spreads, as well as the condition of the cells. The cells and their GFP (or RFP) signals should be checked under a microscope every day.
Critical: If the virus expresses genes that are toxic to the cells at a higher expression level, apply more volume of supernatant on day 0 at higher MOI and collect supernatant by day 5.
TROUBLESHOOTING
-
42)
If you need to concentrate a large amount of virus, prepare 10, 60–70% confluent 150 mm dishes of B7GG cells before proceeding with concentration steps (Stage V). Otherwise go directly to either step 47 for pseudotyping (Stage IV) or step 68 for titration (Stage VI).
Critical: The number of amplifications required depends on the purpose and design of the experiments. For example, a single amplification should be enough for most in vitro experiments. In contrast, for in vivo tracing experiments, we usually prepare 10, 15 cm dishes for virus concentration and make many stocks of a single lot to ensure consistent results.
-
43)
Aspirate the media from the 10, B7GG dishes, and add medium and virus supernatant to each 150 mm dish to an MOI of 0.4–0.5. For example, mix the 1st supernatant and 2nd supernatant (from 3 dishes at steps 35 and 36), split the mixed supernatant into 10 parts, apply it (~15 ml) to each 150 mm dish, and then add 9 ml of fresh 10% FBS/DMEM. Maintain the dishes in a humidified atmosphere of 3% CO2 at 35°C. Restart numbering of days and define this day as stage III day 0.
-
44)
Five to six hours later, aspirate the media from the dish, and add 24 ml of pre-warmed media. Incubate for 3 days.
-
45)
On day 3, add 3 ml of fresh pre-warmed medium to the dishes and swirl the dishes gently. Incubate for 1–2 days.
-
46)
On day 4 or 5, collect the supernatant from the rabies virus-infected 150 mm dishes and filtrate it with a 0.45 μm filter.
TROUBLE SHOOTING
Stage IV. Pseudotype virus with EnvA or EnvB
-
47)
Prepare 10, 15cm dishes of 60% confluent BHK-EnvA cells or BHK-EnvB cells (this is stage V Day 0). Prepare BHK-EnvA (BHK-EnvARGCD cell) or BHK-EnvB cells to produce EnvA-pseudotyped or EnvB-pseudotypted G-deleted rabies virus, respectively. With the exception of the cell line used, the procedure for pseudotyping with EnvB is the same as that for pseudotyping with EnvA. Thus the procedure continues describing EnvA only.
-
48)
Aspirate medium from 10, 15cm dishes of BHK-EnvA cells, apply supernatant of unpseudotyped G-deleted rabies virus to get MOI = 0.5–0.6. For example, apply 10 ml of filtered supernatant of unpseudotyped G-deleted rabies virus to each dish, and then apply 10 ml of fresh 10% FBS/DMEM.
-
49)
Maintain the dishes in a humidified atmosphere of 3% CO2 at 35°C for 6 hr.
-
50)
Five to six hr later, aspirate the medium from infected BHK-EnvA cell dishes and rinse the dishes with PBS (without Ca, Mg) twice to remove unpseudotypted viruses. Tilt and swirl the plates to wash out any unpseudotypted viruses from the walls of the plates.
CRITICAL STEP: Minimizing remnants of starter supernatant of unpseudotyped rabies viruses is crucial to making high purity pseudotyped rabies virus.
-
51)
Remove PBS, apply pre-warmed 5 ml of 0.25% trypsin-EDTA and incubate the dish at 37°C for 3 min to destroy residual rabies G.
CRITICAL STEP: This step is essential for the production of well-pseudotyped virus.
-
52)
Detach the cells completely by pipetting, apply 5 ml of 10% FBS/DMEM and collect the cells into a 50 ml conical tube. To collect the remaining cells on the dish, apply an additional 5 ml of media to the dish, and transfer the cell suspension into the 50 ml conical tubes.
-
53)
Centrifuge the tube at 200 g for 3 min and remove the supernatant to remove remnants of native rabies virus G-enveloped, unpseudotyped G-deleted rabies viruses.
-
54)
Re-suspend the cells with media and plate the cell suspension onto 10, new 15cm dishes at 23 ml per dish. Maintain the dishes in a humidified atmosphere of 3% CO2 at 35°C overnight.
-
55)
On day 1, remove the media to remove both unpseudotyped viruses and dead cells. Add 23 ml of fresh pre-warmed medium. Incubate for 2 days.
CRITICAL STEP: The first supernatant may be contaminated with the initially applied unpseudotyped viruses.
-
56)
On day 3, collect the supernatant from the 10 virus-infected 15 cm dishes, and filter it through a 0.45 μm filter. If you need to concentrate the supernatant, please see section IV above and follow steps 47–57.
Stage V. Concentrate virus
-
57)
Apply 35 ml of the supernatant to each of 6 centrifuge tubes in the hood. Make sure to prepare a proper counter-balance.
Critical: For the 1st centrifugation, use the Beckman SW28 rotor. This rotor gives 35 ml × 6 tubes = 210 ml total capacity.
-
58)
Spin down the viral supernatant at 70,000 g for 2 hr at 4°C. Use the Beckman SW28 rotor at 19,400 RPM.
-
59)
Stop the centrifuge, move the centrifuged tubes back to the hood and gently remove the supernatant by pouring it off and allowing the remaining liquid to drain by resting the inverted tubes on paper towels. Remove all liquid from the pellet using an aspirator. The pellet should be barely visible as a small translucent spot.
-
60)
Re-suspend each pellet in 300 μl Hanks’ Balanced Salt Solution (HBSS), wrap the top of the tubes in Parafilm and agitate the tubes on a low speed vortex for 5 seconds, 5 times.
-
61)
For the 2nd centrifugation, place 2.5 ml of HBSS/20% sucrose in a round-bottom tube that is suitable for a Beckman SW55 rotor.
-
62)
Gently pipette the viral suspension up and down and transfer 1.8 ml of the viral suspension from the 6 tubes (300 μl ×6) on top of the sucrose cushion.
Critical: Avoid creating air bubbles.
-
63)
Rinse the tubes with 200 μl of HBSS to recover any remaining rabies virus and transfer the suspension to the sucrose cushion again. Make sure to prepare a proper counter-balance.
-
64)
Spin at 50,000 g for 2 hr at 4°C. Use the SW55 rotor at 21,000 RPM.
-
65)
Two hr later, stop the 2nd centrifugation, move the centrifuged tubes back to the hood, gently remove the supernatant as described at step 59, and re-suspend the viral pellets in 100 μl of cold HBSS.
-
66)
Wrap the tube with parafilm, agitate the tube on a low speed vortex for 3 seconds, 7–10 times, and keep the tube at 4°C for 1 hr.
-
67)
Gently pipette the viral suspension up and down and make aliquots of the virus (3–5 μl each) and freeze at −80°C for future use.
Critical: Avoid the production of bubbles during pipetting. PAUSEPOINT Concentrated virus can be frozen at −80°C for at least 3–4 years. However, avoid freeze-and-thaw cycles of frozen stocks as this reduces the viral titer.
Stage VI. Determine viral titer
-
68)
To titer unpseudotyped viruses, prepare HEK293t cells at 3.0 × 105 cells/ml and seed 500 μl of the cell suspension in each well of a 24-well plate, to get 1.5 × 105 cells/well in a final volume of 500 μl per well (this corresponds to stage VI day 0). To titer pseudotyped viruses, prepare both HEK293t cells and HEK293t-TVA cells for EnvA-pseudotyped viruses or HEK293t-TVB cells for EnvB-pseudotyped viruses on 24-well plates at 1.5 × 105 cells/well.
Critical: Pseudotyped viruses need to be tested for purity by infecting HEK293t cells.
-
69)On Stage VI day 1, make a tenfold serial dilution of the viral preparation (from a dilution of 10−3 to a dilution of 10−11) of the viral preparation. The actual range to test will depend on the concentration of each viral preparation and must be determined empirically. For example, you could test the following concentrations:
Viral preparation Containing A 2 μl of virus stock in 1998 ul of media containing 10% FBS. (10−3) B 100 μl of A in 900 μl of media (10−4) C 100 μl of B in 900 μl of media (10−5) D 100 μl of C in 900 μl of media (10−6) E 100 μl of D in 900 μl of media (10−7) F 100 μl of E in 900 μl of media (10−8) G 100 μl of F in 900 μl of media (10−9) H 1000 μl of media (control: no virus) CRITICAL STEP: Change tips every dilution. Avoid transferring volumes <10 μl to minimize dilution errors.
-
70)
Add 250 μl of each viral dilution (A–H) to the cells gently (final medium volume: 750ul) and incubate the cells in a humidified atmosphere of 3% CO2 at 35°C. GFP fluorescence should become visible 24–36 h after infection.
Critical: 35°C, 3% CO2 conditions can reduce cell division.
-
71)
On Stage VI day 3, determine the number of infected cells.
-
72)
Calculate the biological titer (infectious units/ml).
To do this, start by finding wells containing 10–100 GFP+ cells. Count the number of GFP+ cells in the wells. Then proceed, with calculating the average for them.
For example, if the numbers of GFP+ cells are 95 and 85 in the 10−7 wells (E), the average is 90 cells/well. This means that 90 virus particles exist in the well E.
Thus, the virus concentration of the well E is 90/0.75 (virus particles/ml)
The concentration of the tube E is 90/0.75 × 3 = 3.6 × 102 (virus particles/ml)
-
Then the titer of the original stock becomes 3.6 × 102 × 107 (virus particles/ml) = 3.6 × 109 (infectious units/ml).
CRITICAL STEP: If you use wells at higher infection efficiency for titration, these cells are infected with multiple virions. From these wells, it is not possible to determine the titer because the number of virions is not equal to the number of GFP+ cells in those wells. Thus, use a well which has 10–100 GFP+ cells that were considered to be infected with single virions.
TROUBLESHOOTING
TIMING
Steps 1–20 : Design and construction of rabies virus vector: 1–3 weeks
Steps 21–37 : Recovery of G-deleted rabies virus from cDNA: 10–11 days
Steps 38–46 : Amplification of G-deleted rabies virus: 5–6 days
Steps 47–56 : Pseudotyping of G-deleted rabies virus with EnvA or EnvB: 3–4 days
Steps 57–67 : Concentration of virus by ultracentrifugation: 1 day
Steps 68–72 : Titration of virus: 3 days
Troubleshooting
Troubleshooting advice can be found in Table 3.
Table 3.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 4Aiii and 4Diii | No correctly sized band for the transgene | Incorrect annealing temperature or primers. | Set a different annealing temperature or design a new set of primers. |
| 17 | No recovery | The genomic plasmid or helper plasmids could be wrong. | Check 260/280 absorbance ratio with spectrophotometer. Check plasmids with restriction digest and gel electrophoresis. Sequence plasmids. |
| 37 | No recovery | Cell conditions could be bad. | Check the media and reagents. Prepare new culture media. Try another lot of FBS which allows proper cell proliferation and virus amplification. |
| 37 | Low titer | The MOI at which you applied virus supernatant is low | Apply supernatant at higher MOI on day 0. For further amplification, use a lower splitting ratio, such as 1:2. |
| 41 | Low titer | Cell conditions could be bad. | Check the media and reagents. Use fresh culture media. Try another lot of FBS which allows proper cell proliferation and virus amplification. Restart from an earlier passage of B7GG cells. |
| 46 | Low titer | The MOI at which you applied virus supernatant is low | Apply virus supernatant at higher MOI on day 0. |
| 46 | Low titer | Cell conditions could be bad. | Check the media and reagents. Use fresh culture media. Try another lot of FBS which allows proper cell proliferation and virus amplification. Restart from an earlier passage of B7GG cells. |
| 72 | Low titer | The MOI at which you applies virus supernatant is low | Apply supernatant at higher MOI on day 0. |
| 72 | Low titer | Cell conditions could be bad. | Check the media and reagents. Use fresh culture media. Try another lot of FBS which allows proper cell proliferation and virus amplification. |
| 72 | Low titer | The cells or viruses are old. | Restart from an earlier passage of B7GG cells and viruses. Both cells and viruses obtained from their laboratory of origination should be immediately expanded into a large number of low-passage aliquots that can be stored (See Box 2). |
| 72 | Low titer | The titer of the starter stock is low. | Use one 100 mm dish for the first amplification, and then scale up to 15 cm dishes. |
ANTICIPATED RESULTS
Our protocols produce consistent outcomes when appropriate attention is paid to the conditions of the producer cells (B7GG cells, BHK-EnvA cells and BHK-EnvB cells), to the passage number of cells and viruses, and to the storage of generated viruses. We routinely produce concentrated stocks of unpseudotyped rabies viruses and EnvA- or EnvB-pseudotyped rabies viruses at 109 infectious units/ml and 108 infectious units/ml, respectively13. Unpseudotyped rabies viruses at higher than 5 × 108 infectious units/ml and pseudotyped rabies viruses at higher than 3 × 107 infectious units/ml work well for in vivo circuit tracing experiments8–13, 15. Dilutions of viruses (at 1/10 – 1/100) allow sparse labeling of neurons, depending on the design and purpose of the experiments. Injection of unpseudotyped G-deleted rabies viruses will label long-range projection neurons in a retrograde direction, while VSV-G-pseudotyped rabies viruses will locally infect neurons around an injection site6, 8, 13, 15. When TVA or TVB is introduced in a particular cell type or a single cell (e.g. by using a cell type-specific promoter, Cre-, FLP- or tTA-dependent system, bridge proteins, electroporation or targeted patching in vitro or in vivo), EnvA- or EnvB-pseudotyped rabies viruses can be used to selectively infect the TVA- or TVB-expressing cells, respectively. With gene transfer of G, transcomplementation of G-deleted rabies virus makes it possible to spread G-deleted rabies virus to presynaptic neurons that are directly connected to G-expressing starter cells. It should be noted that only monosynaptically-connected neurons are infected and labeled with G-deleted rabies virus following transcomplementation. The high-resolution circuit tracing can be combined with functional assays monitoring or manipulating neural activity and gene expression. These studies will make it possible to link structure to function of neural circuits.
Acknowledgments
We thank Karl-Klaus Conzelmann, John A.T. Young, Ian R. Wickersham, Takuma Mori, Jiwon Choi, Nicholas R. Wall and Ali H. Cetin for discussions, and Beatriz Virgen, Karine von Bochmann and Mauricio De La Parra for technical assistance. F.O. is thankful to Noriko Osakada for constant encouragement and support. We are grateful for the support from the National Institutes of Health (MH063912, NS069464, and EY520568: E.M.C.), the Kavli Institute for Brain and Mind at UC San Diego (E.M.C.), the Gatsby Charitable Foundation (E.M.C.), the Japan Society for the Promotion of Science (F.O.), the Kanae Foundation for the Promotion of Medical Science (F.O.), the Uehara Memorial Foundation (F.O.), the Naito Foundation (F.O.) and the Pioneer Fund (F.O.).
References
- 1.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenkiel BR, Ehlers MD. Molecular genetics and imaging technologies for circuit-based neuroanatomy. Nature. 2009;461:900–907. doi: 10.1038/nature08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scanziani M, Hausser M. Electrophysiology in the age of light. Nature. 2009;461:930–939. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- 4.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2011;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods. 2007;4:47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickersham IR, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen DD, Wickersham IR, Callaway EM. Retrograde tracing with recombinant rabies virus reveals correlations between projection targets and dendritic architecture in layer 5 of mouse barrel cortex. Front Neural Circuits. 2007;1:5. doi: 10.3389/neuro.04.005.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nassi JJ, Callaway EM. Specialized circuits from primary visual cortex to V2 and area MT. Neuron. 2007;55:799–808. doi: 10.1016/j.neuron.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Young JA, Callaway EM. Selective viral vector transduction of ErbB4 expressing cortical interneurons in vivo with a viral receptor-ligand bridge protein. Proc Natl Acad Sci U S A. 2010;107:16703–16708. doi: 10.1073/pnas.1006233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci U S A. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshel JH, Mori T, Nielsen KJ, Callaway EM. Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron. 2010;67:562–574. doi: 10.1016/j.neuron.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osakada F, et al. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, Callaway EM. Monosynaptic inputs to ErbB4-expressing inhibitory neurons in mouse primary somatosensory cortex. J Comp Neurol. 2011;519:3402–3414. doi: 10.1002/cne.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nhan HL, Callaway EM. Morphology of superior colliculus- and middle temporal area-projecting neurons in primate primary visual cortex. J Comp Neurol. 2012;520:52–80. doi: 10.1002/cne.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Haubensak W, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonehara K, et al. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- 19.Miyamichi K, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rancz EA, et al. Transfection via whole-cell recording in vivo: bridging single-cell physiology, genetics and connectomics. Nat Neurosci. 2011;14:527–532. doi: 10.1038/nn.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479:61–66. doi: 10.1038/nature10538. [DOI] [PubMed] [Google Scholar]
- 22.Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Vivar C, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takatoh J, et al. New modules are added to vibrissal premotor circuitry with the emergence of exploratory whisking. Neuron. 2012;77:346–360. doi: 10.1016/j.neuron.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia I, Huang L, Ung K, Arenkiel BR. Tracing synaptic connectivity onto embryonic stem cell-derived neurons. Stem Cells. 2012;30:2140–2151. doi: 10.1002/stem.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin L, et al. Imaging light responses of retinal ganglion cells in the living mouse eye. J Neurophysiol. 2013 doi: 10.1152/jn.01043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, et al. Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2013;110:9106–9111. doi: 10.1073/pnas.1306912110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- 31.Ugolini G. Advances in viral transneuronal tracing. J Neurosci Methods. 2010;194:2–20. doi: 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nhan HL, Callaway EM. Morphology of superior colliculus- and middle temporal area-projecting neurons in primate primary visual cortex. J Comp Neurol. 2012;520:52–80. doi: 10.1002/cne.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mebatsion T, Konig M, Conzelmann KK. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–951. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 35.Etessami R, et al. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J Gen Virol. 2000;81:2147–2153. doi: 10.1099/0022-1317-81-9-2147. [DOI] [PubMed] [Google Scholar]
- 36.Weible AP, et al. Transgenic targeting of recombinant rabies virus reveals monosynaptic connectivity of specific neurons. J Neurosci. 2010;30:16509–16513. doi: 10.1523/JNEUROSCI.2442-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mank M, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5:805–811. doi: 10.1038/nmeth.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohkura M, Sasaki T, Kobayashi C, Ikegaya Y, Nakai J. An improved genetically encoded red fluorescent Ca2+ indicator for detecting optically evoked action potentials. PLoS One. 2012;7:e39933. doi: 10.1371/journal.pone.0039933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akemann W, Mutoh H, Perron A, Rossier J, Knopfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods. 2010;7:643–649. doi: 10.1038/nmeth.1479. [DOI] [PubMed] [Google Scholar]
- 41.Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 2012;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marvin JS, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan EM, et al. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnus CJ, et al. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 48.Gradinaru V, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnard RJ, Elleder D, Young JA. Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion. Virology. 2006;344:25–29. doi: 10.1016/j.virol.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Seidler B, et al. A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc Natl Acad Sci U S A. 2008;105:10137–10142. doi: 10.1073/pnas.0800487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taniguchi H, et al. A resource of cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnell MJ, McGettigan JP, Wirblich C, Papaneri A. The cell biology of rabies virus: using stealth to reach the brain. Nat Rev Microbiol. 2010;8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- 58.Finke S, Mueller-Waldeck R, Conzelmann KK. Rabies virus matrix protein regulates the balance of virus transcription and replication. J Gen Virol. 2003;84:1613–1621. doi: 10.1099/vir.0.19128-0. [DOI] [PubMed] [Google Scholar]
- 59.Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez F, Boue S, Izpisua Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet. 2011;12:231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 61.Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wickersham IR, Sullivan HA, Seung HS. Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons. Nat Protoc. 2010;5:595–606. doi: 10.1038/nprot.2009.248. [DOI] [PubMed] [Google Scholar]
- 63.Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- 64.Schnell MJ, et al. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci U S A. 2000;97:3544–3549. doi: 10.1073/pnas.050589197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mebatsion T, Conzelmann KK. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci U S A. 1996;93:11366–11370. doi: 10.1073/pnas.93.21.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato S, et al. Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein. Hum Gene Ther. 2007;18:1141–1151. doi: 10.1089/hum.2007.082. [DOI] [PubMed] [Google Scholar]
- 67.Mazarakis ND, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 68.Lafon M. Rabies virus receptors. J Neurovirol. 2005;11:82–87. doi: 10.1080/13550280590900427. [DOI] [PubMed] [Google Scholar]
- 69.Ryan MD, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. Embo J. 1994;13:928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vagner S, Galy B, Pyronnet S. Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2001;2:893–898. doi: 10.1093/embo-reports/kve208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasegawa K, Cowan AB, Nakatsuji N, Suemori H. Efficient multicistronic expression of a transgene in human embryonic stem cells. Stem Cells. 2007;25:1707–1712. doi: 10.1634/stemcells.2006-0813. [DOI] [PubMed] [Google Scholar]
- 72.Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther. 2000;1:376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- 73.Le Mercier P, Jacob Y, Tanner K, Tordo N. A novel expression cassette of lyssavirus shows that the distantly related Mokola virus can rescue a defective rabies virus genome. J Virol. 2002;76:2024–2027. doi: 10.1128/JVI.76.4.2024-2027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Symons RH. Small catalytic RNAs. Annual review of biochemistry. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- 75.Gaudin Y, Tuffereau C, Segretain D, Knossow M, Flamand A. Reversible conformational changes and fusion activity of rabies virus glycoprotein. J Virol. 1991;65:4853–4859. doi: 10.1128/jvi.65.9.4853-4859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaudin Y, Ruigrok RW, Knossow M, Flamand A. Low-pH conformational changes of rabies virus glycoprotein and their role in membrane fusion. J Virol. 1993;67:1365–1372. doi: 10.1128/jvi.67.3.1365-1372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]